Abstract
In the last decades, there has been increased interest in the field of quality of life in mental disorders in general, and particularly in schizophrenia. In addition, the appearance of the atypical antipsychotic drugs (amisul pride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone) with different therapeutic and side-effect profiles, has promoted a greater interest in assessing the quality of life of schizophrenic patients. In this paper we will briefly summarize the difficulties in assessing quality of life in schizophrenic patients, as well as the results concerning their quality of life and the influence of psychopathology, especially negative and depressive symptoms, on it. We will also review data from recent clinical trials showing the impact of antipsychotic treatments and their side effects upon quality of life.
Keywords: quality of life, schizophrenia, antipsychotic, side effect
Abstract
Durante las últimas décadas se ha producido un creciente interés en el campo de la calidad de vida en los trastornos mentales en general, y en la esquizofrenia en particular. Por otra parte, la aparición de los medicamentos antipsicóticos atípleos (amilsupríde, aripiprazol, clozapina, olanzapina, quetiapina, risperidona y ziprasidona) con diferentes perfiles terapéuticos y efectos secundarios, ha promovido un mayor interés a la hora de evaluar la calidad de vida en los pacientes que padecen esquizofrenia. En este trabajo se analizan brevemente las dificultades que conlleva la evaluación de la calidad de vida en pacientes con esquizofrenia además de los resultados acerca de su calidad de vida y la influencia de psicopatología, especialmente síntomas negativos y depresivos, sobre ésta. También se revisan resultados de ensayos clínicos recientes que muestran el impacto del tratamiento antipsicótico y de sus efectos secundarios sobre la calidad de vida.
Abstract
L'intérêt pour la qualité de vie au cours des troubles mentaux en général et de la schizophrénie en particulier s'est accru ces 10 dernières années. L'émergence de médicaments antipsychotiques atypiques (amisulpride, aripiprazole, cloza-pine, olanzapine, quétiapine, rispéridone et zipra-sidone) dotés de profils d'effets thérapeutiques et d'effets secondaires différents a favorisé l'intérêt croissant dans l'évaluation de la qualité de vie des patients schizophrènes. Nous allons rapidement résumer dans cet article les difficultés d'évaluation de la qualité de vie des patients schizophrènes, ainsi que les résultats de la qualité de vie et l'influence de la psychopathologie sur celle-ci, en particulier les symptômes dépressifs et négatifs. Nous allons aussi revoir des données d'études cliniques récentes qui montrent l'impact des traitements antipsychotiques et leurs effets indésirables sur la qualité de vie.
Quallty of life is defined by the World Health Organization1 as “Individuals' perceptions of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards, and concerns.” In the last two decades, there has been increasing interest in quality of life in schizophrenic patients, since schizophrenia is a severe, disabling, lifelong disorder, associated with severe social and occupational dysfunction. Furthermore, the development of atypical antipsychotics with broader efficacy and lower incidence of extrapyramidal side effects than typical neuroleptics has promoted greater interest from the patient's perspective.2
Measurement of quality of life in schizophrenic patients
Quality of life measurement is based on the principle of applying medical care and interventions, taking into account patients' right of autonomy, which necessarily includes their opinion both during diagnostic evaluation and while formulating their care plan.3 However, there are still doubts as to whether patients with schizophrenia are capable of self-assessment of their quality of life, because of their cognitive deficits and lack of insight into their illness.4 Lehman et al5 have demonstrated convergent validity in the perception of quality of life between patients and clinicians, but they have also recommended caution regarding the validity of quality of life assessments made by severely mentally ill patients. Browne et al6 summarized the view of several authors, and stated that clinical evaluation of quality of life obtained from reports of psychiatric patients is desirable, since selfreports can be influenced by persistent psychotic symptoms, the idiosyncratic views and values of these patients, and by the adaptation to adverse circumstances. Skantze et al7 showed that schizophrenic patients feel, experience, and are able to report their social deficits, which supports the thesis that quality of life can be assessed subjectively. Lehman8,9 has demonstrated that it is indeed feasible to collect statistically reliable quality of life data from chronic mental patients, and concluded that subjective quality of life assessments can be applied to such patients. Nonetheless, he remained uncertain about the validity of patients' judgments of their welfare, and about how discrepancies between patients and clinicians could best be resolved. Such discrepancies have been reported by Sainfort et al10 using the Wisconsin Quality of Life Questionnaire (W-QOL)11 in a sample of 40 schizophrenic patients from Wisconsin. The W-QOL attempts to address the issue of validity by questioning not only the patient, but also the clinician and the family. Sainfort et al10 have shown little agreement between welfare ratings made by service providers and patients in any domain but symptoms.
Nevertheless, the questions about the validity of patients' self-assessment of their quality of life should detract us, under no circumstances, from the clinical duty to discuss and negotiate every aspect of treatment with patients, and to incorporate their views in service developments.
The level of quality of life of schizophrenic patients
Reviewing the various studies in the literature concerning the quality of life of schizophrenic patients, we have found considerable differences in the methodology applied, thus making it difficult to establish comparisons. However, it can be concluded that quality of life of schizophrenic patients is characterized, in general, by the following aspects2:
It is worse than that of the general population and that of other physically ill patients.
Young people, women, married persons, and those with a low level of education report a better quality of life.
The longer the length of the illness, the worse the quality of life.
Psychopathology, especially negative and depressive syndromes, correlates negatively with quality of life.
Fewer side effects and the combination of psychopharmacological and psychotherapeutic treatment improve quality of life.
Patients integrated in community support programs demonstrate a better quality of life than those who are institutionalized.
Quality of life and antipsychotics
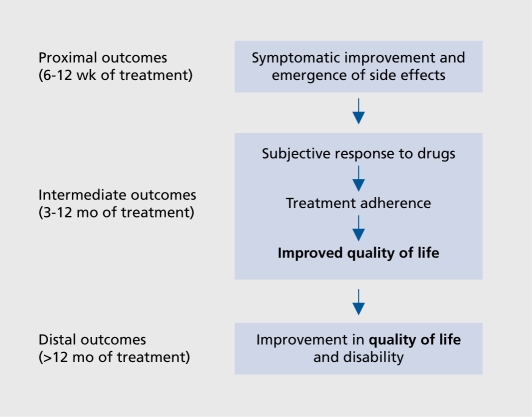
Although the concept of outcome in psychiatry has been widened beyond symptom improvement to include quality of life, management of side effects, and subjective response to drugs,3 (Figure 1) and that the quality of life should be considered as important as psychopathology13 has been emphasized, relatively few studies on the impact of antipsychotic drugs have reported data on this outcome.
