Abstract
Brain areas implicated in the stress response include the amygdala, hippocampus, and prefrontal cortex. Traumatic stress can be associated with lasting changes in these brain areas. Traumatic stress is associated with increased cortisol and norepinephrine responses to subsequent stressors. Antidepressants have effets on the hippocampus that counteract the effects of stress. Findings from animal studies have been extended to patients with post-traumatic stress disorder (PTSD) showing smaller hippocampal and anterior cingulate volumes, increased amygdala function, and decreased medial prefrontal/anterior cingulate function. In addition, patients with PTSD show increased cortisol and norepinephrine responses to stress. Treatments that are efficacious for PTSD show a promotion of neurogenesis in animal studies, as well as promotion of memory and increased hippocampal volume in PTSD.
Keywords: positron emission tomography, depression, stress, post-traumatic stress disorder
Abstract
El estrés traumático surte efectos muy diversos sobre la función y la estructura cerebrales. Las regiones cerebrales implicadas en la respuesta al estrés son la amígdala (núcleo amigdalino), el hipocampo y la corteza prefrontal. Los sistemas neuroquímicos, como el cortisol y la noradrenalina, desempeñan una misión crítica en la respuesta al estrés. Estas regiones cerebrales influyen sobre la respuesta al estrés y sobre la memoria, lo que subraya la interrelación entre la memoria y la respuesta al estrés traumático. Los antidepresivos actúan sobre el hipocampo y contrarrestan el efecto del estrés. Los estudios sobre pacientes con trastorno por estrés postraumático (TEPT) revelan alteraciones en las regiones cerebrales implicadas en los estudios con animales como la amígdala, el hipocampo y la corteza prefrontal, así como en los sistemas neuroquímicos de respuesta al estrés, entre ellos el cortisol y la noradrenalina. Los tratamientos con eficacia frente al TEPT promueven la neurogénesis en los estudios con animales y también aumentan la memoria, y el volumen hipocámpico en el TEPT. Se requieren nuevos estudios para evaluar los mecanismos neurales de la respuesta terapéutica en el TEPT.
Abstract
Le stress traumatique exerce une grande variété d'effets sur la fonction et la structure cérébrales. Les aires cérébrales impliquées dans la réponse au stress comprennent l'amygdale, l'hippocampe et le cortex préfrontal. Les systèmes neurochimiques, incluant le cortisol et la norépinéphrine, jouent un rôle critique dans la réponse au stress. Ces aires cérébrales influent sur la mémoire et sur la réponse au stress traumatique, soulignant ainsi les interactions existant entre les deux. Les effets des antidépresseurs sur l'hippocampe compensent les effets du stress. Les études chez les patients atteints de trouble stress post-traumatique (ESPT) montrent des modifications des aires cérébrales impliquées au cours des études animales, telles l'amygdale, l'hippocampe et le cortex préfrontal, ainsi que des modifications des systèmes neurochimiques de réponse au stress comme le cortisol et la noradrénaline. Les traitements efficaces dans l'ESPT entraînent une activation de la neurogenèse chez l'animal de même qu'une amélioration de la mémoire et une augmentation du volume de l'hippocampe dans l'ESPT. Il faudra d'autres études pour évaluer les mécanismes neuronaux dans la réponse thérapeutique au cours de l'ESPT.
Effects of traumatic stress
Traumatic stressors such as early trauma can lead to posttraumatic stress disorder (PTSD), which affects about 8% of Americans at some time In their lives,1 as well as depression,2,3 substance abuse,1,4 dissociation,5 personality disorders,6,7 and health problems.8 For many trauma victims, PTSD can be a lifelong problem.9 The President's New Freedom Commission Report highlights the Importance of providing services for mental disorders related to early trauma.10-12 However, the development of effective treatments is limited by gaps in knowledge about the underlying neurobiological mechanisms that mediate symptoms of traumarelated disorders like PTSD. This paper reviews preclinical and clinical studies on the effects of traumatic stress on the brain.
Normal development of the brain across the lifespan
To understand how traumatic stress occurring at different stages of the life cycle interacts with the developing brain, it is useful to review normal brain development. The normal human brain undergoes changes in structure and function across the lifespan from early childhood to late life. Understanding these normal developmental changes is critical for determining the difference between normal development and pathology, and how normal development and pathology interact.
Although the bulk of brain development occurs in utero, the brain continues to develop after birth. In the first 5 years of life there is an overall expansion of brain volume related to development of both gray matter and white matter structures; however, from 7 to 17 years of age there is a progressive increase in white matter (felt to be related to ongoing myelination) and decrease in gray matter (felt to be related to neuronal pruning) while overall brain size stays the same.13-16 Gray matter areas that undergo the greatest increases throughout this latter developmental epoch include frontal cortex and parietal cortex.17,18 Basal ganglia decrease in size, while corpus callosum,19,20 hippocampus, and amygdala21-23 appear to increase in size during childhood, although there may be developmental sex-laterality effects for some of these structures.24 Overall brain size is 10% larger in boys than girls during childhood.24
During the middle part of life (from age 20 to 70) there is a gradual decrease in caudate,25 diencephalon,25 and gray matter,25,26 which is most pronounced in the temporal27 and frontal cortex,26 with enlargement of the ventricles26,27 and no change in white matter.25,26 Studies have not been able to document changes in hippocampal volume in normal populations during this period.27 After menopause in women at about the age of 50, however, there are changes in reproductive hormones, such as decreased levels of estrogen. Since estrogen promotes neuronal branching in brain areas such as the hippocampus,28 a loss of estrogen may lead to changes in neuronal structure. Although the effects of menopause on the brain have not been well studied, it is known that sex hormones also affect brain function and circuitry29; therefore, the changes in sex hormones with menopause will presumably affect brain function, as well as possibly structure. There is some evidence in super-elderly individuals (age >70) for modest reductions in hippocampal volume with late stages of aging.27,30 More robust findings have included increased ventricular volume and reduction in gray matter, temporal lobe, and cerebellum volumes with normal aging, that begins before the age of 70.25,27,31-33
Therefore, trauma at different stages in life will presumably have different effects on brain development. The few studies that have looked at this issue do suggest that there are differences in the effects of trauma on neurobiology, depending on the stage of development at which the trauma occurs. Studies in this area, however, have been limited.
Neurobiology of PTSD
PTSD is characterized by specific symptoms, including intrusive thoughts, hyperarousal, flashbacks, nightmares, and sleep disturbances, changes in memory and concentration, and startle responses. Symptoms of PTSD are hypothesized to represent the behavioral manifestation of stress-induced changes in brain structure and function. Stress results in acute and chronic changes in neurochemical systems and specific brain regions, which result in longterm changes in brain “circuits,” involved in the stress response.34-37 Brain regions that are felt to play an important role in PTSD include hippocampus, amygdala, and medial prefrontal cortex. Cortisol and norepinephrine are two neurochemical systems that are critical in the stress response (Figure 1.)
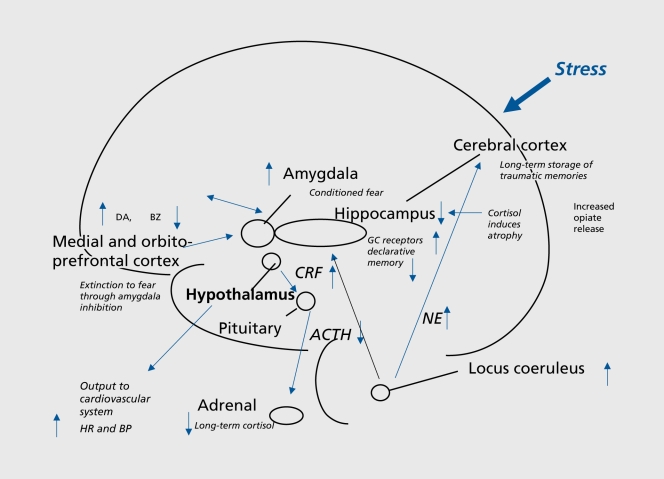
Figure 1. Lasting effects of trauma on the brain, showing long-term dysregulation of norepinephrine and Cortisol systems, and vulnerable areas of hippocampus, amygdala, and medial prefrontal cortex that are affected by trauma. GC, glucocorticoid; CRF, corticotropin-releasing factor; ACTH, adrenocorticotropin hormone; NE, norepinephrine; HR, heart rate; BP, blood pressure; DA, dopamine; BZ, benzodiazapine; GC, glucocorticoid.
