Abstract
Although gender is increasingly perceived as a key determinant in health and illness, systematic gender studies in medicine are still lacking. For a long time, cardiovascular disease (CVD) has been seen as a “male” disease, due to men's higher absolute risk compared with women, but the relative risk in women of CVD morbidity and mortality is actually higher: Current knowledge points to important gender differences in age of onset, symptom presentation, management, and outcome, as well as traditional and psychosocial risk factors. Compared with men, CVD risk in women is increased to a greater extent by some traditional factors (eg, diabetes, hypertension, hypercholesterolemia, obesity,) and socioeconomic and psychosocial factors also seem to have a higher impact on CVD in women. With respect la differences in CVD management, a gender bias in favor of men has to be taken into account, in spite of greater age and higher comorbidity in women, possibly contributing to a poorer outcome. Depression has been shown to be an independent risk factor and consequence of CVD; however, concerning gender differences, The results have been inconsistent. Current evidence suggests that depression causes a greater increase in CVD incidence in women, and that female CVD patients experience higher levels of depression than men. Gensier aspects should be more intensively considered, both in further research on gender differences in comorbid depresion, and in cardiac treatment and rehabilitation, with the goal of making secondary prevention more effective.
Keywords: gener, cardiovascular disease, depression, risk factor, socioeconomic status, social support, job strain
Abstract
Aunque el género se percibe cada vez más como un factor determinante en la salud y la enfermedad, aun faltan estudios sistemáticos en medicina. Por mucho tiempo la enfermedad cardiovascular (ECV) ha sido considerada como una enfermedad del “hombres,” debido al mayor riesgo absoluto de los hombres en comparación con la mujeres; pero en realidad es mayor el riesgo relativo de morbi-mortalidad de la ECV en las mujeres. El conocimiento en este momento apunta a importantes diferencias de género en la edad de aparición, la presentación sintomática, el manejo y la evolución, como también a los factores de riesgo tradicionales y psicosociales. En comparación con los hombres, el riesgo de ECV en las mujeres es más importante debido en gran medida a algunos factores tradicionales (por ejemplo, diabetes, hipertensión, hipercolesterolemia, obesidad) y factores socioeconómicos y psicosociales, los que también parecen tener un mayor impacto en la ECV de las mujeres. Con respecto a las diferencias en el manejo de la ECV, debe tenerse en cuenta un sesgo de género a favor de los hombres, a pesar de una mayor edad y mayor comorbilidad en las mujeres, lo que posiblemente contribuye a una peor evolución. La depresión se ha considerado un factor de riesgo independiente y una consecuencia de la ECV; sin embargo, en relación con las diferencias por género, los resultados todavia no han sido consistentes. La evidencia actual sugiere que la depresión causa un mayor aumento en la incidencia de ECV en las mujeres y a su vez que las pacientes con ECV presentan mayor frecuencia de depresión que los hombres. Los aspectos relacionados con el género deben ser más ampliamente considerados, tanto en futuras investigaciones en diferencias de género en la depresión comórbida como en el tratamiento y rehabilitación, con el objetivo de ralizar una prevención secundaria más efectiva.
Abstract
Bien que le sexe soit de plus en plus perçu comme un déterminant clé de la santé et de la maladie, les études systématiques en médecine concernant les différences entre les deux genres sont toujours insuffisantes. La maladie cardiovasculaire (MCV) a depuis longtemps été considérée comme une maladie “masculine”, à cause du risque absolu plus élevé chez l'homme que chez la femme. Le risque relatif de morbidité et de mortalité de MCV est en réalité plus élevé chez la femme. L'état actuel de nos connaissances souligne des différences importantes entre les sexes au niveau de l'âge de début, des premières manifestations symptomtiques, de la prise en charge et de l'évolution ainsi que des facteurs de risque classiques et psychosociaux. Certains facteurs de risque traditionnels (comme le diabète, l'hypertension, l'hypercholestérolémie, l'obésité) augmentent de façon plus importante le risque de MCV chez la femme que chez l'homme. Les facteurs socio-économiques et psychosociaux semblent aussi avoir un impact plus important sur la MCV chez la femme. Étant donné les différences de prise en charge de la MCV, il faut prendre en compte un parti pris en faveur des hommes, bien que chez les femmes, un âge plus élevé et une comorbidité plus importante contribuent probablement à de moins bons résultats. Il a été démontré que la dépression est un facteur de risque indépendant et une conséquence de la MCV; les résultats ont cependant été contradictoires en ce qui concerne les différences entre les sexes. Les données actuelles indiquent que la dépression induit une augmentation importante de l'incidence de la MCV chez la femme, et que les femmes atteintes de MCV présentent des taux de dépression plus élevés que les hommes. Il faudrait prendre en compte plus intensivement les différences entre les sexes, à la fois dans les recherches futures sur la dépression comorbide et dans le traitement et la réadaptation cardiaques, dans le but de rendre plus efficace la prévention secondaire.
Gender - a key determinant of health
One of the more robust factors in explaining differences in morbidity and mortality is gender. in contrast to the term “sex,” “gender” is a multidimensional construct including biological/genetic, psychological, and social differences between men and women. Although gender is based on biology, and biological factors in men and women may affect behavior and vulnerability differently, these factors do not influence the entire scope of gender-related behavior, emotions, and attitudes. Beyond genetic and biological differences, gender refers to the socially constructed roles for men and women, Implicating different social norms and expectations. These define which emotions, behaviors, and attitudes are typical and desirable for males and females. They even result in classifying disorders as male and female, such as “male” heart disease and “female” depression.1
Although traditional gender norms have changed during the last three decades, and concepts of being male and female have become more individualistic, normative notions of typical male and female attributes still remain influential in social perception and evaluation,2 including health care (gender bias).
In medical and epidemiological research, the terms “gender” and “sex” are often used interchangeably, suggesting that psychosocial and biological attributes inevitably covary,3 but even in the case of depression, where a sex difference is consistently found, biology alone cannot provide a complete explanation.4 Piccinelli and Wilkinson5 even state that genetic and biological factors have only a minor role in the emergence of gender differences in depression. In depression, the preponderance of women is obviously better explained by stressors related to social roles. Likewise, the lower prevalence of depression in men may be less due to biological causes than to male-typed Illness behavior, such as the male-based symptoms of depression, which are conventionally not defined as depressive symp_ toms: aggression, irritability, anger attacks, abusive behavior, and drug addiction.6,7
Beyond biological sex, gender is a basic principle of societal organization, structuring social roles and the access to personal, social, and economic resources differently for men and women. It has been found that social struc_ tural and psychosocial determinants generally tend to be more Important for women's health, whereas behavioral determinants tend to be more important for men's health.8,9 Sociological stress research has become one of the most commonly used explanations of gender differences in health, assuming that susceptibility to psycho_ logical or physical breakdown is shaped largely by inequalities in life opportunities emerging from the orga_ nization of gender, class, race, and age. Women are underprivileged in several aspects, and generally suffer from poorer health and greater distress than men,10 including mood and anxiety disorders and a variety of chronic conditions. Two global hypotheses have been posed to explain this: differential exposure and differential vulnerability. The differential exposure hypothesis suggests that women report higher levels of health problems because of their reduced access to the material and social conditions of life that foster health, and due to the greater stress associated with their gender and marital roles. The differential vulnerability hypothesis refers to the possibility of women's higher reactivity or responsiveness to the life events and stressors.11,12 However, as patterns and magnitude of gender differences in health vary according to the symptoms/disorders and phase of the life cycle, explanations of these differences need to also consider these conditions.12
Gender and cardiovascular disease
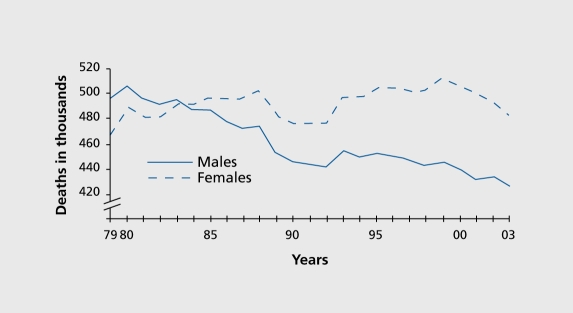
Coronary heart disease (CHD) is the leading cause of morbidity and mortality in the more economically developed areas of the world, being two to five times more common in men than in women in the younger age groups.13 CHD risk increases with age in both men and women, but shows a more prominent increase in women older than 50. Despite better medical treatment of CHD, It remains the leading killer of women.14 In Europe, about 55% of all female deaths are caused by cardiovascular disease (CVD), especially CHD and stroke, compared with 44% of all male deaths.15 Age-adjusted mortality for CVD has continuously declined in the last four decades, but to a lesser extent in women than in men. In fact, the temporal trend of the incidence of CVD even shows a rise in women (Figure 1) 16,17. This has been mainly attributed to a decrease in myocardial infarction incidence in younger men, with a concomitant increase in older women.16 Recent data even suggest an increased incidence in women under the age of 5418
Figure 1. Cardiovascular disease mortality trends for males and females, United States: 1 979-2003. 16 Reproduced from reference 17: American Heart Association. Heart and Stroke Statistics 2006 update. Available at: www.american-heart.org/presenter.jhtml identifier=3018163. Accessed December 2006. Copyright © American Heart Association 2006.