Figure 1. Outcomes in schizophrenia. Modified from reference 12: Awad AG, Voruganti LNP, Heslegrave RJ. A conceptual model of quality of life in schizophrenia: description and preliminary clinical validation. Quai Life Res. 1997;6:21-26. Copyright © Kluwer Academic Publishers 1997.
The appearance of the atypical antipsychotic drugs (aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone) with different therapeutic and side-effect profiles promoted further studies and a greater interest in assessing the quality of life of schizophrenic patients (Table I). However, as stated by Corrigan et al,34 findings on this topic are contradictory; just about half of the studies demonstrated that, in comparison with typical antipsychotics, atyplcals significantly Increase the quality of life of schizophrenia-spectrum patients. The inconsistency of the results may be due to the following factors:
Table I. Quality of life in clinical trials with antipsychotic drugs. AMI, amisulpride; CAPS, conventional antipsychotics; CLZP, clozapine; HAL, haloperidol; FLU, flupenthixol; LA-RISP, long-acting risperidone; MLDL, Munich Quality of Life Dimensions; OLZ, olanzapine (OLZ-L, olanzapine 5±2.5 mg/d, OLZ-M, olanzapine 10±2.5 mg/d; OLZ-H, olanzapine 15±2.5 mg/d); PSYCH-BASE, Psychiatric Status You Currently Have-Baseline version; QLS, Qualtiy of Life Scale; QOLI, Quality of Life Interview; QUET, quetiapine; RISP, risperidone; SF-36, Short-Form 36; SIP, Sickness Impact Profile; SQLQ, Sevilla Quality of Life Questionnaire; SWN, Subjective Well-Being under Neuroleptic Treatment; UC, usual care; WHOQOL-BREF, World Health Organization Quality of Life [Brief] (continued on pages 112 and 113).
| Author, year | Country | Antipsychotic drug | Design | Patients | Instruments | Results |
| Carrière et al, 200014 | France | Amisulpiride (400-1200 mg/d) Haloperidol (10-30 mg/d) | 4-month double-blind, randomized Inpatients | Baseline: 199 (94 AMI, 105 HAL) 4-month:129 (70 AMI, 59 HAL) | QLS | AMI>HAL |
| Colonna et al, 200015 | France | Amisulpiride (200-800 mg/d) Haloperidol (5-20 mg/d) | 12-month open-label, randomized Outpatients | Baseline: 488 (370 AMI, 118 HAL) 12-month: 322 (253 AMI, 69 HAL) | QLS | AMI>HAL in QLS total score and in Intrapsychic Foundations, Interpersonal Relations and Instrumental Role domain scores |
| Rosenheck et al 199716, | USA (multicenter) | Clozapine (100-900 mg/d) Haloperidol (5-30 mg/d) | 12-month open-label, randomized Treatment-resistant inpatients | Baseline: 423 (205 CLZP, 218 HAL) 1-year: 178 (117 CLZP, 61 HAL) | QLS | CLZPglt;HAL among patients who completed their assigned treatment |
| Essock et al, 199617 | USA (Connecticut) | Clozapine Usual care | 2-year open-label, randomized Treatment-resistant inpatients | Baseline: 227 (138 CLZP, 89 UC) | QOLI | CLZP=UC |
| Meltzer et al, 199018 | USA (Cleveland) | Clozapine | 6-month open-label Treatment-resistant patients | Baseline: 38 | QLS | Significant improvement in all QLS scores |
| Strakowski et al, 200519 | USA | Olanzapine Haloperidol | 1-year double-blind, randomized | 195 first episode (100 OLZ,95 HAL) | Sf-36 | OLZ = HAL Significant improvement in5 SF-36 subscales and in the mental summary scale |
| Naber et al, 200520 | Germany (multicenter) | Olanzapine (mean dose: 162 mg/d) | 26-week randomized | 99 (49 OLZ, 50 CLZP) | MLDL SWN | OLZ = CLZP |
| Clozapine (mean dose: 209 mg/d) | double-blind | |||||
| Giner et al, 200421 | Spain (multicenter) | Olanzapine (mean dose: 13.5 mg/d) | 12-month open label | 372 | SQLQ QoLI | Significant improvement in both QoL scales |
| Revicki et al, 199922 | International (11 countries) | Olanzapine (5-20 mg/day) Haloperidol (5-20 mg/day) | 52-week double-blind, randomized - 6 week acute phase - 46 week extension phase | Baseline: 1159 (787 OLZ, 372 HAL) 6-week: 600 OLZ, 228 HAL Outpatients | QLS SF-36 | Acute phase: QLS: OLZ>HAL in total, Intrapsychic Foundations and Interpersonal Relation scores. SF-36: OLZ>HAL in MCS, V and MH Extension phase: QLS; OLZ>HAL in total, Intrapsychic Foundations and Instrumental Role scores SF-36: OLZ = HAL |
| Hamilton et al 199823 | USA (multicenter) | Olanzapine (5±2.5 mg/d; 10±2.5 mg/d; 15±2.5 mg/d) Haloperidol (15±5 mg/d) Placebo | 24-week double-blind, randomized, placebo controlled inpatients | Data from 76 patients (16 OLZ-L, 16 OLZ-M, 22 OLZ-H, 12 HAL, 10PBO) | QLS | OLZ-M & OLZ-H: significant improvements on QLS total and all subscale scores OLZ-H>PBO on QLS total score OLZ-M>PBO on QLS total and all subscale scores |
| Montes et al, 200324 | Spain (multicenter - EFESO study) | Olanzapine (5-30 mg/d) Risperidone (1.5-9.5 mg/d) Haloperidol (3.8-30 mg/d) | 6-month open label without randomization | 182(114 OLZ, 31 RISP, HAL 37) | EuroQol | OLZ and RlSP>HAL on EuroQol-VAS scores |
| Gureje et al, 200325 | Australia and New Zealand | Olanzapine (10-20 mg/day) Risperidone (4-8 mg/day) | 30-week double-blind, randomized | Baseline: 65 (32 OLZ, 33 RISP) 30 week: 29 (17 OLZ, 12 RISP) In and outpatiens | QLS SF-36 | QLS: OLZ > RISP in the Intrapsychic Foundations subscale score SF-36: OLZ > RISP in the Role Emotional subscale score |
| Ritchie et al, 200326 | UK | Olanzapine (9.9 mg/d) Risperidone (1.7 mg/d) | Open-label, randomized | Baseline: 66 (36 OLZ, 30 RISP) End: 32 OLZ, 22 RISP | WHOQOL- BREF | Significant improvements in OLZ-group in physical. psychological and health satisfaction domains OLZ>RISP in the Psychological domain score |
| Voruganti et al, 200227 | Canada | Olanzapine (15-40 mg/d) Risperidone (2-8 mg/d) Quetiapine (200-800 mg/d) | Naturalistic, single-blind 2-6 years | Baseline: 150 (50 OLZ, 50 RISP, 50 QUET) End: 118 (44 OLZ, 43 RISP, 31 QUET) | QLS | Significant improvements across all groups OLZ=RlSP=QUET |
| Ho et al, 199928 | USA (Iowa) | Olanzapine (144 mg/day) Risperidone (57 mg/day) | 6-month open-label | Baseline: 42 (21 OLZ, 21 RISP) 6-month: 26 (13 OLZ, 13 RISP) Inpatients | PSYCH-BASE | OLZ = RISP |
| Tran et al, 199729 | International (9 countries) | Olanzapine (10-20 mg/d) Risperidone (4-12 mg/d) | 28-week double-blind, randomized in- and outpatients | Baseline: 339 (172 OLZ, 167 RISP) 28-week | QLS | OLZ & RISP: significant improvements on QLS total and all subscale scores OLZ>RISP on QLS interpersonal relations score |
