Abstract
Bipolar disorder is characterized by a combination of state-related changes in psychological functíon that are restricted to illness episodes, coupled with trait-related changes that persist through periods of remission, irrespective of symptom status. This article reviews studies that have investigated the brain systems involved in these state-and trait-related changes, using two techniques: (i) indirect measures of neurocognitive function, and (ii) direct neuroimaging measures of brain function during performance of a cognitive task. Studies of neurocognitive function in bipolar disorder indicate deficits in three core domains: attention, executive function, and emotional processing. Functional imaging studies implicate pathophysiology in distributed neural circuitry that includes the prefrontal and anterior cingulate cortices, as well as subcortical limbic structures including the amygdala and the ventral striatum. Whilst there have been clear advances in our understanding of brain changes in bipolar disorder, there are limited data in bipolar depression, and there is limited understanding of the influence of clinical variables including medication status, illness severity, and specific symptom dimensions.
Keywords: bipolar disorder, depression, mania, executive function, attention, emotion, prefrontal cortex
Abstract
El trastorno bipolar se caracteriza por la combinación de cambios en la función psicológica estado-dependiente, la que está restringida a los episodios de la enfermedad, y cambios rasgo-dependientes que persisien a través de los períodos de remisión independiente de la sintomatología. En este artículo se revisan estudios que han investigado los sistemas cerebrales involucrados en estes cambios esiado y rasgo-dependienies utilizando dos técnicas: 1) mediciones indirectas de la función neurocognitiva y 2) mediciones directes con neuroimágenes de la función cerebral durante el rendimiento frente a una prueba cognitiva. Los estudios de la función neurocognitiva en el trastorno bipolar revelan déficit en très áreas principales: atención, función ejecutiva y procesamiento emocional. Los estudios de imágenes funcionales incorporan la fisiopatología de circuitos neurales distribuidos en las cortezas prefrontal y del cíngulo anterior, como también de estructuras límbicas subcorticales que incluyen la amigdala y el esiriado ventral. Aunque han existido claras evidencias acerca de nuestra comprensión de los cambios cerebrales en el trastorno bipolar, hay datos limitados para la depresión bipolar, y hay una comprensión reducida de la influencia de variables clínicas como la medicación, la gravedad de la enfermedad y las dimensiones de síntomas específicos.
Abstract
Les troubles bipolaires sont caractérisés par une association de modifications psychologiques liées à l'état qui sont limitées aux épisodes de la maladie, et de modifications « trait » qui persistent lors des périodes de rémission, indépendamment du statut thymique. Cet article passe en revue les études qui ont utilisé deux techniques pour observer les mécanismes cérébraux impliqués dans ces modifications irait et état dépendantes: 1) des mesures indirectes de la fonction neurocognitive et 2) des mesures directes par neuro-imagerie de la fonction cérébrale pendant l'exécution d'une tâche cognitive. Les études de la fonction neurocognitive dans les troubles bipolaires montrent des déficits dans trois domaines clés: l'attention, les fonctions executives et les processus émotionnels. Les études d'imagerie fonctionnelle impliquent la physiopaihologie de la distribution des circuits neuronaux, dont les cortex cingulaire antérieur et pré frontal, comme celle des structures limbiques sous-corticales, dont l'amygdale et le striatum ventral. Les connaissances sur les modifications cérébrales dans la maladie bipolaire ont bien progressé mais les données sur la dépression bipolaire sont peu nombreuses et l'influence de variables cliniques comme le traitement, la sévérité de la maladie et l'importance des symptômes spécifiques est mal comprise.
The bipolar states of mania and depression have a clear impact on cognitive function. The clinical criteria for mania include distractibility, inappropriate speech and behavior, increased goal-directed behavior, and a tendency to make decisions associated with potential painful consequences.1 The depressive state is also characterized by cognitive changes, including a lack of concentration, difficulty making decisions, motor slowing, and changes in memory. Understanding the brain changes that, accompany these illness states is an important target for psychiatric neuroscience, for a number of reasons. The identification of illness markers for bipolar disorder will facilitate the early detection of bipolar episodes, which may spare patients and their families considerable distress. Earlier detection of the illness itself is also of significant, benefit: bipolar disorder continues to be frequently misdiagnosed as Major Depressive Disorder in individuals without, a clear history of manic episodes,2,3 with the consequence that, patients may be maintained on a suboptimal medication regime until their bipolar diathesis is noticed. Second, characterizing the profile of brain dysfunction in bipolar disorder will also help identify novel targets for pharmacological treatment, and may eventually allow identification of individuals at high risk for developing bipolar disorder. Third, the assessment of cognitive performance in patients with bipolar disorder can also be useful in the management of individual patients, in order to assess functional capacity (eg, ability to concentrate or problem-solve), and to chart, progressive changes occurring with advancing chronicity, acute treatment, or prophylactic medication.
The purpose of the present, article is to summarize psychological and ncurobiological studies that, have sought, to characterize the profile of brain dysfunction in bipolar disorder. In the first, part of the review, we will examine the evidence from studies of neurocognition in bipolar disorder. Neuropsychological testing with standardized assessment, procedures can provide indirect, measures of activity in brain systems, based on validatory research from neuroimaging, studies of human lesion patients, and translational data from nonhuman species.4 Although the index of underlying brain function is indirect, the advantage of this approach is that neuropsychological testing is relatively inexpensive, and can be readily administered in a clinical or hospital setting. In the second part of the review, we will examine studies that have used brain imaging techniques, chiefly functional magnetic resonance imaging (fMRI),to directly quantify the neural abnormalities associated with bipolar disorder.
