Abstract
The major findings regarding the genetics of stress response and stress-related disorders are: (i) variations in genes involved in the sympathetic system or in the hypothalamic-pituitary-adrenocortical axis are associated with altered stress responses; (ii) genes related to the renin-angiotensin-aldosterone system or inflammation/immune response show associations with cardiovascular disorders; (iii) genes involved in monoaminergic neurotransmitter systems are associated with bipolar disorder and unipolar depression. The vast majority of these association studies followed a conventional hypothesis-driven approach, restricting the gene selection to established candidates. This very conservative approach retarded our understanding of the complex interplay between genetic factors, stress response, and stress-related disorders. Chip-based whole-genome technologies will open up access to new unbiased and statistically efficient approaches that will help to identify new candidate genes, which should be thoroughly validated in clinical and preclinical confirmatory studies. This, together with the use of new text- and information-mining tools, will bring us closer to integrating all the findings into sophisticated models delineating the pathways from genes to stress response and stress-related disorders.
Keywords: stress, cardiovascular disorder, bipolar disorder, unipolar depression, genetics, sympathetic system, hypothalamic-pituitary-adrenocortical axis, renin-angiotensin-aldosterone system
Abstract
Los descuhrimentos principales en la genética de y de los trastornos relacionados con el estrés son: i) las variaciones de los genes implicados en el sistema simpático o en el eje hipotálamo-hipófisis-corteza suprarrenal están asociadas con respuestas alteradas al estrés; ii) los genes relacionados con el sistema renina-angiotensina-aldosterona o la respuesta inflamatoria/inmunitaria están asociados a los trastornos cardiovasculares; iii) los genes implicados en los sistemas neurotransmisores monoami-nérgicos están asociados al trastorno bipolar y a la depresión unipolar. La inmensa mayoría de estos estudios de asociación siguieron un enfoque convencional, impulsado por hipótesis, lo que restringe la selección de genes candidatos conocidos. Este método tan conservador ha retrasado el conocimiento de la interrelación compleja entre los factores genéticos, la respuesta al estrés y los trastornos relacionados con éste. Las tecnologías de chip para el estudio de todo el genoma abrirán las puertas a métodos nuevos, objectivos y eficaces, lo que estadísticamente permitirá identificar nuevos genes candidatos que serán validados minuciosamente en estudios confirmatorios clínicos y preclínicos. Todo ello, sumado al uso de nuevos instrumentos para la explotación de texto e información, nos ayudará a integrar todos los datos dentro de modelos complejos que delimiten las vías desde los genes hasta la respuesta al estrés y los trastornos relacionados con el estrés.
Abstract
Voici les principaux résultats sur la génétique de la réponse au stress et des troubles liés au stress: 1) les variations des gènes impliqués dans le système sympathique ou l'axe hypothalamo-hypophyso-sur-rénalien sont associées à des anomalies de la réponse au stress; 2) les gènes liés au système rénine-angiotensine-aldostérone ou à une la réponse inflammatoire/immune sont associés avec des maladies cardiovascuiaires; 3) les gènes impliqués dans les systèmes de neurotransmission monoaminergiques sont associés aux troubles bipolaires et à la dépression unipolaire. La grande majorité de ces études d'association a suivi une approche conventionnelle hypothético-déductive, limitant donc la sélection des gènes aux candidats établis. Cette approche très conservatrice a retardé notre compréhension des interactions complexes entre les facteurs génétiques, la réponse au stress et les troubles liés au stress. Les technologies de puce à ADN sur le génome entier ouvriront la voie à de nouvelles approches non biaisées et statistiquement efficaces qui permettront d'identifier de nouveaux gènes candidats. Ces derniers devront être minutieusement validés dans des études cliniques et précliniques de confirmation. Ces technologies, associées à de nouveaux outils d'analyse des textes et des informations, nous permettront d'intégrer plus facilement tous les résultats dans des modèles sophistiqués précisant les voies qui vont des gènes à la réponse au stress et aux troubles liés au stress.
Recent advances In molecular genetics have stimulated basic and clinical research, and opened up access to hypothesis-driven and unbiased genetic approaches. With knowledge of the genes Involved in complex basic functions like the stress response, and of multifactorial diseases like stress-related disorders, we can Improve our understanding of the mechanisms and moderators Involved In the biology of normal and altered stress response, which In turn will help to Identify new drug targets and Interventions for stress-related disorders.
Stress response and stress-related disorders
Though there is no generally accepted definition, stress Is usually defined as a state of disturbed homeostasis evoking a multiplicity of somatic and mental adaptive reactions, which are summarized as stress response aiming to reconstitute the initial homeostasis or allostasis,1 ie, a new level of homeostasis after successful adaptation.2 The pioneer of stress research, Hans Selye, claimed a stimulus-independent nonspeciflcity of the stress response3,4 which has been criticized by others.1,5,6 Nevertheless, different kinds of stressors, physical and psychosocial, lead equivocally to a rapid activation of the sympathetic nervous system followed by a stimulation of the hypothalamlc-pitultary-adrenocortical (HPA) axis. Successful coping with stress Implies an appropriate regulation of the stress response and an effective termination when the stress is over or the Individual has adapted to the new conditions.
The perception of a stressful situation activates a large number of neuronal circuits In the prefrontal cortex and limbic system, Including the hypothalamus, where the sympathetic nervous system Is activated; this In turn leads to a widespread release of noradrenalin from the post-ganglionic fibers and to the release of adrenalin (and noradrenalin) from the adrenal medulla. Additionally, the parvocellular neurons of the hypothalamus are stimulated to secrete the neuropeptides corticotropin-releasing hormone (CRH) and vasopressin (AVP) Into the portal vessel system to activate the synthesis and release of corticotropin (ACTH) from the anterior pituitary. ACTH, In turn, stimulates the adrenal cortex to synthesize and release glucocorticoids, In particular Cortisol (In humans). These hormones have a multiplicity of functions, which are necessary for the adaptation to acute stress, but can be pathogenic when the organism Is persistently exposed. Therefore, a fine-tuned regulation of the sympathetic system and of the HPA axis is essential to avoid the development of a pathological dysregulation that can progress to stress-related disorders, which can be defined as illnesses whose causation, onset, or development Is substantially Influenced by stress and Its neurobiological correlates. Among others, cardiovascular disorders such as hypertension and coronary artery disease, as well as psychiatric diseases such as bipolar disorder and unipolar depression, are examples of stress-related disorders that will be discussed in this review.
The main central structure for the regulation of the autonomic nervous system Is the hypothalamus, which receives Input from cortical and subcortical structures, as well as from peripheral receptors and organs. The primary regulatory elements of the HPA axis are the corticosteroid receptors, glucocorticoid receptors (GR), and mineral corticold receptors7 (for details see ref 8).