| Hertling et aI 200330, | Germany and Austria | Risperidone (2-6 mg/d) Flupenthixol (4-12 mg/d) | 24-week double-blind, randomized | Baseline: 153 (77 RISP, 76 FLU) 24-week: 107 (56 RISP, 51 FLU) In and outpatients | EuroQuol-VAS | Significant improvement in EuroQuol-VAS in both groups; RISP=FLU |
| Bobes et al, 199831 | Spain (multicenter) | Risperidone (3-12 mg/d) | 8-month open-label outpatients | Baseline: 318 Month-8: 264 | SF-36 | At end point significant improvement in all SF-36 subscale scores and summary measures |
| Nasrallah et al, 200532 | USA | Long-acting risperidone (25, 50,75 mg/2w) Placebo | 12-week, randomized, double-blind, placebo-controlled | LA-RISP (93 25 mg/2w, 97 50 mg/2w, 87 75 mg/2w) PBO (92) | SF-36 | LA-RISP>PBO in bodily pain, general health, social functioning, role emotional and mental health At week 12 LA-RISP 25 mg/2w reached normal values in all 5F-36 scales but physical functioning |
| Velligan et al, 200333 | USA (Texas) | Quetiapine Conventional antipsychotics | 6-month open-label, rater-blinded, randomized | Baseline; 40 (20 QUET, 20 CAPS) 6-month: 27 (14 QUET, 13 CAPS) | QLS | QUET>CAPS |
The instruments employed: despite the fact that the QLS35 was specifically designed to assess the deficit syndrome of schizophrenia, most studies, including clinical trials, have employed the QLS as a measure of quality of life, even thought its is a “clinician-rated” instrument and does not incorporate the subjective views of patients themselves.
Clinical trials do not always accurately reflect psychiatric routine treatment of patients.
Illness-related differences, treatment, and many other factors affecting participants may influence quality of life outcomes.
Three naturalistic comparative studies have been recently published,36-38 comparing quality of life outcomes between atypical and typical antipsychotics in schizophrenic patients. Two of them36,37 suggest that atypical antipsychotics have several advantages over typicals in quality of life outcomes, while the other demonstrates the opposite.
The first36 was a cross-sectional study including 78 schizophrenic outpatients stabilized on risperidone or olanzapine, and 55 patients stabilized on typical antipsychotics. Quality of life was assessed employing the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q)39 and the QLS35 at baseline. After adjusting for daily doses, duration of treatment, subjective tolerability, and adjuvant antidepressants, atypicals showed greater improvements in quality of life than typicals. No significant differences were found in quality of life outcomes between atypicals.
The second one was the SOHO study (Pan-European Schizophrenia Outpatient Health Outcomes)37 that included a total of 10 972 patients from 10 European countries. Quality of life was assessed employing the EuroQoL-5 Dimensions (EQ-5D).40 After 6 months of treatment, patients in the risperidone, quetiapine, amisulpride, oral typicals, and depot typicals cohorts had a significantly lower quality of life than those in the olanzapine cohort, although the magnitudes of the differences were quite small.
Finally, Kilian et al38 did not find any significant differences in the quality of life of schizophrenic outpatients treated with first- or second-generation antipsychotics. This was a prospective naturalistic trial including 307 schizophrenics who were assessed at 6-month intervals over 2.5 years. Quality of life was assessed using the Quality of Life Interview (QoLI).41 They found that the type of antipsychotic treatment had no significant effect on the improvement of subjective quality of life of the patients.
Studies on quality of life conducted with the different antipsychotics are briefly described below.
Amisulpride
With respect to amisulpride, Carrière et al14 conducted a multicenter, double-blind, randomized study over 4 months. A total of 199 inpatients with a diagnosis of paranoid schizophrenia or schizophreniform disorder (Diagnostic and Statistical Manual of Mental Disorders. 4th ed, DSM-IV 42) were assessed using the QLS.35 Patients were randomized to receive oral amisulpride (400 to 1200 mg/d) (n=94) or haloperidol (10-30 mg/d) (n=105). Quality of life was improved in both groups, but the improvement was significantly greater with amisulpride than with haloperidol.
Colonna et al15 studied the long-term efficacy and safety of amisulpride in a group of 488 schizophrenic patients. They carried out a 12-month open-label, randomized study in which 370 patients were receiving amisulpride (200 to 800 mg/d) and 118 patients haloperidol (5 to 20 mg/d). Patients' quality of life was assessed using the QLS.35 Amisulpride demonstrated significantly greater improvements in total QLS score and in scores on three QLS domains: intrapsychic foundations, interpersonal relations, and instrumental role.
Clozapine
Meltzer et al18 described 38 treatment-resistant schizophrenics from Cleveland who had started on clozapine. Using the QLS,35 they found a significant improvement in the total score between baseline and after 6 months of treatment. There was an increase of 59.9% in the mean score, and in all of the four subscales, those with the largest mean increase being the interpersonal role and intrapsychic aspects (72.2% and 70.8% respectively). In 1992,43 having studied 25 of these 38 patients over a 12-month period, Meltzer reported the same results, ie, a significant improvement in the total score and in all subscales and, furthermore, a greater improvement in the instrumental role function, which reached similar levels to those of interpersonal and intrapsychic aspects. In 1993,44 the group reported results from 96 patients who were admitted to an open trial of clozapine for treatment-resistant schizophrenia at the University Hospital of Cleveland, and demonstrated that quality of life scores only improved in patients who continued clozapine treatment for at least 2 years, which means an improvement of 242%.
