Abstract
Anhedonia refers to the reduced ability to experience pleasure, and has been studied in different neuropsychiatrie disorders. Anhedonia is nevertheless considered as a core feature of major depressive disorder, according to DSM-IV criteria for major depression and the definition of melancholic subtype, and regarding its capacity to predict antidepressant response. Behavioral, electrophysiological, hemodynamic, and interview-based measures and selfreports have been used to assess anhedonia, but the most interesting findings concern neuropharmacological and neuroanatomical studies. The analyses of anhedonic nonclinical subjects, nonanhedonic depressed patients, and depressed patients with various levels of anhedonia seem to favor the hypothesis that the severity of anhedonia is associated with a deficit of activity of the ventral striatum (including the nucleus accumbens) and an excess of activity of ventral region of the prefrontal cortex (including the ventromedial prefrontal cortex and the orbitofrontal cortex), with a pivotal, but not exclusive, role of dopamine.
Keywords: depression, mood, striatum, orbitofrontal, anhedonia, accumbens
Abstract
El término anhedonia se refiere a la disminución de la capacidad de experimentar placer, y ha sido estudiada en diferentes trastornos neuropsíquíá-tricos. Sin embargo, la anhedonia se considera una característica central del trastorno depresivo mayor, de acuerdo con los criterios del DSM-IV para depresión mayor y la definición del subtipo melancólico, respecto a su capacidad para predecir la respuesta antidepresiva. Para evaluar la anhedonia se han empleado mediciones conductuales, electro fisiológicas, hemodinámicas y otras basadas en entrevistas y auto-reportes, pero los hallazgos más interesantes se relacionan con estudios neurofar-macológicos y neuroanatómícos. El análisis de sujetos no clínicos con anhedonia, de pacientes depresivos sin anhedonia y de pacientes depresivos con diferentes grados de anhedonia parecen favorecer la hipótesis que postula que la gravedad de la anhedonia se asocia con un déficit de actividad del estriado ventral (incluyendo el núcleo accumbens) y un exceso de actividad de la región ventral de la corteza prefrontal (incluyendo la corteza prefron-tal ventromedíalyla corteza orbítofrontal), con un papel central aunque no exclusivo de la dopamina.
Abstract
L'anhédonie est définie par une capacité diminuée à éprouver du plaisir. Présente dans différents troubles neuropsychiatriques, elle est néanmoins considérée comme un symptôme clé de la dépression selon les critères DSM-IV de l'épisode dépressif majeur, fait partie intégrante du caractère mélancolique de l'épisode dépressif et est proposée comme facteur prédictif de la réponse aux antidépresseurs. L'anhédonie a été évaluée par des mesures comportementales, électrophysiologiques, hémodynamiques ainsi que par l'interrogatoire et l'autoévaluation. Les résultats des études neuropharmacologiques et neuroanatomiques sont néanmoins les plus intéressants. L'analyse de sujets anhédoniques non cliniques, de patients déprimés nonanhédoniques et de patients déprimés ayant des degrés variés d'anhédonie, est en faveur de l'hypothèse d'une corrélation entre la sévérité de l'anhédonie et un déficit de l'activité du striatum ventral (noyau accumbens compris), un excès d'activité de la région ventrale du cortex prefrontal (cortex prefrontal ventromédian et cortex orbitofrontal compris), et un rôle central, mais non exclusif, de la dopamine.
The concept of anhedonia
Anhedonia refers to the reduced ability to experience pleasure.1 It has had an important place in many aspects of psychopathology since it was first described in the previous century,2 and is still a feature of several types of psychiatric disorders and maladaptive behaviors.3-5 Anhedonia has been the most extensively studied in major depression,6 but, as it also constitutes one important negative symptom of schizophrenia, much literature has also been devoted to anhedonia in psychosis.3,7 Anhedonia has in fact been studied in a large range of neuropsychiatrie disorders, including substance use disorder,8-10 Parkinson's disease,11 overeating,12 and various risky behaviors.13
Anhedonia is nevertheless considered to be a core feature of major depressive disorder as, for example, the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV)14 requires that either depressed mood or anhedonia be present to propose this diagnosis. Furthermore, lack of reactivity and anhedonia are key diagnostic criteria for the DSM-IV melancholic subtype of major depression,14 and presence of anhedonia has been shown to be predictive of antidepressant response.15
The absence of diagnostic specificity could be regarded as a limiting factor when trying to define anhedonia as a pivotal feature of major depressive disorder. The development of the “endophenotype” concept may help to overtake such limits, on the basis of three notions.16
Patients with psychiatrie disorders could differ from healthy individuals quantitatively more than qualitatively. Furthermore, the detected disorder could be more extensively understood if the genetic and environmental risk factors are being related to the disorder through intermediate phenotypes. Lastly, endophenotypes might be unspecific, being based on abnormal neurobiological mechanisms that can be shared by various psychiatric disorders, these usually being defined as complex, polyfactorial disorders.