Investigation of the profile of brain changes in bipolar disorder has been hampered by a number of factors. First, it has become apparent that, in bipolar disorder, there is a combination of both state-related changes during illness episodes, and more enduring trait-related changes which persist, through periods of symptom remission.5 This accumulating evidence for trait deficits in bipolar disorder contrasts with the original Kraepelinian concept, of bipolar disorder, which highlighted the apparent recovery of function between episodes (in contrast to the chronic deteriorating course of schizophrenia dementia praecox). Of course, the identification of trait markers for bipolar disorder raises the possibility that neurocognitive variables may represent illness endophenotypes, related to underlying genetic liability.6 A second complication pertains to the state-related changes: whilst, the manic and depressive states of bipolar disorder have some neuropsychological similarities, they also have some important differences. For example, the florid disinhibition and risk-taking during mania are highly reminiscent, of the effects of brain lesions to the orbitofrontal cortex, as we will discuss further below. A related third point, is that, multiple domains of cognitive function are clearly disrupted in bipolar disorder, including attention, executive function, emotional processing, and memory,7-8 and these cognitive domains may be differentially affected by state and trait variables. Finally, the current, literature on bipolar disorder has failed to consistently control for a range of clinical factors that may putatively impact, on neurocognition, including medication status,9 comorbidities including substance use disorders,10 and specific symptom dimensions such as suicidally11 or insomnia.12
Cognition in bipolar disorder
The current, article will focus on three areas of neuropsychological function that have been studied in detail in bipolar disorder: attention, executive function and emotional processing. For in-depth reviews, the reader is referred to refs 7, 8, and 13.
Attention
Attention refers to our ability to selectively and flexibly process some of the information in the environment, at the expense of other information. The attcntional system of the human brain can be decomposed into several distinct, mechanisms, including those responsible for selective attention, attentional shifting, and sustained attention.14 The former two processes have considerable overlap with executive function, and will be considered in the following section. Sustained attention has been widely studied in bipolar disorder, and relates to the capacity to maintain selective processing over prolonged periods of time (“vigilance”). Sustained attention can be measured with continuous performance tests (CPTs) including the Rapid Visual Information Processing task in the Cambridge Neuropsychological Automated Testing Battery (CANTAB) assessment.15 In these tasks, the subject, must, monitor a stream of stimuli (eg, digits) for target stimuli that are specified by the experimenter. The target, stimuli occur infrequently and unpredictably, and healthy subjects typically show a deterioration in target detection as the task progresses.
CPT target, detection is reliably impaired during the manic state,16,17 and was one of the strongest predictors of the manic state across a wide-ranging neuropsychological assessment.16 As well as deficits in target detection, manic patients also committed more false alarms - responses to nontargct stimuli.16,18 This is likely to reflect clinical symptoms of impulsivity and disinhibition in the manic state.
The deficit, in target, detection (but not impulsive errors) was shown to persist, in remitted patients with bipolar disorder, albeit at, a somewhat, less severe level compared with mania.5,19-21 The deficit, in euthymic patients was unrelated to subclinical symptoms,19,22 which are common in remitted patients and may impact, upon functional capacity and cognitive performance. In fact, after controlling for subclinical symptoms, target detection was the only variable to remain significantly impaired in euthymic cases across a wide-ranging neuropsychological assessment. Target detection impairment, was present in a subset, of patients who were early in the illness course, but, also correlated with disease duration and number of episodes.19 The sustained attention deficit, in euthymic cases was also present, regardless of the working memory load of the CPT.23 It, is likely that, the deficit is also exacerbated during depressed states,24 although, as discussed below, there are currently limited data in bipolar depression.
Executive function
Executive function refers to a collection of higher-level psychological processes that, enable the flexible organization of behavior, including planning, working memory, inhibitory control, strategy development, and attentional shifting.25 These processes arc intimately associated with the integrity of the prefrontal cortex. Specifically, executive control has been mapped to a lateral prefrontal system comprising the dorsolateral and ventrolateral prefrontal cortices.26 Executive dysfunction may impact upon functional capacity, that is, patients' ability to complete everyday tasks and work-related activities, and it also impacts more broadly upon quality of life.
Patients during a manic episode show significant impairments in a variety of classic measures of executive function, like the Wisconsin Card Sort. Test, the Stroop test, and the Tower of London27-29 (although notably, the effect size for the deficits was less than for sustained attention and verbal memory in the study by Clark et al).16 A number of studies have also reported executive impairments in bipolar depression; for example, on the ID-ED task of attentional shifting, which aims to dissect some component processes of the Wisconsin Card Sort, Test.30 Basso et al31 reported significant deficits on Verbal Fluency and the Trail Making Test, (part B) in bipolar groups tested in either depressed, manic, or mixed episodes, with no significant differences between groups. A study in unmedicated patients reported significantly worse performance on the Wisconsin Card Sort Test, the Trail Making Test, and the Stroop test in bipolar depressed patients compared with a group of unipolar depressed cases who were matched for duration and severity of illness.32
These deficits in bipolar depression appear highly sensitive to symptom severity: an earlier study by Sweeney et al33 compared depressed bipolar, depressed unipolar, and manic bipolar patients on a number of tasks from the CANTAB assessment. Whilst executive deficits were pronounced in the manic patients, the two depressed groups were not, significantly impaired on the Tower of London planning and the ID-ED attentional shifting task. The depressed groups in that, study showed only moderate symptom scores on the HDRS. A recent study in a group of unmedicated depressed patients with bipolar II diagnoses also detected reasonably intact neurocognitive function across measures of executive control, memory, decision-making and impulsivitv,34 sec also ref 35).
Thus, impaired executive function is consistently reported in the manic and depressed states of bipolar disorder, but appears to be highly sensitive to mood state and symptom/illness severity. From these observations, we might, not anticipate executive deficits to persist, in euthymia, and indeed, a number of studies have suggested that executive deficits are not an invariable feature of the recovered state.10,19-36 However, a number of studies have detected executive deficits in remitted patients, after controlling carefully for residual mood symptoms that, are common in bipolar outpatient populations.9,37-40 The presence or absence of executive deficits in remitted cohorts may depend upon a variety of clinical factors including illness severity, illness duration, and medication effects. In addition, it is possible that, the size of the battery may affect performance - results in very long test assessments could be attributable to an overriding deficit, in sustained attention rather than executive control.
