Abstract
The mind involves the whole body, and two-way communication between the brain and the cardiovascular, immune, and other systems via neural and endocrine mechanisms. Stress is a condition of the mind-body interaction, and a factor in the expression of disease that differs among individuals. It is not just the dramatic stressful events that exact their toll, but rather the many events of daily life that elevate and sustain activities of physiological systems and cause sleep deprivation, overeating, and other health-damaging behaviors, producing the feeling of being “stressed out.” Over time, this results in wear and tear on the body, which is called “allostatic load,” and it reflects not only the impact of life experiences but also of genetic load, individual lifestyle habits reflecting items such as diet, exercise, and substance abuse, and developmental experiences that set life-long patterns of behavior and physiological reactivity. Hormones associated with stress and allostatic load protect the body in the short run and promote adaptation by the process known as allostasis, but in the long run allostatic load causes changes in the body that can lead to disease. The brain is the key organ of stress, allostasis, and allostatic load, because it determines what is threatening and therefore stressful, and also determines the physiological and behavioral responses. Brain regions such as the hippocampus, amygdala, and prefrontal cortex respond to acute and chronic stress by undergoing structural remodeling, which alters behavioral and physiological responses. Translational studies in humans with structural and functional imaging reveal smaller hippocampal volume in stress-related conditions, such as mild cognitive impairment in aging and prolonged major depressive illness, as well as in individuals with low self-esteem. Alterations in amygdala and prefrontal cortex are also reported. Besides Pharmaceuticals, approaches to alleviate chronic stress and reduce allostatic load and the incidence of diseases of modern life include lifestyle change, and policies of government and business that would improve the ability of individuals to reduce their own chronic stress burden.
Keywords: stress, stress hormone, allostasis, hippocampus, amygdala, prefrontal cortex
Abstract
La mente se extiende a todo el cuerpo y a la comunicación bilateral entre el cerebro y los aparatos cardiovascular, sistema inmunitario y otros a través de mecanismos neurales y endocrinos. El estrés es un estado de interacción entre la mente y el cuerpo e interviene en la expresión diferente de la enfermedad entre las personas. No son únicamente los sucesos estresantes más llamativos los que cuestan más, sino más bien los múltiples episodios de la vida cotidiana que elevan y sostienen la actividad de los sistemas fisiológicos y determinan una privación del sueño, sobrealimentación y otras conductas dañinas para la salud que producen una sensación de “agotamiento por estrés”. Con el tiempo, el organismo desgasta por la llamada “carga alostática”, que refleja no sólo el impacto de las experiencias vitales sino también de la carga genética, de los hábitos personales de vida -que traducen aspectos como la alimentación, el ejercicio y el abuso de sustancias- y de las experiencias del desarrollo que fijan los patrones duraderos de conducta y reactividad fisiológica. Las hormonas asociadas al estrés y a la carga alostática protegen el organismo a corto plazo y fomentan la adaptación a través de un proceso llamado alostasia pero, a la larga, la carga alostá-sica determina cambios corporales que pueden causar enfermedades. El cerebro es el órgano destinatario del estrés, la alostasia y la carga alostática, porque decide qué información resulta amenazadora y, en consecuencia, estresante y determina, además, las respuestas fisiológicas y conductuales. Las regiones cerebrales, como el hipocampo, la amígdala (núcleo amigdalino) y la corteza prefrontal, responden al estrés agudo y crónico sometiéndose a una remodelación estructural que modifica las respuestas comportamen-talesl y fisiológicas. Los estudios translacionales de imágenes estructurales y funcionales de seres humanos revelan un volumen hipocámpico más reducido en los estados de estrés, por ejemplo una ligera alteración cognitiva con el envejecimiento y el trastorno depresivo mayor prolongado, asi como entre las personas que se subestiman. Se han descrito también alteraciones de la amígdala y de la corteza prefrontal. Además del enfoque farmacéutico, las medidas para aliviar el estrés crónico y reducir la carga alostásica así como la incidencia de las enfermedades de la vida moderna se basan en cambios en los hábitos de vida y políticas gubernamentales y empresariales para mejorar la capacidad del individuo y reducir la carga propia y crónica del estrés.
Abstract
L'esprit implique le corps entier, et il existe une intercommunication entre le cerveau, les systèmes cardio-vasculaire, immunitaire et d'autres, par des mécanismes neuraux et endocriniens. Le stress est la manifestation d'une interaction entre l'esprit et le corps et un facteur d'expression de maladies qui diffère selon les individus. Les événements de la vie quotidienne, plus que les stress aigus ou intenses de la vie, élèvent et entretiennent les niveaux d'activité des systèmes physiologiques, entraînant privation de sommeil, boulimie et autres comportements néfastes pour la santé qui donnent le sentiment d'être «dépassé par les événements». Avec le temps, ceci entraîne une usure du corps appelée «charge allostatique», qui reflète non seulement l'impact des expériences de la vie mais aussi la charge génétique, les habitudes de vie quotidienne comme le régime, l'exercice, l'usage de drogues et le vécu au cours du développement qui mettent en place tout au long de la vie des schémas de comportement et de réactivité physiologique. Les hormones associées au stress et à la charge allostatique protègent le corps à court terme et favorisent l'adaptation par un procédé nommé allostase mais à long terme, les modifications somatiques dues à la charge allostatique peuvent entraîner l'apparition d'une maladie. Le cerveau est l'organe clé du stress, de l'allostase et de la charge allostatique car il détermine ce qui est menaçant et donc stressant ainsi que les réponses physiologiques et comportementales. Les régions cérébrales comme l'hippocampe, l'amygdale et le cortex préfrontal répondent au stress aigu et chronique par un remodelage structural qui modifie les réponses physiologiques et comportementales. Des études réalisées chez l'homme par imagerie structurale et fonctionnelle montrent que le volume de l'hippocampe est diminué dans des situations de stress comme il l'est dans les déficits cognitifs légers dus à l'âge, les dépressions majeures prolongées et les individus qui se sous-estiment Des altérations de l'amygdale et du cortex préfrontal sont aussi rapportées. Outre les traitements pharmacologiques, les modifications du mode de vie, les politiques gouvernementales et de travail pouvant améliorer la capacité individuelle à réduire la charge de stress chronique de chacun, sont autant d'approches visant à alléger le stress chronique et réduire la charge allostatique et l'incidence des maladies liées à la vie moderne.
“Stress” Is a commonly used word that generally refers to experiences that cause feelings of anxiety and frustration because they push us beyond our ability to successfully cope. “There Is so much to do and so little time!” Is a common expression. Besides time pressures and daily hassles at work and home, there are stressors related to economic insecurity, poor health, and interpersonal conflict. More rarely, there are situations that are life-threatening - accidents, natural disasters, violence - and these evoke the classical "fight or flight" response. In contrast to daily hassles, these stressors are acute, and yet they also usually lead to chronic stress in the aftermath of the tragic event.