The corticotropin-releasing factor (CRF)/hypothalamic-pituitary-adrenal (HPA) axis system plays an important role in the stress response. CRF is released from the hypothalamus, with stimulation of adrenocorticotropic hormone (ACTH) release from the pituitary, resulting in glucocorticoid (Cortisol in man) release from the adrenal, which in turn has a negative feedback effect on the axis at the level of the pituitary, as well as central brain sites including hypothalamus and hippocampus. Cortisol has a number of effects which facilitate survival. In addition to its role in triggering the HPA axis, CRF acts centrally to mediate fear-related behaviors,38 and triggers other neurochemical responses to stress, such as the noradrenergic system via the brain stem locus coeruleus.39 Noradrenergic neurons release transmitter throughout the brain; this is associated with an increase in alerting and vigilance behaviors, critical for coping with acute threat.40-42
Studies in animals showed that early stress has lasting effects on the HPA axis and norepinephrine. A variety of early stressors resulted in increased glucocorticoid response to subsequent stressors.43-45 Maternally deprived rats had decreased numbers of glucocorticoid receptors in the hippocampus, hypothalamus, and frontal cortex.46 Stressed animals demonstrated an inability to terminate the glucocorticoid response to stress,47,48 as well as deficits in fast-feedback of glucocorticoids on the HPA axis, which could be related to decreased glucocorticoid receptor binding in the hippocampus.49 Early postnatal adverse experiences increase hypothalamic CRF messenger ribonucleic acid (mRNA), median eminence CRF content, and stress-induced glucocorticoid50 and ACTH release.46 These effects could be mediated by an increase in synthesis of CRH mRNA following stress.51 In nonhuman primates, adverse early experiences resulted in long-term effects on behaviors, as well as elevated levels of CRF in the cerebrospinal fluid.52 Exposure to chronic stress results in potentiation of noradrenergic responsiveness to subsequent stressors and increased release of norepinephrine in the hippocampus and other brain regions.42
Preclinical and clinical studies have shown alterations in memory function following traumatic stress,53 as well as changes in a circuit of brain areas, including hippocampus, amygdala, and medial prefrontal cortex, that mediate alterations in memory.54 The hippocampus, a brain area involved in verbal declarative memory, is very sensitive to the effects of stress. Stress in animals is associated with damage to neurons in the CA3 region of the hippocampus (which may be mediated by hypercortisolemia, decreased brain-derived neurotrophic factor (BDNF), and/or elevated glutamate levels) and inhibition of neurogenesis.55-60 High levels of glucocorticoids seen with stress were also associated with deficits in new learning.61,62
Antidepressant treatments have been shown to block the effects of stress and/or promote neurogenesis.58,63-66 Animal studies have demonstrated several agents with potentially beneficial effects on stress-induced hippocampal damage. It has been found that phenytoin blocks the effects of stress on the hippocampus, probably through modulation of excitatory amino acid-induced neurotoxicity.67 Other agents, including tianeptine, dihydroepiandosterone (DHEA), and fluoxetine have similar effects.63,64,66,68-73 These medications may share a common mechanism of action through upregulation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) that may lead to regulation of expression of specific target genes involved in structural modeling of the hippocampus. Such treatment effects on BDNF and trkB messenger ribonucleic acid (mRNA), can have long-term effects on brain structure and function. There is new evidence that neurogenesis is necessary for the behavioral effects of antidepressants,74,75 although this continues to be a source of debate.72,76
The hippocampus demonstrates an unusual capacity for neuronal plasticity and regeneration. In addition to findings noted above related to the negative effects of stress on neurogenesis, it has recently been demonstrated that changes in the environment, eg, social enrichment or learning, can modulate neurogenesis in the dentate gyrus of the hippocampus, and slow the normal age-related decline in neurogenesis.77,78 Rat pups that are handled frequently within the first few weeks of life (picking them up and then returning them to their mother) had increased type II glucocorticoid receptor binding which persisted throughout life, with increased feedback sensitivity to glucocorticoids, and reduced glucocorticoid-mediated hippocampal damage in later life.79 These effects appear to be due to a type of “stress inoculation” from the mothers' repeated licking of the handled pups.80 Considered together, these findings suggest that early in the postnatal period there is a naturally occurring brain plasticity in key neural systems that may “program” an organism's biological response to stressful stimuli. These findings may have implications for victims of childhood abuse.
Long-term dysregulation of the HPA axis is associated with PTSD, with low levels of Cortisol found in chronic PTSD in many studies81-86 and elevations in CRF.82,87 Not all studies, however, have found lower Cortisol levels in PTSD.88-91 Exposure to a traumatic reminder appears to be associated with a potentiated release of Cortisol in PTSD.92 The few studies of the effects of early stress on neurobiology conducted in clinical populations of traumatized children have generally been consistent with findings from animal studies. Research in traumatized children has been complicated by issues related to psychiatric diagnosis and assessment of trauma.93 Some studies have not specifically examined psychiatric diagnosis, while others have focused on children with trauma and depression, and others on children with trauma and PTSD. Sexually abused girls (in which effects of specific psychiatric diagnosis were not examined) had normal baseline Cortisol and blunted ACTH response to CRF,94 while women with childhood abuse-related PTSD had hypercortisolemia.95 Another study of traumatized children in which the diagnosis of PTSD was established showed increased levels of Cortisol measured in 24-hour urines.96 Emotionally neglected children from a Romanian orphanage had elevated Cortisol levels over a diurnal period compared with controls.97 Maltreated school-aged children with clinicallevel internalizing problems had elevated Cortisol compared with controls.98 Depressed preschool children showed increased Cortisol response to separation stress.99 Adult women with a history of childhood abuse showed increased suppression of Cortisol with low-dose (0.5 mg) dexamethasone.100 Women with PTSD related to early childhood sexual abuse showed decreased baseline Cortisol based on 24-hour diurnal assessments of plasma, and exaggerated Cortisol response to stressors (traumatic stressors101 more than neutral cognitive stressors).102 We also found that patients with PTSD had less of an inhibition of memory function with synthetic Cortisol (dexamethasone) than normal subjects.103 Adult women with depression and a history of early childhood abuse had an increased Cortisol response to a stressful cognitive challenge relative to controls,104 and a blunted ACTH response to CRF challenge.105 These findings show longterm changes in stress responsive systems. Early in development, stress is associated with increased Cortisol and norepinephrine responsiveness, whereas with adulthood, resting Cortisol may be normal or low, but there continues to be increased Cortisol and norepinephrine responsiveness to stressors. In addition, early stress is associated with alterations in hippocampal morphology which may not manifest until adulthood, as well as increased amygdala function and decreased medial prefrontal function.
Cognitive function and brain structure in PTSD
Studies in PTSD are consistent with changes in cognition and brain structure. Multiple studies have demonstrated verbal declarative memory deficits in PTSD.53,106-108
Patients with PTSD secondary to combat109-113 and childhood abuse114,115 were found to have deficits in verbal declarative memory function based on neuropsychological testing. Studies, using a variety of measures (including the Wechsler Memory Scale, the visual and verbal components of the Selective Reminding Test, the Auditory Verbal Learning Test, Paired Associate Recall, the California Verbal New Learning Test, and the Rivermead Behavioral Memory Test), found specific deficits in verbal declarative memory function, with a relative sparing of visual memory and IQ.109-113,115-124 These studies have been conducted in both patients with PTSD related to Vietnam combat,109-113,116,119-121,123 rape,117 the Holocaust,124-126 adults with early childhood abuse,115 and traumatized children.118 One study in adult rape survivors showed that verbal declarative memory deficits are specifically associated with PTSD, and are not a nonspecific effect of trauma exposure.117 Another study of women with early childhood sexual abuse in which some, but not all, of the patients had PTSD, showed no difference between abused and nonabused women,127 while another study was not able to show a difference between Vietnam veterans with and without PTSD.128 Other types of memory disturbances studied in PTSD include gaps in memory for everyday events (dissociative amnesia),129 deficits in autobiographical memory,130 an attentional bias for trauma-related material,131-140 and frontal lobe-related impairments.141 These studies suggest that traumas such as early abuse with associated PTSD result in deficits in verbal declarative memory. It is not clear if cognitive deficits in early abuse survivors are specific to PTSD and are not related to the non-specific effects of abuse.
These effects were specific to verbal (not visual) memory, and were significant after controlling for IQ. Some of these studies used neuropsychological tests of declarative memory, such as the Wechsler Memory Scale (WMS) and Selective Reminding Test (SRT), that have been validated as sensitive to loss of neurons in the CA3 region of the hippocampus in epileptics who underwent hippocampal resection.142,143 Vietnam veterans with PTSD were originally shown by us to have 8% smaller right hippocampal volume based on magnetic resonance imaging (MRI) relative to controls matched for a variety of factors such as alcohol abuse and education (P<0.05); smaller volume was correlated with deficits in verbal declarative memory function as measured with the Wechsler Memory Scale.144 A second study from our group showed a 12% reduction in left hippocampal volume in 17 patients with childhood abuse-related PTSD compared with 17 case-matched controls, that was significant after controlling for confounding factors.145 Smaller hippocampal volume was shown to be specific to PTSD within the anxiety disorders, and was not seen in panic disorder.146 Gurvits et al147 showed bilateral hippocampal volume reductions in combat-related PTSD compared with combat veterans without PTSD and normal controls. Combat severity was correlated with volume reduction. Stein et al148 found a 5% reduction in left hippocampal volume. Other studies in PTSD have found smaller hippocampal volume and/or reductions in iV-acetyl aspartate (NAA), a marker of neuronal integrity.149-153 Studies in childhood154-156 and new-onset157,158 PTSD did not find hippocampal volume reduction, although reduced NAA (indicating loss of neuronal integrity) was found in medial prefrontal cortex in childhood PTSD.159 In a recent meta-analysis we pooled data from all of the published studies and found smaller hippocampal volume for both the left and the right sides, equally in adult men and women with chronic PTSD, and no change in children.160 More recent studies of holocaust survivors with PTSD did not find a reduction in hippocampal volume, although PTSD patients who developed PTSD in response to an initial trauma had smaller hippocampal volume compared with those who developed PTSD after repeated trauma, suggesting a possible vulnerability of smaller hippocampal volume.161 Two independent studies have shown that PTSD patients have deficits in hippocampal activation while performing a verbal declarative memory task,149,162 although it is unclear if this is a deficit in activation or higher hippocampal blood flow at baseline. Both hippocampal atrophy and hippocampal-based memory deficits reversed with treatment with the selective serotonin reuptake inhibitor (SSRI) paroxetine, which has been shown to promote neurogenesis (the growth of neurons) in the hippocampus in preclinical studies.163 In addition, treatment with the anticonvulsant phenytoin led to an improvement in PTSD symptoms164 and an increase in right hippocampal and right cerebral volume.165 We hypothesize that stress-induced hippocampal dysfunction may mediate many of the symptoms of PTSD which are related to memory dysregulation, including both explicit memory deficits as well as fragmentation of memory in abuse survivors. It is unclear at the current time whether these changes are specific to PTSD, whether certain common environmental events (eg, stress) in different disorders lead to similar brain changes, or whether common genetic traits lead to similar outcomes.