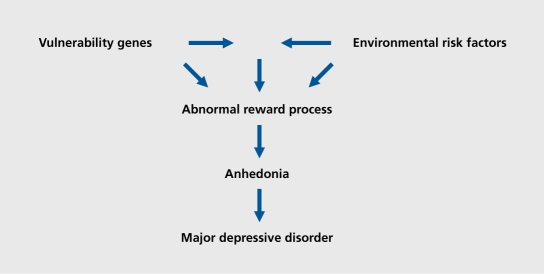
These endophenotypes, involving more directly the neurobiological and neuropsychological aspects of the disease, could help to link the potential risk factors more directly to major depression (Figure 1). There are different required qualities to use a trait as an endophenotype, such as sensitivity and specificity, heritability, presence in unaffected relatives, state-independence, biological plausibility, sound psychometric properties, and feasibility. Even if there are practically no endophenotypes meeting all these criteria, the biological plausibility of anhedonia in major depressive disorder is the matter of this review, and the first required quality to constitute a valid endophenotype is the validity of its assessment.
Figure 1. The role of anhedonia as an intermediate phenotype (endophenotype) between the involved risk factors and depression.
Assessing anhedonia
An emotion involves physiologic arousal, appraisal, subjective experience, expression, and goal-directed behavior.17 Anhedonia can therefore be measured in various ways (for an extensive review see Franken et al18). Behavioral ,19 electrophysiological,20hemodynamic,21 interview-based measures,22 and self-reports are cited in research devoted to anhedonia in major depressive disorder (MDD). For example, depressed patients show higher sweet taste perception thresholds in response to sucrose solutions,23 with significantly reduced reward responsiveness, partly because of difficulty in integrating reinforcement history over time.24
Rewards serve to elicit approach and consummatory behaviors, increase the frequency and intensity of the behaviors, maintain the behaviors, prevent their extinction, and induce subjective feelings of pleasure or positive emotional states.25 Reward is therefore a key concept in assessing anhedonia, and the basis of the majority of animal studies devoted to its neurobiological mechanisms.
Self-questionnaires have been more frequently used in clinical practice and research, for obvious reasons of simplicity. Various instruments are available, including the 61 -item instrument Chapman Physical Anhedonia Scale the (PAS)22 and its revised form, the Revised Physical Anhedonia Scale (R-PAS),28 the Fawcett-Clark Pleasure Scale (FCPS),27 and the Snaith-Hamilton Pleasure Scale (SHPS or SH APS).18,28 These instruments all assess hedonic capacity (see for example Table I), but their psychometric properties, and frequently their analyzed dimensions, are different.29 Nevertheless, the predictive validity of these instruments seems to be good; for example, individuals with higher scores on self-report measures of anhedonia report lower hedonic responses to emotioneliciting pictures,30 positive emotional scripts,31 and sucrose solutions,23 and are less responsive on measures of heart rate and facial expressions in response to emotion-eliciting slides.30
Table I. Items of the Snaith-Hamilton Pleasure Scale.28 .
| 1. I would enjoy my favourite television or radio programme |
| 2. I would enjoy being with family or close friends |
| 3. I would find pleasure in my hobbies and pastimes |
| 4. I would be able to enjoy my favorite meal |
| 5. I would enjoy a warm bath or refreshing shower |
| 6. I would find pleasure in the scent of flowers or the smell of a fresh sea breeze or freshly baked bread |
| 7. I would enjoy seeing other people's smiling faces |
| 8. I would enjoy looking smart when I have made an effort with my appearance |
| 9. I would enjoy reading a book magazine or newspaper |
| 10. I would enjoy a cup of tea or coffee or my favorite drink |
| 11. I would find pleasure in small things e.g bright sunny day a telephone call from a friend |
| 12. I would be able to enjoy a beautiful landscape or view |
| 13. I would get pleasure from helping others |
| 14. I would feel pleasure when I receive praise from other people |
Neural basis of normal positive emotion perception
As previously described in detail,32 feeling a normal emotion requires the identification of the emotional significance of a stimulus (appraisal), then the production of an affective state (production), which can be regulated at different levels (regulation). These three steps can be considered as being organized through two different systems, with a reciprocal functional relationship.