The older age at onset of CVD in women (70 years) com_ pared with men (60 years), probably related to estrogen deficiency post-menopause, correlates with an increase in comorbid diseases and consequently an increase in mortality; 38% of women die within 1 year of an initial unrecognized myocardial infarction, compared with 25% of men.19
Until the last decade, CVD in women had been underestimated because of lower prevalence rates in younger age groups, and due to the image of CVD as a male disorder, with the consequence that these disorders have been largely underdiagnosed in women. With regard to cardiological research, either the study populations exclu_ slvely consisted of males, the gender distribution was not specified, or the number of females included was too small to enable conclusions to be drawn about gender differences in risk factors. It was simply assumed that the knowledge derived from studies on men was applicable to women, whether It concerned biological or psychosocial risk factors. Gender bias in constructing hypotheses on risk factors led to numerous methodological pitfalls and false conclusions; for example, It was assumed that men were harmed by work stress, while women were protected by being at home.20 Now, the situation has changed, and several recent controlled cohort studies in men and women are available, which indicate important gender differences in clinical presentation, disease management, and outcome, as well as biological and psychosocial risk factors.
Gender differences in CHD symptoms, management, and outcome
Women with acute myocardial infarction (MI) tend to present with atypical symptoms such as abdominal pain, dyspnea, nausea, back and neck pain, Indigestion, palpitations, and unexpected fatigue, rather than clearly defined chest pain, which is the typical male complaint and probably better recognized by physicians.21,22 Regarding the delay in help-seeking, It has been noted that women underestimate their risk of CHD because the general public still perceives CHD as primarily a health problem for men.23 Misconceptions about risk and symptoms, as well as lack of Immediate help for older women living alone, may result in late arrival in the emergency room. This might be the explanation for earlier reports noting that women were less likely to be referred for diagnostic and therapeutic procedures, and that younger women had higher rates of death during hospitalization after acute MI compared with men of the same age (<50 years: 6.1% vs 2.9%).24 Moreover, serious comorbidities are more common in older women, and may limit treatment options. indeed, lower rates of specific treatments for women have been reported, but some authors suggest that It is not clear whether gender differences in treatment would have consequences for outcome. However, despite an increasing awareness of CHD in women, outcome in women remains worse than in men; eg, hospital mortality rates for acute MI are 16% for women and 11% for men.25 The mortality for bypass surgery in women is twice that for men; they have higher rates of hospital readmission (32.6% vs 21.3%) and a decreased 5-year survival rate (42% vs 58 %).21 Although the poor prognosis for women after MI is mostly attributed to their worse baseline characteristics, these differences do not account for the total gender difference in clinical outcome.26 Poor clinical outcome in women has to also be attributed to psychosocial adjustment, which has been shown to be worse in women than in men in terms of quality of life, anxiety, and depression, probably explaining their increased mortality risk.27
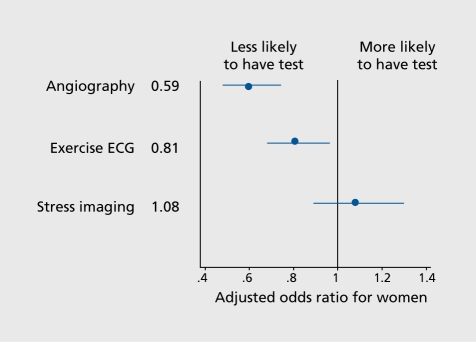
In summary, the management of CVD in men and women is obviously different, and these differences are partly due to a gender bias in favor of men. While some studies did not find a gender bias in the management and outcome of patients with acute coronary artery disease,28 unstable angina,29 and in selection for coronary angiography and revascularization early after MI,30 others did.31,32 For example, in a large European study, Daley et al32 Identified significant gender bias at multiple levels in the investigation and management of stable angina (Figure 2)
Figure 2. Effect of gender on the investigation and management of new-onset stable angina from the Euro Heart Survey of Stable Angina.31 Adjusted ORs and 95% Cls for women compared with men are shown. ORs were adjusted for age, gender, comorbidity, duration of symptoms 6 months, use of two antianginal drugs, severity of symptoms, and availability of invasive facilities at the enrolling center. Additional variables included performance or nonperformance of exercise ECG and result of exercise ECG (positive vs negative or inconclu_ sive). OR, odds ratio; ECG, electrocardiogram Reproduced from ref 32: Daley C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical out_ come of stable angina. Circulation. 2006;113:490-498. Copyright ©American Heart Association 2006.
Female patients were referred significantly less often for either noninvasive or invasive investigation than male patients, and were less likely to undergo revascularization or optimal secondary preventive medication, even in the presence of confirmed coronary disease. They were twice as likely to suffer death or nonfatal MI within a 1year follow-up period.
In an Irish population of 15 590 patients with ischemic heart disease, compared with male patients, female patients were less likely to receive a secondary preventive medication (ß-blocker, aspirin, anglotensin-converting enzyme inhibitor). However, they were more likely to receive anxiolytics (benzodiazepines).33 A similar gender bias was detected by a Scottish study,34 where gender difference was independent of age, adverse circumstances, and comorbidities, and even increased over time. Gender bias has also been reported with respect to cardiac rehabilitation. Most studies report poorer program uptake, poorer adherence, and higher dropout rates for women than for men, although data indicate that women show Improvements the same as or greater than those of men.26 This seems not only due to psychosocial barriers in women themselves (low self-esteem, multiple care-giving roles, economic concerns), but also to less strong recommendations for rehabilitation. Gender stereotypes in medicine may have fatal consequences as in the case of CVD, and the lack of secondary preventive medication in women may additionally expose them to a higher risk of MI and death, and may be one reason for the slower decline in female mortality rates.