Emotional processing
Whilst, the dorsal and lateral aspects of the prefrontal cortex are typically associated with relatively “cold” executive processing, the orbital sector of the prefrontal cortex is linked to a distinct set of emotional “hot.” processes including reward evaluation, risky decision-making, and impulse control.4,41 Following damage to this region, lesion patients typically show changes in emotional behavior, as well as reward-driven and impulsive judgment.42,43 These changes are highly reminiscent, of some of the behaviors seen during manic episodes, leading to the hypothesis that mania may be selectively associated with a disruption of orbitofrontal function.16,29-44 In addition, researchers have recently begun to use broader tests of emotion processing in bipolar groups, for example, tasks assessing the recognition of emotional facial expressions.45-47 In the healthy brain, these emotional tasks recruit a limbic neural system that comprises the orbital and medial parts of the prefrontal cortex (including the subgenual cingulate cortex) as well as subcortical structures including the amygdala and ventral striatum.48,49
There is accumulating evidence that, patients with bipolar disorder tested in manic states show impaired performance on measures of risk-taking and emotional decision-making. These tasks are based upon gambling scenarios, and have been validated as measures of orbitofrontal cortex integrity through studies in human lesion patients.50 Clark et al16 reported a moderate deficit, in mania, on the Iowa Gambling Task, although a recent follow-up study in a larger group of manic patients (n=45) found a more substantial decision-making impairment that was correlated with ratings of (lack of) insight.51 On the Cambridge Gamble Task, manic patients were found to display poor probabilistic judgment, and increased deliberation times compared with healthy controls.36 The former deficit was correlated with symptom ratings on the Young Mania. Scale, consistent with a statesensitive marker.
During manic episodes, bipolar patients also showed deficits in impulse control on Go-No Go tasks and CPTs, as discussed above. Murphy et al29 examined performance on an affective variant of the Go-No Go procedure that, used positive and negative-valcnccd words. As well as making more commission errors to No Go stimuli, the manic group also showed a mood-congruent attentional bias, responding faster to the positive words (eg, SUCCESS) than the negative words (eg, GUILT). An affective bias in the opposite direction (ie, preferential processing of negative information) has been demonstrated in major depressive disorder,52,53 a finding that has also been reported in bipolar depression.54
Patients with bipolar depression did not show the tendency of manic patients to make more Go-No Go commission errors.30 In terms of decision-making, our study in bipolar II depression (with a mean MontgomeryAsberg Depression Rating Scale [MADRS] score of 24, in the moderately depressed range) found no effects on the Cambridge Gamble Task.34 However, a recent study in more severely depressed bipolar I patients (mean Hamilton score of 25) did indicate deficits on this test in probabilistic judgment, and deliberation times,30 similar to those reported in mania.44 In euthymic cases, two studies reported intact, performance on the Iowa Gambling Task and the Cambridge Gamble Task,19,36 and similarly, recognition of emotional facial expressions appears fairly intact during testing in remission.46,47 Thus, during remission, emotional processing and decision-making functions seem to recover substantially, indicating that these may be predominantly state-related changes. Whether these changes are restricted to the manic state (perhaps indicative of an orbitofrontal ”lesion“ syndrome), or occur in both mania and bipolar depression, is difficult, to ascertain at the current time given the small number of studies examining these processes in bipolar depression.
Brain imaging in bipolar disorder
Structural and functional brain imaging studies in bipolar disorder lend direct, support to the indications of prefrontal cortical pathophysiology from studies of neurocognition. Classic studies of patients with secondary mood disturbance as a consequence of organic pathology like stroke or tumor reported increased prevalence of depressed mood following damage to the left, frontal cortex and the left, basal ganglia.55,56 Cases of secondary mania arc unsurprisingly less common than poststroke depression, but, are reported to show the reverse pattern of laterality, associated with right-lateralized damage to the frontal cortex and basal ganglia.57 These data highlight the connectivity between the frontal cortex and basal ganglia, and this frontostriatal circuitry is thought, to support, many aspects of attentional, executive and emotional function.58 Neurological patients with basal ganglia pathology (eg, Parkinson's disease and Huntington's disease) also show elevated levels of depression, compared with other patient, groups with disorders matched for level of disability59
In bipolar disorder, structural brain abnormalities in the prefrontal cortex have been confirmed in postmortem studies60,61 and with structural MRI. For example, the subgenual portion of the anterior cingulate cortex was reduced in volume in patients with bipolar disorder with a family history of affective disorder.62 Recent, studies using voxel-based morphometry (VBM) have also reported gray-matter reductions in the prefrontal cortex in patients with bipolar disorder.63-65 In light of the evidence for structural brain changes in prefrontal cortex,the following sections will consider the evidence for changes in functional brain activity in bipolar disorder.
Imaging studies of mania
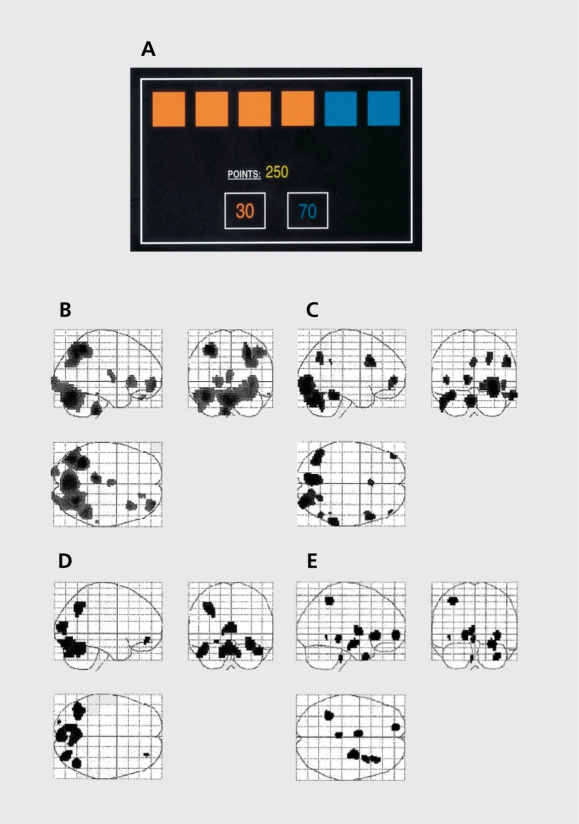
Data from neurocognitive studies indicate widespread impairments in executive, attentional, and emotional function during the manic phase of bipolar disorder. Functional imaging studies that scanned manic patients at, rest, have indicated changes in blood flow and metabolism, particularly in the orbitofrontal cortex.62,66-67 The disadvantage of these resting state studies is that it is not, possible to control for thought content during scanning, and increased activity may relate to aspects of the manic state like flights of ideas. Consequently, recent work has scanned patients while they perform a neuropsychological task, in order to investigate task-related neural activity. For example, Rubinsztein et al68 scanned a small number of manic patients with positron emission tomography while they performed a variant, of the Cambridge Gamble Task in the scanner (Figure 1). Blocks of a decision-making task were contrasted against blocks of a control task that was matched for visual and motor demands, but, without the requirement, for risk assessment, and decision-making. Compared with demographicallymatched healthy controls, the manic cases showed a dysregulation of medial and ventral prefrontal circuitry during risky decision-making.