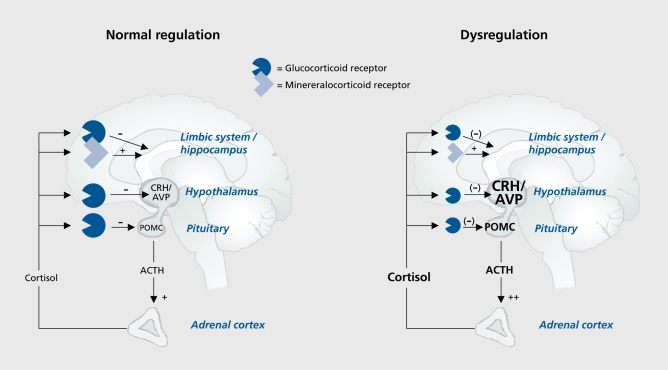
As Indicated In the left panel of Figure 1, activation of the HPA axis leads to the secretion of Cortisol (In humans), which Induces a negative feedback Inhibition to CRH and AVP (at the level of the hypothalamus) and to ACTH (at the level of the anterior pituitary). Impaired corticosteroid signaling results In an attenuation of the negative feedback Inhibition, which could result In the failure to sufficiently suppress CRH and AVP release from the hypothalamus and ACTH from the anterior pituitary, which in turn leads to chronically elevated levels of Cortisol (Figure 1, right panel). The attenuated negative feedback Inhibition can be most sensitively diagnosed with a neuroendocrine challenge test of the HPA axis, the combined dexamethasone (dex)/CRH test.9 In this test, the stimulating effects of 100 ug Intravenous human CRH upon ACTH and Cortisol are examined under the suppressive action of 1.5 mg of dexamethasone.10,11 This test is sensitive to impaired GR signaling at the pituitary level, as well as to the effects of Increased secretion of the hypothalamic neuropeptides CRH and AVP, which is a consequence of impaired central GR signaling.8,12,13
Figure 1. Model for normal and impaired regulation of the HPA axis. HPA, hypothalamic-pituitary-adrenocortical; CRH, corticotropin-releasing hormone; AVP, arginin-vasopressin; POMC, pro-opiomelanocortin; ACTH, adrenocorticotropic hormone.
Impaired HPA axis regulation during an acute episode Is the most consistent laboratory finding In depression and bipolar disorder (see refs 13 to 15 for reviews), which corresponds to the concept of stress-related disorders. Accordingly, the majority of depressed patients exhibit an exaggerated ACTH and Cortisol response to the combined dex/CRH test (Figure 2).
Figure 2. Cortisol response to the combined dex/CRH test is elevated in depression (AUC, P<.001) suggesting dysregulation of the HPA axis due to impaired glucocorticoid signaling. Dex, dexamethasone; CRH, corticotropin-releasing hormone; HPA, hypothalamic-pituitary-adrenocortical; AUC, area under the curve.
These alterations were shown to normalize after successful antidepressant treatment,11,16-18 suggesting that altered HPA axis regulation and Its normalization Is Involved In the pathogenesis of and recovery from depression, respectively.
Genetics of stress response
Evidence for herltabillty Is a prerequisite for the Involvement of genetic factors. The most efficient way for eval_ uatlng heritability Is twin studies comparing phenotypical similarity between monozygotic and dizygotic twins. Twin data are available for the Trier Social Stress Test (TSST),19 which Is a standardized procedure for the assessment of the psychosocial stress response. Briefly, this test comprises a public speaking task involving a mock job interview and a mental arithmetic task. Subjects are asked to prepare a presentation for promoting their candidacy for a position that is tailored to their education. After the preparation time, subjects give their presentation in front of a panel of judges who are evaluating the talk. After 5 minutes, subjects are requested to perform an unexpected mental arithmetic task for a further 5 minutes. HPA axis activity (plasma ACTH and Cortisol and/or salivary Cortisol) Is evaluated before and after the tasks as well as during recovery. Federenko and coworkers20 reported a herltabillty estimate (h2) of 0.32 for the plasma Cortisol response to the TSST in 33 monozygotic and 25 dizygotic twin pairs, suggesting moderate herltabillty, but this Increased up to 0.98 In two repetitions of the test. Herltabillty estimates for ACTH and salivary Cortisol were distinctly smaller In the first test session, but increased markedly In the repeated test sessions. A previous study by KIrschbaum and coworkers21 with 13 monozygotic and 11 dizygotic twin pairs also reported only marginal herltabillty for the sailvary Cortisol response to a single administration of the TSST. High heritability was observed for salivary Cortisol after stimulation with 100 µg human CRH (without dex suppression) and no herltabillty was found for the salivary Cortisol response to strenuous physical exercise (ergometer activity).21
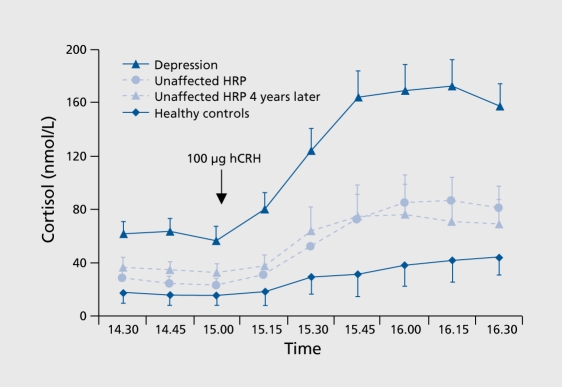
No heritability data are available for the combined dex/CRH test. However, In the Munich Vulnerability Study,22,23 the combined dex/CRH test was conducted In healthy first-degree relatives of patients with a major depressive disorder, who are assumed to carry a genetic vulnerability for affective disorders. These so-called high-risk probands (HRPs) are characterized by a moderately elevated hormonal response to the combined dex/CRH test, which was significantly higher compared with controls without a personal or familial history of psychiatric disorders, but less pronounced compared with the response in acutely depressed patients. Modell and coworkers24 replicated these findings In still unaffected HRPs who were re-examined in a follow-up Investigation about 4 years later (Figure 3), suggesting that this trait-like impaired regulation of the HPA system could reflect the genetic vulnerability for affective disorders in these subjects.
Figure 3. Cortisol response to the combined dex/CRH test is moderately elevated in high risk probands for affective disorders (AUC, P<.05), which was stable over time at the group level (AUC, P=.758) as well as at the individual level (Pearson correlation, r=.51, P<.05) in a follow-up investigation 4 years later. Dex, dexamethasone; CRH, corticotropin-releasing hormone; AUC, area under the curve.
Despite the statistical evidence for a considerable heritability of the stress response, the number of significant genetic findings Is small, and the conclusiveness rather limited. The findings are summarized in Table I. Due to the Importance of the HPA system for the stress response, which is primarily regulated by GR, the GR gene has been proposed as the primary candidate for the genetic association studies. Significant associations between GR and psychosocial stress response were reported, but only when a haplotype approach is applied25 or when male subjects are separately analyzed (Kumsta and Wust, 2006; personal communication). Further genetic associations, not yet replicated, are reported for the γ-aminobutyric acid (GABA) A 6 receptor subunit gene26 and for an nonsynonymous exon single-nucleotide polymorphism (SNP) of the micro-opiold receptor 1 (MOR) gene.27
Table I. Genetic associations with stress response in human paradigms. GABA, γ-aminobutyric acid; ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; HPA, hypothalamic-pituitary-adrenal.