Gender differences in traditional cardiac risk factors
Traditional cardiac risk factors are assumed to be essentially the same for men and women, although Important quantitative differences in physiology and pathology have been observed. Women have smaller artery dimension, different electrical properties, and different plaque composition and development.35 Men have generally less favorable cardiac risk factors than women (eg, smoking, low-fiber diet, low vitamin C levels, and high blood viscosity20); on the other hand, diabetes mellltus, hypertension, smoking, hypercholesterolemia, and obesity have been shown to contribute more to women's than men's CVD risk.
Most significant is diabetes, which results in a 3- to 7-fold increased CVD risk in women compared with a 2- to 3fold elevated risk in men. Diabetes negates the presumed gender-protective effect of estrogen in premenopausal women.21 It is estimated that two thirds of all deaths in diabetic patients are due to CVD.19
Hypertension, a major CVD risk factor for both sexes, is more prevalent in women than in men after the age of 65. Contrary to earlier belief, women do not tolerate the effects of hypertension on the cardlovasular and renal systems better than men do.24
In women younger than 50 years, smoking is the leading cause of CVD. Although the prevalence of smokers is still slightly higher in men than in women, the decline in tobacco use among women is less evident than in men.36 In fact, in younger women there may even be an increase rather than a decrease, and this may explain the increased incidence rates of CVD.36-38 This risk in young female smokers is additionally elevated by the use of oral contraceptives.39
Hypercholesterolemia plays a central role in the development of CVD in men and women, with a linear relationship between low-density lipoprotein (LDL) levels and risk for CVD, particularly in women less than 65 years. Additionally, low high-density lipoprotein (HDL) levels in women over 65 years convey a greater risk than in men. 19,40
Obesity, and particularly central obesity, more prevalent in men up to the age of 45 and in women over the age of 45, increases the CVD risk specifically in women41 and is associated with diabetes, hypertension, and dyslipldemia, as well as other lifestyle risk factors such as physical inactivity and poor diet.
The abovementioned risk factors account for only approximately 40% of the variance of CVD.
Gender differences in psychosocial cardiac risk factors
Since the late 1950s, the role of potential psychosocial risk factors in the development and outcomes of CVD have been extensively studied. Type A personality (excess aggression, impatience, and competitiveness) and more recently type D personality (inhibition of negative emotions in social situations), depression and anxiety, low socioeconomic status, lack of social support, social isola_ tion, and chronic work stress have all been evoked. While these factors were initially believed to indirectly increase CVD by affecting the traditional risk factors (reinforcing unhealthy lifestyle behaviors), numerous prospective cohort studies have also demonstrated direct effects via mechanisms such as disturbed autonomic and neuroendocrine regulation.42,43 In a critical evaluation of a number of systematic reviews on the association between psychosocial risk factors and CHD, Bunker et al44 found strong and consistent evidence of an independent causal association between depression, social isolation, and lack of social support and the occurrence and prognosis of CHD, whereas a causal association with regard to type A behavior, hostility, anxiety disorders, chronic life events, and work-related stressors was less evident. When psychosocial risk factors occur in combination, and they tend to cluster together (for example, high levels of chronic stress and social isolation), the rate of subsequent cardiac events is 4-fold higher, independently of pre-existing CHD.42 The above findings come predominantly from studies in men; knowledge of gender-specific risk factor profiles remains limited, although some population-based prospective studies such as the Framingham Study,45 the WHO MONICA study (Monitoring trends and determinants in cardiovascular disease),46 the Stockholm Heart Epidemiology Program,47 and the Whitehall II study48 have included women.
With regard to gender differences in CVD incidence and mortality, there is consistent evidence that low socioeconomic status, as defined by occupational position, income, or education, is not only a major psychosocial risk factor in men, but also in women. In women, the social gradient seems to be even stronger than in men.26,49,50 Less than 8 years of education contributed to a 4-fold risk of women (compared with women with 12 and more years of education) of developing CHD over a 14-year follow-up period; even after adjustment for other coronary risk factors, level of education remained a significant predictor.51
A strong gradient in CHD by years of education was also confirmed by the Swedish Women's Lifestyle and Health Cohort Study in a 10-year follow-up period.52 Several studies focussing on a life course approach to socioeconomic position found that socioeconomic disadvantage in childhood and in later life were both associated with increased CHD risk in women (4-fold53,54), and a twofold risk of dying from CHD in men.55 The fact that unhealthy lifestyles (the traditional CHD risk factors) are more prevalent in men and women with low socioeconomic status did not explain the different effects of social status on CHD risk and outcome: traditional CHD risk factors explain about 33% to 50% of the risk associated with the social CHD gradient (higher rates in lower employment grades).56,57
The risk gradient in CHD has been ascribed to psychosocial stressors of the work environment, mainly referring to Karasek's job strain model (high demands-low control) and Siegrist's effort-reward imbalance model.58,59 Findings indicate odds ratios (OR) from 1.2 to 5.0 with respect to job strain, and from 1.5 to 6.1 with respect to effort-reward imbalance. These OR seem higher for men than for women, but whether this is due to scarce data in women or to other reasons remains unresolved. While low job control in the Whitehall II study was related to a higher risk of newly reported CHD during 5-year followup for males and females,60 other studies revealed only weak associations between psychosocial work characteristics and risk of CHD in women.52,61,62 For example, the Framingham Offspring Study63 did not find any support for high job strain as a significant risk factor for CHD or death, either in women or in men within a 10-year followup period. Contrary to expectation, and unlike men, women with “active” job strain (high demands-high control) had a 2.8-fold risk of CHD compared with women with high job strain (high demands-low control). This may be due to more difficulties in adopting new social roles for women when traditional expectations remain normative as well Recent evidence suggests that women who are employed in male-dominated jobs (such as higher management or mechanical jobs) have a 2-fold risk of myocardial infarction compared with those in female-dominated jobs (such as nursing).64
With regard to employment, employed men as well as women are healthier than their unemployed counterparts, even after adjustment for low income and low level of education. The relationship between employment and CHD risk is complex in women. Findings indicate that, although women of all occupational levels were protected against CHD relative to those performing home duties, the protective effect of employment seems to be more pronounced in women in professional and managerial occupations than those in blue-collar occupations.26 However, there is evidence that employed women with children have an increased risk of CHD, perhaps because of the double load of work and family, which can result in anger and frustration due to low control over their lives.65 In the Framingham Study,66 performing both work and family duties was associated with increased CHD incidence, in particular in working women who had raised three or more children. Recent results suggest the same conclusion: the Stockholm Female Coronary Risk Study67 indicates that women's double exposure to stress from work and family was accompanied by the highest risk and the worst prognosis in CHD. Other predictors of CHD risk in women are marital stress68 and caring for a disabled or ill spouse. As the findings from the Nurses' Health Study69 show, women carers (>9 hours per week) had a significantly increased risk of fatal CHD or nonfatal MI infarction that was independent of age, smoking, exercise, alcohol intake, body mass index, history of hypertension, diabetes, and other covariates.
Other gender differences in psychosocial risk factors had been demonstrated concerning life events. While events occurring at work and at home affect risk increase in women, work-related events seem to influence men to a greater extent,70,71 a finding which is due to the fact that men consider their role at work as central, thus making them more vulnerable to job stressors. In the case of bereavement, most of the studies report a brief increase in CHD mortality during the first months after bereavement for men and women, and then a later slight increase in mortality in men.26 Loss of a spouse might be more disruptive for men, because they lose their only confidant, and seem to be more affected by a decrease in social network and social support than women, who usually have larger social networks.