A ventral system (including the amygdala, insula, ventral striatum, and ventral regions of the anterior cingulate gyrus and prefrontal cortex), could be more specifically involved in the identification of the emotional significance of environmental stimuli, and the production of affective states. This system could also be in charge of automatic regulation and mediation of autonomic responses to emotive stimuli and contexts accompanying the production of affective states.
A dorsal system (including the hippocampus and dorsal regions of the anterior cingulate gyrus and prefrontal cortex), on the other hand, could be more important for effortful rather than automatic regulation of affective states, probably through executive functions, including selective attention and planning.
The basis of hedonic feelings has been more specifically studied through different paradigms. Euphoric response to dextroamphetamine,33 cocaine-induced euphoria,34 monetary reward,35,36 and even pleasurable responses to music,37 pictures,38 and attractive faces,39 have been associated with activity within the nucleus accumbens, ventral caudate, and ventral putamen, and, in studies devoted to the neurobiology of pleasure, with dopamine release in the ventral caudate and putamen.
The ventral striatum, and particularly the nucleus accumbens,40 may indeed have a priority role according to studies in both animals and humans, in behavioral responses to, anticipation of, and/or monitoring of errors in the prediction of reward.41,42 The nucleus accumbens appears to respond to the emotional intensity and self-relatedness of a variety of stimuli, independent of their valence,43 with both positive and negative valences possibly processed along a rostrocaudal gradient.44 The nucleus accumbens receives projections from midbrain regions (such as the ventral tegmental area), from regions involved in emotion (such as the amygdala, orbitofrontal cortex, and medial prefrontal cortex), from motor regions (such as the dorsal caudate and globus pallidus), and from regions involved in memory (such as the hippocampus).45 The accumbens also indirectly projects to cortical regions including the cingular and medial prefrontal cortex, the ventral pallidum, the thalamus, the amygdala, and the hypothalamus.46-48 Many of these regions are also implicated in emotion processing, suggesting a network of tightly anatomically and functionally connected regions.49
The orbitofrontal cortex is a nexus for sensory integration, the modulation of autonomic reactions, and anticipation in learning, prediction and decision-making for emotional and reward-related behaviours.50 Ncuroimaging studies have found that the reward value,51 and the expected reward value,52 and even the subjective pleasantness of food53 and other reinforcers are represented in the orbitofrontal cortex. The orbitofrontal cortex receives input from the five classic sensor}' modalities: gustatory, olfactory, somatosensory, auditory, and visual,54 and also receives visceral sensory information. This large variety of inputs makes the orbitofrontal cortex one of the most polymodal regions in the entire cortical mantle.53 The orbitofrontal cortex has direct reciprocal connections with other brain structures, including the amygdala, cingulate cortex, insula/operculum, hypothalamus, hippocampus, striatum, periaqueductal grey, and dorsolateral prefrontal cortex. Hence, the orbitofrontal cortex may have an important role for representing incentive salience, hedonic impact, and subjective hedonic experience, ic, constituting the link between reward and hedonic experience.53
It has been shown that the human amygdala is a key structure for extracting the affective significance from external stimuli,55 responds preferentially to emotionally valenced faces, for fearful but also for happy faces, and rapidly habituates to them.56 According to discrepant findings,57,58 the amygdala could be considered as reacting more intensively for negative stimuli, explaining its major function in fear and anxiety.
The anterior cingular cortex was not activated by transient happiness induced by recalling positive life events and looking at happy human faces.59 On the other hand, individual differences in the ability to accurately detect emotional signals, interoceptively or exteroceptive ly, may be at least in part a function of the degree to which the anterior cingulate cortex participates in the experiential processing and response to emotion cues.60
The ventromedial prefrontal cortex (VMPFC) has been implicated21 in the generation of an abstract representation of the rewarding value of a stimulus by attending to its context,61 and the learning of contingencies based on the outcome of a rewarding situation.35 By contrast, lateral areas of the ventral prefrontal cortex may be less involved in hedonic emotions, responding to avcrsive rather than rewarding stimuli.62,63
Some other regions might have a more obvious role in negative and/or distressing emotions rather than hedonic experiences, such as the insula.64,65 Recall-generated sadness was associated with significantly greater increases in activity in the vicinity of the anterior insular cortex, suggesting that this region participates in the emotional response to potentially distressing cognitive or interoceptive sensory stimuli.