| Genes | Chromosomal position | Results |
| Psychosocial stress response | ||
| Glucocorticoid receptor (GR, NR3C1) | 5q31.3 | Combined Bcll and N363S polymorphisms associated with salivary cortisol response to psychosocial stress (Trier Social Stress Test, TSST) in male mono- and dizygotic twins25; replicated in male unrelated subjects but not in female subjec (Kumsta and Wüst, 2006; personal communication) |
| GABA(A) α6 receptor subunit (GABRA6) | 5q34 | T1521C polymorphism associated with ACTH, cortisol, and blood pressure response to psychosocial stress (TSST) in healthy subject26 |
| Opioid receptor µ1 (OPRM1) | 6q24-q25 | A118G polymorphism associated with cortisol response to psychosocial stress (modified TSST) in healthy subjects27 |
| Endocrine HPA challenge tests | ||
| Glucocorticoid receptor (GR, NR3C1) | 5q31.3 | Bcll and N363S polymorphisms associated with ACTH and cortisol suppression after oral low-dose dexamethasone (dexamethasone suppression test) in elderly subjects28,29 |
| Angiotensin-converting enzyme (ACE) | 17q23.3 | Insertion/deletion polymorphism associated with hormonal response to the combined dexamethasone suppression/CRH stimulation test in acute major depression30,31 |
| Brain-derived neurotrophic factor (BDNF) | 11p13 | Val66Met polymorphism associated with ACTH and cortisol response to the combined dexamethasone suppression/CRH stimulation test in acute depression23 |
Additional evidence for an Involvement of the GR gene in the genetics of the stress response has been provided by two other studies (Table I) employing a low-dose dex suppression test In elderly subjects.28,29 In this test, plasma Cortisol levels after oral administration of dex are Interpreted as an Indicator for GR sensitivity, which is the major regulator of the stress hormone activity at the pituitary level Two other studies In patients suffering from major depression30,31 reported associations between the angiotensin-converting enzyme (ACE) gene and the hormonal response to the combined dex suppresslon/CRH stimulation test, which Is the most sensitive challenge test for evaluating stress hormone regulation. ACE is Involved in the so-called reninangiotensin cascade of water regulation, which in turn affects blood volume and blood pressure. A recent study observed an association between the combined dex/CRH test and brain-derived neurotrophic factor (BDNF) in depressed patients, which has been interpreted as evidence for an involvement of a reduced neuroplasticity in the development of disturbed HPA axis regulation.23
Taken together, there are only a limited number of studies examining the association between candidate genes and the stress response. Besides genes involved in the sympathetic (ACE) or HPA axis-mediated (GR) stress response, further genes constituting different biological systems implicated in emotional regulation26 and neuroplasticity (BDNF) have been examined. However, the results show only moderate effect sizes, although heritability estimates suggest a strong involvement of genetic factors. Further evidence for genes involved in the regulation of the stress response could be provided by clinical studies investigating genetic vulnerability factors for stress-related disorders. These genetic risk factors are assumed to be responsible for an inappropriate response to repeated and/or continuous stress and thus for mediating the vulnerability for stress-related disorders.
Genetics of stress-related disorders
A large number of diseases can be understood as stress-related disorders, and most of them are characterized by an at least moderate heritability. In this review, we focus on the most prevalent stress-related disorders, hypertension and coronary artery disease, as examples of cardio-vascular disorders, and on bipolar disorder and unipolar depression as examples of psychiatric disorders.
Cardiovascular disorders are the leading cause of mortality in the Western world, and are projected to become the leading cause of disease burden worldwide in 2020.32 Essential hypertension is the most common cardiovascular disorder, with a lifetime prevalence of above 50% in most western communities, affecting approximately f billion individuals worldwide33; heritability estimates around 30% have been reported.34 Myocardial infarction is a serious outcome of coronary artery disease. Twin studies suggest that the risk for myocardial infarction is fairly heritable, with a heredity estimate of 60% in females and 26% in males.35
A large number of case-control association studies in essential hypertension are available (Table IIa) focussing on a number of candidate gene systems. The majority of findings have been obtained with candidates from the sympathetic system, including adrenergic genes, genes of the renin-angiotensin-aldosterone system (RAAS), and genes involved in vascular regulation. Despite the large number of studies, only a few associations can be regarded as convincing, including the associations with the angiotensinogen (AGT), aldosterone synthase (CYP11B2), and with the renin (REN) gene, all involved in the RAAS.
Table IIa. Replicated findings of genetic associations with hypertension. 5-HT, serotonin; SAH, SA hypertension-associated homolog.
| Genes | Chromosomal position | Results |
| Adrenergic system | ||
| β2-adrenoceptor (ADRB2) | 5q31-q32 | Significant associations reported in Caucasian36,37 and Asian populations,38 but also several negative findings39 |
| β3adrenoceptor (ADRB3) | 8p12-p11.2 | Significant associations reported in Caucasian population40 and in male type 2 diabetics41 |
| Renin-angiotensin-aldosterone system | ||
| Angiotensin-converting enzyme (ACE) | 17q23.3 | Significant small to moderate effects,42-45 but also several negative reports40,46-48 |
| Angiotensinogen (AGT) | 1q42-q43 | Largest number of positive studies,47,49,50 but also some negative findings51 |
| Aldosterone synthase (CYP11B2) | 8q21-q22 | More positive52-56 than negative57 reports |
| Angiotensin (AT1) receptor (AGTR1) | 3q21-q25 | Mixed results, positive findings49 as well as negative reports44 |
| α Adductin (ADD1) | 4p16.3 | Mixed results, positive findings58 as well as negative reports51 |
| Atrial natriuretic peptide (NPPA, NPPB) | 1p36.2 | Less positive findings59 than negative reports60,61 |
| Renin (REN) | 1q32 | Predominance of positive findings than negative reports62-64 |
| 11β-hydroxisteroid dehydrogenase 2 (HSD11B2) | 16q22 | Weak positive effects are reported65,66 |
| Vascular system | ||
| Endothelin 1 (EDN1) | 6p24.1 | Significant association with blood pressure in obese subjects;67,68 some evidence for association with hypertension69; in interaction with 5-HTR2A |
| Nitric oxide synthase (NOS3) | 7q36 | Less positive findings70 than negative reports71,72 |
| Other genes | ||
| D2 receptor (DRD2) | 11q23 | Associated with hypertension73 and with elevated blood pressure in personality disorder74 |
| G protein β3 subunit (GNB3) | 12p23 | Less positive findings75 than negative reports51,54,76 |
| SAH (ACSM3) | 16p13.11 | Mixed resuits, positive findings77 as well as negative reports78 |
Several studies report gene x gene interaction effects, eg, between the endothelin f (EDN1) and serotonin receptor 2a (5HTR2A) genes,69 and between the ACE, aldosterone synthase (CYP11B2), and α adductin (ADD1) genes.42 Several candidate genes from other biological systems (eg, DRD2, GNB3, ACSM3) have been proposed, but no unambiguous conclusion can yet be drawn from the findings from these studies.
As for hypertension, a large number of genetic association studies have also been conducted for coronary artery disease. However, the results are more difficult to interpret than in hypertension, since different clinical conditions, including myocardial infarction and arteriosclerosis/stenosis, are integrated as coronary artery disease. Most candidate genes showing replicable associations have been derived from the concept of inflammation as a major risk factor for coronary heart disease. Convincing evidence for genetic associations has been reported for genes involved in innate immunity or genes moderating the inflammatory reaction, such as leukotrienes and lymphotoxins (Table IIb).