The most common stressors are therefore ones that operate chronically, often at a low level, and that cause us to behave in certain ways. For example, being “stressed out” may cause us to be anxious and or depressed, to lose sleep at night, to eat comfort foods and take in more calories than our bodies need, and to smoke or drink alcohol excessively Being stressed out may also cause us to neglect to see friends, or to take time off or engage in regular physical activity as we, for example, sit at a computer and try to get out from under the burden of too much to do. Often we are tempted to take medications - anxiolytics, sleep-promoting agents - to help us cope, and, with time, our bodies may increase in weight...
The brain is the organ that decides what is stressful and determines the behavioral and physiological responses, whether health-promoting or health-damaging. And the brain is a biological organ that changes under acute and chronic stress, and directs many systems of the body-metabolic, cardiovascular, immune - that are involved in the short- and long-term consequences of being stressed out. What does chronic stress do to the body and brain? This review summarizes some of the current information, placing emphasis on how the stress hormones can play both protective and damaging roles in brain and body, depending on how tightly their release is regulated, and it discusses some of the approaches for dealing with stress in our complex world.
Definition of stress, allostasis, and allostatic load
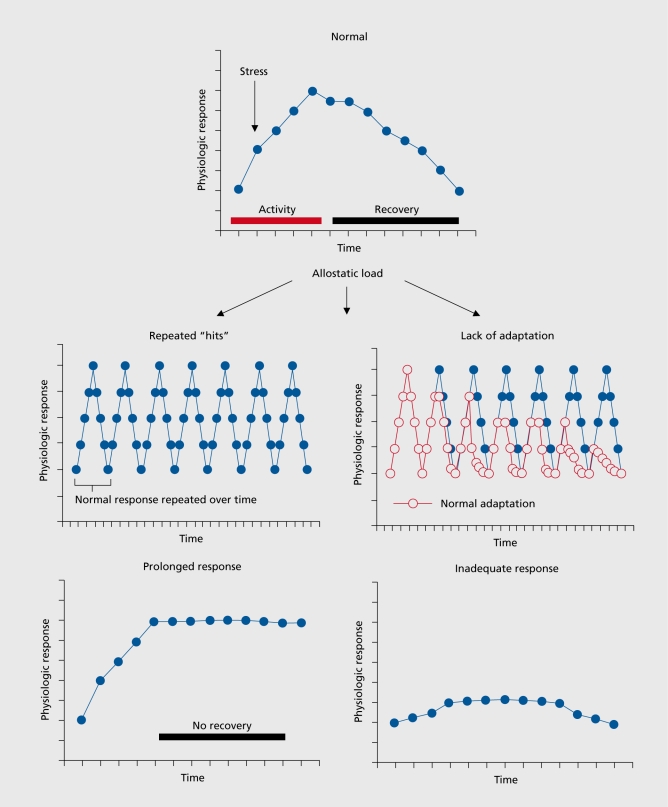
“Stress” is an ambiguous term, and has connotations that make it less useful in understanding how the body handles the events that are stressful. Insight into these processes can lead to a better understanding of how best to intervene, a topic that will be discussed at the end of this article. There are two sides to this story1: on the one hand, the body responds to almost any event or challenge by releasing chemical mediators -eg, catecholamines that increase heart rate and blood pressure -that help us cope with the situation; on the other hand, chronic elevation of these same mediators -eg, chronically increased heart rate and blood pressure -produce chronic wear and tear on the cardiovascular system that can result, over time, in disorders such as strokes and heart attacks. For this reason, the term “allostasis” was introduced by Sterling and Eyer2 to refer to the active process by which the body responds to daily events and maintains homeostasis (allostasis literally means “achieving stability through change”). Because chronically increased allostasis can lead to disease, we introduced the term “allostatic load or overload” to refer to the wear and tear that results from either too much stress or from inefficient management of allostasis, eg, not turning off the response when it is no longer needed.1,3,4 Other forms of allostatic load are summarized in Figure 1, and involve not turning on an adequate response in the first place, or not habituating to the recurrence of the same stressor, and thus dampening the allostatic response.
Figure 1. Four types of allostatic load. The top panel illustrates the normal allostatic response, in which a response is initiated by a stressor, sustained for an appropriate interval, and then turned off. The remaining panels illustrate four conditions that lead to allostatic load: top left- repeated “hits” from multiple stressors; top right- lack of adaptation; bottom left- prolonged response due to delayed shut down; and bottom right - inadequate response that leads to compensatory hyperactivity of other mediators (eg, inadequate secretion of glucocorticoid, resulting in increased levels of cytokines that are normally counter-regulated by glucocorticoids). Reproduced from reference 1 ; McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998; 338:171-179. Copyright © Massachusetts Medical Society 1998.
Protection and damage as the two sides of the response to stressors
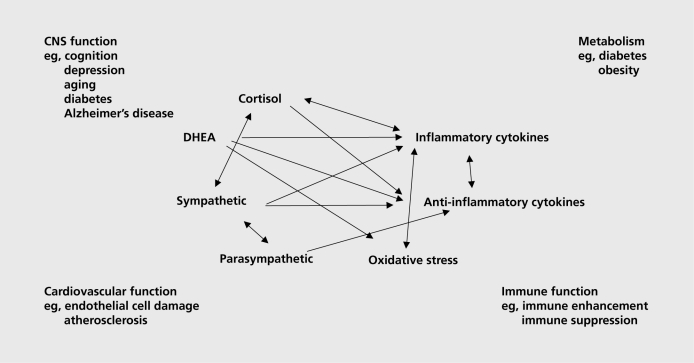
Thus, protection and damage are the two contrasting sides of the physiology involved in defending the body against the challenges of daily life, whether or not we call them “stressors.” Besides adrenaline and noradrenaline, there are many mediators that participate in allostasis, and they are linked together in a network of regulation that is nonlinear (Figure 2), meaning that each mediator has the ability to regulate the activity of the other mediators, sometimes in a biphasic manner.
Figure 2. Nonlinear network of mediators of allostasis involved in the stress response. Arrows indicate that each system regulates the others in a reciprocal manner, creating a nonlinear network. Moreover, there are multiple pathways for regulation- eg, inflammatory cytokine production is negatively regulated via anti-inflammatory cytokines as well as via parasympathetic and glucocorticoid pathways, whereas sympathetic activity increases inflammatory cytokine production. Parasympathetic activity in turn, restrains sympathetic activity DHEA, dehydroepiandrosterone.