The meaning of findings related to deficits in memory and the hippocampus in PTSD, and questions related to the relative contribution of genetic and environmental factors, has become an important topic in the field of PTSD and stress research. There are three possible models, taking into account genetic or environmental factors, which have been proposed to explain smaller hippocampal volume in PTSD: Model A (Environment), Model B (Environment and Genetic), and Model C (Genetic).166-169 In Model C (Genetic), smaller hippocampal volume represents a premorbid risk factor for PTSD. In support of this model Pitman and colleagues170 have demonstrated that lower premilitary IQ is associated with combat-related PTSD, as well as finding a correlation between PTSD symptoms and hippocampal volume in twin brothers.151 Model A (Environment) states that stress leads to damage or inhibition of neurogenesis via hypercortisolemia, decreased BDNF, or increased glutamate. Model B (Environment/Genetic) states that a combination of environmental and genetic factors leads to deficits in hippocampal function and structure. Showing that an intervention like medication changes hippocampal volume and cognition would provide support for at least a partial contribution of the environment to the outcomes of interest.
In addition to the hippocampus, other brain structures have been implicated in a neural circuitry of stress, including the amygdala and prefrontal cortex. The amygdala is involved in memory for the emotional valence of events, and plays a critical role in the acquisition of fear responses. The medial prefrontal cortex includes the anterior cingulate gyrus (Brodmann's area [BA] 32) and subcallosal gyrus (area 25) as well as orbitofrontal cortex. Lesion studies demonstrated that the medial prefrontal cortex modulates emotional responsiveness through inhibition of amygdala function. Conditioned fear responses are extinguished following repeated exposure to the conditioned stimulus in the absence of the unconditioned (aversive, eg, electric shock) stimulus. This inhibition appears to be mediated by medial prefrontal cortical inhibition of amygdala responsiveness.
Animal studies also show that early stress is associated with a decrease in branching of neurons in the medial prefrontal cortex.171 Rauch and colleagues found smaller volume of the anterior cingulate based on MRI measurements in PTSD172; we have replicated these findings in women with abuse and PTSD.160 An important question is whether these effects are reversible with treatment.
Neural circuits in PTSD
Brain imaging studies have shown alterations in a circuit including medial prefrontal cortex (including anterior cingulate), hippocampus, and amygdala in PTSD. Many of these studies have used different methods to trigger PTSD symptoms (eg, using traumatic cues) and then look at brain function. Stimulation of the noradrenergic system with yohimbine resulted in a failure of activation in dorsolateral prefrontal, temporal, parietal, and orbitofrontal cortex, and decreased function in the hippocampus.173 Exposure to traumatic reminders in the form of traumatic slides and/or sounds or traumatic scripts was associated with an increase in PTSD symptoms, decreased blood flow, and/or failure of activation in the medial prefrontal cortex/anterior cingulate, including Brodmann's area 25, or subcallosal gyrus, area 32 and 24, as measured with positron emission tomography (PET) or functional MRI (fMRI).174-183 Other findings in studies of traumatic reminder exposure include decreased function in hippocampus,176 visual association cortex,176,180 parietal cortex,176,179,180,184 and inferior frontal gyrus,176,179,180,184 and increased function in amygdala,181,184 posterior cingulate,174,176,177,180 and parahippocampal gyrus.174,176,178 Shin and colleagues found a correlation between increased amygdala function and decreased medial prefrontal function with traumatic reminders,181 indicating a failure of inhibition of the amygdala by the medial prefrontal cortex that could account for increased PTSD symptoms with traumatic reminders. Other studies found increased amygdala and parahippocampal function and decreased medial prefrontal function during performance of an attention task,182 increased posterior cingulate and parahippocampal gyrus and decreased medial prefrontal and dorsolateral prefrontal function during an emotional Stroop paradigm,185 and increased amygdala function with exposure to masked fearful faces.186 Retrieval of emotionally valenced words187 (eg “rape-mutilate”) in women with PTSD from early abuse resulted in decreases in blood flow in an extensive area which included orbitofrontal cortex, anterior cingulate, and medial prefrontal cortex (BA 25, 32, and 9), left hippocampus, and fusiform gyrus/inferior temporal gyrus, with increased activation in posterior cingulate, left inferior parietal cortex, left middle frontal gyrus, and visual association and motor cortex.188 Another study found a failure of medial prefrontal cortical/anterior cingulate activation, and decreased visual association and parietal cortex function, in women with abuse and PTSD relative to women with abuse without PTSD, during performance of the emotional Stroop task (ie, naming the color of a word such as “rape”).189 We recently found increased amygdala activation with classical fear conditioning (pairing a shock and a visual stimulus), and decreased medial prefrontal cortex function with extinction, in abuse-related PTSD.190 The findings described above point to a network of related regions mediating symptoms of PTSD, including medial prefrontal cortex, anterior cingulate, hippocampus, amygdala, posterior cingulate, parietal, visual association, and dorsolateral prefrontal cortex.191
Fewer brain imaging studies have been performed in children with PTSD. Several studies have shown alterations in electroencephalogram (EEG) measures of brain activity in children with a variety of traumas who were not selected for diagnosis compared with healthy children. About half of the children in these studies had a psychiatric diagnosis. Abnormalities were located in the anterior frontal cortex and temporal lobe and were localized to the left hemisphere.192,193 Two studies have found reductions in brain volume in children with trauma and PTSD symptoms.154,155 One group did not find reductions in hippocampal volume, either at baseline or over a longitudinal period,154,156 while another group found an 8.5% reduction in hippocampal volume that was not significant after controlling for smaller brain volumes in the PTSD group.155 One study used single-voxel proton magnetic resonance spectroscopy (proton MRS) to measure relative concentration of NAA and creatinine (a marker of neuronal viability) in the anterior cingulate of 11 children with maltreatment-related PTSD and 11 controls. The authors found a reduction in the ratio of NAA to creatinine in PTSD relative to controls.159 Studies have also found smaller size of the corpus callosum in children with abuse and PTSD relative to controls.154 as well as larger volume of the superior temporal gyrus.194 In a study of abused children in whom diagnosis was not specified, there was an increase in T2 relaxation time in the cerebellar vermis, suggesting dysfunction in this brain region.195 The reason for differences in findings between adults and children are not clear; however, factors such as chronicity of illness or interaction between trauma and development may explain findings to date.
In summary, dysfunction of a circuit involving the medial prefrontal cortex, dorsolateral prefrontal cortex, and possibly hippocampus and amygdala during exposure to traumatic reminders may underlie symptoms of PTSD. These studies have primarily assessed neural correlates of traumatic remembrance, while little has been done in the way of utilizing cognitive tasks as probes of specific regions, such as memory tasks as probes of hippocampal function.
MRI assessment of brain abnormalities in PTSD and trauma spectrum disorders
Findings of smaller hippocampal volume appear to be associated with a range of trauma related psychiatric disorders, as long as there is the presence of psychological trauma. We have used MRI to show smaller hippocampal volume in PTSD,144,145,149,196 depression,197 depression with early abuse,198 borderline personality disorder (BPD) with early abuse,199 and Dissociative Identity Disorder (DID) with early abuse.200 The greatest magnitude of difference was seen in the DID patients, who had unusually severe early childhood sexual abuse histories. We did not find changes in hippocampal volume in patients with panic disorder without a history of abuse (suggesting that findings are not generalized to other anxiety disorders).201 We found smaller amygdala volume in BPD with early abuse199 and increased amygdala volume in depression.197,202 Patients with depression had smaller orbitofrontal cortex volume with no changes in anterior cingulate (BA 32) or medial prefrontal cortex (BA 25).203 More recently, we found smaller anterior cingulate volume in women with abuse and PTSD relative to controls.204
Neural circuits in women with abuse and PTSD
We have used PET to study neural circuits of traumarelated disorders in women with early abuse and a variety of trauma spectrum mental disorders. Initially we studied women with abuse and PTSD.54,205-208 We initially measured brain activation with a paragraph-encoding task in conjunction with PET 0-15 water measurement of brain blood flow. Women with abuse and PTSD showed a failure of hippocampal activation during the memory task relative to controls.149 Women with abuse and PTSD in this study also had smaller hippocampal volume measured with MRI relative to both women with abuse without PTSD and nonabused non-PTSD women. The failure of hippocampal activation was significant after controlling differences in hippocampal volume as well as accuracy of encoding. In another study we measured neural correlates of exposure to a personalized script of childhood sexual abuse. Women with abuse and PTSD showed a failure of medial prefrontal and hippocampal activation relative to abused women without PTSD.176 Women with abuse and PTSD also showed a failure of medial prefrontal and hippocampal function during recall of paired word associates with traumaticemotional content (eg, “rape-mutilate”),188 and decreased medial prefrontal function during an emotional Stroop task with trauma-content words.209 Other studies showed a failure of medial prefrontal activation in women with BPD and early abuse during an abandonment script.210 Women with BPD and abuse had increased psychophysiological responses to abandonment scripts relative to trauma scripts, while women with PTSD and abuse had the opposite pattern,211 indicating differential responding in those two disorders in spite of the common exposure to early abuse.