Neuropharmacology of anhedonia in major depressive disorder
There is considerable evidence that dopamine has a core role in the brain reward system.66 Indeed, dopamine is released in animal models of behaviors that involve the brain reward system such as food intake or expectation, sex, and drug self-administration. More precisely, dopamine release from the nucleus accumbens, during exposure to a novel food, is modulated by various characteristics of the stimulus and motivational state.67
Accordingly, dopamine D2 receptor blockers inhibit drug self-administration and conditioned place preference with psychostimulants, while a D2 agonist is self-administered in monkeys.68 Dopamine release in the nucleus accumbens may underlie approach responses and guidance towards positive incentives (ie, motivation).69 It has been alternatively proposed that dopamine docs not mediate reinforcement directly, but instead constitutes a higher-order sensorimotor integrator, relating primary reinforcers (such as palatable food) and response initiation, maintenance and selection,70 therefore stressing the concepts of “wanting” instead of “liking.”71 Others argue that neurons from the mesocorticolimbic dopamine pathway are serving to induce approach behaviors for consumption, positive reinforcement, and learning, all of which lead the organism to adapt to the environment. Interestingly, dopamine neurons do not predict aversive stimuli like pain,72 showing that dopamine is relatively specific for the detection of potentially rewarded stimuli. When confronting neuropharmacology with neuroanatomy, it was shown that primary rewards increase dopamine release, mainly in the shell of the nucleus accumbens, whereas secondary rewards increase it in the medial prefrontal cortex and nucleus accumbens core. This distinction might be important for anhedonia in major depressive disorder, as both processes could be involved, either decreased feelings of rewards (duc a to reduced dopamine firing in the shell) or abnormal capacity to distinguish the incentive value of a conditioned appetitive stimulus (due to reduced dopamine firing in the medial prefrontal cortex and the core of the nucleus accumbens). The essential role of dopamine in the brain reward system does not mean that it has an independent role, nor does it imply that dopamine is the final common pathway to getting the reward effect. For example, the nucleus accumbens contains opioid receptors which also mediate reward.73 Opioid antagonists decrease reward behavior,74 and block the stimulation of dopamine release in the nucleus accumbens shell when exposed to various drugs and palatable food,75 and could directly modulate sexual motivation.76
Glutamate also has a significant role in the reward system, for example via the subiculum, a hippocampal structure containing glutamatergic neurons, that projects to the nucleus accumbens.77 Accordingly, N-methyl-D-aspartic acid (NMDA) produces conditioned place preference in rats, an effect which is reversed by a NMDA antagonist.78 The activation of NMDA may be more specifically responsible for shortening the reaction time for the responses to stimuli predictive of reward.79
Serotonin has a recognized effect on the modulation of dopamine and opioid release,80 and therefore could have a regulatory role in the reward process. For example, serotonin reuptake inhibitors raise the threshold for brain stimulation reward,81 and reduce firing rate of dopamine neurons in the ventral tegmental area.82
The above list of neurotransmitters potentially involved in hedonic capacity is not exhaustive, as, for example, acetylcholine and cholecystokynin also modulate glutamate and dopamine release, and thus participate in the modulation of the related behaviors or emotions.83,84
Neural basis of trait anhedonia in nondepressed subjects
Assessing a normal range of individual differences regarding hedonic capacity in front of a set of pictures with positive valence, Harvey et al85 found that trait anhedonia severity was negatively correlated with the volume of the anterior caudate and ventral striatum, and was positively correlated with the activity of the VMPFC for the processing of positive information.