Figure 1. Brain responses during risky decision-making in patients with mania, patients with depression, and healthy controls. A: Screen display for the Risk Task. Subjects are instructed that a token has been hidden at random under one of the six boxes. They must guess whether the token is hidden under a red or blue box, in order to win points. The less likely option (blue, in this example) is also associated with a higher win value, to create conflict between reward and uncertainty. Blocks of decision-making were contrasted with a visuomotor baseline condition. B: Activations during decision-making in healthy controls. C: Activations during decision-making in patients with mania. D: Activations during decision-making in patients with major depressive disorder. E: Areas of increased activity in healthy controls relative to patients with mania. Reproduced from ref 68: Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision-making in mania; a PET study. Brain. 2001;124:2550-2263. Copyright © Oxford University Press 2001 .
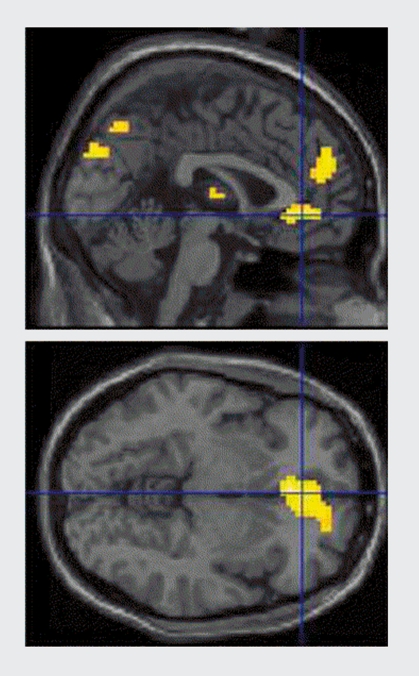
Using fMRI, Altshuler et al, scanned manic patients during performance of a Go-No Go task that required the suppression of impulsive responses. Compared with matched healthy controls, the manic patients showed blunted activation of the right, lateral orbitofrontal cortex,69 a region that is thought, to be critical for inhibitory control. The affective variant of the Go-No Go task used by Murphy et al29 has also been adapted for fMRI by Elliott et al.70 In a comparison of manic patients and healthy controls, the manic group showed reduced activity in ventrolateral prefrontal cortex during blocks of affective Go-No Go against, a nonemotional control condition. Manic patients also showed evidence of increased activity in the medial orbitofrontal cortex during blocks when positive stimuli were distractors (ie, to be ignored, (Figure 2). Thus, the available evidence clearly indicates pathophysiology in the ventral, orbital sectors of the prefrontal cortex. This pathophysiology is perhaps better described as a dysregulation, rather than a more simplistic “lesion” account, underlying the changes in emotional processing in the manic state.
Figure 2. Brain responses during the affective Go-No Go task in patients with mania and healthy controls. This section shows areas of increased activity in patients relative to controls, in blocks when positive (happy) words were distracters compared with blocks where neutral valenced words were distracters, consistent with increased brain activity to mood-congruent, task-irrelevant information. Reproduced from ref 70: Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1 163-1 170. Copyright © Elsevier 2004.
Imaging studies of euthymia/remission
A number of studies have examined remitted patients with bipolar disorder in similar imaging protocols to those employed in mania. The Stroop test, where color words (eg, RED) are presented in congruent or incongruent inks, has been widely validated for use in neuroimaging. This paradigm yields a robust signal in the anterior cingulate cortex during presentation of incongruent, stimuli, where the natural tendency to read the color word must, be overriden. Gruber et al71 reported reduced anterior cingulate activity in remitted bipolar patients compared with controls, which may indicate a failure to recruit prefrontal cortex during effortful executive control.72-74 Remitted bipolar patients have also been reported to show deactivation in orbital and medial prefrontal cortex during the incongruent Stroop blocks,72-75 an effect that was also seen in manic and depressed bipolar groups, suggesting a trait, marker of pathophysiology in the orbitofrontal cortex.
Other studies using emotional tasks such as recognition of emotional facial expressions have reported abnormally increased subcortical limbic activity in remitted patients with bipolar disorder.76-77 limbic hyperactivity has also been reported during nonemotional tasks, for example, during performance of a sustained attention task,78 and a serial reaction time task with implicit sequences.79 These findings suggest, that patients with bipolar disorder may recruit, emotional (“hot”) neural systems in the processing of emotionally neutral “cold” material. These findings are consistent with studies showing high trait, emotionality in bipolar patients using psychological mood manipulations.80,81
Imaging studies of bipolar depression
The limited number of imaging studies in bipolar depression have also highlighted changes in prefrontal and subcortical activity A resting state positron-emission tomography (PET) study in a notably large group (n=43) of patients with bipolar depression reported decreases in prefrontal cortical metabolism, and increases in subcortical metabolism, compared with healthy controls.82 Both of these effects were correlated with depressive severity on the Hamilton scale. Using cognitive activation designs, decreased activation (or reduced deactivation) in the prefrontal cortex has also been reported, where attentional or executive tasks have been employed.77 In addition, resting state activation in the subgenual cingulate region was positively correlated with target detection performance on a CPT performed outside the scanner.83 Decreased blood flow in medial prefrontal cortex was also reported during a sad mood induction in remitted and depressed patients with type 1 bipolar disorder,84 although this study did not, include a healthy comparison group.