In another project we studied 19 physically healthy women including women with a history of severe childhood sexual abuse and the diagnosis of current PTSD (N=8) and women without childhood abuse or PTSD (N=11).212 All subjects underwent PET measurement of cerebral blood flow and psychophysiology measurement of heart rate and skin conductance during habituation, acquisition, and extinction conditions, on a single day, with scanning during a control condition on another day separated by 1 week from the active condition. Subjects were randomly assigned to undergo either the active condition or the control condition first (ie, active-control or control-active). Subjects were told at the beginning of the study that they would be exposed to electric shocks and viewing images on a screen during collection of PET and psychophysiology data. During habituation subjects were exposed to a blue square on a screen (conditioned stimulus [CS]), 4 seconds in duration, followed by 6 seconds of a blank screen. CS exposure was repeated eight times at regular intervals over 80 seconds in two separate blocks separated by 8 minutes. One PET image of brain blood flow was obtained starting from the beginning of each of the blocks. During active fear acquisition exposure to the blue square (CS) was paired with an electric shock to the forearm (unconditioned stimulus [UCS]).Subjects had 8 paired CS-UCS presentations at 10-second intervals for each of two blocks. With extinction subjects were again exposed to the blue squares (CS) with out shock (“active” extinction). On a second day subjects went through the same procedure with electric shocks delivered randomly when the blue square was not present (unpaired CS-UCS) (an equal number as on day 1) during scans 3 and 4, which served as a control for active fear acquisition.
PTSD subjects had increased symptoms of anxiety, fear, dissociation, distress, substance use disorders (SUDs), and PTSD at all time points during both study days relative to non-PTSD. Acquisition of fear was associated with increased skin conductance (SC) responses to CS exposure during the active versus the control conditions in all subjects. There was increased SC for PTSD during the first CS-UCS presentation. Extinction of fear was associated with increased skin conductance (SC) responses to CS exposure during the active versus the control conditions in all subjects. When PTSD and non-PTSD subjects were examined separately, SC levels were significantly elevated in non-PTSD subjects undergoing extinction following the active compared with the control condition during session one.
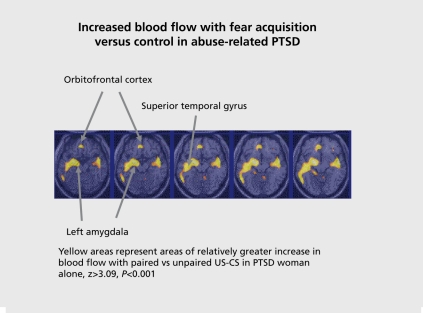
PTSD subjects showed activation of the bilateral amygdala during fear acquisition compared with the control condition. Non-PTSD subjects showed an area of activation in the region of the left amygdala. When PTSD subjects and control subjects were directly compared, PTSD subjects showed greater activation of the left amygdala during the fear conditioning condition (pairing of US and CS) relative to the random shock control than healthy women. Other areas that showed increased activation with fear acquisition in PTSD included bilateral superior temporal gyrus (BA 22), cerebellum, bilateral inferior frontal gyrus (BA 44, 45), and posterior cingulate (BA 24). Fear acquisition was associated with decreased function in medial prefrontal cortex, visual association cortex, and medial temporal cortex, inferior parietal lobule function, and other areas. Extinction of fear responses was associated with decreased function in the orbitofrontal and medial prefrontal cortex (including subcallosal gyrus, BA 25, and anterior cingulate BA 32), visual association cortex, and other areas, in the PTSD subjects, but not in the controls. Amygdala blood flow with fear acquisition was negatively correlated with medial prefrontal blood flow with fear extinction (increased blood flow in amygdala correlated with decreased blood flow in medial prefrontal cortex) in all subjects (r=-0.48; P<0.05). Increased amygdala blood flow with fear acquisition was positively correlated with PTSD (r=0.45), anxiety (r=0.44) and dissociative (r=0.80) symptom levels in PTSD (but not non-PTSD) subjects. There was a negative correlation between medial prefrontal blood flow during extinction and anxiety as measured with the Panic Attack Symptom Scale (PASS) during extinction in the PTSD group only, which was significant after correction for multiple comparisons (r=-0.90; P=0.006).190 This study was consistent with increased amygdala function with fear acquisition, and decreased medial prefrontal (anterior cingulate) function during extinction in PTSD. This is consistent with the model of an overactive amygdala and a failure of medial prefrontal cortex to extinguish, or shut off, the amygdala, when the acute threat is no longer present.
Treatment of PTSD
Intervening soon after the trauma is critical for long-term outcomes, since with time traumatic memories become indelible and resistant to treatment.213 Early treatments are not necessarily effective. For instance, studies have shown that Critical Incident Stress Debriefing (CISD) can be associated with a worsening of outcome relative to no treatment at all.214 Pharmacological treatment of chronic PTSD has shown efficacy originally for imipramine,215 amitriptyline,216 and phenalzine,215 and later for brofaramine,217 paroxetine,218,219 and sertraline.220 Selective serotonin reuptake inhibitors (SSRIs) and tianeptine are now recommended as first-line treatment for PTSD.221-226
The utility of early treatment is also demonstrated by animal studies showing that pretreatment before stress with antidepressants reduces chronic behavioral deficits related to stress.227,228 Antidepressants, including both norepinephrine and serotonin reuptake inhibitors, as well as gabapentine and phenytoin, promote nerve growth (neurogenesis) in the hippocampus, while stress inhibits neurogenesis.63,64,66,69,71,75,229 This is important because hippocampal neurogenesis has been shown to be required for antidepressant response.74
Few studies have examined the effects of pharmacological treatment on brain structure and function in patients with trauma-related mental disorders. We studied a group of patients with depression and found no effect of fluoxetine on hippocampal volume, although there were increases in memory function230 and hippocampal activation measured with PET during a memory encoding task. Depressed patients with a history of childhood trauma were excluded, and we subsequently have found hippocampal volume reductions at baseline in women with early abuse and depression but not in women with depression without early abuse;198 this suggests that the study design of excluding patients with early trauma may account for the negative result. Other studies in depression showed that smaller hippocampal volume was a predictor of resistance to antidepressant treatment.231 Smaller orbitofrontal cortex volume is associated with depression; one study in geriatric depression found smaller orbitofrontal cortex volume, while length of antidepressant exposure was correlated with larger orbitofrontal volume.232
Several studies have looked at functional brain imaging response to antidepressants in depression. Single photonemission computed tomography (SPECT) blood flow studies in depression showed that antidepressants increased anterior cingulate, right putamen, and right thalamus function.233 SPECT Xenon-133 studies showed reduced prefrontal function at baseline in depression, with treatment responders showing reduced perfusion in prefrontal cortex compared with non-responders after treatment.234 In a fluorodeoxyglucose (FDG) PET study of brain function patients with depression treated with fluoxetine who had a positive response to treatment had limbic and striatal decreases (subgenual cingulate, hippocampus, insula, and pallidum) and brain stem and dorsal cortical increases (prefrontal, parietal, anterior, and posterior cingulate) in function. Failed response was associated with a persistent 1-week pattern and absence of either subgenual cingulate or prefrontal changes.235 Sertraline resulted in an increase in middle frontal gyrus activity in depression measured with PET FDG, as well as increased function in right parietal lobe and visual association cortex.236 Successful paroxetine therapy of depression was associated with increased glucose metabolism measured with PET in dorsolateral, ventrolateral, and medial aspects of the prefrontal cortex, parietal cortex, and dorsal anterior cingulate. Areas of decreased metabolism were noted in both anterior and posterior insular regions (left) as well as right hippocampal and parahippocampal regions.237 In another PET FDG study, at baseline, subjects with depression had higher normalized metabolism than controls in the prefrontal cortex (and caudate and thalamus), and lower metabolism in the temporal lobe. With treatment with paroxetine, subjects with depression had metabolic changes in the direction of normalization in these regions.238 A PET FDG study of patients with depression and controls showed that at baseline, the mean metabolism was increased in the left and right lateral orbital cortex/ventrolateral prefrontal cortex (PFC), left amygdala, and posterior cingulate cortex, and decreased in the subgenual anterior cingulate cortex (ACC) and dorsal medial/dorsal anterolateral PFC in depressives relative to controls. Following treatment with antidepressants, metabolism significantly decreased in the left amygdala and left subgenual ACC. The metabolic reduction in the amygdala and right subgenual ACC appeared largely limited to those subjects who both responded to treatment and remained well at 6 months' follow-up.239 Another study showed that antidepressant treatment of depression resulted in a decrease in amygdala activation with emotional faces as measured with fMRI.240 In summary, studies show changes in limbic and prefrontal cortical regions with successful antidepressant treatment of depression.