These results therefore confirm the relevancy of the brain reward system, showing the importance of the ventral striatum in reward behaviors and pleasurable experiences, in accordance with other studies.86,87 The VMPFC is involved in the cognitive aspects of emotional processing.88,89 It is proposed that VMPFC activity could reflect a cortical compensatory mechanism for an underactive subcortical/striatal response to pleasant stimuli.21,85 The literature supports the idea that the VMPFC not only monitors the rewarding value of stimuli/responses, but also represents one's upcoming emotional states/reactions.90
An alternative explanation for an overactive VMPFC in trait anhedonia could be that this region inhibits emotional processing performed by other limbic structures, which in turn would lead to reduced emotional experiencing. VMPFC activation in anhedonic nonclinical individuals would therefore reflect such corticolimbic inhibitory process, more or less specific to positively valenced stimuli.85
Specifically assessing the neural basis of anhedonia in depression is challenging, as anhedonia and mood disorders constitute entangled but not equivalent concepts, frequently difficult to distinguish.
Neural basis of anhedonia in major depressive disorder
Studies of depressed patients32 have demonstrated reduced density (number of glial cells) and volume (in structural neuroimaging studies), but increased activity (for functional neuroimaging approaches) of regions involved in the identification of emotional stimuli and the generation of emotional behavior. In the opposite way, decreased activity of regions involved in the effortful regulation of emotional behavior is observed. The subgenual cingulate gyrus, the ventrolateral prefrontal cortex, the amygdala, the anterior insula, the ventral striatum, and the thalamus therefore have relative increased activity (when corrected for volume reduction), while a decrease in activity is observed in the dorsomedial and the dorsolateral prefrontal cortices. Interestingly, this pattern of activity reverses after recovery from a major depressive episode.91 Such modifications concern both positive and negative emotions, and hence are not specific to the capacity to recognize and feel pleasant emotions, ie, anhedonia. Pleasant and unpleasant emotions could represent opposite ends of a pleasure continuum, or alternatively, the two motivational systems could be independent of one another.91 Some structures might be equally solicited for pleasant and unpleasant emotions92 (such as the thalamus, hypothalamus, midbrain, and medial prefrontal cortex) but others may not.
The role of the amygdala is a heuristic example of the relative specificity of one neuroanatomical structure in anhedonia. Decreased volume of the amygdala may participate in the restricted emotional range observed in anhedonic depressed patients (because of the secondary reduced capacity to prioritize emotional valence of stimuli), whereas relative hyperactivity of the amygdala would favor a bias toward the perception of negative emotions (because the amygdala may globally react more intensively for negative stimuli).
Negative correlations were reported between anhedonia severity and response in subcortical regions, including the ventral striatum (and thus the nucleus accumbens), in a neuroimaging study specifically analyzing anhedonia in depressed individuals engaged in a cognitive task.93 This result is in accordance with three other studies showing: (i) a correlation between a psychomotor-anhedonia symptom cluster and lower metabolism in the anteroven tral caudate/putamen in depressed subjects94 ; (ii) the lack of linear increase in right putamen response to facial expressions of increasing happiness observed in depressed patients compared with healthy comparison subjects95 ; and (iii) negative correlations between anhedonia and activity in larger areas of the striatum in depressed patients.21 In other words, patients with the smallest amount of ventral striatal activation report the least interest and pleasure in, and subsequent performance of activities. It has been proposed that the paucity of ventral striatal activation observed in depressed patients may relate more to the translation of motivational information into behavior than to affective evaluation or encoding per se, which is consistent with a model of the nucleus accumbens as the limbic-motor interface.93,96 Individuals with MDD may have supersensitive behavioral and pharmacological responses to d-amphet amine compared with controls.97,98 This hypersensitive response correlated with the severity of anhedonic symptoms, providing further support for the involvement of the brain reward system, and dopamine, in major depressive disorder. The role of the nucleus accumbens is so widely accepted in anhedonia as a pivotal concept of major depressive disorder, that deep brain stimulation was recently proposed to three patients in order to alleviate anhedonia in severe refractory major depression.99
Positive correlations were observed between anhedonia severity and VMPFC activity.93 The response to pleasant stimuli was also associated with an increased VMPFC response in depressed individuals.100,101 In another study analyzing patients with MDD and variable level of anhedonia,21 positive correlations were found between responses to happy stimuli and activity in a larger area of the VMPFC (extending to the anterior cingulate and the orbitofrontal cortex).21 The increased and decreased responses of VMPFC to happy and sad stimuli respectively in MDD, compared with neutral stimuli, but a reversed pattern of response in healthy volunteers, led to the interpretation that the increased activity of the VMPFC in anhedonic depressed patients is because they arc attending more closely to happy stimuli, in an unsuccessful attempt to get into a happy mood.21
It might be somewhat artificial to describe the potential role of each brain region when depicting the organization of anhedonia, although for reasons of clarity it is difficult to avoid, considering the close relationships linking these areas. For example, the dissociation of function between the VMPFC and striatum in response to happy stimuli, in anhedonically depressed individuals, needs to take into account their reciprocal connections. The “hypofrontality” hypothesis of depression suggests that the primary deficit may be in the VMPFC, but the VMPFC could be compensating for an underactive subcortical/striatal response. 21,102 The development of diffusion tensor imaging studies might help to further understand the connections between these different key brain areas.