Malhi et al85 scanned patients with bipolar depression on an affect, generation task where they viewed captioned emotional pictures. In comparison with healthy controls, the bipolar depressed group showed enhanced subcortical activation in the amygdala, thalamus, and basal ganglia. A comparable finding was reported by Chen et al,86 who used a facial expression task in two groups of bipolar patients with depression and mania. The bipolar depressed group displayed relative increases in subcortical limbic activity in response to happy faces.
These findings of subcortical/limbic hyperreactivity are consistent with the findings discussed above in the remitted phase. Notably, this pattern of neural response may also be capable of distinguishing bipolar disorder from major depressive disorder. Lawrence et al87 directly compared neural activity to emotional facial expressions in bipolar disorder and major depressive disorder. The bipolar group were stable outpatients who had subclinical depressive symptoms. This study found increases in amygdala and subcortical limbic activity predominantly to mild happy, but also to fearful, facial expressions. Thus, the imaging studies in bipolar depression to date indicate a pattern of decreased prefrontal activity during cognitive challenge paradigms, coupled with a relative hyperactivity of subcortical limbic structures. There is clearly a need for further studies comparing neural activity across illness states in bipolar disorder, and contrasting these effects against major depressive disorder. In addition, there are few neuroimaging studies in unmedicated patients, and studies may benefit from using longitudinal designs in addition to the more standard parallel-groups designs.
Relevance for treatment
Cognitive effects of bipolar medications
Studies examining cognitive function and neural systems in bipolar disorder are typically confounded by medication status. It is common for patients in research studies to be maintained on mood-stabilizing medications, and many studies also include subgroups of patients receiving neuroleptics, antidepressants and sedatives. These medications may act directly to influence cognitive function in either a beneficial or detrimental manner. A number of studies have investigated the effects of lithium medication on cognition, (reviewed in refs 88, 89). These studies have employed a variety of designs, cither comparing bipolar patients on and off lithium medication,90 comparing lithium-treated euthymic patients against, controls,91 or studying the effects of lithium versus placebo in healthy volunteers.92-94 These studies have shown reliable effects on psychomotor speed, consistent, with frequent complaints of mental slowing from patients. There is also some evidence for impaired learning and memory function, but higher-level executive function and attention appear to be spared, and there is no evidence for cumulative effects of long-term treatment.88 A number of the neuropsychological studies in euthymic bipolar patients have also performed post-hoc analyses to examine potential confounding effects of lithium treatment19-95 and have generally found patients receiving lithium to perform similarly to those not receiving lithium.
There is less evidence for cognition-enhancing or-impairing effects of other mood stabilizers. Two studies have reported that the use of antipsychotic medications was associated with deficits in executive function9,95-; an effect that remained present after controlling for levels of psychosis, and applied equally to atypical and conventional antipsychotics.9 It is generally thought that selective serotonin reuptake inhibitor (SSRI) medication does not induce significant cognitive impairment,96 but benzodiazepines and anticholinergic drugs may have some detrimental effects, mainly on psychomotor speed and memory rather than higher-level executive function.97,98 It is also difficult to assess the cumulative impact, of polypharmacy on cognitive function.
Predictors of treatment response
Recent, research has begun to use neurocognitive testing and functional imaging to investigate markers associated with treatment response. In major depressive disorder, metabolism in the medial prefrontal cortex prior to initiating treatment has been reported to predict the response to antidepressant medication, although the direction of effect, has been somewhat, inconsistent: several studies have associated a positive response with increased metabolism,99,100 whereas a further study associated a positive response with decreased metabolism in the same region.101 Recent research has begun to examine effects associated with treatment response in bipolar disorder. One study reported decreases in subcortical limbic activity (ventral striatum and amygdala) whilst, at rest, following a positive response to levothyroxine in bipolar depression.102 A recent study also indicated a reduction in dorsolateral prefrontal activity during processing of emotional stimuli, in bipolar depressed patients who responded to sleep deprivation and light, therapy.103 Future work may also fruitfully examine treatment response in relation to neurocognitive variables, as these are considerably more amenable for use in clinical settings compared with fMRI. In bipolar disorder, there is clear evidence that neurocognitive abnormalities adversely affect functional outcomes.104,105 A recent study reported that two neurocognitive indices of executive control (Stroop score and verbal fluency) predicted the time to remission in first, episode bipolar disorder,106 although this group included a mixture of patients in manic and depressed states. Further research is clearly required to examine neurocognitive and neuroimaging predictors of response to pharmacotherapy, and also to psychological treatments in bipolar disorder.
Conclusions
Evidence from neurocognitive testing indicates a complex array of neuropsychological impairments in patients with bipolar disorder. A surge of complimentary studies using functional brain imaging indicate that these deficits may be associated with pathophysiology in a neural system comprising the prefrontal and anterior cingulate cortex, as well as subcortical limbic regions including the amygdala and ventral striatum. A detailed description of this cognitive and neurobiological profile has been elusive, due to a combination of both state-and trait-related changes in bipolar disorder.
In principle, three distinct profiles may exist. An abnormality may be a state-related deficit that recovers fully during periods of remission, but is similarly affected by both manic and depressive episodes. We have presented evidence that executive dysfunction may adhere to this profile, associated with reduced neural activation in the dorsal and lateral aspects of the prefrontal cortex. However, it should be noted that executive dysfunction in bipolar disorder is heterogeneous, and this deficit, can persist in some patients, probably as a function of clinical features such as illness severity and possibly medication status.