Glucocorticoids produced by the adrenal cortex in response to acetylcholine (ACTH) from the pituitary gland is the other major “stress hormone.” Pro- and anti-inflammatory cytokines are produced by many cells in the body; they regulate each other and are, in turn, regulated by glucocorticoids and catecholamines. Whereas catecholamines can increase proinflammatory cytokine production, glucocorticoids are known to inhibit this production.5 Yet, there are exceptions - proinflammatory effects of glucocorticoids that depend on dose and cell or tissue type.6,7 The parasympathetic nervous system also plays an important regulatory role in this nonlinear network of allostasis, since it generally opposes the sympathetic nervous system and, for example, slows the heart and also has anti-inflammatory effects.8,9
What this nonlinearity means is that when any one mediator is increased or decreased, there are compensatory changes in the other mediators that depend on time course and level of change of each of the mediators. Unfortunately, we cannot measure all components of this system simultaneously, and must rely on measurements of only a few of them in any one study. Yet the nonlinearity must be kept in mind in interpreting the results.
Stress in the natural world
The operation of allostasis in the natural world provides some insight into how animals use this response to their own benefit or for the benefit of the species. As an example of allostasis, in spring, a sudden snowstorm causes stress to birds and disrupts mating, and stress hormones are pivotal in directing the birds to suspend reproduction, to find a source of food, and to relocate to a better mating site, or at least to delay reproduction until the weather improves.10 As an example of allostatic load, bears preparing to hibernate for the winter eat large quantities of food and put on body fat to act as an energy source during the winter.11 This accumulation of fat is used, then, to survive the winter and provide food for gestation of young; this is in contrast to the fat accumulation that occurs in bears that are captive in zoos and eating too much, partially out of boredom, while not exercising.4 The accumulation of fat under these latter conditions can be called “allostatic overload,” referring to a condition that is associated with pathophysiology. However, allostatic overload can also have a useful purpose for the preservation of the species, such as In migrating salmon or the marsupial mouse, which die of excessive stress after mating- the stress, and allostatic load, being caused for salmon, In part, by the migration up the rapidly flowing rivers, but also because of physiological changes that represent accelerated aging.12-14 The result is freeing up food and other resources for the next generation. In the case of the marsupial mouse, it is only the males that die after mating, due apparently to a response to mating that reduces the binding protein, corticosteroid-binding globulin (CBG), for glucocorticoids and renders them much more active throughout the body.15
Being “stressed out”, especially sleep deprivation and its consequences
The common experience of being “stressed out” has as its core the elevation of some of the key systems that lead to allostatic load- Cortisol, sympathetic activity, and proinflammatory cytokines, with a decline in parasympathetic activity. Nowhere is this better illustrated than for sleep deprivation, which is a frequent result of being “stressed out.” Sleep deprivation produces an allostatic overload that can have deleterious consequences.
Sleep restriction to 4 hours of sleep per night increases blood pressure, decreases parasympathetic tone, increases evening Cortisol and insulin levels, and promotes increased appetite, possibly through the elevation of ghrelin, a proappetitive hormone, and decreased levels of leptin.16-18 Proinflammatory cytokine levels are increased, along with performance in tests of psychomotor vigilance, and this has been reported to result from a modest sleep restriction to 6 hours per night.19 Reduced sleep duration was reported to be associated with increased body mass and obesity in the NHANES study20
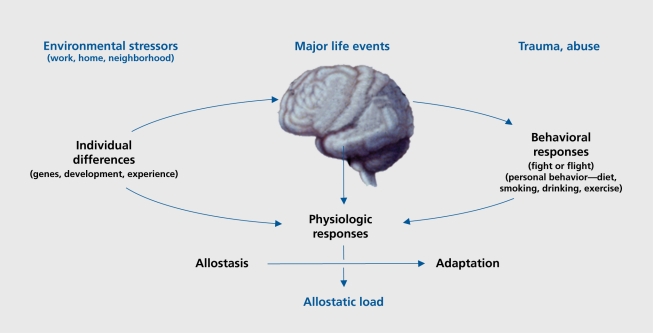
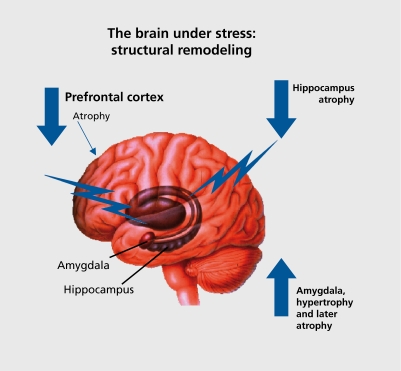
Sleep deprivation also causes cognitive impairment. The brain is the master regulator of the neuroendocrine, autonomic, and immune systems, along with behaviors that contribute to unhealthy or health lifestyles, which, in turn, influence the physiological processes of allostasis (Figure 3).2 Alterations in brain function by chronic stress can, therefore, have direct and indirect effects on the cumulative allostatic overload. Allostatic overload resulting from chronic stress in animal models causes atrophy of neurons in the hippocampus and prefrontal cortex, brain regions involved in memory, selective attention, and executive function, and causes hypertrophy of neurons in the amygdala, a brain region involved in fear and anxiety, as well as aggression.21 Thus, the ability to learn and remember and make decisions may be compromised by chronic stress, and may be accompanied by increased levels of anxiety and aggression.
Figure 3. Central role of the brain in allostasis and the behavioral and physiological response to stressors. Reproduced from reference 1: McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171-179. Copyright © Massachusetts Medical Society 1998.
Although sleep deprivation has not yet been studied In terms of all these aspects, there Is Increasing evidence, not only for cognitive impairment resulting from sleep restriction, but also for altered levels of cytokines, oxidative stress markers, glycogen levels, and structural changes in the form of reduced dentate gyrus neurogenesis.