Conclusions
Although it is difficult to disentangle the specific role of anhedonia in major depressive disorder, imaging studies have clearly shown that the severity of anhedonia is correlated, in depressed patients, with a deficit of activity of the ventral striatum (reflecting decreased function of the nucleus accumbens, probably as a primary event) and an excess of activity of ventral region of the prefrontal cortex (concerning an increased function of the VMPFC and the orbitofrontal cortex, probably as a secondary phenomenon). It is not yet possible to prove that the deficits or excesses of activity are primary or secondary, but the analyses of anhedonic nonclinical subjects, nonanhedonic depressed patients, and depressed patients with various levels of anhedonia seems to favor this way of thinking. This oversimplified way of assessing the role of two major structures in anhedonia as a pivotal symptom of depression also has to be confronted with the complexity of the concept of anhedonia. Indeed, in order to get a pleasurable, hedonic feeling, a large number of steps have to be efficient, such as arousal (being able to globally detect potentially rewarded stimuli), appraisal (having the capacity to detect which specific stimuli are hedonically relevant), and expression of this emotion (being detected). The role of dopamine and the ventral striatum in anhedonia, as a symptom of depression, is nevertheless a largely replicated finding; this does not mean that they explain the trait, but more likely, that they are definitely involved...among others.
References
- 1.Ribot T. The Psychology of Emotions. London, UK: W. Scott; 1897 [Google Scholar]
- 2.Myerson A. Anhedonia. . Am J Psychiatry. 1923;2:87–103. [Google Scholar]
- 3.Andreasen NC., Olsen S. Negative versus positive schizophrenia: definition and validation. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 4.Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- 5.Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 6.Klein D. Depression and anhedonia. In: Clark C, Fawcett J, eds. Anhedonia and Affect Deficit States. New York, NY: PMA Publishing; 1984 [Google Scholar]
- 7.Blanchard JJ., Horan WP., Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. J Abnorm Psychol. 2001;110:363–371. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed SH., Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF., Le Moal M. Drug addiction dysregulation of reward and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND., Fowler JS., Wang GJ., Goldstein RZ. Role of dopamine the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 11.Isella V., lurlaro S., Piolti R., et al. Physical anhedonia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:1308–1311 . doi: 10.1136/jnnp.74.9.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis C., Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Comp Psychiatry. 2002; 43:189–194. doi: 10.1053/comp.2002.32356. [DOI] [PubMed] [Google Scholar]
- 13.Franken IHA., Zijlstra C., Muris P. Are nonpharmacological induced rewards related to anhedonia? A study among skydivers. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:297–300. doi: 10.1016/j.pnpbp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 15.Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 16.Gorwood P., Wohl M., Le Strat Y., Rouillon F. Gene-environment interactions in addictive disorders: epidemiological and methodological aspects. C R Biol. 2007;330:329–338. doi: 10.1016/j.crvi.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Plutchik R. Emotions: A general psychoevolutionary theory. In: Scerer KC, Ekman P, eds. Approaches to Emotion. Hillsdale, NJ: Lawrence Erlbaum; 1984:197–219. [Google Scholar]
- 18.Franken IH., Rassin E., Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Pizzagalli DA., Jahn AL., O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubai S., Pierson A., Jouvent R. Focussed attention in anhedonia: a P3 study. Psychophysiology. 2000;37:711–714. [PubMed] [Google Scholar]
- 21.Keedwell PA., Andrew C., Williams SC., Brammer MJ., Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Chapman LJ., Chapman JP., Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 23.Berlin I., Givry-Steiner L., Lecrubier Y., Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 24.Pizzagalli DA., losifescu D., Hallett LA., Ratner KG., Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008. In press doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF. Hedonic valence dopamine and motivation. Mol Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- 26.Chapman LJ., Chapman JP. The Revised Physical Anhedonia Scale. Madison, Wl: University of Wisconsin; 1982 [Google Scholar]
- 27.Fawcett J., Clark DC., Scheftner WA., Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 28.Snaith RP., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 29.Leventhal AM., Chasson GS., Tapia E., Miller EK., Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson ML., Katkin ES. Visceral perception anhedonia and emotion. Biological Psychology. 1996;42:131–145. doi: 10.1016/0301-0511(95)05151-1. [DOI] [PubMed] [Google Scholar]