Table IIb. Replicated findings of genetic associations with coronary artery disease.
| Genes | Chromosomal position | Results |
| Innate immunity | ||
| CD14 molecule (CDI4) | 5q31.1 | Significant associations with myocardial infarction,79-81 but also negative reports82,83 |
| Toll-like receptor 4 (TLR4) | 9q32-q33 | Significant associations reported for acute coronary events84 and myocardial infarction85,86 but not with coronary stenosis87 |
| Leukotrienes | ||
| Arachidonate 5-lipoxygenase-activating protein (ALOX5AP) | 13q12 | Evidence for an association with myocardial infarction88,89 ateriosclerosis90 |
| Leukotriene A4 hydrolase (LTA4H) | 12q22 | Significant association with ethnicity-specific risk, for myocardial infarction fferent ethnic samples91 |
| Other genes | ||
| Lymphotoxine α (LTA) | 6p21.3 | Significant association with myocardial infarction in Japanese populations92,93 as well with arteriosclerosis in Caucasians,94 but also negative reports95,96 |
| Galectin 2 (LGALS2) | 22q13.1 | Associated with myocardial infarction97; protein interacts with LTA |
The number of positive results outweighs the negative findings, and most effect sizes were in an at least moderate range. Nevertheless, not all candidate genes derived from potent endophenotypes show convincing associations. One example of this divergence is lipoprotein A, which has been identified as a potent vulnerability factor for coronary artery disease,98 even though there is only a little evidence for a genetic association of the lipoprotein A (LPA) gene. Further gene candidates have been derived from studies in mendelian disorders involving premature coronary artery diseases such as familial hypercholesterolemia, familial defective apolipoprotein B (APOB), sitosterolemia, and Tangier disease. An overview of these findings is provided by Watkins and Farrall.99 However, the translation of these findings to multifactorial cardiovascular disorders is limited.
Besides cardiovascular diseases, bipolar disorder and unipolar depression are further examples of burdensome stress-elated disorders with a distinct heritability and a high prevalence in the general population, especially unipolar depression, which is projected to become the second leading cause for disease burden in 2020.32 Lifetime prevalence of bipolar disorder is around 1% according to population-based epidemiological studies in Europe100 as well as in the US,101 while lifetime prevalence of unipolar depression is distinctly higher, with a similar rate of 17% in Europe and in the USA. Twin studies suggest a high heritability for bipolar disorder, with heritability estimates, h2, ranging between 80% and 90%, and a moderate heritability for unipolar depression with h2 between 33% and 42%. 102
Most candidate genes for association studies with bipolar disorder and unipolar depression have been derived from neurotransmitter systems involved in antidepressant drug action. Only some of the findings could be consistently replicated, Including associations between the monoamlnoxldase A (MAOA)103 and catechol-o-methyl-transferase (COMT) gene and bipolar disorder and tryptophan hydroxllase 2 (TPH2) gene and unipolar depression (Table III). Further conclusive evidence exists for an Involvement of the D-aminoacidoxidase activator DAOA (G72)/G30 locus In the susceptibility for bipolar disorder, but also for schizophrenia. A large number of studies have examined the genetic associations between polymorphisms In the serotonin (5-HT) transporter (SLC6A4) gene and bipolar disorder and unipolar depression. Most attention focused on a functional Insertion/deletion polymorphism In the promoter region to SLC6A4, known as 5HTTLPR. Despite several positive results, the number of negative replications Is Increasing, and the relevance of this polymorphism for the susceptibility to bipolar disorder or unipolar depression Is meanwhile being challenged.
Table III. Replicated findings of genetic associations with bipolar disorder and unipolar depression. 5-HT, serotonin.
| Genes | Chromosomal position | Results |
| Bipolar disorder | ||
| Monoaminoxidase A (MAOA) | 5q31.3 | Significant associations with a modest effect size confirmed by meta-analyses103,104 suggesting greatest effects in female patients |
| Catechol-o-methyltransferase (COMT) | 22q11.21 | Meta analysis revealed a modest effect size105,106 and has been suggested as a common susceptibility gene for bipolar disorder and schizophrenia107 |
| 5-HT transporter (SLC6A4) | 17q11.1-q12 | A number of positive studies108-111 confirmed in meta-analyses,112,113 but also negative studies for 5-HTTLPR114 one negative meta-analysis105 |
| D-aminoacidoxidase activator DAOA (G72) / G30 | 13q33-q34 | Several positive reports with polymorphisms in the proximity of these nested, genes,7,115-117 but also with schizophrenia, suggesting a common susceptibility locus118 |
| Brain-derived neurotrophic factor (BDNF) | 11p13 | Family-based association studies showed significant effects119,120 but most replication studies were negative121-124; one study suggested association with a subgroup of patients displaying rapid cycling124 |
| P2X ligand-gated ion channel 7 (P2RK7) | 12q24 | Significant associations reported125,126 |
| Unipolar depression | ||
| Tryptophan hydroxilase 2 (TPH2) | 12q21.1 | Significant associations with major depression127,128 and suicide129 |
| 5-HT transporter (SLC6A4) | 17q11.1-q12 | More depressive symptoms in carriers of the short 5-HTTLPR allele,130,131 but also negative reports114,132 |
| Glucocorticoid receptor (NR3C1) | 5q31.3 | Bcll and ER22/23EK polymorphisms associated with susceptibility to recurrent unipolar depression133 |
| P2X ligand-gated ion channel 7 (P2RK7) | 12q24 | Significant associations with unipolar depression reported134,135 |
Besides SLC6A4, P2X ligand-gated Ion channel 7125 Is the only gene showing replicated effects for susceptibility to both bipolar disorder and unipolar depression. This gene codes for a cation-selective Ion channel expressed In central glial cells as well as In neurons, and Is assumed to regulate Immune function and neurotransmitter release.136,137
In summary, genetic association studies In stress-related disorders have provided evidence for an involvement of several other genes not identified by basic genetic studies on stress response. Since an inappropriate response to repeated and/or continuous stress mediates the susceptibility to stress-related disorders, these genes are also assumed to moderate the stress response. We have reviewed genetic association studies in hypertension, coronary artery disease, bipolar disorder, and unipolar depression. Due to the large and rapidly increasing number of publications, it is impossible to provide a complete overview. However, we have tried to summarize the most consistent and most frequently discussed findings. It is important to note that different classes of candidate genes have been investigated in the four diagnostic groups reported in this review, despite their common relationship to stress and inappropriate stress response. While candidate genes in hypertension and coronary artery disease are primarily related to the RAAS and to inflammation/immune response, respectively, the majority of candidate genes in bipolar disorder and unipolar depression are derived from monoaminergic neurotransmitter systems. This makes it clear that our actual knowledge of the complex interplay between genetic factors, altered stress response, and stress-related disorders is still limited, and that further research and new approaches are required to improve our understanding of these complex functions.
Conclusion and outlook
The summarized findings do not provide an exhaustive and satisfying answer about the genetics of stress response and stress-related disorders. Many single findings are still unconnected, and the restriction of the gene selection to established candidates has retarded our understanding of the complex interplay between genetic factors, stress response, and stress-related disorders. Sophisticated models, especially those aiming to integrate the findings from basic and clinical research as well as from the different types of stress-related disorders, are required to close the gap in our knowledge. The new chip-based whole-genome technologies, Affymetrix GeneChip and Illumina Genotyping BeadChip, are powerful tools for this endeavor. With this technology, the advantages of an unbiased approach as provided by linkage analysis, and the statistical power of association studies are combined to identify new candidate genes. However, results from unbiased approaches are always preliminary, and require validation in confirmatory studies. This means that independent replication studies are needed, but also clinical studies taking gene x gene and gene x environment interactions into account. For causal inferences, preclinical experiments are required, including (conditional) genetic modification and the development of specific compounds as research tools for the protein targets. Finally, text- and information-mining tools, which are already available but have to be further developed, will be very helpful to integrate all findings into sophisticated models delineating the pathways from genes to stress response and stress-related disorders. There is still a long way to go - but the prerequisites for success are more present than ever.