The second profile of deficit, is the trait marker: an impairment that is present during acute episodes but, which also persists during periods of remission. There is reasonable evidence that deficits in target detection on sustained attention (CPT) tasks adhere to a trait profile. Trait deficits may occur as a consequence of repeated illness episodes (as may be the case for executive dysfunction), or may predate the onset, of the illness and be associated with genetic liability to bipolar disorder. Ongoing research in high-risk populations, such as the unaffected first-degree relatives of bipolar probands, may identify neurocognitive markers associated with bipolar vulnerability, but studies so far have been inconclusive and limited by small sample sizes.107-109
The third profile is of a state-related marker that is restricted to either the manic or the depressive episodes. We have presented some evidence that deficits in risk assessment, emotional decision-making, and impulsive responding are pronounced during the manic episodes, and these may represent objective, quantifiable indicators of the classic manic symptoms of disinhibition and behavior with harmful consequences (eg, spending sprees and sexual indiscretions). It is likely that, these deficits are linked to dysregulation of the orbitofrontal cortex. The degree to which these changes are restricted to mania is equivocal currently, given the lack of data in bipolar depression. Functional imaging studies in bipolar depression have indicated a hyperreactivity of subcortical limbic systems, such that emotionally neutral material may be processed in an emotional manner. Whilst, it is promising that this phenomenon may show specificity to bipolar disorder compared with major depressive disorder,87 it is not, yet fully clear whether this effect, is restricted to bipolar depression or could represent, a trait marker.
The clinical implications of this program of research are substantial. By identifying illness markers that, are selectively associated with bipolar disorder, we may be able to diagnose patients earlier in the course of the illness, and at an earlier stage during illness episodes, with considerable benefits to long-term functional outcome and quality of life. Cognitive and imaging variables have the potential to predict, treatment response in symptomatic patients, and possibly to predict which course of treatment (eg, pharmacotherapy versus psychotherapy) may be best, suited to individual patients. This raises the possibility that these instruments should be incorporated into routine clinical management. The findings discussed in this review illustrate one of the current aims for the development of the DSM-V (to be released in 2011), which is the need to translate research findings from basic and clinical neuroscience into a system of psychiatric classification based on pathophysiological and etiological processes.110,111
Contributor Information
Luke Clark, Department of Experimental Psychology, University of Cambridge, Downing Street, Cambridge, United Kingdom; Behavioural and Clinical Neuroscience Institute, University of Cambridge, United Kingdom.
Barbara J. Sahakian, Department of Psychiatry, University of Cambridge School of Clinical Medicine, Addenbrooke's Hospital, Cambridge, United Kingdom; Behavioural and Clinical Neuroscience Institute, University of Cambridge, United Kingdom.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision. Washington, DC: American Psychiatric Association. 2000 [Google Scholar]
- 2.Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52:51–55. doi: 10.1176/appi.ps.52.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Ghaemi SN., Ko JY., Goodwin FK. “Cade's disease” and beyond: misdiagnosis, antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47:125–134. doi: 10.1177/070674370204700202. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain SR., Sahakian BJ. The neuropsychology of mood disorders. Curr Psychiatry Rep. 2006;8:458–463. doi: 10.1007/s11920-006-0051-x. [DOI] [PubMed] [Google Scholar]
- 5.Clark L., Goodwin GM. State-and trait-related deficits in sustained attention in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:61–68. doi: 10.1007/s00406-004-0460-y. [DOI] [PubMed] [Google Scholar]
- 6.Glahn DC., Bearden CE., Niendam TA., Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Bearden CE., Hoffman KM., Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 8.Quraishi S., Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 9.Frangou S., Donaldson S., Hadjulis M., Landau S., Goldstein LH. The Maudsley Bipolar Disorder Project: executive dysfunction in bipolar disorder I and its clinical correlates. Biol Psychiatry. 2005;58:859–864. doi: 10.1016/j.biopsych.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 10.van Gorp WG., Altshuler L., Theberge DC., Wilkins J., Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. A preliminary study. Arch Gen Psychiatry. 1998;55:41–46. doi: 10.1001/archpsyc.55.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Swann AC., Dougherty DM., Pazzaglia PJ., Pham M., Steinberg JL., Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- 12.Harvey AG., Schmidt DA., Scarna A., Semler CM., Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 13.Clark L., Sahakian BJ. Neuropsychological and biological approaches to understanding bipolar disorder. In: Jones S, Bentall RP, eds. The Psychology of Bipolar Disorder. Oxford, UK: Oxford University Press. 2006 [Google Scholar]
- 14.Posner Ml., Petersen SE. The attention system of the human brain. Ann Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 15.Sahakian B., Jones G., Levy R., Gray J., Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;1 54:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- 16.Clark L., Iversen SD., Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- 17.Sax KW., Strakowski SM., Zimmerman ME., DelBello MP., Keck PE., Jr., Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Swann AC., Pazzaglia P., Nicholls A., Dougherty DM., Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 19.Clark L., Iversen SD., Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 20.Liu SK., Chiu CH., Chang CJ., Hwang TJ., Hwu HG., Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry. 2002;1 59:975–982. doi: 10.1176/appi.ajp.159.6.975. [DOI] [PubMed] [Google Scholar]
- 21.Wilder-Willis KE., Sax KW., Rosenberg HL., Fleck DE., Shear PK., Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. 2001;3:58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- 22.Najt P., Glahn D., Bearden CE., et al. Attention deficits in bipolar disorder: a comparison based on the Continuous Performance Test. Neurosci Lett. 2005;379:122–126. doi: 10.1016/j.neulet.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Harmer CJ., Clark L., Grayson L., Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologic. 2002;40:1586–1590. doi: 10.1016/s0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 24.van den Bosch RJ., Rombouts RP., van Asma MJ. What determines continuous performance task performance? Schizophr Bull. 1996;22:643–651 . doi: 10.1093/schbul/22.4.643. [DOI] [PubMed] [Google Scholar]