With respect to proinflammatory cytokines, IL-β messenger ribonucleic acid (mRNA) levels in brain are reported to increase following sleep deprivation by gen tie handling and to be higher in daytime (during the normal sleep period in rodents) than in darkness (during the normal activity time for rodents).22 Closely related to inflammatory processes through the actions of reduced nicotinamide adenine nucleotide phosphate (NADPH) oxidase23,24 is oxidative stress involving the generation of free radicals. Sleep deprivation in mice for 72 hours by the “flowerpot” or platform method has been reported to increase oxidative stress in hippocampus, as measured by increased lipid peroxidation and increased ratios of oxidized to reduced glutathione.25
Another noteworthy effect of sleep deprivation is regulation of the level of glycogen, found predominantly in white matter, which is reported to decrease by as much as 40% in rats deprived of sleep for 24 hours by novelty and gentle handling, and reversed by recovery sleep.26,27 It is noteworthy that glycogen in astrocytes is able to sustain axon function during glucose deprivation in central nervous system (CNS) white matter.28
Sleep deprivation in rats using a treadmill for 96 hours has been reported to decrease proliferation of cells in the dentate gyrus of the hippocampal formation by as much as 50 %.29 A similar effect has also been reported by keeping rats in a slowly rotating drum, but here again, there is a question of how much physical activity and physical stress may have contributed to the suppression of cell proliferation.30 Nevertheless, sleep restriction by novelty exposure, a more subtle method, prevented the increased survival of new dentate gyrus neurons promoted by spatial training in a Morris water maze.31
Indeed, with respect to memory and cognitive performance, there are numerous reports of impairments following sleep deprivation. For example, sleep deprivation by the platform (or flowerpot) method resulted in impaired retention of passive avoidance memory, a context-dependent fear memory task,25 as well as impaired performance of spatial memory in the Morris water maze32 and a reduction in longterm potentiation in the CA1 region of the hippocampus.33
Sleep deprivation by gentle stimulation or novelty in the aftermath of contextual fear conditioning has been reported to impair memory consolidation.34 Moreover, a 6-hour period of total sleep deprivation by novelty exposure impaired acquisition of a spatial task in the Morris water maze.35 Furthermore, a 4-hour period of sleep deprivation by gentle stimulation impaired the latephase long-term potentiation (LTP) in the dentate gyrus 48 hours later, but had the opposite effect of enhancing latephase LTP in the prefrontal cortex.36
Sleep deprivation has also been associated with increases in fighting behavior after deprivation of rapid eye movement (REM) sleep;37 there is also a report of increased aggression in the form of muricide after phencyclidine administration after sleep deprivation.38 These findings may be related to the finding of increased aggression among cagemates in rats subjected to 21 days of 6 hours per day of chronic restraint stress during the resting period when some sleep deprivation may occur.39 Interestingly, a 12-hour sleep deprivation that is applied by using a slowly rotating drum which minimizes physical stress, but does produce locomotor activity, reversed the decreased open-field behavior induced by a single social defeat.40
Key role of the brain in response to stress
The brain is the key organ of the stress response because it determines what is threatening, and therefore, stressful, and also controls the behavioral and physiological responses that have been discussed earlier in this article (see Figure 3). There are enormous individual differences in the response to stress, based upon the experience of the individual early in life and in adult life. Obviously, positive or negative experiences in school, at work, or in romantic and family interpersonal relationships can bias an individual towards either a positive or negative response in a new situation. For example, someone who has been treated badly in a job by a domineering and abusive supervisor and/or has been fired will approach a new job situation quite differently than someone who has had positive experiences in employment.
Early life experiences perhaps carry an even greater weight in terms of how an individual reacts to new situations. Early life physical and sexual abuse imposes a life- long burden of behavioral and pathophysiological problems.41,42 Cold and uncaring families produce long-lasting emotional problems in children.43 Some of these effects are seen on brain structure and function, and in the risk for later depression and post-traumatic stress disorder (PTSD).44-46
Animal models have been useful in providing insights into behavioral and physiological mechanisms. Early life maternal care in rodents is a powerful determinant of life-long emotional reactivity and stress hormone reactivity, and increases in both are associated with earlier cognitive decline and a shorter lifespan.47,48 Effects of early maternal care are transmitted across generations by the subsequent behavior of the female offspring as they become mothers, and methylation of deoxyribonucleic acid (DNA) on key genes appears to play a role in this epigenetic transmission.49 Furthermore, in rodents, abuse of the young is associated with an attachment, rather than an avoidance, of the abusive mother, an effect that increases the chances that the infant can continue to obtain food and other support until weaning.50 Moreover, other conditions that affect the rearing process can also affect emotionality in offspring. For example, uncertainty in the food supply for rhesus monkey mothers leads to increased emotionality in offspring and possibly an earlier onset of obesity and diabetes. 51
So far, we have emphasized the important role of the environment and experiences of individuals in the health outcomes, but clearly genetic differences also play an important role. Different alleles of commonly occurring genes determine how individuals will respond to experiences. For example, the short form of the serotonin transporter is associated with a number of conditions such as alcoholism, and individuals who have this allele are more vulnerable to respond to stressful experiences by developing depressive illness.52 In childhood, individuals with an allele of the monoamine oxidase A gene are more vulnerable to abuse in childhood and more likely to them- selves become abusers and to show antisocial behaviors compared with individuals with another commonly occurring allele.53 Yet another example is the consequence of having the Val66Met allele of the brain-derived neurotrophic factor (BDNF) gene on hippocampal volume, memory, and mood disorders.54-57
The brain as a target of stress
The hippocampus
One of the ways that stress hormones modulate function within the brain is by changing the structure of neurons.
The hippocampus is one of the most sensitive and malleable regions of the brain, and is also very important in cognitive function. Within the hippocampus, the input from the entorhinal cortex to the dentate gyrus is ramified by the connections between the dentate gyrus and the CA3 pyramidal neurons. One granule neuron innervates, on the average, 12 CA3 neurons, and each CA3 neuron innervates, on the average, 50 other CA3 neurons via axon collaterals, as well as 25 inhibitory cells via other axon collaterals. The net result is a 600-fold amplification of excitation, as well as a 300-fold amplification of inhibition, that provides some degree of control of the system.58
As to why this type of circuitry exists, the dentate gyrus (DG)-CA3 system is believed to play a role in the memory of sequences of events, although longterm storage of memory occurs in other brain regions.59 However, because the DG-CA3 system is so delicately balanced in its function and vulnerability to damage, there is also adaptive structural plasticity, in that new neurons continue to be produced in the dentate gyrus throughout adult life, and CA3 pyramidal cells undergo a reversible remodeling of their dendrites in conditions such as hibernation and chronic stress.58,60,61 The role of this plasticity may be to protect against permanent damage. As a result, the hippocampus undergoes a number of adaptive changes in response to acute and chronic stress.
One type of change involves replacement of neurons. The subgranular layer of the dentate gyrus contains cells that have some properties of astrocytes (eg, expression of glial fibrillary acidic protein) and which give rise to granule neurons.62,63 After BrdU administration to label DNA of dividing cells, these newly born cells appear as clusters in the inner part of the granule cell layer, where a substantial number of them will go on to differentiate into granule neurons within as little as 7 days. In the adult rat, 9000 new neurons are born per day, and survive with a half -life of 28 days.64 There are many hormonal, neurochemical, and behavioral modulators of neurogenesis and cell survival in the dentate gyrus including estradiol, insulin-like growth factor (IGF)-1, antidepressants, voluntary exercise, and hippocampal-dependent learning.65-67 With respect to stress, certain types of acute stress and many chronic stressors suppress neurogenesis or cell survival in the dentate gyrus, and the mediators of these inhibitory effects include excitatory amino acids acting via N-methyl-D-aspartate (NMDA) receptors and endogenous opioids.68
Another form of structural plasticity is the remodeling of dendrites in the hippocampus. Chronic restraint stress causes retraction and simplification of dendrites in the CA3 region of the hippocampus.58,69 Such dendritic reorganization is found in both dominant and subordinate rats undergoing adaptation of psychosocial stress in the visible burrow system, and it is independent of adrenal size.70
What this result emphasizes is that it is not adrenal size or presumed amount of physiological stress per se that determines dendritic remodeling, but a complex set of other factors that modulate neuronal structure. Indeed, in species of mammals that hibernate, dendritic remodeling is a reversible process, and occurs within hours of the onset of hibernation in European hamsters and ground squirrels, and it is also reversible within hours of wakening of the animals from torpor.60,61,71 This implies that reorganization of the cytoskeleton is taking place rapidly and reversibly, and that changes in dendrite length and branching are not “damage,” but a form of structural plasticity.