Rosenheck et al16 conducted a comparative study of clozapine and haloperidol in refractory schizophrenic inpatients. They carried out a randomized, 1-year double-blind study at 15 Veterans Affairs medical centers. A total of 423 patients (clozapine = 205 and haloperidol = 218) were assessed using the QLS.35 After 1 year, 117 clozapine-treated patients and 61 haloperidol-treated patients continued their assigned treatment. In these patients, clozapine was significantly better than haloperidol in improving patients' quality of life.
In 1996, Essock et al17 failed to find superiority of clozapine over conventional antipsychotics on patients' quality of life. Their study was the first randomized costeffectiveness trial of clozapine. It was a 2-year open-label randomized study comparing clozapine with usual care in schizophrenic or schizoaffective treatment-resistant inpatients. A total of 227 patients (138 in the clozapine group and 89 in the usual care group) were assessed using the Quality of Life Interview (QoLI).14 Clozapine did not significantly affect patients' quality of life. By the 8th month of treatment, both groups experienced equivalent improvements in the QoLI global satisfaction score.
Olanzapine
Hamilton et al23 evaluated the impact of treatment with olanzapine compared with haloperidol and placebo on quality of life in schizophrenic inpatients. They conducted a double-blind randomized study, with a 6-week acute phase and an extension phase of 46 weeks for the responders. A total of 335 patients were randomized to one of the following groups: olanzapine 5±2.5 mg/d, olanzapine 10±2.5 mg/d, olanzapine 15±2.5 mg/d, haloperidol 15±5 mg/d, and placebo. Data at extension week 24 was reported in their paper. Quality of life was assessed employing the QLS.35 At end point, no significant changes in the QLS total and subscale scores were observed for the placebo, olanzapine low-dose, or haloperidol groups. Moreover, significant improvements were observed for olanzapine medium and high doses. The olanzapine medium-dose group demonstrated significant greater improvements in all QLS scores than the placebo group. The olanzapine high-dose group showed greater improvement in QLS total score compared with the placebo treatment group.
The impact of olanzapine on quality of life has also been compared with the impact of haloperidol in a 6-week, double-blind randomized multicenter trial with a longterm extension (46 weeks).22 A total of 1159 outpatients with a diagnosis of schizophrenia or schizophreniform or schizoaffective disorder and scores on the BPRS ≥18 were assessed using the QLS.35 The Medical Outcomes Study Short Form 36-item (SF-36)45 was also administered, only in English-speaking countries. Questionnaires were administered at baseline and at the end of the acute phase of the study (week 6). In the extension phase they were administered every 8 weeks.
At the end of acute phase, data from 828 patients (600 in the olanzapine group and 228 in the haloperidol group) were obtained. Olanzapine-treated patients showed significantly greater improvements in QLS total, intrapsychic foundations, and interpersonal relations scores compared with the haloperidol group. Using the criteria of a 20% increase as clinically meaningful improvement in QLS total scores, 38% of olanzapinetreated patients showed clinical improvement in quality of life compared with 27% in the haloperidol group. Results in the SF-36 were similar; the olanzapine group demonstrated significantly greater improvements in mental component summary scores and in general health perception, vitality, and mental health subscale scores. At the end of extension phase (week 52) results in the QLS were almost identical; patients in the olanzapine group showed statistically greater improvements in QLS total, intrapsychic foundations, and instrumental role scores than haloperidol-treated patients. However, in the SF-36 no statistically significant differences were obtained between the treatment groups.
In an other study, Giner et al21 found that 1 year after switching to olanzapine, due to lack of efficacy or intolerance, the quality of life, assessed by means of the Sevilla Quality of Life Questionnaire (SQLQ)46 and the Lehman's Quality of Life Interview,41 of the 372 schizophrenic patients included in the study had improved. Strakowski et al19 compared the quality of life improvement in 195 patients with first-episode schizophrenia for up to 1 year following randomization to either olanzapine or haloperidol in a double-blind clinical trial. Quality of life was assessed using the SF-36. They found that both antipsychotics showed similar improvements on the SF-36. Specifically, significant improvement was observed for the following SF-36 subscales: bodily pain, general health, social functioning, role emotional, and mental health, and in the Mental Summary Scale. Similarly, Naber et al20 found that olanzapine had no significant difference from clozapine regarding improvements on the Subjective Well-Being under Neuroleptic Treatment scale (SWN)47 and on the Munich Life Dimension List (MLDL).48
Olanzapine and risperidone
Recently, an interesting study comparing the safety, effectiveness, and quality of life of olanzapine, risperidone, and conventional antipsychotics in first-episode schizophrenia has been conducted.24 Patients were taken from the EFESO study (a Spanish-multicenter, phaseIV, observational, 6-month open-label study). Patients' quality of life was assessed by means of the EuroQol (EQ-5D).40 Data from 114 patients receiving olanzapine, 31 receiving risperidone, and 37 on conventional antipsychotics were obtained. Mean doses were as follows: olanzapine 13.5 mg/d, risperidone 5.4 mg/d, and haloperidol 12.4 mg/d. After 6 months of treatment, improvement in EuroQol-VAS scores was significantly greater in olanzapine and risperidone-treated patients than in those receiving haloperidol.
Gureje et al25 conducted a multicenter, 30-week, doubleblind study comparing the efficacy, safety, use of health care resources, level of functioning, and quality of life between olanzapine and risperidone. Sixty-five patients, either inpatients or outpatients, with a diagnosis of schizophrenia or schizophreniform disorder (DSM-IV criteria42) and scores on the Brief Psychiatric Rating Scale (BPRS) greater than 36 were randomized to receive olanzapine 10 to 20 mg/day (n=32) or risperidone 4 to 8 mg/day (n=33). Quality of life was assessed using the QLS35 and the SF-36.45 A total of 29 patients (17 in the olanzapine group and 12 in the risperidone group) completed the study At the end of the 30 weeks, olanzapinetreated patients had statistically significant greater improvement compared with the risperidone-treated patients in the QLS intrapsychic foundation subscale and in the SF-36 Role Emotional subscale. The olanzapinetreated group reported statistically significant improvement from baseline to end point in QLS total score, in all QLS subscales except the instrumental role, and in all SF36 scales but the role physical. For the risperidone-treated group statistically significant improvement was only achieved for the SF-36 bodily pain scale.
Ritchie et al26 compared the impact on quality of life of a switch from conventional antipsychotics to risperidone or olanzapine in 66 elderly patients with schizophrenia (mean age 69.6 years). Quality of life was measured using the World Health Organization Quality of Life [Brief] scale (WHOQOL-BREF).49 Olanzapine-treated patients significantly improved from baseline in the WHOQOL-BREF physical, psychological, and health satisfaction domains, whereas risperidone-treated patients did not show significant improvements in any quality of life domain. Treatment with olanzapine was associated with a better response over risperidone on the psychological domain of the WHOQOL-BREF.