- 25.Stuss DT., Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Ann Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 26.Robbins TW. Dissociating executive functions of the prefrontal cortex. In: Roberts AC, Robbins TW, Weiskrantz L, eds. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford, UK: Oxford University Press. 1998 [Google Scholar]
- 27.McGrath J., Scheldt S., Welham J., Clair A. Performance on tests sensitive to impaired executive ability in schizophrenia, mania and well controls: acute and subacute phases. Schizophr Res. 1997;26:127–137. doi: 10.1016/s0920-9964(97)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br J Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Murphy FC., Sahakian BJ., Rubinsztein JS., et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 30.Rubinsztein JS., Michael A., Underwood BR., Tempest M., Sahakian BJ. Impaired cognition and decision-making in bipolar depression but no 'affective bias' evident. Psychol Med. 2006;36:629–639. doi: 10.1017/S0033291705006689. [DOI] [PubMed] [Google Scholar]
- 31.Basso MR., Lowery N., Neel J., Purdie R., Bornstein RA. Neuropsychological impairment among manic, depressed, and mixed-episode inpatients with bipolar disorder. Neuropsychology. 2002;16:84–91. doi: 10.1037//0894-4105.16.1.84. [DOI] [PubMed] [Google Scholar]
- 32.Borkowska A., Rybakowski JK. Neuropsychological frontal lobe tests indicate that bipolar depressed patients are more impaired than unipolar. Bipolar Disord. 2001;3:88–94. doi: 10.1034/j.1399-5618.2001.030207.x. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney JA., Kmiec JA., Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 34.Taylor Tavares JV., Clark L., Cannon DM., Erickson K., Drevets WC., Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Torrent C., Martinez-Aran A., Daban C., et al. Cognitive impairment in bipolar II disorder. Br J Psychiatry. 2006;189:254–249. doi: 10.1192/bjp.bp.105.017269. [DOI] [PubMed] [Google Scholar]
- 36.Rubinsztein JS., Michael A., Paykel ES., Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- 37.Ferrier IN., Stanton BR., Kelly TP., Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246–251. doi: 10.1192/bjp.175.3.246. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Aran A., Vieta E., Reinares M., et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 39.Mur M., Portella MJ., Martinez-Aran A., Pifarre J., Vieta E. Persistent neuropsychological deficit in euthymic bipolar patients: executive function as a core deficit. J Clin Psychiatry. 2007;68:1078–1086. doi: 10.4088/jcp.v68n0715. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JM., Gallagher P., Hughes JH., et al. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- 41.Roiser JP., Rubinsztein JS., Sahakian BJ. Cognition in depression. Psychiatry. 2003;2:43–47. [Google Scholar]
- 42.Berlin HA., Rolls ET., Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 43.Clark L., Manes F. Social and emotional decision-making following frontal lobe injury. Neurocase. 2004;10:398–403. doi: 10.1080/13554790490882799. [DOI] [PubMed] [Google Scholar]
- 44.Murphy FC., Rubinsztein JS., Michael A., et al. Decision-making cognition in mania and depression. Psychol Med. 2001;31:679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- 45.Harmer CJ., Grayson L., Goodwin GM. Enhanced recognition of disgust in bipolar illness. Biol Psychiatry. 2002;51:298–304. doi: 10.1016/s0006-3223(01)01249-5. [DOI] [PubMed] [Google Scholar]
- 46.Lembke A., Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;1 59:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- 47.Venn HR., Gray JM., Montagne B., et al. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disord. 2004;6:286–293. doi: 10.1111/j.1399-5618.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 48.Phillips ML., Drevets WC., Rauch SL., Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 49.Phillips ML., Drevets WC., Rauch SL., Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–414. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 50.Bechara A., Tranel D., Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 51.Adida M., Clark L., Pomietto P., et al. Impaired decision-making associated with lack of insight in bipolar mania. Bipolar Disord. In press. doi: 10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 52.Gotlib IH., Krasnoperova E., Yue DN., Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 53.Mathews A., Ridgeway V., Williamson DA. Evidence for attention to threatening stimuli in depression. Behavr Res Ther. 1996;34:695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 54.Lyon HM., Startup M., Bentall RP. Social cognition and the manic defense: attributions, selective attention, and self-schema in bipolar affective disorder. J Abnorm Psychol. 1999;108:273–282. doi: 10.1037//0021-843x.108.2.273. [DOI] [PubMed] [Google Scholar]
- 55.Robinson RG., Kubos KL., Starr LB., Rao K., Price TR. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry. 1983;24:555–566. doi: 10.1016/0010-440x(83)90024-x. [DOI] [PubMed] [Google Scholar]
- 56.Starkstein SE., Robinson RG., Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987;110:1045–1059. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- 57.Robinson RG., Boston JD., Starkstein SE., Price TR. Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry. 1988;145:172–178. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- 58.Alexander GE., DeLong MR., Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 59.Ehmann TS., Beninger RJ., Gawel MJ., Riopelle RJ. Depressive symptoms in Parkinson's disease: a comparison with disabled control subjects. J Geriatr Psychiatry Neurol. 1990;3:3–9. doi: 10.1177/089198879000300102. [DOI] [PubMed] [Google Scholar]
- 60.Cotter D., Mackay D., Chana G., Beasley C., Landau S., Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 61.Rajkowska G., Halaris A., Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 62.Drevets WC., Price JL., Simpson JR., Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 63.Lyoo IK., Kim MJ., Stoll AL., et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Larson MP., DelBello MP., Zimmerman ME., Schwiers ML., Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 65.Lochhead RA., Parsey RV., Oquendo MA., Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 66.Blumberg HP., Stern E., Ricketts S., et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 67.Goodwin GM., Cavanagh JT., Glabus MF., Kehoe RF., O'Carroll RE., Ebmeier KP. Uptake of 99mTc-exametazime shown by single photon emission computed tomography before and after lithium withdrawal in bipolar patients: associations with mania. Br J Psychiatry. 1997;170:426–430. doi: 10.1192/bjp.170.5.426. [DOI] [PubMed] [Google Scholar]