Lack of social support and social isolation have proven to be major long-term predictors of mortality from all causes, including CHD. Although social support has been examined by a variety of methods, the results have been remarkably consistent.72 The relative risk (RR) of CHD incidence owing to lack of social support is 2- to 3-fold, independent of conventional and sociodemographic CHD predictors.42 Social support can have direct effects on CHD risk, and can also act as a buffer by moderating the effect of adverse life events, job strain, anger, and depression on CHD incidence. Lack of social support at work is particularly associated with increased risk of CHD.73 Again, empirical evidence is more consistent for men than for women in this respect. independently of work, the risk of fatal CHD was up to 3.7 times higher among women lacking social ties than those who had them,74 whereas no consistent association was found for women in a Finnish study.75 Single mothers in particular, as they are exposed to a combination of several psychosocial stressors and behavioral risk factors, have been shown to be at higher risk for CVD than mothers with partners.76 Being lonely during the day was associated with higher MI or CHD mortality in housewives at 20year follow-up, as reported by the Framingham Study.45 For both men and women, social support (measured by being married) has been shown to be an independent predictor for survival rates and recurrent infarction in CHD patients.77,78 However, women with CHD tended to report less support than did men with regard to information about the disease, rehabilitation and self-help groups, assistance with household duties, and encouragement from their spouses.79,80
Personality characteristics such as Type A behavior have been investigated as psychosocial stressors in CHD research. Overall, data on Type A behavior have not been conclusive, and the attention has more recently focused on hostility and anger, resulting again in mixed findings.81 However, the literature shows a relationship between anger and CHD. One of the first prospective studies in this respect, the Framingham Offspring Study,63 found that trait anger, hostility, and symptoms of anger were independent risk factors for incident CHD in men, but not in women. This finding was supported by a population-based study by Haas et al.82 In contrast, other studies indicate that hostility is an independent CHD risk factor for nonfatal MI and recurrent events in postmenopausal women with CHD.83,84 In a prospective community study in older men, anger was associated with a 2- to 3-fold increase in CHD risk with evidence for a dose-response relationship,85 and in a study in young men followed up over 36 years, anger was prospectively related to a 3-fold RR of premature CHD.86
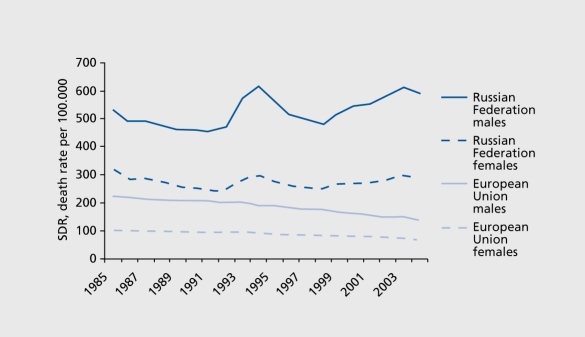
The role of psychosocial factors in CVD, particularly gender-role aspects and socioeconomic factors, is dramatically documented by the rapid increase in CVD mortality among middle-aged men in Eastern Europe in the late 1990s during the transition from a socialist to a market economy.87 Although these results are correlational and do not allow causal interpretation, they suggest that societal transition may have adverse long-term effects, particularly on men (Figure 3)
Figure 3. Trends in ischemic heart disease in the Russian Federation and the European Union by gender. Adapted from: http://data.euro.who.int/hfamb. Copyright © World Health Organization 2006.
In a comparative study in Lithuanian and Swedish men,88 traditional CVD risk factors (systolic blood pressure, smoking, dyslipidemia) did not differ, but striking differences in psychosocial CHD risk factors were found: Lithuanian men reported significantly more job strain, lower social support, lower social integration, less effective coping, lower self-esteem, and more vital exhaustion and depression than Swedish men; they were 4 times more likely to die from CHD than their Swedish counterparts. A similar pattern of findings was reported with regard to CVD morbidity in women from Eastern European countries. This would suggest that women's strategies for coping with severe stress (asking for assistance) may be more cardioprotective than men's coping strategies. Men faced with unexpected socioeconomic stressors (loss of work, job insecurity) and faced with threats to the male role (as breadwinner) tend to cope by excessive alcohol use, smoking, and social withdrawal89,90
Gender, depression, and CHD
Like CHD, depression is a major health problem, with a lifetime prevalence of approximately 15 %.91 By the year 2020, it is estimated that disability worldwide will be determined largely by depression and heart disease.92 It is known that major depression is twice as common in women as in men.93,94 The female predominance begins in adolescence and persists into middle age and early old age.95,96 The reasons for this gender difference are not fully understood. A substantial part can be attributed to gender role-related stressors to which women are more exposed than men, such as low socioeconomic status, lack of power, role overload, and sexual abuse, and associated psychological attributes such emotion-focused coping styles, interpersonal orientation and related vulnerability, anxiety, and lowered self-esteem. The differences between men and women reflect differences in endocrine stress reactions, and might influence processes leading to depression.5,96
Lower prevalence rates in males may be due to their better social position, but also to under- or misdiagnosing because of typical male illness behavior, including externalizing coping styles (aggressiveness, antisocial behavior, alcohol misuse), which often mask depressive symptoms in men. As externalizing symptoms are not included in depression inventories,6,8 depression in men may be underestimated, and this may also be true for the association between depression and CVD.
With respect to comorbidity, etiologic and prognostic studies indicate that depression may be a cause or a consequence of CVD, thus supporting a bidirectional relationship. Major depression has been identified as a prominent psychosocial risk factor in CVD incidence for initially healthy men and women, with a RR of 1.5 to 2.0, independent of traditional risk factors.72,97,98 However, as Rugulies97 concluded from his meta-analysis, clinical depression has a stronger effect size in predicting CVD than depressive mood. The association between depression and CVD may have several mechanisms, including coronary-prone behavior and noncompliance, hypercortisolism, and autonomic dysregulation. Among patients already suffering from CVD, 17% to 27% have major depression when diagnosed according to DSM criteria during the first year after MI, and a significantly larger percentage (up to 87%) has subsyndromal symptoms of depression. In patients with MI or unstable angina pectoris, those who had been diagnosed as depressed had a 3-fold risk of dying compared with nondepressed patients, indicating that depression is an independent predictor of mortality as well.99 Although the importance of depression in CVD is well documented, it remains largely underdiagnosed. According to recent data from a survey of cardiovascular physicians, 50% of the respondents were unaware of depression as an independent cardiac risk factor, 71% asked less than half their patients with CVD about depression, and 79% used no standard screening method to diagnose depression.100
Gender differences in depression as a risk factor for CVD
There are very few studies which address depression as a primary risk factor in the development of CVD in gender-balanced samples. Wassertheil-Smoller et al101 did not find an association between baseline depression score and MI, but reported a significantly (25%) increased mortality risk for women who had a 5-unit increase in depression score (measured with the Center for Epidemiological Studies Depression Scale, CES-D) during a 4.5-year follow-up period. In the National Health and Nutrition Examination Survey,102 CVD mortality was only related to depression in men, with a RR of 2.34 compared with nondepressed men, while depression had no effect on CVD mortality in women. However, it was associated with an increased risk of CVD in women as well. In contrast, another study found an effect of depressive symptoms and CVD death only in women.103 Penninx et al104 investigated the effects of recent-onset and chronic depression on CVD events in a prospective cohort study in men and women ≥65 years over 5 years. Newly depressed older men (depressed at baseline, not earlier, CES-D), but not women, were twice as likely to have a CVD event as those who were never depressed. This association remained significant after adjusting for CVD risks. In men, recent onset of depression was a better predictor of CVD than was chronic depression. In a similar study on the effects of depression (CES-D) on heart failure,105 depression was found to be an independent risk factor for heart failure in elderly women, but not elderly men. Whether the under-representation of men was due to death before commencement of the study, to different help-seeking behavior of depressed men and women, or to other processes, remains unclear. Diabetes and childhood maltreatment have been investigated with regard to factors affecting the relationship between gender, depression, and CVD differently for men and women. Depression is common in diabetic patients, particularly in women, with a prevalence of 28% (vs 18% in men).106 Depression rates double in the presence of diabetes, and depressed diabetic women have more rapid development of CVD than nondepressed diabetic women.107 Whether this association also holds true for men remains unclear. Concerning childhood maltreatment, a greater impact of traumatic experiences on the development of depression in women and a greater impact on CHD in men was postulated, but could not be confirmed, in a representative sample of more than 5000 adults.108 Childhood maltreatment was associated with an almost 9-fold increase in CVD in women only, and with a significant increase in lifetime depression in both men and women. Although depression and CVD were correlated, depression did not contribute to the occurrence of CVD in women.