The impact of switching from conventional to novel antipsychotic drugs on quality of life was also studied by Voruganti et al.27 One hundred and fifty schizophrenic or schizoaffective patients (DSM-IV42) considered suitable for a switch, based on inadequate control of symptoms, subjective reports of side effects, or clinicians' concerns about the risk for adverse effects, were consecutively switched to risperidone (50 patients), olanzapine (50), and quetiapine (50). Patients were followed up for a period between 2 and 6 years. Quality of life was assessed by means of the QLS35 and the Sickness Impact Profile (SIP)modified version. At the end of follow-up 118 patients remained on novel antipsychotic (44 on olanzapine, 43 on risperidone, and 31 on quetiapine). Scores on the QLS and the SIP-modified version improved uniformly in the three groups after the switch. There were no significant differences between the three novel antipsychotics.
In another comparative open-label study, Ho et al28 did not find differential effects of risperidone and olanzapine on patients' quality of life. They included 42 schizophrenic (DSM-IV criteria42) inpatients; 21 of them were started on risperidone (mean baseline dose 5.7 mg/day) and the remaining 21 on olanzapine (mean baseline dose 14.4 mg/day) based on the treating psychiatrist's decision. Quality of life was assessed using the Psychiatric Status You Currently Have-Baseline version (PSYCH-BASE)50 and its longitudinal follow-up version, the PSYCH-UP. The PSYCH-BASE is a structured interview with eight quality of life indices: occupational impairment, financial dependence, impairment in performance of household duties, relationship impairment with family members and with friends, enjoyment of recreational activities, satisfaction, and overall psychosocial functioning. A total of 26 patients (13 in each group) completed the 6-month followup interview. At follow-up there were no statistically differential effects between the two treatments on the eight quality of life indices. Significant improvements at time of follow-up were reported on overall psychosocial functioning in the risperidone group and on impairment in performance of household duties in the olanzapine group. Tran et al29 compared olanzapine with risperidone in an international, 28-week, double-blind, randomized study. Three hundred and thirty nine (olanzapine n=172, risperidone n=167) schizophrenic, schizophreniform, or schizoaffective patients (DSM-IV criteria42) were assessed using the QLS.35 In both treatment groups, statistically significant improvements were observed on the QLS total score and on the four subscales from baseline to end point. Olanzapine demonstrated significant greater improvement in QLS interpersonal relations subscale scores than risperidone.
Risperidone
Bobes et al31 studied the effect of risperidone monotherapy maintenance treatment on the quality of life of 318 schizophrenic outpatients (The ICD-10 Classification of Mental and Behavioral Disorders, Clinical descriptions and diagnostic guidelines, ICD-1051 criteria) who had been previously treated with other neuroleptics. Quality of life was assessed employing the SF-36.48 At month 8, significant improvement was observed in all SF-36 scale scores and in the summary measures. The greatest improvement was observed in the role emotional scale, followed by the role physical and the social functioning.
Hertling et al30 compared the impact of risperidone and flupenthixol upon the quality of life of schizophrenic inpatients and outpatients with mainly negative symptoms. They conducted a binational (Germany and Austria), 24-week, randomized, double-blind trial in which 72 patients received risperidone and 72 flupenthixol. Patients' subjective quality of life was assessed using the EuroQuolVisual Analogue Scale40 and the Patient Satisfaction Questionnaire.52 After 24 weeks of treatment, EuroQuo-lVAS score significantly increased in both groups without significant differences between them. In the Patient Satisfaction Questionnaire, flupenthixol demonstrated significant greater improvements in “feeling more able to cope with stress,” “feeling more relaxed,” and “feeling more able to achieve something” than risperidone.
Nasrallah et al32 evaluated the quality of life of schizophrenic patients under treatment with long-acting, injectable risperidone versus placebo. They assessed a total of 369 schizophrenic patients (92 receiving placebo, 93 25 -mg, 97 50 -mg, and 87 75 -mg/2 weeks of long-acting risperidone) using the SF-36.45 After 12 weeks of doubleblind randomized treatment, patients in the long-acting risperidone group improved significantly in the bodily pain, general health, social functioning, role-emotional and mental health SF-36 scales compared with patients in the placebo group.
Quetiapine
Velligan et al33 studied the effectiveness of quetiapine versus conventional antipsychotic in improving quality of life as measured by the QLS.35 They conducted an open-label, rater-blinded study in which 40 stable schizophrenic outpatients were randomly assigned to continue taking their traditional antipsychotic medication or to be switched to quetiapine for a period of 6 months. In the quetiapine group six patients dropped out and in the conventional antipsychotic group seven did. Quetiapine-treated patients had better quality of life scores during the follow-up period than those in the conventional group.
Ziprasidone ani aripiprazole
These are the newest antipsychotics on the market, and have been demonstrated to be efficacious in a broad spectrum of symptomatology and to have a good tolerance profile. Preliminary data concerning their impact on the patients' quality of life have been presented at different meetings in the last 2 years, showing significantly greater improvements than conventional antipsychotics. However, definitive data for any of them have not yet been published.
Antipsychotic side effects and quality of life
Antipsychotics have a wide range of adverse effects that may negatively affect the quality of life of schizophrenic patients and their compliance with treatment. Ritsner et al36 analyzed the impact of side effects of antipsychotic treatment on the quality of life of 161 schizophrenic inpatients stabilized on typical and atypical antipsychotics. Quality of life was measured using the Quality of Life Enjoyment and Satisfaction Questionnaire (QLES-Q).39 Patients with adverse events reported significantly less satisfaction with subjective feelings and general activities than patients without adverse events. The side effects with the most significant negative impact were sleep disturbance, fatigue, tachycardia, tremor, sexual dysfunction, headache, polyuria, dizziness, hypertension/hypotension, dyskinetic movements, and constipation/diarrhea. Surprisingly, patients did not differ significantly in satisfaction with medication. No significant differences between typical and atypical antipsychotics were found in the quality of life index and specific life domains. In addition, no significant correlations were found between daily doses or duration of treatment and quality of life. Multiple regression analysis showed that adverse side effects accounted for only 3.2% of the variance in quality of life ratings, which is significantly less than other clinical and psychosocial parameters.
Several studies have evaluated the impact of some side effects on quality of life of schizophrenic patients, but the results are contradictory, especially concerning extrapyramidal side effects (EPS). While some studies have found negative influence of EPS on quality of life,6,12,22 others have not found any53,54 (Table II).