Selected abbreviations and acronyms
- ACTH
adrenocorticotropic hormone, corticotropin
- AVP
(arginin) - vasopressin
- CRH
corticotropin-releasing hormone
- DEX
dexamethasone
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenocortical
- MR
mineral corticoid receptor
- RAAS
renin-angiotensin-aldosterone system
- TSST
Trier Social Stress Test
Contributor Information
Marcus Ising, Max Planck Institute of Psychiatry, Munich, Germany.
Florian Holsboer, Max Planck Institute of Psychiatry, Munich, Germany.
REFERENCES
- 1.Pacak K., Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–148. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 3.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 4.Selye H. Stress and the general adaptation syndrome. BMJ. 1950;4667:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrousos GP., Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 6.Mason JW. A re-evaluation of the concept of “non-specificity” in stress theory. J Psychiatr Res. 1971;8:323–333. doi: 10.1016/0022-3956(71)90028-8. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher J., Jamra RA., Freudenberg J., et al. Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- 8.de Kloet ER., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 9.Holsboer F., von Bardeleben U., Wiedemann K., Müller OA., Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST non-suppression. Biol Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- 10.Heuser IJ., Yassouridis A., Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psych Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 11.Zobel AW., Nickel T., Sonntag A., Uhr M., Holsboer F., Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression: a prospective study. J Psych Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 12.Holsboer F., Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 13.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 14.Pariante CM., Thomas SA., Lovestone S., Makoff A., Kerwin RW. Do antidepressants regulate how Cortisol affects the brain? Psychoneuroendocrinology. 2004;29:423–447. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Raison CL., Miller AH. When not enough is too much: the role of insufficient gluco-corticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 16.Zobel AW., Yassouridis A., Frieboes RM., Holsboer F. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry. 1999;156:949–951. doi: 10.1176/ajp.156.6.949. [DOI] [PubMed] [Google Scholar]
- 17.Ising M., Künzel HE., Binder EB., Nickel T., Modell S., Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Ising M., Horstmann S., Kloiber S., et al. Combined Dex/CRH test predicts treatment response in Major Depression - a potential biomarker? Biol Psychiatry. 2006 Nov 21 [Epub Ahead of Print] doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Kirschbaum C., Pirke KM., Hellhammer DH. The Trier Social Stress Test - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 20.Federenko IS., Nagamine M., Hellhammer DH., Wadhwa PD., Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J Clin Endocrinol Metab. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- 21.Kirschbaum C., Wust S., Faig HG., Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrinol Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- 22.Holsboer F., Lauer CJ., Schreiber W., Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- 23.Schüle C., Zill P., Baghai TC., et al. Brain-derived neurotrophic factor VaI66Met polymorphismand dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Modell S., Lauer CJ., Schreiber W., Huber J., Krieg JC., Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- 25.Wüst S., van Rossum EFC., Federenko I., Koper JW., Kumsta R., Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical response to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- 26.Uhart M., McCaui ME., Oswald LM., Choi L., Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Mol Psychiatry. 2004;9:998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- 27.Chong RY., Oswald L., Yang X., Uhart M., Lin PI., Wand GS. The micro-opioid receptor polymorphism A118G predicts Cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- 28.Huizenga NA., de Lange P., Koper JW., et al. Human adrenocorticotropin-secreting pituitary adenomas show frequent loss of heterozygosity at the glucocorticoid receptor gene locus. J Clin Endocrinol Metab. 1998;83:917–921. doi: 10.1210/jcem.83.3.4648. [DOI] [PubMed] [Google Scholar]
- 29.van Rossum EF., Koper JW., van den Beld AW., et al. Identification of the Bcll poly-morphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf). 2003;59:585–592. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- 30.Baghai TC., Schule C., Zwanzger P., et al. Hypothalamic-pituitary-adrenocortical axis dysregulation in patients with major depression is influenced by the insertion/deletion polymorphism in the angiotensin I-converting enzyme gene. Neurosci Lett. 2002;328:299–303. doi: 10.1016/s0304-3940(02)00527-x. [DOI] [PubMed] [Google Scholar]
- 31.Baghai T., Binder EB., Schüle C., et al. Polymorphisms in the angiotensin converting enzyme gene are associated with unipolar depression, ACE activity, and hypercortisolism. Mol Psychiatry. 2006 Apr 22 [Epub Ahead of Print] doi: 10.1038/sj.mp.4001884. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. The Global Burden of Disease Study: a useful projection of future global health? J Publ Health Med. 2000;22:518–524. doi: 10.1093/pubmed/22.4.518. [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention Hypertension. http://www.cdc.gov/nchs/fastats/hypertens html. Accessed 2002. [Google Scholar]
- 34.Ward R. Familial aggregation and genetic epidemiology of blood pressure In: Laragh JH, Benner BM, eds. Hypertension. 2nd ed. New York, NY: Raven Press. 1995:67–88. [Google Scholar]
- 35.Jorde LB., Carey JC., Bamshad MJ., White RL. Medical Genetics. 3rd ed. St. Louis, Minn: Mosby. 2003 [Google Scholar]
- 36.Tïmmermann B., Mo R., Luft FC., et al. Beta-2 adrenoceptor genetic variation is associ-ated with genetic predisposition to essential hypertension: the Bergen Blood Pressure Study. Kidney Int. 1998;53:1455–1460. doi: 10.1046/j.1523-1755.1998.00926.x. [DOI] [PubMed] [Google Scholar]
- 37.Tomaszewski M., Brain NJ., Charchar FJ., et al. Essential hypertension and beta2-adrenergic receptor gene: linkage and association analysis. Hypertension. 2002;40:286–91. doi: 10.1161/01.hyp.0000029105.21202.fe. [DOI] [PubMed] [Google Scholar]