Gender differences in depression as e prognostic factor in CHD
Women have a rate of depression twice that of men in the cardiac patient population, as well as in the general population.109 Several studies have shown that women after MI and coronary artery bypass surgery had more severe depressive symptoms than men, and these persisted longer110 and affected women's prognosis more detrimentally.111 Studies agree that the occurrence of post-MI depression occurs unrelated to the severity of MI and other medical factors.112 Younger women in particular (60 years or under) had a depression risk that was 3.1 times higher than that of the reference group of men older than 60.113 According to a large 5-year Norwegian study follow-up with 23 693 participants,112 men and women differed in their long-term outcome after MI: women showed a higher risk for anxiety and depression (measured with the Hospital Anxiety and Depression Rating Scale) in the first 2 years after MI than men, which is followed by a significant symptom reduction. In men, the risk for depression increased after 2 years postMI. These data lend support to the impact of gender-specific coping strategies as a significant factor mediating MI outcome. Although the coping levels of CHD patients have rarely been investigated, evidence indicates that male CHD patients, like men in general, have more limited strategies for coping with stressful life events than women, and tend to deny depression and anxiety, which may result in a worsening their adaptation.114-116
Marital status and social network have also been explored as potential mediators of the link between CVD and depression. Being single has been found to increase the risk of post-MI depession in men, whereas unmarried women or those living alone were less likely to be depressed. 98,117,119 These findings are consistent with the fact that the protective health effects of marriage are notably stronger for men than for women.119
Social networks, in relation to recurrent CVD events were investigated in the Stockholm Female Coronary Risk Study.120 It was demonstrated that two or more depressive symptoms (BDI) and lack of social integration (number and function of social contacts) contributed independently to a relapse of CVD (cardiovascular death, MI or revascularization procedures, eg, percutaneous luminal angioplasty and coronary artery bypass grafting) within 5 years.
Conclusions
Due to the lack of studies in gender-balanced populations and randomized clinical studies including a larger number of women, current knowledge of gender-related risk profiles in CVD and comorbid depression is limited. Nevertheless, there is evidence for significant gender differences in some aspects (Table I), which points to several disadvantages for women with respect to risk factors, CVD management, and outcome.
Table I. Evidence of gender differences in cardiovascular disease (CVD) and depression.
| Gender differences in | Scientific evidence |
| Cardiac physiology | Certain |
| Cardiac pathophysiology | Certain |
| Age of CVD onset | Certain |
| CVD risk | Certain |
| CVD symptoms | Certain |
| Traditional CVD risk factors | Certain |
| Psychosocial CVD risk factors | Probable |
| Depression as a CVD risk factor | Probable |
| CVD management | Probable |
| CVD outcome | Certain |
| Depression as a consequence of CVD | Probable |
Groups with a particularly high risk of CVD are single mothers with low socioeconomic status, working mothers with low employment grades, and older women who live alone and have little social support. At the same time, these groups are more vulnerable to depression. Depression in otherwise healthy subjects seems to increase the risk of CVD more strongly in women, and women with CVD possibly experience higher levels of depression and lower levels of social support than men. However, single male patients also seem to be prone to a poorer outcome of CVD.
While in general, depression has been shown to be an independent risk factor and consequence of CVD, the question as to whether the impact of depression on the development and progression of CVD differs as a function of gender is still unresolved.
There is a need for more systematic gender studies in CVD and comorbid depression, and for the development of gender-related biopsychosocial explanatory models. Prospective studies are needed, because gender bias is of high clinical and public health importance. There is also a need for improving the detection of depression in CVD patients, and for paying more attention to the rate of CVD in patients with major depression. It may be that depression in male CVD patients is underdiagnosed, because males tend to deny their depressive symptoms and compensate for them with attitudes and behavior such as anger, hostility, cynicism, and social withdrawal. in summary, gender-related issues have to be taken into account, not only in detecting CVD and depression, but also in treatment and rehabilitation programs, with the goal of better meeting the specific needs of men and women, improving the prevention of CVD.
Selected abbreviations and acronyms
- CHD
coronary heart disease
- CVD
cardiovascular disease
- MI
myocardial infarction
REFERENCES
- 1.Curry P., O'Brian M. The male heart and the female mind: a study in the gendering of antidepressants and cardiovascular drugs in advertisements in Irish medical publication. Soc Sci Med. 2006;62:1970–1977. doi: 10.1016/j.socscimed.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 2.Glick P., Lameiras M., Fiske ST., et al Bad but bold: ambivalent attitudes toward men predict gender inequality in 16 nations. J Pers Soc Psychol. 2004;86:713–728. doi: 10.1037/0022-3514.86.5.713. [DOI] [PubMed] [Google Scholar]
- 3.Davidson KW., Trudeau KJ., Roosmalen van E., Stewart M., Kirkland S. Gender as a health determinant and implications for health education. Health Educ Behav. Discussion 744-746. 2006;33:731–743. doi: 10.1177/1090198106288043. [DOI] [PubMed] [Google Scholar]
- 4.Hankin BL., Abramson LY. Development of gender differences in depression: an elaborated cognitive vulnerability-transactional stress theory. Psychol Bull. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- 5.Piccinelli M., Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 6.Rutz W., von Knorring L., Pihlgren H., Rihmer Z., Walinder J. Prevention of male suicides: lessons from the Gotland study. Lancet. 1995;345:524. doi: 10.1016/s0140-6736(95)90622-3. [DOI] [PubMed] [Google Scholar]
- 7.Môller-Leimkùhler AM., Bottlender R., StrauB A., Rutz W. is there evidence for a male depressive syndrome in inpatients with major depression?. J Affect Disord. 2004;80:87–93. doi: 10.1016/S0165-0327(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 8.Môller-Leimkùhler AM., Heller J., Paulus NC. Subjective well-being and 'male depression' in male adolescents. J Affect Disord. 2006 Sep 8 [Epub Ahead of Print] doi: 10.1016/j.jad.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Denton M., Prus S., Walters V. Gender differences in health: a Canadian study of the psychosocial, structural and behavioural determinants of health. Soc Sci Med. 2004;58:2585–2600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Rahman O., Strauss G., Gertler P., Ashley D., Fox K. Gender differences in adult health: an international comparison. Gerontologist. 1994;34:463–469. doi: 10.1093/geront/34.4.463. [DOI] [PubMed] [Google Scholar]