Fewer studies have looked at the effects of pharmacological treatment on the brain in anxiety disorders. One PET FDG study showed that caudate function decreased with treatment of obsessive compulsive disorder with antidepressants.241 Paroxetine resulted in a decrease in glutamate/glutamine measured with magnetic resonance spectroscopy (MRS) in children with obsessive-compulsive disorder (OCD).242 Patients with PTSD were shown to have an increase in hippocampal volume and memory function with paroxetine,163 and increased right hippocampal and right cerebral volume with phenytoin.165 No published studies have looked at the effects of pharmacological treatment on brain function in PTSD, or on sensitive markers of brain chemistry like NAA.
Figure 2. Neural correlates of fear conditioning in women with abuse and PTSD. There was increased amygdala activation with fear acquisition using a classical conditioning paradigm relative to nonPTSD abused women. PTSD, post-traumatic stress disorder.
Brain biomarkers like NAA represent an objective marker of neural plasticity. To date psychiatry has relied on subjective reports as the gold standard. However, this is limited by self-reporting and the subjective interpretations of symptoms and response to treatment. Brain markers of antidepressant response may provide a complementary approach to assessing response to treatment, as well as providing insight into the mechanisms of treatment response. Our group is trying to look at mechanisms in the brain underlying treatment response in PTSD.
Effects of pharmacotherapy on brain function and structure in PTSD
We have begun to assess the effects of pharmacotherapy on brain structure and function in PTSD.243 We recently assessed the effects of phenytoin on brain structure and function. Studies in animals show that phenytoin, which is used in the treatment of epilepsy and is known to modulate glutamatergic function, blocks the effects of stress on the hippocampus.67 We studied nine patients with PTSD in an open-label function before and after treatment with phenytoin. Phenytoin resulted in a significant improvement in PTSD symptoms.164 Phenytoin also resulted in increases in both right hippocampal volume and right hemisphere volume.165 These findings indicate that phenytoin has an effects on PTSD symptoms as well as brain structure in PTSD patients.
We have assessed the effects of open4abel paroxetine on memory and the hippocampus in PTSD. Male and female patients with symptoms of PTSD were medication-free for at least 4 weeks before participation in the study. Twenty-eight patients were found to be eligible and started the medication phase. Of the total patient sample five patients did not finish due to noncompliance; 23 patients completed the study.
Before patients started the medication phase, neuropsychological tests were administered, including the Wechsler Adult Intelligence Scale - Revised, WAISR (arithmetic, vocabulary, picture arrangement, and block design test), two subtests of the Wechsler Memory ScaleRevised.WMS-R, including logical memory (free recall of two story narratives, which represents verbal memory) and figural memory (which represents visual memory and involved reproduction of designs after a 6-second presentation); and the verbal and visual components of the Selective Reminding Test, SRT.
Paroxetine was prescribed in the first visit after the pre-treatment assessments. All patients started open-label with a dose of 10 mg daily and were titrated up to 20 mg in 4 days.
Paroxetine treatment resulted in a mean 54% reduction in PTSD symptoms as measured with mean changes from baseline on the CAPS total score (P<0.005) among study completers. Improvement was equally strong on all symptom cluster scores (Reexperiencing, Avoidance/Numbing, Hyperarousal). Treatment also resulted in significant improvements in verbal declarative memory as measured with the WMS-R paragraph recall for delayed recall (P<0.005) and percent retention (80.2 to 91.1; P=0.003), but not immediate recall. Improvements were significant on all subscales of the Verbal Component of the SRT; including long-term recall and delayed recall.
Repeated measures ANOVA with side as the repeated measure showed a main effect for treatment related to a 4.6% increase in mean hippocampal volume (1857.3 mm3 [SD 225.6] to 1906.2 mm3, [SD 243.2]) with treatment (F=8.775 df=1.36; P=0.005). Increased hippocampal volume was seen for both left (5.6%) (1807.6 mm3 [SD 255.5] to 1909.3 mm3 [SD 236.9]) and right (3.7%) (1906.9 mm3 [SD 195.8] to 1976.7 mm3 [SD 249.6]) hippocampus. There was no change in whole brain volume with treatment. Increase in hippocampal volume was significant after adding whole brain volume before and after treatment to the model.
Discussion
Traumatic stress has a broad range of effects on brain function and structure, as well as on neuropsychological components of memory. Brain areas implicated in the stress response include the amygdala, hippocampus, and prefrontal cortex. Neurochemical systems, including Cortisol and norepinephrine, play a critical role in the stress response. These brain areas play an important role in the stress response. They also play a critical role in memory, highlighting the important interplay between memory and the traumatic stress response. Preclinical studies show that stress affects these brain areas. Furthermore, antidepressants have effects on the hippocampus that counteract the effects of stress. In fact, promotion of nerve growth (neurogenesis) in the hippocampus may be central to the efficacy of the antidepressants. Studies in patients with PTSD show alterations in brain areas implicated in animal studies, including the amygdala, hippocampus, and prefrontal cortex, as well as in neurochemical stress response systems, including Cortisol and norepinephrine. Treatments that are efficacious for PTSD show a promotion of neurogenesis in animal studies, as well as promotion of memory and increased hippocampal volume in PTSD. Future studies are needed to assess neural mechanisms in treatment response in PTSD.
Selected abbreviations and acronyms
- ACTH
adrenocorticotropic hormone
- BDNF
brain-derived neurotropic factor
- BPD
bipolar disorder
- CRF
corticotropin-releasing factor
- CS
conditioned stimulus
- FDG
fluorodeoxyglucose
- HPA
hypothalamic-pituitary-adrenal
- MRI
magnetic resonance imaging
- mRNA
messenger ribonucleic acid
- NAA
N-acetyl aspartate
- PET
positron emission tomography
- PTSD
post-traumatic stress disorder
- US
unconditioned stimulus
REFERENCES
- 1.Kessler RC., Sonnega A., Bromet E., et al. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Franklin CL., Zimmerman M. Posttraumatic stress disorder and major depressive disorder: Investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189:548–551. doi: 10.1097/00005053-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Prigerson HG., Maciejewski PK., Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment and abuse among men. J Nerv Ment Dis. 2001;189:99–108. doi: 10.1097/00005053-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD., Southwick SM., Darnell A., et al. Chronic PTSD in Vietnam combat veterans: Course of illness and substance abuse. Am J Psychiatry. 1996;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Putnam FW., Guroff JJ., Silberman EK., et al. The clinical phenomenology of multiple personality disorder: a review of 100 recent cases. J Clin Psychiatry. 1986;47:285–293. [PubMed] [Google Scholar]
- 6.Battle CL., Shea MT., Johnson DM., et al. Childhood maltreatment associated with adult personality disorders: findings from the Collaborative Longitudinal Personality Disorders Study. J Personal Disord. 2004;18:193–211. doi: 10.1521/pedi.18.2.193.32777. [DOI] [PubMed] [Google Scholar]
- 7.Yen S., Shea MT., Battle CL., et al. Traumatic exposure and posttraumatic stress disorder in borderline, schizotypal, avoidant, and obsessive-compulsive personality disorders: findings from the collaborative longitudinal personality disorders study. J Nerv Ment Dis. 2002;190:510–518. doi: 10.1097/00005053-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Dube SR., Felitti VJ., Dong M., et al. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 9.Saigh PA., Bremner JD. Posttraumatic Stress Disorder: a Comprehensive Text. Needham Heights, Mass: Allyn & Bacon; 1999 [Google Scholar]
- 10.Druss BG., Goldman HH. Introduction to the Special Section on the President's New Freedom Commission Report. Psychiatr Serv. 2003;54:1465–1466. doi: 10.1176/appi.ps.54.11.1465. [DOI] [PubMed] [Google Scholar]
- 11.Glover RW., Birkel R., Faenza M., et al. New Freedom Commission Report: The Campaign for Mental Health Reform: A new advocacy partnership. Psychiatric Services. 2003;54:1475–1479. doi: 10.1176/appi.ps.54.11.1475. [DOI] [PubMed] [Google Scholar]
- 12.Hogan MF. New Freedom Commission Report - The President's New Freedom Commission: Recommendations to transform mental health care in America. Psychiatric Services. 2003;54:1467–1474. doi: 10.1176/appi.ps.54.11.1467. [DOI] [PubMed] [Google Scholar]
- 13.Durston S., Hulshoff P., Hilleke E., et al. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adoiesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Gïedd JN., Blumenthal J., Jeffries NO., et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 15.Casey BJ., Giedd JN., Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychiatry. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 16.Paus T., Zijdenbos A., Worsley K., et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 17.Sowell ER., Thompson PM., Holmes CJ., et al. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport JL., Giedd JN., Blumenthal J., et al. Progressive cortical change during adolescence in childhood-onset schizophrenia: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- 19.Thompson PM., Giedd JN., Woods RP., et al. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN., Blumenthal J., Jeffries NO. Development of the normal human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 21.Giedd JN., Vaituzis AC., Hamburger SD., et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN., Castellanos FX., Rajapakse JC., et al. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 23.Pfefferbaum A., Mathalon DH., Sullivan EV., et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;34:71–75. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 24.Giedd JN., Snell JW., Lange N., et al. Quantitative magnetic resonance imaging of human brain development: ages 14-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan TL., Archibald SL., Berhow MT., et al. Cerebral structure on MRI, Part I: localization of age-related changes. Biol Psychiatry. 1991;28:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- 26.Pfefferbaum A., Sullivan EV., Rosenbloom MJ., et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan EV., Marsh L., Mathalon DH., et al. Age-related decline in MRI volumes of temporal gray matter but not hippocampus. Neurobiology Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 28.Gould E., WooIIey CS., Frankfurt M., et al. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaywïtz SE., Shaywitz BA., Pugh KR., et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 30.Lupien SJ., de Leon M., de Santi S., et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan EV., Desmond JE., Deshmukh A., et al. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's Syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan TL., Press GA., Hesselink JR. Methods for measuring brain morphologic features on magnetic resonance images: validation and normal aging. Arch Neurol. 1990;47:27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- 33.Lïm KO., Zipursky RB., Watts MC., et al. Decreased gray matter in normal aging: an in vivo magnetic resonance study. J Gerontol: Biol Sci. 1992;47:B26–B30. doi: 10.1093/geronj/47.1.b26. [DOI] [PubMed] [Google Scholar]