- 31.Fiorito ER., Simons RF. Emotional imagery and physical anhedonia. Psychophysiology. 1994;31:513–521. doi: 10.1111/j.1469-8986.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 32.Phillips ML., Drevets WC., Rauch SL., Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 20031;54:504–514 Review.. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 33.Drevets WC., Gautier C., Price JC., et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 34.Breiter HC., Gollub RL., Weisskoff RM. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 35.Knutson B., Fong GW., Adams CM., Varner JL., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty J., Kringelbach ML., Rolls ET., Hornak J., Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 37.Blood AJ., Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lane RD., Reiman EM., Ahern GL., Schwartz GE., Davidson RJ. Neuroanatomical correlates of happiness sadness and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 39.Aharon I., Etcoff N., Ariely D., Chabris CF., O'Connor E., Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 40.Schlaepfer TE., Cohen MX., Frick C., et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 41.Knutson B., Fong GW., Adams CM., Varner JL., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 42.McClure SM., York MK., Montague PR. The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 43.Phan KL., Taylor SF., Welsh RC., Ho SH., Britton JC., Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds SM., Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- 45.Nauta WJ., Domesick VB. Afferent and efferent relationships of the basal ganglia. Ciba Found Symp. 1984;107:3–29. doi: 10.1002/9780470720882.ch2. [DOI] [PubMed] [Google Scholar]
- 46.Jones DL., Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res. 1980;188:93–105. doi: 10.1016/0006-8993(80)90559-4. [DOI] [PubMed] [Google Scholar]
- 47.Kelley AE., Stinus L. The distribution of the projection from the parataenial nucleus of the thalamus to the nucleus accumbens in the rat: an autoradiographic study. Exp Brain Res. 1984;54:499–512. doi: 10.1007/BF00235475. [DOI] [PubMed] [Google Scholar]
- 48.Mogenson GJ., Swanson LW., Wu M. Neural projections from nucleus accumbens to globus pallidus substantia innominata and lateral preopticlateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 50.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 51.O'Doherty J., Rolls ET., Francis S., et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 52.Gottfried JA., O'Doherty J., Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 53.Kringelbach ML., O'Doherty J., Rolls ET., Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 54.Carmichael ST., Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 55.Irwin W., Davidson RJ., Lowe MJ., Mock BJ., Sorenson JA., Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport. 1996;7:1765–1769. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- 56.Breiter HC., Etcoff NL., Whalen PJ., et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996 Nov; 17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 57.Morris JS., Friston KJ., Bûchel C., et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 58.Ketter TA., Andreason PJ., George MS., et al. Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry. 1996;53:59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- 59.George MS., Ketter TA., Parekh PI., Horwitz B., Herscovitch P., Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 60.Lane RD., Reiman EM., Axelrod B., Yun LS., Holmes A., Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 61.Elliott R., Friston KJ., Dolan RJ. Dissociable neural responses in human reward systems. J Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Doherty J., Kringelbach ML., Rolls ET., Hornak J., Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 63.Small DM., Gregory MD., Mak YE., Gitelman D., Mesulam MM., Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 64.Reiman EM., Lane RD., Ahern GL., et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 65.Lane RD., Reiman EM., Ahern GL., Schwartz GE., Davidson RJ. Neuroanatomical correlates of happiness sadness and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 66.Wise MG. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 67.Bassareo V., Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 68.Naranjo CA., Tremblay LK., Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 69.Koob GF. Drug addiction: the yin and yang ofhedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 70.Salamone JD., Cousins MS., Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 71.Berridge KC. Food reward: brain substrates of wanting and liking. Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 72.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27 . doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Zadina JE., Martin-Schild S., Gerall AA., et al. Endomorphins: Novel endogenous l-opiate receptor agonists in regions of high u-opiate receptor density. Ann NY Acad Sci. 1999;877: 136–144. doi: 10.1111/j.1749-6632.1999.tb07885.x. [DOI] [PubMed] [Google Scholar]