- 11.McDonough P., Walters V. Gender and health: reassessing patterns and explanations. SocSciMed. 2001;52:547–559. doi: 10.1016/s0277-9536(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 12.Matthews S., Manor O., Power C. Social inequalities in health: are there gender differences?. Soc Sci Med. 1999;48:49–60. doi: 10.1016/s0277-9536(98)00288-3. [DOI] [PubMed] [Google Scholar]
- 13.Jackson R., Chambless L., Higgins M., et al Sex difference in ischemic heart disease mortality and risk factors in 46 communities: an écologie analysis. WHO MONICA Project, and ARIC Study. Cardiovasc Risk Factors. 1997;7:43–54. [Google Scholar]
- 14.Mosca L., Appel LJ., Benjamin EJ., Berra K., Chandra-Strobos N., et al Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672–693. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 15.European Cardiovascular Statistics 2005. Available at: www.heartstats.org/1570. 2006 Accesssed December [Google Scholar]
- 16.Tunstall-Pedoe H., Kuulasma K., Mahonen M., Tolonen H., Ruokokoski E., Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 17.American Heart Association. Heart and Stroke Statistics 2006 update. Available at: www.americanheart.org/presenter.jhtml identifier=3018163. 2006 Accessed December [Google Scholar]
- 18.Lôwel H., Meisïnger C., Heïer M., et al Geschlechtsspezifische Trends on plôtzlichem Herztod und akutem Herzînfarkt. Ergebnisse des bevôlkerungsbasïerten KORA/MONICA-Augsburg Herzïnfarktregïsters 1985-1998. Dtsch Med Wochenschr. 2002;44:2311–2316. doi: 10.1055/s-2002-35181. [DOI] [PubMed] [Google Scholar]
- 19.Bello N., Mosca L. Epidemiology of coronary heart disease in women. Progr Cardiovasc Dis. 2004;46:287–295. doi: 10.1016/j.pcad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 21.Eastwood JA., Doering LV. Gender differences in coronary artery disease. J Cardiovasc Nurs. 2005;20:430–351 . doi: 10.1097/00005082-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gold LD., Krumholz HM. Gender differences in treatment of heart failure and acute myocardial infarction. A question of quality or epidemiology?. Cardiol Rev. 2006;14:180–186. doi: 10.1097/01.crd.0000194093.53005.f0. [DOI] [PubMed] [Google Scholar]
- 23.Hart PL. Women's perceptions of coronary heart disease: an integrative review. J Cardiovasc Nurs. 2005;20:170–176. doi: 10.1097/00005082-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarino V., Parsons L., Every NR., Barron HV., Krumholz HM. Sex-based differences in early mortality after myocardial infarction. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 25.American Heart Association. Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association. . 2001 [Google Scholar]
- 26.Brezinka V., Kittel F. Psychosocial factors of coronary heart disease in women: a review. Soc Sci Med. 1995;10:1351–1365. doi: 10.1016/0277-9536(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 27.Carney RM., Freedland KE., Smith L., Lustman PJ., Jaffe AS. RIeation of depression and mortality after myocardial infarction in women. Circulation. 1991;84:1876–1877. [PubMed] [Google Scholar]
- 28.Raine RA., Black NA., Bowker TJ., Wood DA. Gender differences in the management and outcome of patients with acute coronary artery disease. J Epidemiol Community Health. 2002;56:791–797. doi: 10.1136/jech.56.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Ami T., Gilutz H., Porath A., Sosna G., Liel-Cohen N. No gender difference in the clinical management and outcome of unstable angina. isr Med Assoc J. 2005;7:228–232. [PubMed] [Google Scholar]
- 30.Krumholz HM., Douglas PS., Lauer MS., Paternak RC. Selection of patients for coronary angiography and coronary revascularization early after myocardial infarction: is there evidence for a gender bias?. Ann intern Med. 1992;116:875–790. doi: 10.7326/0003-4819-116-10-785. [DOI] [PubMed] [Google Scholar]
- 31.Shaw LJ., Miller DD., Romeis JC., KargI D., Younis LT., Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann intern Med. 1994;120:559–566. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Daley C., Clemens F., Lopez Sendon JL., et al Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 33.Williams D., Bennett K., Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2002;55:604–608. doi: 10.1046/j.1365-2125.2003.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson CR., Hannaford PC., Williams D. Evidence for inequalities in the management of coronary heart disease in Scotland. Heart. 2005;91:630–634. doi: 10.1136/hrt.2004.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legato MJ. Gender-specific physiology: how real is it? How important is it?. int J Fertil. 1997;42:19–29. [PubMed] [Google Scholar]
- 36.Women and smoking: a report of the surgeon general. Executive summary. MMRW Recomm Rep, 2002;51:1–30. [PubMed] [Google Scholar]
- 37.Ulmer H., Diem G., Bischof HP., Ruttmann E., Concin H. Recent trends and sociodemographic distribution of cardiovascular risk factors: results from two population surveys in the Austrian WHO CONDO demonstration area. Wien Klin Wochenschr. 2001;113:573–579. [PubMed] [Google Scholar]
- 38.Màhônen MS., McElduff P., Dobson AJ., Kuulasmaa KA., Evans AE. Current smoking and the risk of non-fatal myocardial infaction in the WHO MONICA Project populations. Tob Control. 2004;13:244–250. doi: 10.1136/tc.2003.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castelli WP. Cardiovascular disease: pathogenesis, epidemiology, and risk among users of oral contraceptives who smoke. Am J Obstet Gynecol. 1999;180:349–356. doi: 10.1016/s0002-9378(99)70695-2. [DOI] [PubMed] [Google Scholar]
- 40.Polk MD., Naqvï TZ. Cardiovascular disease in women: sex differences in presentation, risk factors, and evaluation. Curr Cardiol Rep. 2005;7:166–172. doi: 10.1007/s11886-005-0072-9. [DOI] [PubMed] [Google Scholar]
- 41.Kenchaiah S., Gaziano JM., Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am. 2004;88:1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Rozanski A., Blumenthal JA., Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 43.Hemingway H., Marmot M. Psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. BMJ. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunker SJ., Colquhoun DM., Esler MS., et al “Stress” and coronary heart disease: psychosocial risk factors. Med J Aust. 2003;178:272–276. doi: 10.5694/j.1326-5377.2003.tb05193.x. [DOI] [PubMed] [Google Scholar]
- 45.Eaker ED., Pinky J., Castelli WP. Myocardial infarction and coronary death among women: pychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–864. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 46.Tunstall-Pedoe H., Kuulasmaa K., Amouyel P., Arveiler D., Rajakangas AM., Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 47.Reuterwall C., Hallqvist J., Ahlbom A., et al Higher relative, but lower absolute risks of myocardial infarction in women than in men: analysis of some major risk factors in the SHEEP study. J int Med. 1999;246:161–174. doi: 10.1046/j.1365-2796.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuper H., Marmot M., Hemingway H. Systematic review of prospective cohort studies of psychosocial risk factors in the etiology and prognosis of coronary heart disease. Semin Vas Med. 2002;2:267–314. doi: 10.1055/s-2002-35401. [DOI] [PubMed] [Google Scholar]
- 49.Morrison C., Woodward M., Leslie W., Tunstall-Pedoe H. Effect of socioeconomic group on incidence of, management of, and survival after myocardial infarction and coronary death: analysis of community coronary event register. BMJ. 1997;314:541. doi: 10.1136/bmj.314.7080.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallqvist J., Lundberg M., Didrichsen F., Ahlbom A. Socioeconomic differences in tisk of myocardial infarction 1971-1994 in Sweden: time trends, relative risks and population attributable risks. int J Epidemiol. 1998;27:410–415. doi: 10.1093/ije/27.3.410. [DOI] [PubMed] [Google Scholar]