- 38.Ranade K., Shue WH., Hung YJ., et al. The glycine allele of a glycine/arginine poly-morphism in the beta2-adrenergic receptor gene is associated with essential hypertension in a population of Chinese origin. Am J Hypertens. 2001;14:1196–1200. doi: 10.1016/s0895-7061(01)02213-0. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann SM., Nicaud V., Tiret L., et al. Polymorphisms of the beta2 - adrenoceptor (ADRB2) gene and essential hypertension: the ECTIM and PEGASE studies. J Hypertens. 2002;20:229–235. doi: 10.1097/00004872-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Tonolo G., Melis MG., Secchi G., Atzeni MM., Angius MF., Carboni A., et al. Association of Trp64Arg beta 3-adrenergic-receptor gene polymorphism with essential hypertension in the Sardinian population. J Hypertens. 1999;17:33–38. doi: 10.1097/00004872-199917010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Ringel J., Kreutz R., Distler A., Sharma AM. The Trp64Arg polymorphism of the beta3-adrenergic receptor gene is associated with hypertension in men with type 2 diabetes mellitus. Am J Hypertens. 2000;13:1027–1031. doi: 10.1016/s0895-7061(00)00290-9. [DOI] [PubMed] [Google Scholar]
- 42.Staessen JA., Wang JG., Brand E., et al. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–1358. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Agachan B., Isbir T., Yilmaz H., Akoglu E. Angiotensin converting enzyme l/D, angïo-tensinogen T174M-M235T and angiotensin II type 1 receptor A1166C gene polymorphisms in Turkish hypertensive patients. Exp Mol Med. 2003;35:545–549. doi: 10.1038/emm.2003.71. [DOI] [PubMed] [Google Scholar]
- 44.Giner V., Poch E., Bragulat E., Oriola J., Gonzalez D., Coca A., et al. Reninangiotensin system genetic polymorphisms and salt sensitivity in essential hypertension. Hypertension. 2000;35:512–517. doi: 10.1161/01.hyp.35.1.512. [DOI] [PubMed] [Google Scholar]
- 45.Kario K., Hoshide S., Umeda Y., et al. Angiotensinogen and angiotensin-converting enzyme genotypes, and day and night blood pressures in elderly Japanese hypertensives. Hypertens Res. 1999;22:95–103. doi: 10.1291/hypres.22.95. [DOI] [PubMed] [Google Scholar]
- 46.Dzida G., Sobstyl J., Puzniak A., Golon P., Mosiewicz J., Hanzlik J. Polymorphisms of angiotensin-converting enzyme and angiotensin II receptor type 1 genes in essential hypertension in a Polish population. Med Sci Monit. 2001;7:1236–1241. [PubMed] [Google Scholar]
- 47.Tiret L., Blanc H., Ruidavets JB., et al. Gene polymorphisms of the renin-angiotensin system in relation to hypertension and parental history of myocardial infarction and stroke: the PEGASE study. Projet d'Etude des Genes de l'Hypertension Arterielle Severe a moderee Essentielle. J Hypertens. 1998;16:37–44. doi: 10.1097/00004872-199816010-00007. [DOI] [PubMed] [Google Scholar]
- 48.Zaman MM., Yoshiike N., Date C., et al. Angiotensin converting enzyme genetic polymorphism is not associated with hypertension in a cross-sectional sample of a Japanese population: the Shibata Study. J Hypertens. 2001;19:47–53. doi: 10.1097/00004872-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Castellano M., Glorioso N., Cusi D., Sarzani R., Fabris B., Opocher G., et al. Genetic polymorphism of the renin-angiotensin-aldosterone system and arterial hypertension in the Italian population: the GENIPER Project. J Hypertens. 2003;21:1853–1860. doi: 10.1097/00004872-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Sato N., Katsuya T., Rakugi H., et al. Association of variants in critical core promoter element of angiotensinogen gene with increased risk of essential hypertension in Japanese. Hypertension. 1997;30:321–325. doi: 10.1161/01.hyp.30.3.321. [DOI] [PubMed] [Google Scholar]
- 51.Larson N., Hutchinson R., Boerwinkle E. Lack of association of 3 functional gene variants with hypertension in African Americans. Hypertension. 2000;35:1297–1300. doi: 10.1161/01.hyp.35.6.1297. [DOI] [PubMed] [Google Scholar]
- 52.Casiglia E., Tikhonoff V., Mazza A., et al. C-344T polymorphism of the aldosterone synthase gene and blood pressure in the elderly: a populationbased study. J Hypertens. 2005;23:1991–1996. doi: 10.1097/01.hjh.0000183119.92455.a7. [DOI] [PubMed] [Google Scholar]
- 53.Komiya I., Yamada T., Takara M., et al. Lys(173)Arg and -344T/C variants of CYP11B2 in Japanese patients with low-renin hypertension. Hypertension. 2000;35:699–703. doi: 10.1161/01.hyp.35.3.699. [DOI] [PubMed] [Google Scholar]
- 54.Pamies-Andreu E., Ramirez-Lorca R., Stiefel Garcia-Junco P., et al. Renin-angiotensin-aldosterone system and G-protein beta-3 subunit gene polymorphisms in salt-sensitive essential hypertension. J Hum Hypertens. 2003;17:187–191. doi: 10.1038/sj.jhh.1001534. [DOI] [PubMed] [Google Scholar]
- 55.Tiago AD., Badenhorst D., Nkeh B., et al. Impact of renin-angiotensin-aldosterone system gene variants on the severity of hypertension in patients with newly diagnosed hypertension. Am J Hypertens. 2003;16:1006–1010. doi: 10.1016/j.amjhyper.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Tsukada K., Ishimitsu T., Teranishi M., et al. Positive association of CYP11B2 gene polymorphism with genetic predisposition to essential hypertension. J Hum Hypertens. 2002;16:789–793. doi: 10.1038/sj.jhh.1001484. [DOI] [PubMed] [Google Scholar]
- 57.Tsujita Y., Iwai N., Katsuya T., et al. al. Lack of association between genetic polymorphism of CYP11B2 and hypertension in Japanese: the Suita Study. Hypertens Res. 2001;24:105–109. doi: 10.1291/hypres.24.105. [DOI] [PubMed] [Google Scholar]
- 58.Cusi D., Barlassina C., Azzani T., et al. Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. Lancet. 1997;349:1353–1357. doi: 10.1016/S0140-6736(97)01029-5. [DOI] [PubMed] [Google Scholar]
- 59.Kato N., Sugiyama T., Morita H., et al. Genetic analysis of the atrial natriuretic peptide gene in essential hypertension. Clin Sci (Lond). 2000;98:251–258. [PubMed] [Google Scholar]
- 60.Cheung BM., Leung R., Shiu S., Tan KC., Lau CP., Kumana CR. Hpall polymorphism in the atrial natriuretic peptide gene and hypertension. Am J Hypertens. 1999;12:524–527. doi: 10.1016/s0895-7061(98)00267-2. [DOI] [PubMed] [Google Scholar]
- 61.Rahmutula D., Nakayama T., Soma M., et al. Association study between the variants of the human ANP gene and essential hypertension. Hypertens Res. 2001;24:291–294. doi: 10.1291/hypres.24.291. [DOI] [PubMed] [Google Scholar]
- 62.Chiang FT., Hsu KL., Tseng CD., Lo HM., Chern TH., Tseng YZ. Association of the renin gene polymorphism with essential hypertension in a Chinese population. Clin Genet. 1997;51:370–374. doi: 10.1111/j.1399-0004.1997.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 63.Frossard PM., Lestringant GG., Elshahat Yl., John A., Obineche EN. An Mbol two-allele polymorphism may implicate the human renin gene in primary hypertension. Hypertens Res. 1998;21:221–225. doi: 10.1291/hypres.21.221. [DOI] [PubMed] [Google Scholar]
- 64.Frossard PM., Lestringant GG., Malloy MJ., Kane JP. Human renin gene Bgll dimorphism associated with hypertension in two independent populations. Clin Genet. 1999;56:428–33. doi: 10.1034/j.1399-0004.1999.560604.x. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal AK., Giacchetti G., Lavery G., et al. CA-Repeat polymorphism in intron 1 of HSD11B2: effects on gene expression and salt sensitivity. Hypertension. 2000;36:187–194. doi: 10.1161/01.hyp.36.2.187. [DOI] [PubMed] [Google Scholar]
- 66.White PC., Agarwal AK., Li A., et al. Possible association but no linkage of the HSD11 B2 gene encoding the kidney isozyme of 11beta-hydroxysteroid dehydrogenase to hypertension in black people. Clin Endocrinol (Oxf). 2001;55:249–252. doi: 10.1046/j.1365-2265.2001.01314.x. [DOI] [PubMed] [Google Scholar]