- 34.Vermetten E., Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment of PTSD. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 35.Bremner JD. Does Stress Damage the Brain? Understanding Trauma-related Disorders from a Mind-Body Perspective. New York, NY: W.W. Norton; 2002 [Google Scholar]
- 36.Pitman RK. Investigating the pathogenesis of posttraumatic stress disorder with neuroïmagïng. J Clin Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- 37.Vermetten E., Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- 38.Arborelius L., Owens MJ., Plotsky PM., et al. The role of corticotropinreleasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 39.Melia KR., Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad sci US A. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremner JD., Krystal JH., Southwïck SM., et al. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Bremner JD., Krystal JH., Southwïck SM., et al. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Abercrombie ED., Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levïne S., Weiner SG., Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;4:297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- 44.Frïde E., Dan Y., Feldon J., et al. Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- 45.Stanton ME., Gutierrez YR., Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 46.Ladd CO., Owens MJ., Nemeroff CB. Persistent changes in CRF neuronal systems produced by maternal separation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM., Krey LC., McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- 48.Sapolsky R., Krey L., McEwen B. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makino S., Schulkin J., Smith MA., et al. Regulation of corticotropinreleasing hormone receptor messenger-ribonucleic acid in the rat-brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- 50.Plotsky PM., Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 51.Makino S., Smith MA., Gold PW. Increased expression of corticotropinreleasing hormone and vasopressin messenger-ribonucleic acid (messenger RNA) in the hypothalamic paraventricular nucleus during repeated stressassociation with reduction in glucocorticoid messenger-RNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 52.Coplan JD., Andrews MW., Rosenblum LA., et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad sci U S A. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elzinga BM., Bremner JD. Are the neural substrates of memory the final common pathway in PTSD? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- 55.Gould E., Tanapat P., McEwen BS., et al. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magarinos AM., McEwen BS., Flugge G., et al. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McEwen BS., Angulo J., Cameron H., et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 58.Nibuya M., Morinobu S., Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sapolsky RM., Uno H., Rebert CS., et al. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 61.Luine V., Villages M., Martinex C., et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 62.Diamond DM., Fleshner M., Ingersoll N., et al. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 63.Malberg JE., Eïsch AJ., Nestler EJ., et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czeh B., Michaelis T., Watanabe T., et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santarellï L., Saxe M., Gross C., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 66.Lucassen PJ., Fuchs E., Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Eur J Neurosci. 2004;14:161–166. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe YE., Gould H., Cameron D., et al. Phenytoin prevents stress and corticosterone induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 68.Garcia R. Stress, metaplasticity, and antidepressants. Curr Moi Med. 2002;2:629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- 69.D'Sa C., Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 70.Duman RS., Heninger GR., Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 71.Duman RS., Malberg JE., Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 72.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 73.McEwen BS., Chattarjï S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14 (suppl 5):S497–502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Santarelli L., Saxe M., Gross C., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe Y., Gould E., Daniels DC., et al. Tianeptine attenuates stressinduced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 76.Henn FA., Vollmayr B. Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry. 2004;56:146–150. doi: 10.1016/j.biopsych.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Kempermann G., Kuhn HG., Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould E., Beylin A., Tanapat P., et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 79.Meaney MJ., Aitken D., van Berkel C., et al. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–769. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 80.Liu D., Diorio J., Day JC., et al. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;8:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 81.Yehuda R., Southwïck SM., Nussbaum EL., et al. Low urinary Cortisol in PTSD. J Nerv Ment Dis. 1991;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Bremner JD., Licinio J., Darnell A., et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delahanty DL., Raîmonde AJ., Spoonster E., et al. Injury severity, prior trauma history, urinary Cortisol levels, and acute PTSD in motor vehicle accident victims. J Anxiety Disord. 2003;17:149–164. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 84.Yehuda R., Kahana B., Binder-Brynes K., et al. Low urinary Cortisol excretion in holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 85.Yehuda R., Teicher MH., Levengood RA., et al. Circadian regulation of basal Cortisol levels in posttraumatic stress disorder. Ann NY Acad Sci. 1994:378–380. doi: 10.1111/j.1749-6632.1994.tb39260.x. [DOI] [PubMed] [Google Scholar]
- 86.Yehuda R., Teicher MH., Trestman RL., et al. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 87.Baker DB., West SA., Nicholson WE., et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 88.Young EA., Breslau N. Saliva Cortisol in posttraumatic stress disorder: A community epidemiologic study. Biol Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Young EA., Toman T., Wïtkowski K., et al. Salivary Cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Mason J., Wang S., Yehuda R., et al. Marked lability of urinary free Cortisol levels in sub-groups of combat veterans with posttraumatic stress disorder. Psychosom Med. 2002;64:238–246. doi: 10.1097/00006842-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Young EA., Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiological community study. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- 92.Elzinga BM., Schmahl CS., Vermetten E., et al. Higher Cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 93.Cïcchettï D., Walker EF. Stress and development: biological and psychological consequences. Dev Psychopathol. 2001;13:413–418. [PubMed] [Google Scholar]
- 94.De Bellis MD., Chrousos GP., Dorn LD., et al. Hypothalamic pituitary adrenal dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 95.Lemieux AM., Coe CL. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 96.De Bellis MD., Baum AS., Keshavan MS., et al. A.E. Bennett Research Award: Developmental traumatology: Part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 97.Gunnar MR., Morison SJ., Chisolm K., et al. Salivary Cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 98.Cïcchettï D., Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- 99.Luby JL., Heffelfinger A., Mrakotsky C., et al. Alterations in stress Cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. Dec 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 100.Stein MB., Yehuda R., Koverola C., et al. Enhanced dexamethasone suppression of plasma Cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 101.Elzinga BM., Schmahl CS., Vermetten E., et al. Increased Cortisol responses to the stress of traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 102.Bremner JD., Vythilingam M., Vermetten E., et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 103.Bremner JD., Vythilingam M., Vermetten E., et al. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (PTSD). Psychiatry Res. 2005;129:1–10. doi: 10.1016/j.psychres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 104.Heim C., Newport DJ., Heit S., et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 105.Heim C., Newport DJ., Bonsall R., et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001 ;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 106.Buckley TC., Blanchard EB., Neill WT. Information processing and PTSD: A review of the empirical literature. Clin Psychol Rev. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 107.Brewïn CR. A cognitive neuroscience account of post-traumatic stress disorder and its treatment. Behav Res Ther. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 108.Golier J., Yehuda R. Neuroendocrine activity and memory-related impairments in posttraumatic stress disorder. Dev Psychopathol. 1998;10:857–869. doi: 10.1017/s0954579498001904. [DOI] [PubMed] [Google Scholar]
- 109.Vasterling JJ., Brailey K., Constans Jl., et al. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 110.Bremner JD., Scott TM., Delaney RC., et al. Deficits in short-term memory in post-traumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 111.Golier J., Yehuda R., Cornblatt B., et al. Sustained attention in combatrelated posttraumatic stress disorder. Integr Physiol Behav Sci. 1997;32:52–61. doi: 10.1007/BF02688613. [DOI] [PubMed] [Google Scholar]