- 74.Van Wolfswindel L., Seiffert WF., Van Ree JM. Catecholamines and endogenous opioids in ventral tegmental self-stimulation reward. Pharmacol Biochem Behav. 1998;30:589–595. doi: 10.1016/0091-3057(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 75.Tanda G., Di Chiara. A dopamine-l1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 76.VanFurth WR., VanRee JM. Sexual motivation: involvement of endogenous opioids in the ventral tegmental area. Brain Res. 1996;729:20–28. doi: 10.1016/s0006-8993(96)00225-9. [DOI] [PubMed] [Google Scholar]
- 77.Sweet KL., Neill DB. Amphetamine injections into the nucleus accumbens enhance the reward of stimulation of thesubiculum. Ann NY Acad Sci. 1999;877:828–830. doi: 10.1111/j.1749-6632.1999.tb09332.x. [DOI] [PubMed] [Google Scholar]
- 78.Panos JJ., Rademacher DJ., Renner SL., Steinpreis RE. The rewarding properties of NMDA and MK-801 (dizocilpine) as indexed by the conditioned place preference paradigm. Pharmacol Biochem Behav. 1999;64:591–595. doi: 10.1016/s0091-3057(99)00155-0. [DOI] [PubMed] [Google Scholar]
- 79.Hauber W., Bohn I., Giertler C. NMDA, but not dopamine D, receptors in the rat nucleus accumbens are involved in guidance of instrumental behavior by stimuli predicting reward magnitude. J Neurosci. 2000;20:6282–6288. doi: 10.1523/JNEUROSCI.20-16-06282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan QS. Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain Res Bull. 2000;51:75–81. doi: 10.1016/s0361-9230(99)00208-7. [DOI] [PubMed] [Google Scholar]
- 81.Lee K., Kornetsky C. Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav. 1998;60:539–544. doi: 10.1016/s0091-3057(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 82.Di Mascio M., Di Giovanni G., Di Matteo V., Prisco S., Esposito E. Selective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental area. Brain Res Bull. 1998;46:547–554. doi: 10.1016/s0361-9230(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 83.Ikemoto S., Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 84.Bush DE., De Sousa NJ., Vaccarino FJ. Self-administration of intravenous amphetamine: effect of nucleus accumbens CCKB receptor activation on fixed-ratio responding. Psychopharmacoiogy. 1999;147:331–334. doi: 10.1007/s002130051176. [DOI] [PubMed] [Google Scholar]
- 85.Harvey PO., Pruessner J., Czechowska Y., Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:703 767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 86.Heinz A., Schmidt LG., Reischies FM. Anhedonia in schizophrenic depressed or alcohol-dependent patients-neurobiological correlates. Pharmacopsychiatry. 1994; 27(suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- 87.Willner P., Hale AS., Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86:37–45. doi: 10.1016/j.jad.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Phan KL., Wager T., Taylor SF., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neurolmage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 89.Wager TD., Phan KL., Liberzon I., Taylor SF. Valence gender and lateralization of functional brain anatomy in emotion: a metaanalysis of findings from neuroimaging. Neuro Image. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 90.Amodio D., Frith C. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 91.Kennedy SH., Evans KR., Kruger S., et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 92.Lane RD., Reiman EM., Bradley MM. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–44. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 93.Epstein J., Pan H., Kocsis JH., et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 94.Dunn RT., Kimbrell TA., Ketter TA., et al. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry. 2002;51:387–399. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- 95.Surguladze S., Brammer MJ., Keedwell P., et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 96.Mogenson GJ., Jones DL., Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 97.Tremblay LK., Naranjo CA., Cardenas L., Herrmann N., Busto UE. Probing the brain reward system in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- 98.Tremblay LK., Naranjo CA., Graham SJ., et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 99.Schlaepfer TE., Cohen MX., Frick C., et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacoiogy. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 100.Mitterschiffthaler MT., Kumari V., Malhi GS., et al. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- 101.Kumari V., Mitterschiffthaler MT., Teasdale JD., et al. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol Psychiatry. 2003;54:777–791. doi: 10.1016/s0006-3223(02)01785-7. [DOI] [PubMed] [Google Scholar]
- 102.Beyer JL., Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]