- 51.Eaker ED. Psychosocial factors in the epidmiology of coronary heart disease in women. Psych iatr Clin North Am. 1989;12:167–173. [PubMed] [Google Scholar]
- 52.Kuper H., Adami HO., Theorell T., Weiderpass E. Psychosocial determinants of coronary heart disease in middle-aged women: a prospective study in Sweden. Am J Epidemiol. 2006;164:349–357. doi: 10.1093/aje/kwj212. [DOI] [PubMed] [Google Scholar]
- 53.Wamala SP., Lynch J., Kaplan GA. Women's exposure to early and later life socioeconomic disadvantage and coronary heart disease risk: the Stockholm Female Coronary Risk Study. int J Epidemiol. 2001;30:275–284. doi: 10.1093/ije/30.2.275. [DOI] [PubMed] [Google Scholar]
- 54.Lawlor DA., Ebrahïm S., Smith GD. Adverse socioeconomic position across the lifecourse increases coronary heart disease risk cumulatively: findings from the British women's heart and health study. J Epidemiol Community Health. 2005;59:785–793. doi: 10.1136/jech.2004.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hart CL., Davey Smith G., Blane D. inequalities in mortality by social class measured at three stages of the life course. Am J Public Health. 1998;88:471–474. doi: 10.2105/ajph.88.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marmot M., Bartley M. Social class and coronary heart disease. in: Stansfeld S, Marmot M, eds. Stress and the Heart. London, UK: BMJ Books. 2002:5–19. [Google Scholar]
- 57.Pekkanen J., Tuomilehto J., Uutela A., Vartiainen E., Nissinen A. Social class, health behaviour, and mortality among men and women in eastern Finland. BMJ. 1995; 311:589–93. doi: 10.1136/bmj.311.7005.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marmot MG., Bosma H., Hemingway H., Brunner E., Stansfeld S. Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet. 1997;350:235–239. doi: 10.1016/s0140-6736(97)04244-x. [DOI] [PubMed] [Google Scholar]
- 59.Peter R., Sïegrïst J. Psychosocial work environment and the risk of coronary heart disease. int Arch Occup Environ Health. 2000;73 (suppl):41–45. doi: 10.1007/pl00014625. [DOI] [PubMed] [Google Scholar]
- 60.Bosma H., Marmot MG., Hemingway H., Nicholsen AC., Brunner E., Stansfeld SA. Low job control and risk of coronary heart disease in Whitehall II (prospective cohort) study. BMJ. 1997;314:558–565. doi: 10.1136/bmj.314.7080.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wamala SP., Mittleman MA., Horsten M., Schenck-Gustafsson K., OrthGômer K. Job stress and the occupational gradient in coronary heart disease risk in women. The Stockholm Female Coronary Risk Study. Soc Sci Med. 2000;51:481–489. doi: 10.1016/s0277-9536(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 62.Chandola T., Brunner E., Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eaker ED., Sullivan LM., Kelly-Hayes M., D'Agostino RB Sr., Benjamin EJ. Does job strain increase the risk for coronary heart disease or death in men and women? The Framingham Offspring Study. Am J Epidemiol. 2004;159:950–958. doi: 10.1093/aje/kwh127. [DOI] [PubMed] [Google Scholar]
- 64.Peter R., Hammarstrom A., Hallqvist J., Siegrist J., Theorell T. SHEEP Study Group. Does occupational gender segregation influence the association of effort-reward imbalance with myocardial infaction in the SHEEP study?. int J Behav Med. 2006;13:34–43. doi: 10.1207/s15327558ijbm1301_5. [DOI] [PubMed] [Google Scholar]
- 65.La Rosa JH. omen, work, and health: employment as a risk factor for coronary heart disease. Am J Obstet Gynecol. 1988;158:1597–1602. doi: 10.1016/0002-9378(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 66.Haynes SG., Feinleib M., Kennel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham Study. III. Eight-year incidence of coronary heart disease. Am J Epidemiol. 1980;111:37–58. doi: 10.1093/oxfordjournals.aje.a112873. [DOI] [PubMed] [Google Scholar]
- 67.Orth-Gômer K., Leineweber C. Multiple stressors and coronary disease in women. The Stockholm Female Coronary Risk Study. Biol Psychol. 2005;69:57–66. doi: 10.1016/j.biopsycho.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Orth-Gômer K., Wamala SP., Horsten M., Schenck-Gustafsson K., Schneiderman N., Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 69.Lee S., Colditz G., Berkam L., Kawachi I. Caregiving to children and grandchildren and risk of coronary heart disease in women. Am J Public Health. 2003;93:1939–44. doi: 10.2105/ajph.93.11.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall EM. Double exposure: the combined impact of the home and work environments on psychosomatic strain in Swedish women and men. int J Health Serv. 1992;22:239–60. doi: 10.2190/7VW4-GE0D-WRKU-Q62V. [DOI] [PubMed] [Google Scholar]
- 71.Theorell T., Tsutsumi A., Hallqvist J., et al Decision latitude, job strain, and myocardial infarction: a study of working men in Stockholm. The SHEEP Study Group. Stockholm Heart Epidemiology Program. Am J Public Health. 1998;88:382–388. doi: 10.2105/ajph.88.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lett HS., Blumenthal JA., Babyak MA., Strauman TJ., Robins C., Sherwood A. Social Support and coronary heart disease: epidmiologic evidence and implications for treatment. Psychosomat Med. 2005;67:869–878. doi: 10.1097/01.psy.0000188393.73571.0a. [DOI] [PubMed] [Google Scholar]
- 73.Hammar N., Alfredsson L., Johnson JV. Job strain, social support at work, and incidence of myocardial infarction. Occup Environ Med. 1998;55:548–553. doi: 10.1136/oem.55.8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orth-Gômer K., Johnson JV. Social network interaction and mortality. A six year follow-up study of a random sample of the Swedish population. J Chronic Dis. 1987;40:949–57. doi: 10.1016/0021-9681(87)90145-7. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan GA., Salonen JT., Cohen RD., Brand RJ., Syme SL., Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. Am J Epidemiol. 1988;128:370–380. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- 76.Young LE., Cunningham SL., Buist DS. Lone mothers are at higher risk for cardiovascular disease compared with partnered mothers. Data from the National Health and Nutrition Examination Survey III (NHANES III). Health Care Women int. 2005;26:604–621. doi: 10.1080/07399330591004845. [DOI] [PubMed] [Google Scholar]
- 77.Chandra V., Szklo M., Goldberg R., Tonascia J. The impact of marital status on survival after an acute myocardial infarction: a population-based study. Am J Epidemiol. 1983;117:320–325. doi: 10.1093/oxfordjournals.aje.a113544. [DOI] [PubMed] [Google Scholar]
- 78.Williams RB., Barefoot JC., Califf RM., et al Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA. 1992;267:520–524. [PubMed] [Google Scholar]
- 79.Hildïngh C., Fridlund B. Social network and experiences of social support among women 12 months after their first myocardial infarction. int J Rehab Health. 1997;3:131–142. [Google Scholar]
- 80.Rose GL., Suis J., Green PJ., Lounsbury P., Gordon El. Comparison of adjustment, activity, and tangible social support in men and women patients and their spouses during the six months post-myocardial infarction. Ann Behav Med. 1996;18:264–272. doi: 10.1007/BF02895288. [DOI] [PubMed] [Google Scholar]