- 68.Rubinsztein JS., Fletcher PC., Rogers RD., et al. Decision-making in mania: a PET study. Brain. 2001;124:2550–2263. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 69.Altshuler LL., Bookheimer SY., Townsend J., et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Elliott R., Ogilvie A., Rubinsztein JS., Calderon G., Dolan RJ., Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Gruber SA., Rogowska J., Holcomb P., Soraci S., Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16:349–360. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- 72.Kronhaus DM., Lawrence NS., Williams AM., et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 73.Matsuo K., Kato N., Kato T. Decreased cerebral haemodynamic response to cognitive and physiological tasks in mood disorders as shown by nearinfrared spectroscopy. Psychol Med. 2002;32:1029–1037. doi: 10.1017/s0033291702005974. [DOI] [PubMed] [Google Scholar]
- 74.Monks PJ., Thompson JM., Bullmore ET., et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 75.Blumberg HP., Leung HC., Skudlarski P., et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 76.Blumberg HP., Donegan NH., Sanislow CA., et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 77.Yurgelun-Todd DA., Gruber SA., Kanayama G., Killgore WD., Baird AA., Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 78.Strakowski SM., Adler CM., Holland SK., Mills N., DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 79.Berns GS., Martin M., Proper SM. Limbic hyperreactivity in bipolar II disorder. Am J Psychiatry. 2002;1 59:304–306. doi: 10.1176/appi.ajp.159.2.304. [DOI] [PubMed] [Google Scholar]
- 80.Kruger S., Aida M., Young LT., Goldapple K., Parikh S., Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 81.Mansell W., Lam D. “I won't do what you tell me!”: Elevated mood and the assessment of advice-taking in euthymic bipolar I disorder. Behav Res Ther. 2006;44:1787–1801. doi: 10.1016/j.brat.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Ketter TA., Kimbrell TA., George MS., et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 83.Brooks JO., 3rd, Wang PW., Strong C., et al. Preliminary evidence of differential relations between prefrontal cortex metabolism and sustained attention in depressed adults with bipolar disorder and healthy controls. Bipolar Disord. 2006;8:248–254. doi: 10.1111/j.1399-5618.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 84.Kruger S., Seminowicz D., Goldapple K., Kennedy SH., Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 85.Malhi GS., Lagopoulos J., Ward PB., et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen CH., Lennox B., Jacob R., Calder A., et al. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Lawrence NS., Williams AM., Surguladze S., et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Pachet AK., Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology. 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- 89.Honig A., Arts B., Ponds R., Riedel WJ. Lithium induced cognitive sideeffects in bipolar disorder: a qualitative analysis and implications for daily practice. Int Clin Psychopharmacol. 1999;14:167–171. [PubMed] [Google Scholar]
- 90.Kocsis JH., Shaw ED., Stokes PE., et al. Neuropsychologic effects of lithium discontinuation. J Clin Psychopharmacol. 1993;13:268–275. [PubMed] [Google Scholar]
- 91.Jauhar P., McClure I., Hillary C., Watson A. Psychomotor performance of patients on maintenance lithium therapy. Hum Psychopharmacol. 1993;8:141–143. [Google Scholar]
- 92.Stip E., Dufresne J., Lussier I., Yatham L. A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord. 2000;60:147–157. doi: 10.1016/s0165-0327(99)00178-0. [DOI] [PubMed] [Google Scholar]
- 93.Judd LL. Effect of lithium on mood, cognition, and personality function in normal subjects. Arch Gen Psychiatry. 1979;36:860–866. doi: 10.1001/archpsyc.1979.01780080034010. [DOI] [PubMed] [Google Scholar]
- 94.Judd LL., Hubbard B., Janowsky DS., Huey LY., Takahashi Kl. The effect of lithium carbonate on the cognitive functions of normal subjects. Arch Gen Psychiatry. 1977;34:355–357. doi: 10.1001/archpsyc.1977.01770150113013. [DOI] [PubMed] [Google Scholar]
- 95.Altshuler LL., Ventura J., van Gorp WG., Green MF., Theberge DC., Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Amado-Boccara I., Gougoulis N., Poirier Littre MF., Galinowski A., Loo H. Effects of antidepressants on cognitive functions: a review. Neurosci Biobehav Rev. 1995;19:479–493. doi: 10.1016/0149-7634(94)00068-c. [DOI] [PubMed] [Google Scholar]
- 97.Lane RM., O'Hanlon JF. Cognitive and psychomotor effects of antidepressants with emphasis on selective serotonin reuptake inhibitors and the elderly depressed patient. German J Psychiatry. 1999;2:1–42. [Google Scholar]
- 98.Curran HV. Benzodiazepines, memory and mood: a review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- 99.Mayberg HS., Brannan SK., Mahurin RK., et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 100.Saxena S., Brody AL., Ho ML., Zohrabi N., Maidment KM., Baxter LR., Jr Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 101.George MS., Wassermann EM., Kimbrell TA., et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- 102.Bauer M., London ED., Rasgon N., et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry. 2005;10:456–469. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- 103.Benedetti F., Bernasconi A., Blasi V., et al. Neural and genetic correlates of antidepressant response to sleep deprivation: a functional magnetic resonance imaging study of moral valence decision in bipolar depression. Arch Gen Psychiatry. 2007;64:179–187. doi: 10.1001/archpsyc.64.2.179. [DOI] [PubMed] [Google Scholar]
- 104.Atre-Vaidya N., Taylor MA., Seidenberg M., Reed R., Perrine A., GlickOberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:120–126. [PubMed] [Google Scholar]
- 105.Martinez-Aran A., Vieta E., Colom F., et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 106.Gruber SA., Rosso IM., Yurgelun-Todd D. Neuropsychological performance predicts clinical recovery in bipolar patients. J Affect Disord. 2008;105:253–260. doi: 10.1016/j.jad.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arts B., Jabben N., Krabbendam L., van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2007:1–15. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 108.lark L., Kempton MJ., Scarna A., Grasby PM., Goodwin GM. Sustained attention deficit confirmed in euthymic bipolar disorder, but not in firstdegree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Clark L., Scarna A., Goodwin GM. Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthyrn ic pat ients with un ipolar depression. Am J Psychiatry. 2005;162:1980–1982. doi: 10.1176/appi.ajp.162.10.1980. [DOI] [PubMed] [Google Scholar]
- 110.Hasler G., Drevets WC., Gould TD., Gottesman, II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 111.Phillips ML., Frank E. Redefining bipolar disorder: toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]