- 112.Yehuda R., Keefe RS., Harvey PD., et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- 113.Uddo M., Vasterlîng JJ., Braily K., et al. Memory and attention in posttraumatic stress disorder. J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 114.Bremner JD., Vermetten E., Nafzal N., et al. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD). J Nerv Ment Dis. 2004;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- 115.Bremner JD., Randall PR., Capelli S., et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- 116.Gilbertson MW., Gurvits TV., Lasko NB., et al. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–420. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- 117.Jenkins MA., Langlais PJ., Delis D., et al. Learning and memory in rape victims with posttraumatic stress disorder. Am J Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- 118.Moradi AR., Doost HT., Taghavi MR., et al. Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioural Memory Test. J Child Psychol Psychiatry. 1999;40:357–361. [PubMed] [Google Scholar]
- 119.Roca V., Freeman TW. Complaints of impaired memory in veterans with PTSD. Am J Psychiatry. 1739. 2001;158:1738. doi: 10.1176/appi.ajp.158.10.1738-a. [DOI] [PubMed] [Google Scholar]
- 120.Vasterlîng JJ., Duke LM., Brailey K., et al. Attention, learning, and memory performance and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 121.Barrett DH., Green ML., Morris R., et al. Cognitive functioning and posttraumatic stress disorder. Am J Psychiatry. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- 122.Gil T., Calev A., Greenberg D., et al. Cognitive functioning in posttraumatic stress disorder. J Trauma Stress. 1990;3:29–45. [Google Scholar]
- 123.SachinvaIa N., vonScotti H., McGuire M., et al. Memory, attention, function, and mood among patients with chronic posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 124.Golier JA., Yehuda R., Lupien SJ., et al. Memory performance in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 2002;159:1682–1688. doi: 10.1176/appi.ajp.159.10.1682. [DOI] [PubMed] [Google Scholar]
- 125.Yehuda R., Golier JA., Harvey PD., et al. Relationship between Cortisol and age-related memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30:678–687. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Yehuda R., Golier JA., Tischler L., et al. Learning and memory in aging combat veterans with PTSD. J Clin Exp Neuropsychol. 2005;27:504–515. doi: 10.1080/138033990520223. [DOI] [PubMed] [Google Scholar]
- 127.Stein MB., Hanna C., Vaerum V., et al. Memory functioning in adult women traumatized by childhood sexual abuse. J Trauma Stress. 1999;12:527–534. doi: 10.1023/A:1024775222098. [DOI] [PubMed] [Google Scholar]
- 128.Zalewski C., Thompson W., Gottesman I. Comparison of neuropsychological test performance in PTSD, generalized anxiety disorder, and control Vietnam veterans. Assessment. 1994;1:133–142. doi: 10.1177/1073191194001002003. [DOI] [PubMed] [Google Scholar]
- 129.Bremner JD., Steinberg M., Southwïck SM., et al. Use of the Structured Clinical Interview for DSMIV-Dissociative Disorders for systematic assessment of dissociative symptoms in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- 130.McNally RJ., Lïtz BT., Prassas A., et al. Emotional priming of autobiographical memory in posttraumatic stress disorder. Cogn Emot. 1994;8:351–367. [Google Scholar]
- 131.Cassiday KL., McNally RJ., Zeitlin SB. Cognitive processing of trauma cues in rape victims with posttraumatic stress disorder. Cogn Ther Res. 1992;16:283–295. [Google Scholar]
- 132.Foa EB., Feske U., Murdock TB., et al. Processing of threat related information in rape victims. J Abnorm Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- 133.McNally RJ., Kaspi RJ., Riemann BC., et al. Selective processing of threat cues in posttraumatic stress disorder. J Abnorm Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- 134.McNally RJ., English GE., Lipke HJ. Assessment of intrusive cognition in PTSD: Use of the modified Stroop paradigm. J Trauma Stress. 1993;6:33–41. [Google Scholar]
- 135.Moradi AR., Taghavï R., Neshat-Doost HT., et al. Memory bias for emotional information in children and adolescents with posttraumatic stress disorder: A preliminary study. J Anxiety Disord. 2000;14:521–534. doi: 10.1016/s0887-6185(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 136.Bryant RA., Harvey AG. Processing threatening information in posttraumatic stress disorder. J Abnorm Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- 137.Beck JG., Freeman JB., Shîpherd JC., et al. Specificity of Stroop interference in patients with pain and PTSD. J Abnorm Psychology. 2001;110:536–543. doi: 10.1037//0021-843x.110.4.536. [DOI] [PubMed] [Google Scholar]
- 138.McNeil DW., Tucker P., Miranda R., et al. Response to depression and anxiety Stroop stimuli in posttraumatic stress disorder, obsessive-compulsive disorder and major depressive disorder. J Nerv Ment Dis. 1999;187:512–516. doi: 10.1097/00005053-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 139.Thrasher SM., Dalgleish T., Yule W. Information processing in post-traumatic stress disorder. Behav Res Ther. 1994;32:247–254. doi: 10.1016/0005-7967(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 140.Golier JA., Yehuda R., Lupien SJ., et al. Memory for trauma-related information in Holocaust survivors with PTSD. Psychiatry Res. 2003;121:133–143. doi: 10.1016/s0925-4927(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 141.Beckham JC., Crawford AL., Feldman ME. Trail making test performance in Vietnam combat veterans with and without posttraumatic stress disorder. J Trauma Stress. 1998;11:811–819. doi: 10.1023/A:1024409903617. [DOI] [PubMed] [Google Scholar]
- 142.Sass KJ., Spencer DD., Kim JH., et al. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40:1694–1697. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- 143.Sass KJ., Buchanan CP., Kraemer S., et al. Verbal memory impairment resulting from hippocampal neuron loss among epileptic patients with structural lesions. Neurology. 1995;45:2154–2158. doi: 10.1212/wnl.45.12.2154. [DOI] [PubMed] [Google Scholar]
- 144.Bremner JD., Randall PR., Scott TM., et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bremner JD., Randall PR., Vermetten E., et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Narayan M., Bremner JD., Kumar A. Neuroanatomical substrates of latelife mental. disorders. J G eriatr Psychiatry Neurol. 1999;12:95–106. doi: 10.1177/089198879901200303. [DOI] [PubMed] [Google Scholar]
- 147.Gurvits TG., Shenton MR., Hokama H., et al. Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:192–199. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stein MB., Koverola C., Hanna C., et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 149.Bremner JD., Vythilingam M., Vermetten E., et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD). Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 150.Freeman TW., Cardwell D., Karson CN., et al. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combatrelated posttraumatic stress disorder. Magn Reson Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 151.Gilbertson MW., Shenton ME., Ciszewskï A., et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schuff N., Neylan TC., Lenoci MA., et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.VilIarreal G., Hamilton DA., Petropoulos H., et al. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 154.De Bellis MD., Keshavan MS., Clark DB., et al. A.E. Bennett Research Award: Developmental traumatology: Part II. Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 155.Carrion VG., Weems CF., Elîez S., et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 156.De Bellis MD., Hall J., Boring AM., et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 157.Bonne O., Brandes D., Gilboa A., et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Notestine CF., Stein MB., Kennedy CM., et al. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;51:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 159.De Bellis MD., Keshavan MS., Spencer S., et al. N-acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 160.Kitayama N., Vaccarïno LV., Kutner M., et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 161.Yehuda R., Golier JA., Tischler L., et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: Relation to risk and resilience factors. J Psychiatr Res. Jan 27, 2006 [Epub ahead of print] doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 162.Shin LM., Shin PS., Heckers S., et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 163.Vermetten E., Vythilingam M., Southwick SM., et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bremner JD., MIetzko T., Welter S., et al. Treatment of posttraumatic stress disorder with phenytoin: An open label pilot study. J Clin Psychiatry. 2004;65:1559–1564. doi: 10.4088/jcp.v65n1120. [DOI] [PubMed] [Google Scholar]
- 165.Bremner JD., MIetzko T., Welter S., et al. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: A pilot study. J Psychopharmacol. 2005;19:159–165. doi: 10.1177/0269881105048996. [DOI] [PubMed] [Google Scholar]
- 166.Yehuda R. Are glucocorticoids responsible for putative hippocampal damage in PTSD? How and when to decide. Hippocampus. 2001;11:85–89. doi: 10.1002/hipo.1025. [DOI] [PubMed] [Google Scholar]
- 167.Pitman RK. Hippocampal dimunition in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- 168.Bremner JD. Hypotheses and controversies related to the effects of stress on the hippocampus: An argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder (PTSD). Hippocampus. 2001;11:75–81. doi: 10.1002/hipo.1023. [DOI] [PubMed] [Google Scholar]
- 169.Sapolsky RM. Atrophy of the hippocampus in posttraumatic stress disorder: how and when? Hippocampus. 2001;11:90–91. doi: 10.1002/hipo.1026. [DOI] [PubMed] [Google Scholar]
- 170.ackIin ML., Metzger LJ., Litz BT., et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol. 1998;66:323–326. doi: 10.1037//0022-006x.66.2.323. [DOI] [PubMed] [Google Scholar]