Regarding the mechanism of structural remodeling, adrenal steroids are important mediators of remodeling of hippocampal neurons during repeated stress, and exogenous adrenal steroids can also cause remodeling in the absence of an external stressor. The role of adrenal steroids involve many interactions with neurochemical systems in the hippocampus, including serotonin, γ-aminobutyric acid (GABA), and excitatory amino acids.21,58 Probably the most important interactions are those with excitatory amino acids such as glutamate. Excitatory amino acids released by the mossy fiber pathway play a key role in the remodeling of the CA3 region of the hippocampus, and regulation of glutamate release by adrenal steroids may play an important role.58
Among the consequences of restraint stress is the elevation of extracellular glutamate levels, leading to induction of glial glutamate transporters, as well as increased activation of the nuclear transcription factor, phosphoCREB.72 Moreover, 21d chronic restraint stress (CRS) leads to depletion of clear vesicles from mossy fiber terminals and increased expression of presynaptic proteins involved in vesicle release.73-75 Taken together with the fact that vesicles that remain in the mossy fiber terminal are near active synaptic zones and that there are more mitochondria in the terminals of stressed rats, this suggests that CRS increases the release of glutamate.73
Extracellular molecules play a role in remodeling. Neural cell adhesion molecule (NCAM) and its polysialated-NCAM (PSA-NCAM), as well as L1 are expressed in the dentate gyrus and CA3 region, and the expression of both NCAM, L1, and PSA-NCAM are regulated by 21d CRS.76
Tissue plasminogen activator (tPA, see below) is an extracellular protease and signaling molecule that is released with neural activity and is required for chronic stress-induced loss of spines and NMDA receptor subunits on CA1 neurons.77 Within the neuronal cytoskeleton, the remodeling of hippocampal neurons by chronic stress and hibernation alters the acetylation of microtubule subunits that is consistent with a more stable cytoskeleton,78 and alters microtubule associated proteins, including the phosphorylation of a soluble form of tau, which is increased in hibernation and reversed when hibernation is terminated.71
Neurotrophic factors also play a role in dendritic branching and length in that BDNF +/- mice show a less branched dendritic tree and do not show a further reduction of CA3 dendrite length with chronic stress, whereas wild-type mice show reduced dendritic branching (Magarinos and McEwen, unpublished data). However, there is contradictory information thus far concerning whether CRS reduces BDNF mRNA levels, some reporting a decrease79 and other studies reporting no change.80,81
This may reflect the balance of two opposing forces, namely, that stress triggers increased BDNF synthesis to replace depletion of BDNF caused by stress.82 BDNF and corticosteroids appear to oppose each other - with BDNF reversing reduced excitability in hippocampal neurons induced by stress levels of corticosterone.83
Corticotropin-releasing factor (CRF) is a key mediator of many aspects related to stress.84 CRF in the paraventricular nucleus regulates ACTH release from the anterior pituitary gland, whereas CRF in the central amygdala is involved in control of behavioral and autonomic responses to stress, including the release of tPA that is an essential part of stress-induced anxiety and structural plasticity in the medial amygdala.85 CRF in the hippocampus is expressed in a subset of GABA neurons (Cajal-Retzius cells) in the developing hippocampus, and early life stress produces a delayed effect that reduces cognitive function and the number of CA3 neurons, as well as decreased branching of hippocampal pyramidal neurons.86,87 Indeed corticotropin-releasing hormone (CRH) inhibits dendritic branching in hippocampal cultures in vitro.88
Prefrontal cortex and amygdala
Repeated stress also causes changes in other brain regions, such as the prefrontal cortex and amygdala. Repeated stress causes dendritic shortening in medial prefrontal cortex.89-95 but produces dendritic growth in neurons in amygdala,95 as well as in orbitofrontal cortex.96 Along with many other brain regions, the amygdala and prefrontal cortex also contain adrenal steroid receptors; however, the role of adrenal steroids, excitatory amino acids, and other mediators has not yet been studied in these brain regions. Nevertheless, in the amygdala, there is some evidence regarding mechanism, in that tPA is required for acute stress to activate not only indices of structural plasticity but also to enhance anxiety.97 These effects occur in the medial and central amygdala and not in basolateral amygdala, and the release of CRH acting via CRH1 receptors appears to be responsible.85
Acute stress induces spine synapses in the CA1 region of hippocampus98 and both acute and chronic stress also increases spine synapse formation in amygdala,95-99 but chronic stress decreases it in hippocampus.77 Moreover, chronic stress for 21 days or longer impairs hippocampal-dependent cognitive function58 and enhances amygdala-dependent unlearned fear and fear conditioning,100 which are consistent with the opposite effects of stress on hippocampal and amygdala structure. Chronic stress also increases aggression between animals living in the same cage, and this is likely to reflect another aspect of hyperactivity of the amygdala.39 Behavioral correlates of remodeling in the prefrontal cortex include impairment in attention set shifting, possibly reflecting structural remodeling in the medial prefrontal cortex.95
Translation to the human brain
Much of the impetus for studying the effects of stress on the structure of the human brain has come from the animal studies summarized thus far. Although there is very little evidence regarding the effects of ordinary life stressors on brain structure, there are indications from functional imaging of individuals undergoing ordinary stressors, such as counting backwards, that there are lasting changes in neural activity.101 Moreover, the study of depressive illness and anxiety disorders has also provided some insights. Life events are known to precipitate depressive illness in individuals with certain genetic predispositions.52,102,103 Moreover, brain regions such as the hippocampus, amygdala, and prefrontal cortex show altered patterns of activity in positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), and also demonstrate changes in volume of these structures with recurrent depression: decreased volume of hippocampus and pre frontal cortex and amygdala (Figure 4.).104-106 Interestingly, amygdala volume has been reported to increase in the first episode of depression, whereas hippocampal volume is not decreased.107,108 It has been known for some time that stress hormones, such as Cortisol, are involved in psychopathology, reflecting emotional arousal and psychic disorganization rather than the specific disorder per se.109 We now know that adrenocortical hormones enter the brain and produce a wide range of effects upon it.