- 81.Strike PC., Steptoe A. Psychosocial factors in the development of coronary artery disease. Prog Cardiovasc Dis. 2004;46:337–347. doi: 10.1016/j.pcad.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Haas DC., Chaplin WF., Shimbo D., Pickering TG., Burg M., Davidson KW. Hostility is an independent predictor of recurrent coronary deart disease events in men but not women: results from a population based study. Heart. 2005;91:1609–1610. doi: 10.1136/hrt.2004.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lahad A., Heckbert SR., Koepsell TD., Psaty BM., Patrick LD. Hostility, aggression and the risk of nonfatal myocardial infarction in postmenopausal women. J Psychosom Res. 1997;43:183–195. doi: 10.1016/s0022-3999(96)00369-8. [DOI] [PubMed] [Google Scholar]
- 84.Chaput LA., Adams SH., Simon JA. Hostility predicts recurrent events among post-menopausal women with coronary heart disease. Am J Epidemiol. 2002;126:1092–1096. doi: 10.1093/aje/kwf158. [DOI] [PubMed] [Google Scholar]
- 85.Kawachi I., Sparrow D., Spiro A III., Vokonas P., Weiss ST. A prospective study of anger and coronary heart disease. The Normative Aging Study. Circulation. 1996;94:2090–2095. doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- 86.Chang PP., Ford DE., Meoni LA., Wang NY., Klag MJ. Anger in young men and subsequent premature cardiovascular disease: the precursors study. Arch intern Med. 2002;162:901–906. doi: 10.1001/archinte.162.8.901. [DOI] [PubMed] [Google Scholar]
- 87.Rutz W. Mental health in Europe: problems, advances and challenges. Acta Psychiatr Scand Suppl. 2001;(410):15–20. doi: 10.1034/j.1600-0447.2001.1040s2015.x. [DOI] [PubMed] [Google Scholar]
- 88.Kristenson M., Kucinskiene Z., Bergdahl B., Calkaukas H., Urmonas V., Orth-Gômer K. increased psychosocial strain in Lithuanian versus Swedish men: the LiVicordia study. Psychosom Med. 1998;60:277–282. doi: 10.1097/00006842-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 89.Weidner G., Cain VS. The gender gap in heart disease: lessons from Eastern Europe. Am J Publ Health. 2003;93:768–770. doi: 10.2105/ajph.93.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Môller-Leimkùhler AM. The gender gap in suicide and premature death or: why are men so vulnerable?. Eur Arch Psychiatry Clin Neurosci. 2003;253:1–8. doi: 10.1007/s00406-003-0397-6. [DOI] [PubMed] [Google Scholar]
- 91.Doris A., Ebmeier K., Shajahan P. Depressive illness. Lancet. 1999;354:1369–1375. doi: 10.1016/S0140-6736(99)03121-9. [DOI] [PubMed] [Google Scholar]
- 92.Murray CJ., Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–142. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 93.Weissman MM., Bland R., Joyce PR., Newman S., Weils JE., Wittchen HU. Sex differences in rates of depression: cross national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 94.Kessler RC., McGonagle KA., Swartz M., Blazer DG., Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;2-3:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 95.Mojtabai R., Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34:623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 96.Kùhner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 97.Rudïsch B., Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 98.Rugulies R. Depression as a predictor for coronary heart disease. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 99.S0rensen C., Brandes A., Hendricks O., et al Psychosocial predictors of depression in patients with acute coronary syndrome. Acta Psychiatr Scand. 2005;111:116–124. doi: 10.1111/j.1600-0447.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 100.Feinstein RE., Blumenfield M., Orlowski B., Frishman WH., Ovanessian S. A national survey of cardiovascular physicians' beliefs and clinical care practices when diagnosing and treating depression in patients with cardiovascular disease. Cardiol Rev. 2006;14:164–169. doi: 10.1097/01.crd.0000200977.41695.43. [DOI] [PubMed] [Google Scholar]
- 101.Wassertheil-Smoller S., Shumaker S., Ockene J., Tavalera GA., Greeland P., Cochrane B., et al Depression and cardiovascular sequelae in postmenopausal women. The Women's Health initiative (WHI). Arch intern Med. 2005;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 102.Ferketich Ak., Schwatzaum JA., Frid DJ., Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 103.Mendes de Leon CF., Krumholz HM., Seeman TS., et al Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982-1991. Established Populations for the Epidemiologic Studies of the Elderly. Arch intern Med. 1998;158:2341–2348. doi: 10.1001/archinte.158.21.2341. [DOI] [PubMed] [Google Scholar]
- 104.Penninx BWJH., Guralnik JM., Mendes de Leon CF., et al Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol. 1998;81:988–994. doi: 10.1016/s0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 105.Williams SA., KasI SV., Heiat A., Abramson JL., Krumholz HM., Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community- based study. Psychosomat Med. 2002;64:6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Anderson RJ., Freedlan KE., Clouse RE., Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a metanalysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 107.Clouse RE., lustman PJ., Freedland KE., Griffith LS., McGill JB., Carney RM. Depresion and coronary heart disease in women with diabetes. Psychosomat Med. 2003;65:376–383. doi: 10.1097/01.psy.0000041624.96580.1f. [DOI] [PubMed] [Google Scholar]
- 108.Batten SV., Asian M., Maciejewski PK., Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 109.Frasure-Smith N., Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomat Med. 2005;67(suppl 1):19–25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 110.Drory Y., Kravetz S., Hirschberger G. israel Study Group on First Acute Myocardïcal infarction. Long-term mental health of women after a first acute myocardial infarction. Arch Phys Med Rehabil. 2003;84:1492–1498. doi: 10.1016/s0003-9993(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 111.Frasure-Smith N., Lesperance F., Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 112.Bjerkeset O., Nordahl HM., Mykletun A., Holmen J., DahI AA. Anxiety and depression following myocardial infarction: gender differences in a 5-year prospective study. J Psychosom Res. 2005;58:153–61 . doi: 10.1016/j.jpsychores.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 113.Mallïk S., Spertus JA., Reïd KJ., et al Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 114.Ketterer MW., DenoIIet J., Chapp J., et al Men deny and women cry, but who dies? Do the wages of denial include early ischemic coronary heart disease?. J Psychosom Res. 2004;56:119–123. doi: 10.1016/S0022-3999(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 115.Van Elderen T., Maes S., Dusseldorf D. Coping with coronary heart disease: a longitudinal study. J Psychosom Res. 1999;47:175–183. doi: 10.1016/s0022-3999(99)00024-0. [DOI] [PubMed] [Google Scholar]
- 116.HobfoII SE., Dunahoo CL., Ben-Porath Y., Monnier J. Gender and coping: the dual-axis model of coping. Am J Community Psychol. 1994;22:49–82. doi: 10.1007/BF02506817. [DOI] [PubMed] [Google Scholar]
- 117.Frasure-Smith N., Lesperance F., Juneau M., Talajic M., Bourassa MG. Gender, depression, and one-year prognosis after myocardial infaction. Psychosomat Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 118.AhnIund K., Frodi A. Gender differences in the development of depression. Scand J Psychol. 1996;37:229–237. doi: 10.1111/j.1467-9450.1996.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 119.Kiecolt-Glaser JK., Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 120.Horsten M., Mittleman MA., Wamala SP., Schenck-Gustafsson K., OrthGômer K. Depressive symptoms and lack of social integration in relation to prognosis of CHD in middle-aged women. The Stockholm Female Coronary Risk Study. Eur Heart J. 2000;21:1072–1080. doi: 10.1053/euhj.1999.2012. [DOI] [PubMed] [Google Scholar]