Table II. Antipsychotic side effects and quality of life. MLDL, Munich Quality of Life Dimensions; PGWB, Psychological General Well-Being Index; Q-LES-Q, Quality of Life Enjoyment and Satisfaction Questionnaire; QLS, Quality of Life Scale; QoL, Quality of Life; QoLI, Lehman Quality of Life Interview; SF-12, Short-Form; SF-36, Short-Form 36; SQLS, Schizophrenia Quality of Life Scale; UKU, The UKU Side-Effect Rating Scale; VAS-QoL, Visual Analogue Scale Quality of Life.
| Author, year | Patient (number) | Side effects/Scale | Quality of Life scale | Influence on Quality of Life? |
| Browne, 19966 | 64 | Tardive dyskinesia/Abnormal Involuntary Movements Scale | QLS | Yes |
| Larsen, 199655 | 53 | UKU | PGBW | No |
| Awad, 199712 | 63 | Akathisia (Hillside Akathisia Scale) | Gurin's global QoL | Yes |
| Franz, 199754 | 64 | Extrapyramidal | MLDL | No |
| Revicki, 199922 | 828 | Extrapyremidal/Simpson-Angus Scale | QLS | Yes |
| Kaneda, 200356 | 42 | Hyperprolactinemia | SQLS | No |
| Ritsner, 200336 | 148 | Number of adverse symptoms | Q-LES-Q | Yes |
| Allison, 200357 | 286 | Weight gain | PGWB, VAS-QoL | Yes |
| Strassnig, 200358 | 143 | Body weight / Body mass index | SF-36 | Yes |
| Meyer, 200559 | 1231 | Metabolic syndrome | SF-12 | Yes |
| Olfson, 200560 | 139 | Male sexual dysfunction / Change in Sexual Functioning Questionnaire | QoLI | Yes |
Two studies57,58 have evaluated the impact of weight gain on quality of life among persons with schizophrenia. Both of them demonstrated that weight gain was related to poorer quality of life. Strassnig et al58 studied 143 patients with a diagnosis of schizophrenia, schizoaffective, and psychotic disorder not otherwise specified (NOS). Patients completed the SF-36.45 The authors found that obese patients (BMI ≥30) had worse scores on the following SF-36 scales: physical functioning, gen_ eral health, role emotional and physical health summary component than both overweight (BMI =25-29.9) and healthy weight (BMI <25) subjects. They did not find a correlation between BMI and the mental health summary component. These results were replicated in the CATIE study.59 This study analyzed the influence of the metabolic syndrome on the quality of life of schizophrenic patients using the Short Form-12 (SF-12).They found that those with metabolic syndrome obtained significantly worse scores on physical health than those patients without metabolic syndrome. Allison et al,57 using the Psychological General Well-Being Index (PGWBI),61 found that patients who gained weight had poorer quality of life, general psychological well-being (not statistically significant), self -reported general health, and vitality.
Kaneda56 investigated the impact of prolactin elevation with antipsychotic medications on quality of life in schizophrenic patients. He studied 42 inpatients with chronic schizophrenia using the Schizophrenia Quality of Life Scale (SQLS)62 - Japanese version. He did not find significant correlations between prolactin or testosterone levels and the three subscales of the SQLS, so he concluded that prolactin did not affect subjective quality of life scores directly.
The impact of male sexual dysfunction on quality of life in schizophrenic patients was investigated by Olfson et al.60 A total of 139 patients were assessed using the Changes in Sexual Functioning Questionnaire (CSFQ)63 and a 7-point Likert scale with items from the Quality of Life Interview (QoLI).41 Patients with current sexual dysfunction showed significantly poorer global quality of life and less satisfaction with the amount of enjoyment in their lives than patients without current sexual dysfunction.
In summary, after this comprehensive review of the state of the art in the field of quality of life in schizophrenia, we totally agree with the statement made by Katschnig64 that quality of life is a useful concept and strategy in clinical psychiatry.
Conclusions
Quality of life is nowadays considered in clinical psychiatry as an intermediate and distal outcome, firmly consolidated and broadly demanded by patients, families, clinicians, and institutions.
This outcome is adversely influenced by the presence of clinical symptoms, especially negative and depressive. In this sense, therapeutic interventions upon the whole constellation of schizophrenic symptomatology are of great value in improving patients' quality of life.
Although atypical antipsychotics have demonstrated a broader efficacy profile and better tolerability pattern than conventional ones, results concerning their greater benefits in improving the quality of life of schizophrenic patients are controversial at present.
The impact of extrapyramidal symptoms on the quality of life of schizophrenic patients remains unclear.
Other side effects, such as weight gain and sexual dysfunction, have been shown to be negatively associated with quality of life.
Contributor Information
Julio Bobes, Department of Psychiatry, University of Oviedo, Oviedo, Spain.
Maria Paz Garcia-Portilla, Department of Psychiatry, University of Oviedo, Oviedo, Spain.
Maria Teresa Bascaran, Department of Psychiatry, University of Oviedo, Oviedo, Spain.
Pilar Alejandra Saiz, Department of Psychiatry, University of Oviedo, Oviedo, Spain.
Manuel Bouzoño, Department of Psychiatry, University of Oviedo, Oviedo, Spain.