Figure 4. Brain regions that are involved in perception and response ress, and which show structural remodeling as a result of stress.
In Cushing's disease, there are depressive symptoms that can be relieved by surgical correction of the hypercortisolemia.110,111 Both major depression and Cushing's disease are associated with chronic elevation of Cortisol that results in gradual loss of minerals from bone and abdominal obesity. In major depressive illness, as well as in Cushing's disease, the duration of the illness, and not the age of the subjects, predicts a progressive reduction in volume of the hippocampus, determined by structural magnetic resonance imaging.103,112 Moreover, there are a variety of other anxiety-related disorders, such as PTSD113,114 and borderline personality disorder,115 in which atrophy of the hippocampus has been reported, suggesting that this is a common process reflecting chronic imbalance in the activity of adaptive systems, such as the hypothalamo-pituitary-adrenocortical (HPA) axis, but also including endogenous neurotransmitters, such as glutamate.
Another Important factor In hippocampal volume and function Is glucose regulation. Poor glucose regulation Is associated with smaller hippocampal volume and poorer memory function In Individuals in their 60s and 70s who have “mild cognitive impairment” (MCI),116 and both MCI and type 2, as well as type 1, diabetes are recognized as risk factors for dementia.117-119
Positive affect, self-esteem, and social support
Having a positive outlook on life and good self-esteem appear to have long-lasting health consequences,120 and good social support is also a positive influence on the measures of allostatic load.121 Positive affect, assessed by aggregating momentary experiences throughout a working or leisure day, was found to be associated with lower Cortisol production and higher heart rate variability (showing higher parasympathetic activity), as well as a lower fibrinogen response to a mental stress test.122
On the other hand, poor self-esteem has been shown to cause recurrent increases in Cortisol levels during a repetition of a public speaking challenge in which those individuals with good self-esteem are able to habituate, ie, attenuate their Cortisol response after the first speech.123 Furthermore, poor self-esteem and low internal locus of control have been related to a 12% to 13% smaller volume of the hippocampus, as well as higher Cortisol levels during a mental arithmetic stressor.124,125
Related to both positive affect and self-esteem is the role of friends and social interactions in maintaining a healthy outlook on life. Loneliness, often found in people with low self-esteem, has been associated with larger Cortisol responses to wakening in the morning and higher fibrinogen and natural killer cell responses to a mental stress test, as well as sleep problems.126 On the other hand, having three or more regular social contacts, as opposed to zero to two such contacts, is associated with lower allostatic load scores. 121
Conclusions: what can one do about being stressed out?
If being stressed out has such pervasive effects on the brain as well as the body, what are the ways that Individuals, as well as policymakers in government and business, can act to reduce the negative effects and enhance the ability of the body and brain to deal with stress with minimal consequences? The answers are simple and obvious, but often difficult to achieve.
From the standpoint of the individual, a major goal should be to try to improve sleep quality and quantity, have good social support and a positive outlook on life, maintain a healthy diet, avoid smoking, and have regular moderate physical activity Concerning physical activity, it is not necessary to become an extreme athlete, and seemingly almost any amount of moderate physical activity helps.127,128 Regarding self-esteem, although this is still early in the story, efforts to build self-esteem in individuals might have long-term benefits for physical as well as mental health.
From the standpoint of policy, the goal should be to create incentives at home and in work situations and build community services and opportunities that encourage the development of the beneficial individual lifestyle practices.
As simple as the solutions seem to be, changing behavior and solving problems that cause stress at work and at home is often difficult, and may require professional help on the personal level, or even a change of job or profession. Yet these are important issues because the prevention of later disease is very important for full enjoyment of life, and also to reduce the financial burden on the individual and on society.
Nevertheless, many people often lack the proactive, longterm view of themselves and/or feel that they must maintain a stressful lifestyle and, if they deal with these issues at all, they want to treat their problems with “a pill.” Are there any medications to treat being stressed out? In fact, there are many useful pharmaceutical agents: sleeping pills, anxiolytics, β-blockers, and antidepressants are all drugs that are used to counteract some of the problems associated with being stressed out. Likewise, drugs that reduce oxidative stress or inflammation, block cholesterol synthesis or absorption, and treat insulin resistance or chronic pain can help deal with the metabolic and neurologic consequences of being stressed out. All are valuable to some degree, and yet each one has its side effects and limitations that are based in part on the fact that all of the systems that are dysregulated in allostatic overload are also systems that interact with each other and perform normal functions when properly regulated. Because of the nonlinearity of the systems of allostasis, the consequences of any drug treatment may be either to inhibit the beneficial effects of the systems in question or to perturb other systems that interact with it in a direction that promotes an unwanted side effect. So the best solution would seem to be not to rely solely on such medications and find ways to change lifestyle in a positive direction. Being able to change lifestyle and associated behavior is not just an individual matter, and might become easier with changes via another level of intervention, namely, policies in government and business. The Acheson Report129 from the United Kingdom in 1998 recognized that no public policy should be enacted without considering the implications for health of all citizens. Thus, basic education, housing, taxation, setting of a minimum wage, and policies and programs addressing occupational health and safety and environmental pollution regulations are all likely to affect health via a myriad of mechanisms. At the same time, providing higher-quality food and making it affordable and accessible in poor as well as affluent neighborhoods is necessary for people to eat better, providing they also learn what types of food to eat. Likewise, making neighborhoods safer and more congenial and supportive130 can improve opportunities for positive social interactions and increased recreational physical activity.
However, governmental policies are not the only way to reduce allostatic load. For example, businesses that encourage healthy lifestyle practices among their employees are likely to gain reduced health Insurance costs and possibly a more loyal workforce.131-133 Above all, policymakers and business leaders need to be made aware of their broader Issues of Improving health and preventing disease and the fact that they make economic sense as well as being “the right thing to do.”
Finally, there are programs in existence that combine some of the key elements just described, namely, education, physical activity and social support, along with one other ingredient that is hard to quantify: namely, finding meaning and purpose In life. One such program Is the Experience Corps which takes elderly volunteers and trains them as teachers' assistants for younger children In the neighborhood schools.134 Not only does this program improve the education of the children, It also benefits the elderly volunteers and Improves their physical and mental health.135 This program has now been adopted as a key part of a political campaign for the governorship of the state of Maryland.136 One can only hope that politicians and business leaders will listen to and heed the advice of science, which often Is reinforcing common sense, In helping to address the pervasive problems of stress In our world.
