Abstract
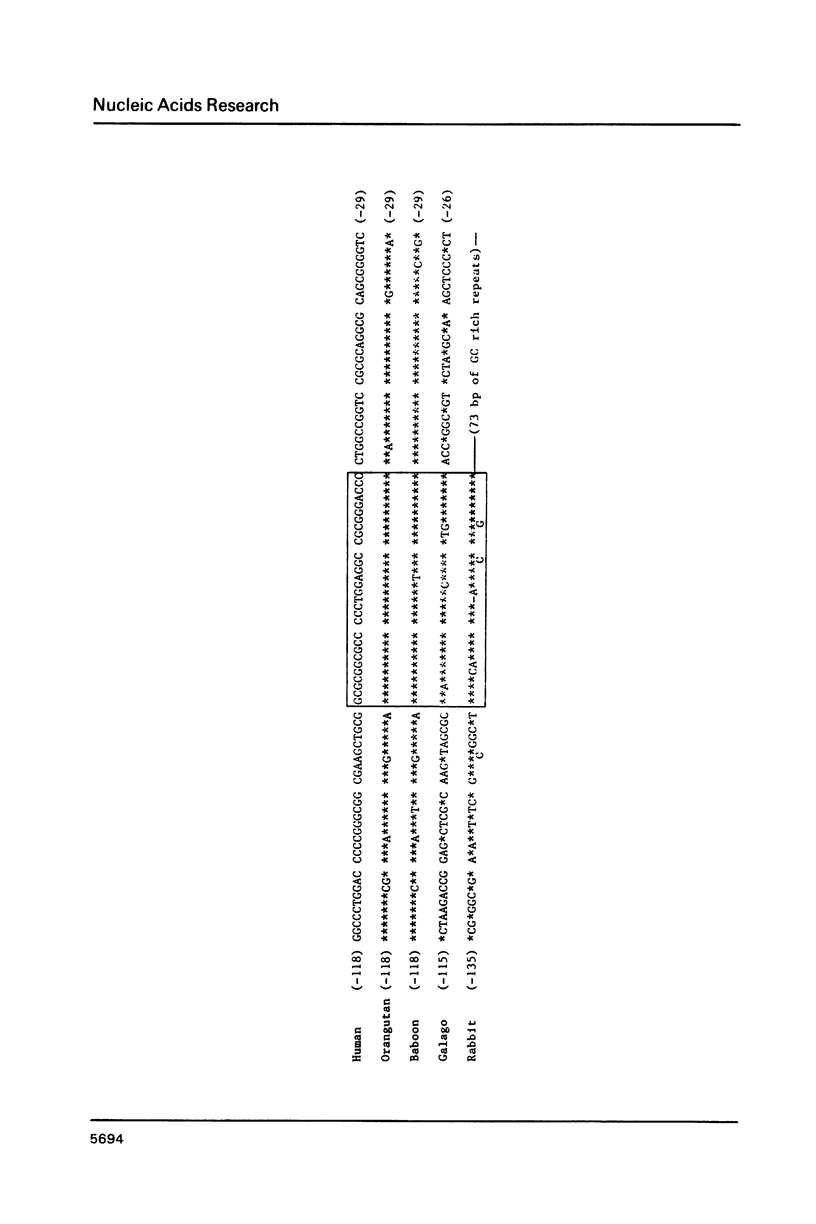
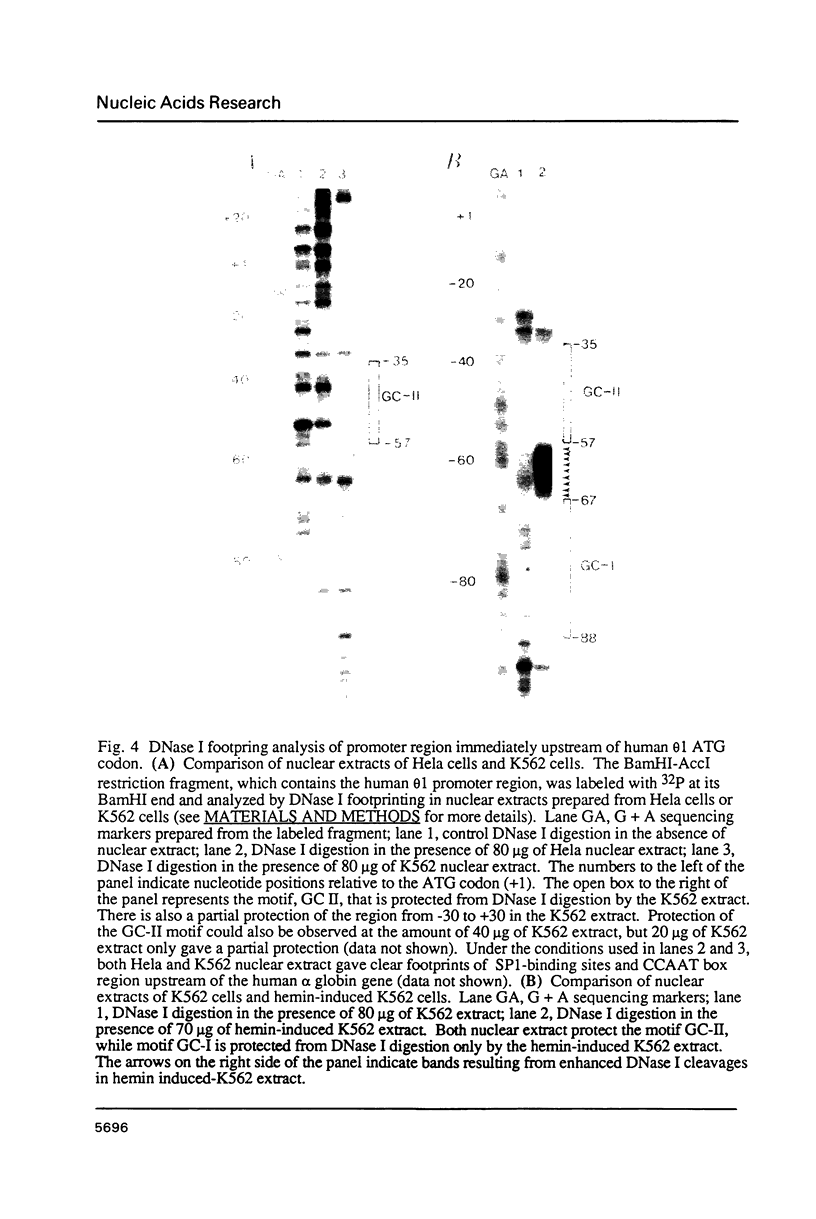
The theta 1 globin gene is an alpha globin-like gene, and started to diverge from the other members of the alpha globin family 260 million years ago. DNA sequencing and transcriptional analysis indicated that it is functional in erythroid cells of the higher primates, but not in prosimians and rabbit. The theta 1 promoter region of higher primates including man consists of GC-rich sequences characteristic of housekeeping gene promoters, and CCAAT and TATA boxes located further upstream. It is shown here that the housekeeping gene promoter-like region of human theta 1 contains two tandemly arranged, GC-rich motifs (GC-I and GC-II). Of these, GC-II interacts with nuclear factor(s) present in the globin-expressing, erythroleukemia cell line K562, before and after hemin induction. GC-I, however, interacts with nuclear factor(s) only present in hemin-induced K562 cells. These factors are different from previously reported erythroid cell-specific factors, and are not detectable in non-erythroid Hela cells. Furthermore, the sequence of the motif GC-I and its location relative to ATG codon have been conserved among all known mammalian theta 1 globin genes. Finally, and most interestingly, the CCAAT box of theta 1 is contained within a 38 bp internal segment of Alu repeat sequence. Immediately upstream from this CCAAT box-containing Alu repeat segment is a 241 bp Alu repeat pointing in the opposite direction. The conservation of this novel arrangement among the higher primates suggests that an inserted Alu family repeat and its flanking genomic sequence have co-evolved, for at least 30 million years, to provide the canonical CCAAT and TATA promoter elements of the theta 1 globin genes in higher primates.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alan M., Grindlay G. J., Stefani L., Paul J. Epsilon globin gene transcripts originating upstream of the mRNA cap site in K562 cells and normal human embryos. Nucleic Acids Res. 1982 Sep 11;10(17):5133–5147. doi: 10.1093/nar/10.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Improved timing of hominoid evolution with a DNA clock. Nature. 1985 Apr 11;314(6011):498–499. doi: 10.1038/314498a0. [DOI] [PubMed] [Google Scholar]