REFERENCES
- 1.The WHOQOL Group. The World Health Organization Quality of Life Assessment (the WHOQOL): position paper from the World Health Organisation. Soc Sci Med. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 2.Bobes J., Garcia-Portilla MP. Quality of life in schizophrenia. In: Katschnig H, Freeman H, Sartorius N, eds. Quality of Life in Mental Disorders. Chichester, UK: John Wiley & Sons Ltd; 2005:153–168. [Google Scholar]
- 3.Bobes J. Current status of quality of life assessment in schizophrenic patients. Eur Arch Psychiatry Clin Neurosc. 2001;251(suppl 2):ll/38–ll/42. doi: 10.1007/BF03035125. [DOI] [PubMed] [Google Scholar]
- 4.Bobes J., Garcia-Portilla MP., Saiz PA., Bascaran T., Bousoño M. Quality of life measures in schizophrenia. Eur Psychiatry. 2005;20:5313–5317. doi: 10.1016/s0924-9338(05)80182-8. [DOI] [PubMed] [Google Scholar]
- 5.Lehman AF., Postrado LT., Rachoba LT. Convergent validation of quality of life assessment for persons with severe mental illness. Qual Life Res. 1993;2:327–333. doi: 10.1007/BF00449427. [DOI] [PubMed] [Google Scholar]
- 6.Browne S., Roe M., Lane A., et al. Quality of life in schizophrenia: relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Acta Psychiatr Scand. 1996;94:118–124. doi: 10.1111/j.1600-0447.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 7.Skantze K., Malm U., Dencker SJ., May PR., Corrigan P. Comparison of quality of life with standard of living in schizophrenic outpatients. Br J Psychiatry. 1992;161:797–801. doi: 10.1192/bjp.161.6.797. [DOI] [PubMed] [Google Scholar]
- 8.Lehman AF. The well-being of chronic mental patients: assessing their quality of life. Arch Gen Psychiatry. 1983;40:369–373. doi: 10.1001/archpsyc.1983.01790040023003. [DOI] [PubMed] [Google Scholar]
- 9.Lehman AF. The effects of psychiatric symptoms on quality of life assessments among the chronically mentally ill. EvaI Prog Planning. 1983;6:143–151. doi: 10.1016/0149-7189(83)90028-9. [DOI] [PubMed] [Google Scholar]
- 10.Sainfort F., Becker M., Diamond R. Judgments of quality of life of individuals with severe mental disorders: patient self-report versus provider perspectives. Am J Psychiatry. 1996; 153:497–502. doi: 10.1176/ajp.153.4.497. [DOI] [PubMed] [Google Scholar]
- 11.Becker M., Diamond R., Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2:239–251. doi: 10.1007/BF00434796. [DOI] [PubMed] [Google Scholar]
- 12.Awad AG., Voruganti LNP., Heslegrave RJ. A conceptual model of quality of life in schizophrenia: description and preliminary clinical validation. Qual Life Res. 1997;6:21–26. doi: 10.1023/a:1026409326690. [DOI] [PubMed] [Google Scholar]
- 13.Naber D., Moritz S., Lambert M., et al. Improvement of schizophrenic patients' subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50:79–88. doi: 10.1016/s0920-9964(00)00166-3. [DOI] [PubMed] [Google Scholar]
- 14.Carrière P., Bonhomme D., Lemperiere T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicenter, double-blind study (the Amisulpride Study Group). Eur Psychiatry. 2000;15:321–329. doi: 10.1016/s0924-9338(00)00401-6. [DOI] [PubMed] [Google Scholar]
- 15.Colonna L., Saleem P., Dondey-Nouvel L., Rein W. and the Amisulpride Study Group. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Int Clin Psychopharmacol. 2000;15:13–22. doi: 10.1097/00004850-200015010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Rosenheck R., Cramer J., Xu W., et al. A comparison of clozapine and haloperidol in hospitalised patients with refractory schizophrenia. N Engl J Med, 1997;337:809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- 17.Essock SM., Hargreaves WA., Covell NH., Goethe J. Clozapine's effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull. 1996;32:683–697. [PubMed] [Google Scholar]
- 18.Meltzer HY., Burnett S., Bastani B., Ramirez LF. Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Comm Psychiatry. 1990;41:892–897. doi: 10.1176/ps.41.8.892. [DOI] [PubMed] [Google Scholar]
- 19.Strakowski SM., Johnson JL., Delbello MP., et al. Quality of life during treatment with haloperidol or olanzapine in the year following a first psychotic episode. Schizophr Res. 2005;78:161–169. doi: 10.1016/j.schres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Naber D., Riedel M., Klimke A., et al. Randomized double blind comparison of olanzapine vs. clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta PsychiatrScand. 2005;111:106–115. doi: 10.1111/j.1600-0447.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Giner J., Bobes J., Cervera S., Leal C., Baca E., Ibáñez E. Impact of olanzapine on quality of life of patients with schizophrenia: one-year follow-up with the Seville Quality of Life Questionnaire. Actas Esp Psiquiatr. 2004;32:1–7. [PubMed] [Google Scholar]
- 22.Revicki DA., Genduso LA., Hamilton SH., Ganoczy D., Beasley CM. Olanzapine versus haloperidol in the treatment of schizophrenia and other psychotic disorders: Quality of life and clinical outcomes of a randomized clinical trial. Qual Life Res. 1999;8:417–426. doi: 10.1023/a:1008958925848. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SH., Revicki DA., Genduso LA., Beasley CM. Olanzapine versus placebo and haloperidol: quality of life and efficacy results of the North American double-blind trial. Neuropsychopharmacology. 1998;18:41–49. doi: 10.1016/S0893-133X(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 24.Montes JM., Ciudad A., Gascon J., Gomez JC., and the EFESO Study Group. Safety, effectiveness, and quality of life of olanzapine in first-episode schizophrenia: a naturalistic study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:667–674. doi: 10.1016/S0278-5846(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 25.Gureje O., Miles W., Keks N., et al. Olanzapine vs risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New Zealand. Schizophr Res. 2003;61:303–314. doi: 10.1016/s0920-9964(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie CW., Chiu E., Harrigan S., et al. The impact upon extra-pyramidal side effects, clinical symptoms and quality of life of a switch from conventional to atypical antipsychotics (risperidone or olanzapine) in elderly patients with schizophrenia. Int J Geriatr Psychiatry. 2003;18:432–440. doi: 10.1002/gps.862. [DOI] [PubMed] [Google Scholar]
- 27.Voruganti L., Cortese L., Owyeumi L., et al. Switching from conventional to novel antipsychotic drugs: results of a prospective naturalistic study. Schizophr Res.. 2002;57:201–208. doi: 10.1016/s0920-9964(01)00309-7. [DOI] [PubMed] [Google Scholar]
- 28.Ho BC., Miller D., Nopoulos P., Andreasen NC. A comparative effectiveness study of risperidone and olanzapine in the treatment of schizophrenia. J Clin Psychiatry. 1999;60:658–663. doi: 10.4088/jcp.v60n1003. [DOI] [PubMed] [Google Scholar]
- 29.Tran PV., Hamilton SH., Kuntz AJ., et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorder. J Clin Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Hertling I., Philipp M., Dvorak A., et al. Flupenthixol versus risperidone: subjective quality of life as an important factor for comliance in chronic schizophrenic patients. Neuropsychology. 2003;47:37–46. doi: 10.1159/000068874. [DOI] [PubMed] [Google Scholar]