Selected abbreviations and acronyms
- ACTH
acetylcholine
- BDNF
brain-derived neurotrophic factor
- CRS
chronic restraint stress
- CRF
corticotropin-releasing factor
- CRH
corticotropin-releasing hormone
- NCAM
neural cell adhesion molecule
REFERENCES
- 1.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 2.Sterling P., Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. New York, NY: John Wiley & Sons; 1988:629–649. [Google Scholar]
- 3.McEwen BS., Stellar E. Stress and the individual: mechanisms leading to disease. Arch Int Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 4.McEwen BS., Wingfield JC. The concept of allostasis in biology and bio-medicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM., Romero LM., Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 6.Dinkel K., MacPherson A., Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 7.MacPherson A., Dinkel K., Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194:376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Borovikova LV., Ivanova S., Zhang M., et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Thayer JF., Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 10.Wingfield JC., Romero LM. Adrenocortical responses to stress and their modulation in free-living vertebrates. In: Coping with the Environment: Neural and Endocrine Mechanisms. Vol. IV. New York, NY: Oxford University Press. 2000:211–234. [Google Scholar]
- 11.Nelson RA. Protein and fat metabolism in hibernating bears. Fed Proc. 1980;39:2955–2958. [PubMed] [Google Scholar]
- 12.Farrell AP. Coronary arteriosclerosis in salmon: growing old or growing fast? Comp Biochem Physiol. 2002;132:723–735. doi: 10.1016/s1095-6433(02)00126-5. [DOI] [PubMed] [Google Scholar]
- 13.Maule AG., Tripp RA., Kaattari SL., Schreck CB. Stress alters immune function and disease resistance in chinook salmon (Oncorhynchus tshawytscha). J Endocrinol. 1989;120:135–142. doi: 10.1677/joe.0.1200135. [DOI] [PubMed] [Google Scholar]
- 14.Gotz ME., Malz CR., Dirr A., et al. Brain aging phenomena in migrating sockeye salmon Oncorhynchus nerka nerka. J Neural Transm. 2005;112:1177–1199. doi: 10.1007/s00702-004-0257-1. [DOI] [PubMed] [Google Scholar]
- 15.Cockburn A., Lee AK. Marsupial femmes fatales. Natural History. 1988;97:40–47. [Google Scholar]
- 16.Leproult R., Copinschi G., Buxton O., VanCauter E. Sleep loss results in an elevation of Cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 17.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel K., Tasali E., Penev P., Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN., Zoumakis E., Bixler EO., et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrin Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 20.Gangwisch JE., Malaspina D., Boden-AIbala B., Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS., Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsycho-pharmacol. 2004;14:S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Taishi P., Chen Z., Obal F Jr., et al. Sleep-associated changes in interleukin1, mRNA in the brain. J Interferon Cytokine Res. 1998;18:793–798. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- 23.Clark RA., Valente AJ. Nuclear factor kappa B activation by NADPH oxidases. Mech Ageing Dev. 2004;125:799–810. doi: 10.1016/j.mad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Tang J., Liu J., Zhou C., et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 25.Silva RH., Abilio VC., Takatsu AL., et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Kong J., Shepel PN., Holden CP., Mackiewicz M., Pack AI., Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- 28.Wender R., Brown AM., Fern R., Swanson RA., Farrell K., Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman-Marin R., Suntsova N., Stewart DR., Gong H., Szymusiak R., McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549. 2:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roman V., Van der Borght K., Leemburg SA., Van der Zee EA., Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Hairston IS., Little MTM., Scanlon MD., et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 32.Youngblood BD., Zhou J., Smagin GN., Ryan DH., Harris RBS. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 33.Kim EY., Mahmoud GS., Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–167. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 34.Graves LA., Heller EA., Pack AI., Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Z., Peng X., Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Romcy-Pereira R., Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- 37.de Paula HMG., Hoshino K. Correlation between the fighting rates of REM sleep-deprived rats and susceptibility to the 'wild running' of audiogenic seizures. Brain Res. 2002;926:80–85. doi: 10.1016/s0006-8993(01)03306-6. [DOI] [PubMed] [Google Scholar]
- 38.Musty RE., Consroe PF. Phencyclidine produces aggressive behavior in rapid eye movement sleep-deprived rats. Life Sciences. 1982;30:1733–1738. doi: 10.1016/0024-3205(82)90307-1. [DOI] [PubMed] [Google Scholar]
- 39.Wood GE., Young LT., Reagan LP., McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 40.Meerlo P., Overkamp GJF., Benning MA., Koolhaas JM., Van Den Hoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- 41.Felitti VJ., Anda RF., Nordenberg D., et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 42.Heim C., Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 43.Repetti RL., Taylor SE., Seeman TE. Risky families: family social environments and the mental and physical health of offspirng. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 44.Kaufman J., Charney DS. Neurobiological correlates of child abuse. Biol Psychiatry. 1999;45:1235–1236. doi: 10.1016/s0006-3223(99)00064-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman J., Plotsky PM., Nemeroff CB., Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 46.Vermetten E., Schmahl C., Lindner S., Loewenstein RJ., Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis D., Diorio J., Liu D., Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 48.Cavigelli SA., McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver ICG., Cervoni N., Champagne FA., et al. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan RM., Landers M., Yeaman B., Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coplan JD., Smith ELP., Altemus M., et al. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- 52.Caspi A., Sugden K., Moffitt TE., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 53.Caspi A., McClay J., Moffitt TE., et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 54.Szeszko PR., Lipsky R., Mentschel C., et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 55.Hariri AR., Goldberg TE., Mattay VS., et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pezawas L., Verchinski BA., Mattay VS., et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang X., Xu K., Hoberman J., et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- 58.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 59.Lisman JE., Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 60.Popov VI., Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- 61.Popov VI., Bocharova LS., Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 62.Seri B., Garcia-Verdugo JM., McEwen BS., AIvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kempermann G., Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- 64.Cameron HA., McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 65.Czeh B., Michaelis T., Watanabe T., et al. Stress-induced changes in cerebral metabolites, hippocampal volume and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aberg MA., Aberg ND., Hedbacker H., Oscarsson J., Eriksson PS. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trejo JL., Carro E., Torres-AIeman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gould E., McEwen BS., Tanapat P., Galea LAM., Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sousa N., Lukoyanov NV., Madeira MD., Almeida OFX., Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 70.McKittrick CR., Magarinos AM., Blanchard DC., Blanchard RJ., McEwen BS., Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Arendt T., Stieler J., Strijkstra AM., et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wood GE., Young LT., Reagan LP., Chen B., McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magarinos AM., Verdugo Garcia JM., McEwen BS. Chronic restraint stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Y., Bezchlibnyk YB., Sun X., Wang JF., McEwen BS., Young LT. Effects of restraint stress on the expression of proteins involved in synaptic vesicle exocytosis in the hippocampus. Neuroscience. 