- 67.Asai T., Ohkubo T., Katsuya T., et al. Endothelin-1 gene variant associates with blood pressure in obese Japanese subjects: the Ohasama Study. Hypertension. 2001;38:1321–1324. doi: 10.1161/hy1101.095333. [DOI] [PubMed] [Google Scholar]
- 68.Tiret L., Poirier O., Hallet V., et al. The Lys198Asn polymorphism in the endothelin-1 gene is associated with blood pressure in overweight people. Hypertension. 1999;33:1169–1174. doi: 10.1161/01.hyp.33.5.1169. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M., Jin JJ., Wu Z., et al. Interaction between serotonin 2A receptor and endothelin-1 variants in association with hypertension in Japanese. Hypertens Res. 2006;29:227–232. doi: 10.1291/hypres.29.227. [DOI] [PubMed] [Google Scholar]
- 70.Lacolley P., Gautier S., Poirier O., Pannier B., Cambien F., Benetos A. Nitric oxide synthase gene polymorphisms, blood pressure and aortic stiffness in normotensive and hypertensive subjects. J Hypertens. 1998;16:31–35. doi: 10.1097/00004872-199816010-00006. [DOI] [PubMed] [Google Scholar]
- 71.Kato N., Sugiyama T., Morita H., et al. Lack of evidence for association between the endothelial nitric oxide synthase gene and hypertension. Hypertension. 1999;33:933–936. doi: 10.1161/01.hyp.33.4.933. [DOI] [PubMed] [Google Scholar]
- 72.Tsujita Y., Baba S., Yamauchi R., et al. Association analyses between genetic polymorphisms of endothelial nitric oxide synthase gene and hypertension in Japanese: the Suita Study. J Hypertens. 2001;19:1941–1948. doi: 10.1097/00004872-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Thomas GN., Tomlinson B., Critchley JA. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension. 2000;36:177–182. doi: 10.1161/01.hyp.36.2.177. [DOI] [PubMed] [Google Scholar]
- 74.Rosmond R., Rankinen T., Chagnon M., et al. Polymorphism in exon 6 of the dopamine D(2) receptor gene (DRD2) is associated with elevated blood pressure and personality disorders in men. J Hum Hypertens. 2001;15:553–558. doi: 10.1038/sj.jhh.1001231. [DOI] [PubMed] [Google Scholar]
- 75.Dong Y., Zhu H., Sagnella GA., Carter ND., Cook DG., Cappuccio FP. Association between the C825T polymorphism of the G protein beta3-subunit gene and hypertension in blacks. Hypertension. 1999;34:1193–1196. doi: 10.1161/01.hyp.34.6.1193. [DOI] [PubMed] [Google Scholar]
- 76.Buchmayer H., Sunder-Plassmann G., HirschI MM., et al. G-protein beta3 subunit gene (GNB3) polymorphism 825C−−>T in patients with hypertensive crisis. Crit Care Med. 2000;28:3203–3206. doi: 10.1097/00003246-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 77.Iwai N., Katsuya T., Mannami T., et al. Association between SAH, an acyl-CoA synthetase gene, and hypertriglyceridemia, obesity, and hypertension. Circulation. 2002;105:41–47. doi: 10.1161/hc0102.101780. [DOI] [PubMed] [Google Scholar]
- 78.Benjafield AV., Iwai N., Ishikawa K., Wang WY., Morris BJ. Overweight, but not hypertension, is associated with SAH polymorphisms in Caucasians with essential hypertension. Hypertens Res. 2003;26:591–595. doi: 10.1291/hypres.26.591. [DOI] [PubMed] [Google Scholar]
- 79.Hohda S., Kimura A., Sasaoka T., et al. Association study of CD14 polymorphism with myocardial infarction in a Japanese population. Jpn Heart J. 2003;44:613–622. doi: 10.1536/jhj.44.613. [DOI] [PubMed] [Google Scholar]
- 80.Hubacek JA., Rothe G., Pit'ha J., et al. C(-260)−−>T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. doi: 10.1161/01.cir.99.25.3218. [DOI] [PubMed] [Google Scholar]
- 81.Shimada K., Watanabe Y., Mokuno H., Iwama Y., Daida H., Yamaguchi H. Common polymorphism in the promoter of the CD14 monocyte receptor gene is associated with acute myocardial infarction in Japanese men. Am J Cardiol. 2000;86:682–684, A8. doi: 10.1016/s0002-9149(00)01054-7. [DOI] [PubMed] [Google Scholar]
- 82.Nauck M., Winkelmann BR., Hoffmann MM., Bohm BO., Wieland H., Marz W. C(-260)T polymorphism in the promoter of the CD14 gene is not associated with coronary artery disease and myocardial infarction in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Am J Cardiol. 2002;90:1249–1252. doi: 10.1016/s0002-9149(02)02845-x. [DOI] [PubMed] [Google Scholar]
- 83.Koch W., Kastrati A., Mehilli J., von BN., Schomig A. CD14 gene -159C/T polymorphism is not associated with coronary artery disease and myocardial infarction. Am Heart J. 2002;143:971–976. doi: 10.1067/mhj.2002.122512. [DOI] [PubMed] [Google Scholar]
- 84.Ameziane N., Beillat T., Verpillat P., et al. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vase Biol. 2003;23:E61–E64. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 85.Edfeldt K., Bennet AM., Eriksson P., et al. Association of hyporesponsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J. 2004;25:1447–1453. doi: 10.1016/j.ehj.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Pasterkamp G., Van Keulen JK., De Kleijn DP. Role of Toll-like receptor 4 in the ini-tiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 87.Yang IA., Holloway JW., Ye S. TLR4 Asp299GIy polymorphism is not associated with coronary artery stenosis. Atherosclerosis. 2003;170:187–190. doi: 10.1016/s0021-9150(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 88.Helgadottir A., Manolescu A., Thorleifsson G., Gretarsdottir S., Jonsdottir H., Thor-steinsdottir U., et al. The gene encoding 5-Iipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 89.Helgadottir A., Gretarsdottir S., St Clair D., et al. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet. 2005;76:505–509. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dwyer JH., Allayee H., Dwyer KM., et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 91.Helgadottir A., Manolescu A., Helgason A., et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet. 2006;38:68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 92.Ozaki K., Ohnishi Y., lida A., et al. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002;32:650–654. doi: 10.1038/ng1047. [DOI] [PubMed] [Google Scholar]
- 93.Iwanaga Y., Ono K., Takagi S., et al. Association analysis between polymorphisms of the lymphotoxin-alpha gene and myocardial infarction in a Japanese population. Atherosclerosis. 2004;172:197–198. doi: 10.1016/j.atherosclerosis.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 94.Laxton R., Pearce E., Kyriakou T., Ye S. Association of the lymphotoxin-alpha gene Thr26Asn polymorphism with severity of coronary atherosclerosis. Genes Immun. 2005;6:539–541. doi: 10.1038/sj.gene.6364236. [DOI] [PubMed] [Google Scholar]
- 95.Clarke R., Xu P., Bennett D., Lewington S., et al. Lymphotoxin-alpha gene and risk of myocardial infarction in 6,928 cases and 2,712 controls in the ISIS case-control study. PLoS Genet. 2006;2:E107. doi: 10.1371/journal.pgen.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamada A., Ichihara S., Murase Y., et al. Lack of association of polymorphisms of the lymphotoxin alpha gene with myocardial infarction in Japanese. J Mol Med. 2004;82:477–483. doi: 10.1007/s00109-004-0556-x. [DOI] [PubMed] [Google Scholar]