- Bark C., Weller P., Zabielski J., Janson L., Pettersson U. A distant enhancer element is required for polymerase III transcription of a U6 RNA gene. Nature. 1987 Jul 23;328(6128):356–359. doi: 10.1038/328356a0. [DOI] [PubMed] [Google Scholar]
- Barnhart K. M., Kim C. G., Banerji S. S., Sheffery M. Identification and characterization of multiple erythroid cell proteins that interact with the promoter of the murine alpha-globin gene. Mol Cell Biol. 1988 Aug;8(8):3215–3226. doi: 10.1128/mcb.8.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. Novel promoter upstream of the human c-myc gene and regulation of c-myc expression in B-cell lymphomas. Mol Cell Biol. 1986 Oct;6(10):3481–3489. doi: 10.1128/mcb.6.10.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Carbon P., Murgo S., Ebel J. P., Krol A., Tebb G., Mattaj L. W. A common octamer motif binding protein is involved in the transcription of U6 snRNA by RNA polymerase III and U2 snRNA by RNA polymerase II. Cell. 1987 Oct 9;51(1):71–79. doi: 10.1016/0092-8674(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Carlson D. P., Ross J. Human beta-globin promoter and coding sequences transcribed by RNA polymerase III. Cell. 1983 Oct;34(3):857–864. doi: 10.1016/0092-8674(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Carlson D. P., Ross J. Point mutation associated with hereditary persistence of fetal hemoglobin decreases RNA polymerase III transcription upstream of the affected gamma-globin gene. Mol Cell Biol. 1986 Sep;6(9):3278–3282. doi: 10.1128/mcb.6.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. F., Raid L., Hardison R. C. Isolation and nucleotide sequence of the rabbit globin gene cluster psi zeta-alpha 1-psi alpha. Absence of a pair of alpha-globin genes evolving in concert. J Biol Chem. 1986 Jan 15;261(2):839–848. [PubMed] [Google Scholar]