- 31.Bobes J., Gutierrez M., Gibert J., Gonzalez MP., Herraiz L., Fernandez A. Quality of life in schizophrenia: long-term follow-up in 362 chronic Spanish schizophrenic outpatients undergoing risperidone maintenance treatment. Eur Psychiatry. 1998;13:158–163. doi: 10.1016/S0924-9338(98)80141-7. [DOI] [PubMed] [Google Scholar]
- 32.Nasrallah HA., Duchesne I., Mehnert A., Janagap C., Eerdekens M. Healthrelated quality of life in patients with schizophrenia during treatment with long-acting, injectable risperidone. J Clin Psychiatry. 2004;65:531–536. doi: 10.4088/jcp.v65n0412. [DOI] [PubMed] [Google Scholar]
- 33.Velligan Dl., Prihoda TJ., Sui D., Ritch JL., Maples N., Miller AL. The effectiveness of quatiapine versus conventional antipsychotics in improving cognitive and functional outcomes in standard treatment settings. J Clin Psychiatry. 2003;64:524–531. doi: 10.4088/jcp.v64n0505. [DOI] [PubMed] [Google Scholar]
- 34.Corrigan PW., Reinke RR., Landsberger SA., Charate A., Toombs GA. The effects of atypical antipsychotic medications on psychosocial outcomes. Schizophr Res. 2003;63:97–101. doi: 10.1016/s0920-9964(02)00379-1. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs DW., Hanlon TE., Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 36.Ritsner M., Gibel A., Perelroyzen G., Kurs R., Jabarin M., Ratner Y. Quality of life outcomes of risperidone, olanzapine, and typical antipscyhotics among schizophrenia patients treated in routine clinical practice. A naturalistic comparative study. J Clin Psychopharmacol. 2004;24:582–591. doi: 10.1097/01.jcp.0000144895.75728.2b. [DOI] [PubMed] [Google Scholar]
- 37.Haro JM., Edgell ET., Novick D., et al. Effectiveness of antipsychotic treatment for schizophrenia: 6-month results of the PanEuropean Schizophrenia Outpatient Health Outcomes (SOHO) study. Acta Psychiatr Scand. 2005;111:220–231. doi: 10.1111/j.1600-0447.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 38.Kilian R., Dietrich S., Toumi M., Angermeyer MC. Quality of life in persons with schizophrenia in out-patient treatment with first- or second-generation antipsychotics. Acta Psychiatr Scand. 2004;110:108–118. doi: 10.1111/j.1600-0047.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 39.Endicott J., Nee J., Harrison W., et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 40.Prieto L., Sacristan JA., Hormaechea JA., Casado A., Badía X., Gómez JC. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr Med Res Opin. 2003;20:827–835. doi: 10.1185/030079904125003674. [DOI] [PubMed] [Google Scholar]
- 41.Lehman AF. A Quality of Life Interview for the chronically mentally ill (QOLI). Eval Prog Planning. 1988;11:51–62. [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 43.Meltzer HY. Dimensions of outcome with clozapine. Br J Psychiatry. 1992;160(suppl 17):46–53. [PubMed] [Google Scholar]
- 44.Meltzer HY., Cola P., Way L., et al. Cost effectiveness of clozapine in neuroleptic-resistant schizophrenia. Am J Psychiatry. 1993;150:1630–1638. doi: 10.1176/ajp.150.11.1630. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE., Sherbourne CD. The MOS 36-ltem Short-Form Health Survey (SF-36). Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 46.Giner J., Ibáñez E., Baca E., Bobes J., Leal C., Cervera S. Desarrollo del Cuestionario Sevilla de Calidad de Vida (CSCV). Actas Luso-Esp Neurol Psiquiatr. 1997;25(supl. 2):11–23. [Google Scholar]
- 47.Naber D. A self-rating to measure subjective effects of neuroleptic drug relationships to objective psychopathology, quality of life, compliance, and other clinical variables. Int Clin Psychopharmacol. 1995;10(suppl. 3):133–138. [PubMed] [Google Scholar]
- 48.Heinisch M., Ludwig M., Bullinger M. Psychometric testing of the “Munchner Lebensqualitäts Dimensionen Liste (MLDL). In: Bullinger M, Ludwig M, Steinbüchel V, eds. Lebensqualität bei Kardiovaskulären Erkrankungen. Göttingen, Toronto, Zürich: Hogrefe Verlag für Psychologie, 1991 [Google Scholar]
- 49.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC. PSYCH-BASE. Iowa City, Ia:The University of Iowa; 1989 [Google Scholar]
- 51.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992 [Google Scholar]
- 52.Hellewell JS. Treatment-resistant schizophrenia: reviewing the options and identifying the way forward. J Clin Psychiatry. 1999;60(suppl 23):14–19. [PubMed] [Google Scholar]
- 53.Larson EB., Gerlach J. Subjective experience of treatment, side-effects, mental state and quality of life in cronic schizophrenic and out-patients treated with depot neuropleptics. Acta Psychiatr Scand. 1996;93:381–388. doi: 10.1111/j.1600-0447.1996.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 54.Franz M., Lis S., Pluddemann K., Gallhofer G. Conventional versus atypical neuroleptics: subjective quality of life in schizophrenic patients. Br J Psychiatry. 1997;170:422–425. doi: 10.1192/bjp.170.5.422. [DOI] [PubMed] [Google Scholar]
- 55.Larsen EB., Gerlach J. Subjective experience of treatment, side-effects, mental state and quality of life in chronic schizophrenic out-patients treated with depot neuroleptics. Acta Psychiatr Scand. 1996;93:381–388. doi: 10.1111/j.1600-0447.1996.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 56.Kaneda Y. The impact of prolactin elevation with antipsychotic medications on subjective quality of life in patients with schizophrenia. Clinical Neuropharmacology. 2003;26:182–184. doi: 10.1097/00002826-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Allison DB., Mackell JA., McDonnell DD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatric Services. 2003;54:565–567. doi: 10.1176/appi.ps.54.4.565. [DOI] [PubMed] [Google Scholar]
- 58.Strassnig M., Brar JS., Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophr Res. 2003;62:73–76. doi: 10.1016/s0920-9964(02)00441-3. [DOI] [PubMed] [Google Scholar]
- 59.Meyer JM., Nasrallah HA., McEvoy JP., et al. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Olfson M., Uttaro T., Carson WH., Tafesse E. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66:331–338. doi: 10.4088/jcp.v66n0309. [DOI] [PubMed] [Google Scholar]
- 61.Dupuy H. The Psychological General Weil-Being (PGWB) Index. In: Wenger N, Mattson M, Furberg C, et al, eds. Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. Greenwich, CT: Le Jacq; 1984 doi: 10.1016/s0002-9149(84)80232-5. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson G., Hesdon B., Wild D., et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–46. doi: 10.1192/bjp.177.1.42. [DOI] [PubMed] [Google Scholar]
- 63.Clayton AH., McGarvey EL., Clavet GJ. The Changes in Sexual Functioning Questionnaire (CSFQ): development, reliability, and validity. Psychopharmacol Bull. 1997;33:731–745. [PubMed] [Google Scholar]
- 64.Katschnig H. How useful is the concept of quality of life in Psychiatry? In: Katschnig H, Freeman H, Sartorius N, eds. Quality of Life in Mental Disorders. Chichester, UK: John Wiley & Sons Ltd; 2005:3–17. [Google Scholar]