- 97.Ozaki K., Inoue K., Sato H., et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature. 2004;429:72–75. doi: 10.1038/nature02502. [DOI] [PubMed] [Google Scholar]
- 98.Danesh J., Collins R., Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 99.Watkins H., Farrall M. Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet. 2006;7:163–173. doi: 10.1038/nrg1805. [DOI] [PubMed] [Google Scholar]
- 100.Jacobi F., Wittchen HU., Holting C., et al. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS). Psychol Med. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- 101.KessIer RC., McGonagle KA., Zhao S., et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 102.Craddock N., Forty L. Genetics of affective (mood) disorders. Eur J Hum Genet. 2006;14:660–668. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- 103.Furlong RA., Ho L., Rubinsztein JS., Walsh C., Paykel ES., Rubinsztein DC. Analysis of the monoamine oxidase A (MAOA) gene in bipolar affective disorder by association studies, meta-analyses, and sequencing of the promoter. Am J Med Genet. 1999;88:398–406. [PubMed] [Google Scholar]
- 104.Preisig M., Bellivier F., Fenton BT., et al. Association between bipolar disorder and monoamine oxidase A gene polymorphisms: results of a multicenter study. Am J Psychiatry. 2000;157:948–955. doi: 10.1176/appi.ajp.157.6.948. [DOI] [PubMed] [Google Scholar]
- 105.Craddock N., Dave S., Greening J. Association studies of bipolar disorder. Bipolar Disord. 2001;3:284–298. doi: 10.1034/j.1399-5618.2001.30604.x. [DOI] [PubMed] [Google Scholar]
- 106.Jones I., Craddock N. Candidate gene studies of bipolar disorder. Ann Med. 2001;33:248–256. doi: 10.3109/07853890108998753. [DOI] [PubMed] [Google Scholar]
- 107.Shifman S., Bronstein M., Sternfeld M., et al. COMT: a common susceptibility gene in bipolar disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;128:61–64. doi: 10.1002/ajmg.b.30032. [DOI] [PubMed] [Google Scholar]
- 108.Ogilvie AD., Battersby S., Bubb VJ., et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- 109.Collier DA., Stober G., Li T., et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- 110.Rees M., Norton N., Jones I., et al. Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT). Mol Psychiatry. 1997;2:398–402. doi: 10.1038/sj.mp.4000256. [DOI] [PubMed] [Google Scholar]
- 111.Kunugi H., Hattori M., Kato T., et al. Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry. 1997;2:457–462. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- 112.Anguelova M., Benkelfat C., Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol Psychiatry. 2003;8:574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- 113.Lasky-Su JA., Faraone SV., Glatt SJ., Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- 114.Mendlewicz J., Massat I., Souery D., et al. Serotonin transporter 5HTTLPR polymorphism and affective disorders: no evidence of association in a large European multi-center study. Eur J Hum Genet. 2004;12:377–382. doi: 10.1038/sj.ejhg.5201149. [DOI] [PubMed] [Google Scholar]
- 115.Chen YS., Akula N., Detera-Wadleigh SD., SchuIze TG., Thomas J., Potash JB., et al. Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33. Mol Psychiatry. 2004;9:87–92. doi: 10.1038/sj.mp.4001453. [DOI] [PubMed] [Google Scholar]
- 116.Hattori E., Liu C., Badner JA., et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet. 2003;72:1131–1140. doi: 10.1086/374822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams NM., Green EK., Macgregor S., et al. Variation at the DAOA/G30 locus influences susceptibility to major mood episodes but not psychosis in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2006;63:366–373. doi: 10.1001/archpsyc.63.4.366. [DOI] [PubMed] [Google Scholar]
- 118.Craddock N., O'Donovan MC., Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neves-Pereira M., Mundo E., Muglia P., King N., Macciardi F., Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sklar P., Gabriel SB., Mclnnis MG., et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brainderived neutro-phic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 121.Hong CJ., Huo SJ., Yen FC., Tung CL., Pan GM., Tsai SJ. Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology. 2003;48:186–189. doi: 10.1159/000074636. [DOI] [PubMed] [Google Scholar]
- 122.Nakata K., Ujike H., Sakai A., et al. Association study of the brain-derived neurotrophic factor (BDNF) gene with bipolar disorder. Neurosci Lett. 2003;337:17–20. doi: 10.1016/s0304-3940(02)01292-2. [DOI] [PubMed] [Google Scholar]
- 123.Oswald P., Del-Favero J., Massat I., Souery D., Claes S., Van BC., et al. al. Non-replication of the brain-derived neurotrophic factor (BDNF) association in bipolar affective disorder: a Belgian patient-control study. Am J Med Genet B Neuropsychiatr Genet. 2004;129:34–35. doi: 10.1002/ajmg.b.30056. [DOI] [PubMed] [Google Scholar]
- 124.Green EK., Raybould R., Macgregor S., et al. Genetic variation of brainderived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry. 2006;188:21–25. doi: 10.1192/bjp.bp.105.009969. [DOI] [PubMed] [Google Scholar]
- 125.Barden N., Harvey M., Gagne B., et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 126.Shink E., Morissette J., Sherrington R., Barden N. A genome-wide scan points to a susceptibility locus for bipolar disorder on chromosome 12. Mol Psychiatry. 2005;10:545–552. doi: 10.1038/sj.mp.4001601. [DOI] [PubMed] [Google Scholar]
- 127.Zhang X., Gainetdinov RR., Beaulieu JM., et al. Loss-of-function mutation in tryptophan hydroxyIase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 128.Zill P., Baghai TC., Zwanzger P., et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- 129.Zill P., Buttner A., Eisenmenger W., Moller HJ., Bondy B., Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry. 2004;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 130.Caspi A., Sugden K., Moffitt TE., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 131.Kendler KS., Kuhn JW., Vittum J., Prescott CA., Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 132.GiIIespie NA., Whitfield JB., Williams B., Heath AC., Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 133.van Rossum EFC., Binder EB., et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 134.Barden N., Harvey M., Shink E., et al. Identification and characterisation of a gene predisposing to both bipolar and unipolar affective disorders. Am J Med Genetics. 2004;130B:122. [Google Scholar]
- 135.Lucae S., Salyakina D., Barden N., Harvey M., Gagne B., Labbe M., et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- 136.Deuchars SA., Atkinson L., Brooke RE., Musa H., Milligan CJ., Batten TF., et al. Neu-ronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wirkner K., Kofalvi A., Fischer W., et al. Supersensitivity of P2X receptors in cerebro-cortical cell cultures after in vitro ischemia. J Neurochem. 2005;95:1421–1437. doi: 10.1111/j.1471-4159.2005.03465.x. [DOI] [PubMed] [Google Scholar]