- 171.Radley JJ., Sisti HM., Hao J., et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 172.Rauch SL., Shin LM., Segal E., et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 173.Bremner JD., Innis RB., Ng CK., et al. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–256. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 174.Bremner JD., Staib L., Kaloupek D., et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lanius RA., Williamson PC., Hopper J., et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 176.Bremner JD., Narayan M., Staib LH., et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lanius RA., Williamson PC., Densmore M., et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 178.Liberzon I., Taylor SF., Amdur R., et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 179.Shin LH., McNally RJ., Kosslyn SM., et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 180.Shin LM., Kosslyn SM., McNally RJ., et al. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–237. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 181.Shin LM., Orr SP., Carson MA., et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 182.SempIe WE., Goyer P., McCormick R., et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared to controls. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 183.Shin LM., Wright CI., Cannistraro PA., et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 184.Rauch SL., van der Kolk BA., Fïsler RE., et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 185.Shin LM., Whalen PJ., Pitman RK., et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 186.Rauch SL., Whalen PJ., Shin LM., et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 187.Bremner JD., Soufer R., McCarthy G., et al. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmacol Bull. 2001;35:55–87. [PubMed] [Google Scholar]
- 188.Bremner JD., Vythilingam M., Vermetten E., et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder (PTSD) related to early childhood sexual abuse. Biol Psychiatry. 2003;53:289–299. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 189.Bremner JD., Vermetten E., Vythilingam M., et al. Neural correlates of the classical color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 190.Bremner JD., Vermetten E., Schmahl C., et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bremner JD. Neuroimaging studies in posttraumatic stress disorder. Curr Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 192.Ito Y., Teicher MH., Glod CA., et al. Increased prevalence of electrophysiological abnormalities in children with psychological, physical and sexual abuse. J Neuropsychiatry Clin Neurosci. 1993;5:401–408. doi: 10.1176/jnp.5.4.401. [DOI] [PubMed] [Google Scholar]
- 193.Schiffer F., Teicher MH., Papanicolaou AC. Evoked potential evidence for right brain activity during the recall of traumatic memories. J Neuropsychiatry Clin Neurosci. 1995;7:169–175. doi: 10.1176/jnp.7.2.169. [DOI] [PubMed] [Google Scholar]
- 194.De Bellis MD., Keshavan MS., Frustaci K., et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 195.Anderson CM., Teicher MH., Polcarï A., et al. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 196.Vythilingam M., Lam T., Morgan CA., et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psych Res: Neuroimaging. 2005;139:89–99. doi: 10.1016/j.pscychresns.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 197.Bremner JD., Narayan M., Anderson ER., et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 198.Vythilingam M., Heim C., Newport CD., et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002; 159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schmahl CG., Vermetten E., Elzinga BM., et al. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psych Res: Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 200.Vermetten E., Schmahl C., Lindner S., et al. Hippocampal and amygdalar volumes in Dissociative Identity Disorder. Am J Psychiatry. 2006;163:1–8. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vythilingam M., Anderson E., Goddard A., et al. Temporal lobe volumes in panic disorder-a quantitative magnetic resonance imaging study. Psych Res: Neuroimaging. 2000;99:75–82. doi: 10.1016/s0925-4927(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 202.Bremner JD., Narayan M., Charney DS. Hippocampus and amygdala pathology in depression: Dr. Bremner and colleagues reply. Am J Psychiatry. 2001;158:653. doi: 10.1176/appi.ajp.158.4.652-a. [DOI] [PubMed] [Google Scholar]
- 203.Bremner JD., Vythilingam M., Vermetten E., et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 204.Kitayama N., Quinn S., Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. Jan 10, 2006 [Epub ahead of print] doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bremner JD., Vermetten E. Stress and development: Behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- 206.Bremner JD. Neuroimaging of childhood trauma. Sem Clin Neuropsychiatry. 2002;7:104–112. doi: 10.1053/scnp.2002.31787. [DOI] [PubMed] [Google Scholar]
- 207.Bremner JD. Effects of traumatic stress on brain structure and function: Relevance to early responses to trauma. In: Cardena E, Croyle K, eds. Acute Reactions to Trauma and Psychotherapy: Multidisciplinary and International Perspective. Binghamton, NY: Haworth Medical Press; 2005 doi: 10.1300/J229v06n02_06. [DOI] [PubMed] [Google Scholar]
- 208.Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiat Clin NA. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 209.Bremner JD., Vermetten E., Vythilingam M., et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 210.Schmahl CG., Elzinga BM., Vermetten E., et al. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol Psychiatry. 2003;54:42–51. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- 211.Schmahl CG., Elzinga BM., Bremner JD. Individual differences in psychophysiological reactivity in adults with childhood abuse. Clin Psychol Psychother. 2002;9:271–276. [Google Scholar]
- 212.First MB., Spitzer RL., Williams JBW., et al. Structured Clinical Interview for DSM-IV-Patient Edition (SCID-P). Washington, DC: American Psychiatric Press; 1995 [Google Scholar]
- 213.Meadows EA., Foa EB. Cognitive-behavioral treatment of traumatized adults. In: Saigh PA, Bremner JD, eds. Posttraumatic Stress Disorder: A Comprehensive Text. Needham Heights, Mass: Allyn & Bacon; 1999:376–390. [Google Scholar]
- 214.Mayou RA., Ehlers A., Hobbs M. Psychological debriefing for road traffic accident victims. Three-year follow-up of a randomised controlled trial. Br J Psychiatry. 2000;176:589–593. doi: 10.1192/bjp.176.6.589. [DOI] [PubMed] [Google Scholar]
- 215.Frank JB., Kosten TR., Giller EL., et al. A randomized clinical trial of phenelzine and imipramine for posttraumatic stress disorder. Am J Psychiatry. 1988;145:1289–1291. doi: 10.1176/ajp.145.10.1289. [DOI] [PubMed] [Google Scholar]
- 216.Davidson J., Kudler H., Smith R., et al. Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry. 1990;47:259–266. doi: 10.1001/archpsyc.1990.01810150059010. [DOI] [PubMed] [Google Scholar]
- 217.Baker DG., Diamond BI., Gillette G., et al. A double-blind, randomized, placebo-controlled, multi-center study of brofaromine in the treatment of post-traumatic stress disorder. Psychopharmacology. 1995;122:386–389. doi: 10.1007/BF02246271. [DOI] [PubMed] [Google Scholar]
- 218.Tucker P., Zaninelli R., Yehuda R., et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 219.Marshall RD., Beebe KL., Oldham M., et al. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 220.Brady KT., Pearlstein T., Asnis GM., et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 221.Foa EB., Davidson JRT., Frances A., et al. The expert consensus guideline series: treatment of posttraumatic stress disorder. J Clin Psychiatry. 1999;60:4–76. [PubMed] [Google Scholar]
- 222.BaIIenger JC., Davidson JR., Lecrubïer Y., et al. Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2000;61:60–66. [PubMed] [Google Scholar]
- 223.Davidson JR. Pharmacotherapy of posttraumatic stress disorder: treatment options, long-term follow-up, and predictors of outcome. J Clin Psychiatry. 2000;61:52–56. [PubMed] [Google Scholar]
- 224.Stein DJ., Seedat S., van der Linden GJ., et al. Selective serotonin reuptake inhibitors in the treatment of posttraumatic stress disorder: a metaanalysis of randomized controlled trials. Int Clin Psychopharmacol. 2000;15:S31–S39. doi: 10.1097/00004850-200008002-00006. [DOI] [PubMed] [Google Scholar]
- 225.Davidson JR. Long-term treatment and prevention of posttraumatic stress disorder. J Clin Psychiatry. 2004;65(suppl 1):44–48. [PubMed] [Google Scholar]
- 226.Davis LL., English BA., Ambrose SM., et al. Pharmacotherapy for posttraumatic stress disorder: a comprehensive review. Exp Opin Pharmacother. 2001;2:1583–1595. doi: 10.1517/14656566.2.10.1583. [DOI] [PubMed] [Google Scholar]
- 227.Sherman AD., Petty F. Additivity of neurochemical changes in learned helplessness and imipramine. Behav Neurol Biol. 1982;35:344–353. doi: 10.1016/s0163-1047(82)90978-5. [DOI] [PubMed] [Google Scholar]
- 228.Petty F., Kramer G., Wilson L. Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacol Biochem Behav. 1992;43:361–367. doi: 10.1016/0091-3057(92)90163-a. [DOI] [PubMed] [Google Scholar]
- 229.Lee H., Kim JW., Yïm SV., et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:725–728. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- 230.Vythilingam M., Vermetten E., Anderson GM., et al. Hippocampal volume, memory and Cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 231.Hsieh MH., McQuoid DR., Levy RM., et al. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatric Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- 232.Lavretsky H., Roybal DJ., Ballmaier M., et al. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–967. doi: 10.4088/jcp.v66n0801. [DOI] [PubMed] [Google Scholar]
- 233.Goodwin GM., Austin MP., Dougall N., et al. State changes in brain activity shown by the uptake of 99mTc- exametazine with single photon emission tomography in major depression before and after treatment. J Affect Disord. 1993;29:245–255. doi: 10.1016/0165-0327(93)90014-b. [DOI] [PubMed] [Google Scholar]
- 234.Nobler MS., Roose SP., Prohovnik I., et al. Regional cerebral blood flow in mood disorders, V: Effects of antidepressant medication in late-life depression. Am J Geriatr Psychiatr. 2000;8:289–296. [PubMed] [Google Scholar]
- 235.Mayberg HS., Brannan SK., Tekell JL., et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 236.Buchsbaum MS., Wu J., Sîegel BV., et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 237.Kennedy SH., Evans KR., Kruger S., et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 238.Brody AL., Saxena S., Stoessel P., et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 239.Drevets WC., Bogers W., Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 240.Sheline Yl., Barch DM., Donnelly JM., et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 241.Baxter LR., Schwartz JM., Bergman KS. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- 242.Rosenberg DR., MacMïIIan SN., Moore GJ. Brain anatomy and chemistry may predict treatment response in paediatric obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2001;4:179–190. doi: 10.1017/S1461145701002401. [DOI] [PubMed] [Google Scholar]
- 243.Bremner JD., Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder (PTSD). Ann NY Acad Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]