2006;14:1139–1148. doi: 10.1016/j.neuroscience.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 75.Grillo CA., Piroli GG., Wood GE., Reznikov LR., McEwen BS., Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 77.Pawlak R., Rao BSS., Melchor JP., Chattarji S., McEwen B., Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchi M., Heidbreder C., Crespi F. Cytoskeletal changes in the hippocampus following restraint stress: role of serotonin and microtubules. Synapse. 2003;49:188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- 79.Smith MA., Cizza G. Stress-induced changes in brain-dervied neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol Aging. 1996;17:859–864. doi: 10.1016/s0197-4580(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 80.Kuroda Y., McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, GAP-43 and MAP2 mRNA expression in the rat hippocampus. Mol Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- 81.Isgor C., Kabbaj M., Akil H., Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–468. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 82.Marmigere F., Givalois L., Rage F., Arancibia S., Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- 83.Hansson AC., Sommer WH., Metsis M., Stromberg I., Agnati LF., Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by Exon IV promoter. J Neuroendocrinol. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- 84.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 85.Matys T., Pawlak R., Matys E., Pavlides C., McEwen BS., Strickland S. Tissue plasminogen activator promotes the effects of corticotropin releasing factor on the amygdala and anxiety-like behavior. Proc Natl Acad Sci U S A. 2004;101:16345–16350. doi: 10.1073/pnas.0407355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunson KL., Eghbal-Ahmadi M., Bender R., Chen Y., Baram TZ. Longterm, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brunson KL., Kramar E., Lin B., et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y., Bender RA., Brunson KL., et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA. 2004; 101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Radley JJ., Rocher AB., Miller M., et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 90.Radley JJ., Sisti HM., Hao J., et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 92.Cook SC., Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 93.Brown SM., Henning S., Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;30:1–9. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 94.Kreibich AS., Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vyas A., Mitra R., Rao BSS., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liston C., Miller MM., Goldwater DS., et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melchor JP., Pawlak R., Strickland S. The tissue plasminogen activator plasminogen proteolytic cascade accelerates amyloid-p (Ap) degradation and inhibits Ap-induced neurodegeneration. J. Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitra R., Vyas A., Chattarji S. Contrasting effects of chronic immobilization and unpredictable stress on spine density in the hippocampus and amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conrad CD., Magarinos AM., LeDoux JE., McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 101.Wang J., Rao H., Wetmore GS., et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.KessIer RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 103.KendIer KS. Major depression and the environment: a psychiatric genetic perspective. Pharmacopsychiatry. 1998;31:5–9. doi: 10.1055/s-2007-979287. [DOI] [PubMed] [Google Scholar]
- 104.SheIine Yl., Sanghavi M., Mintun MA., Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drevets WC., Price JL., Simpson JR Jr., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 106.Sheline Yl., Gado MH., Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 107.Frodl T., Meisenzahl EM., Zetzsche T., et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 108.MacQueen GM., Campbell S., McEwen BS., et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sachar EJ., Hellman L., Roffwarg HP., Halpern FS., Fukushima DK., Gallagher TF. Disrupted 24-hour patterns of Cortisol secretion in psychotic depression. Arch Gen Psychiatry. 1973;28:19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- 110.Murphy BEP. Treatment of major depression with steroid suppressive drugs. J Steroid Biochem Mol Biol. 1991;39:239–244. doi: 10.1016/0960-0760(91)90069-h. [DOI] [PubMed] [Google Scholar]
- 111.Starkman MN., Schteingart DE. Neuropsychiatrie manifestations of patients with Cushing's syndrome. Arch Intern Med. 1981;141:215–219. [PubMed] [Google Scholar]
- 112.Starkman MN., Gebarski SS., Berent S., Schteingart DE. Hippocampal formation volume, memory dysfunction, and Cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 113.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiat Reports. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 114.Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- 115.Driessen M., Hermann J., Stahl K., et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 116.Convit A., Wolf OT., Tarshish C., de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ott A., Stolk RP., Hofman A., van Harskamp F., Grobbee DE., Breteler MMB. Association of diabetes mellitus and dementia: the Rotterdam study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- 118.Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of lateonset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 119.de Leon MJ., Convit A., Wolf OT., et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fIuoro-2-deoxy-D-gIucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pressman SD., Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 121.Seeman TE., Singer BH., Ryff CD., Dienberg G., Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Steptoe A., Wardle J., Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci USA. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirschbaum C., Prussner JC., Stone AA., et al. Persistent high Cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Pruessner JC., Baldwin MW., Dedovic K., et al. Self-esteem, locus of control, hippocampal volume, and Cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 125.Pruessner JC., Hellhammer DH., Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Pers Individ Diff. 1999;27:477–489. [Google Scholar]
- 126.Steptoe A., Owen N., Kunz-Ebrecht SR., Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middleaged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 127.Bernadet P. Benefits of physical activity in the prevention of cardiovascular disease. J Cardiovasc Pharmacol. 1995;25(suppl 1):S3–S8. doi: 10.1097/00005344-199525001-00003. [DOI] [PubMed] [Google Scholar]
- 128.Rovio S., Kareholt I., Helkala E-L., et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 129.Acheson SD. Independent Inquiry into Inequalities in Health Report. London: The Stationery Office; 1998 [Google Scholar]
- 130.Sampson RJ., Raudenbush SW., Earls F. Neighborhoods and violent crime: a multilevel study of collective effects. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 131.Whitmer RW., Pelletier KR., Anderson DR., Baase CM., Frost GJ. A wakeup call for corporate America. J Occup Environ Med. 2003;45:916–925. doi: 10.1097/01.jom.0000086280.38338.83. [DOI] [PubMed] [Google Scholar]
- 132.Pelletier KR. A review and analysis of the clinical- and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite: 1998-2000 update. Am J Health Promotion. 2001;16:107–115. doi: 10.4278/0890-1171-16.2.107. [DOI] [PubMed] [Google Scholar]
- 133.Aldana SG. Financial impact of health promotion programs: a comprehensive review of the literature. Am J Health Promotion. 2001;15:296–320. doi: 10.4278/0890-1171-15.5.296. [DOI] [PubMed] [Google Scholar]
- 134.Frick KD., Carlson MC., Glass TA., et al. Modeled cost-effectiveness of the Experience Corps Baltimore based on a pilot randomized trial. J Urban Health Bull NY Acad Med. 2004;81:106–117. doi: 10.1093/jurban/jth097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fried LP., Carlson MC., Freedman M., et al. Asocial model for health promotion for an aging population: initial evidence on the experience corps model. J Urban Health Bull NY Acad Med. 2004;81:64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abbruzzese R. O'Malley and Brown release detailed plan to support Maryland's aging population. Press Release. Jan. 24, 2006 [Google Scholar]