- Chung J., Sussman D. J., Zeller R., Leder P. The c-myc gene encodes superimposed RNA polymerase II and III promoters. Cell. 1987 Dec 24;51(6):1001–1008. doi: 10.1016/0092-8674(87)90586-1. [DOI] [PubMed] [Google Scholar]
- Clegg J. B. Can the product of the theta gene be a real globin? Nature. 1987 Oct 1;329(6138):465–466. doi: 10.1038/329465a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Pan J., Duncan C. H., Weissman S. M. Transcriptional analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res. 1981 Mar 11;9(5):1171–1189. doi: 10.1093/nar/9.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman S. A., Deininger P. L., LaPorte P., Friedmann T., Geiduschek E. P. Analysis of transcription of the human Alu family ubiquitous repeating element by eukaryotic RNA polymerase III. Nucleic Acids Res. 1981 Dec 11;9(23):6439–6456. doi: 10.1093/nar/9.23.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindlay G. J., Lanyon W. G., Allan M., Paul J. Alternative sites of transcription initiation upstream of the canonical cap site in human gamma-globin and beta-globin genes. Nucleic Acids Res. 1984 Feb 24;12(4):1811–1820. doi: 10.1093/nar/12.4.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Fox M., Schmid C., Shen C. K. Molecular evolution of the human adult alpha-globin-like gene region: insertion and deletion of Alu family repeats and non-Alu DNA sequences. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5970–5974. doi: 10.1073/pnas.80.19.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Perez-Stable C., Deisseroth A., Shen C. K. Characterization of an unique RNA initiated immediately upstream from human alpha 1 globin gene in vivo and in vitro: polymerase II-dependence, tissue specificity, and subcellular location. Nucleic Acids Res. 1985 Sep 11;13(17):6059–6074. doi: 10.1093/nar/13.17.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Perez-Stable C., Wu G. J., Weir B., Tinoco I., Jr, Shen C. K. End-to-end transcription of an Alu family repeat. A new type of polymerase-III-dependent terminator and its evolutionary implication. J Mol Biol. 1985 Jul 5;184(1):7–21. doi: 10.1016/0022-2836(85)90039-7. [DOI] [PubMed] [Google Scholar]
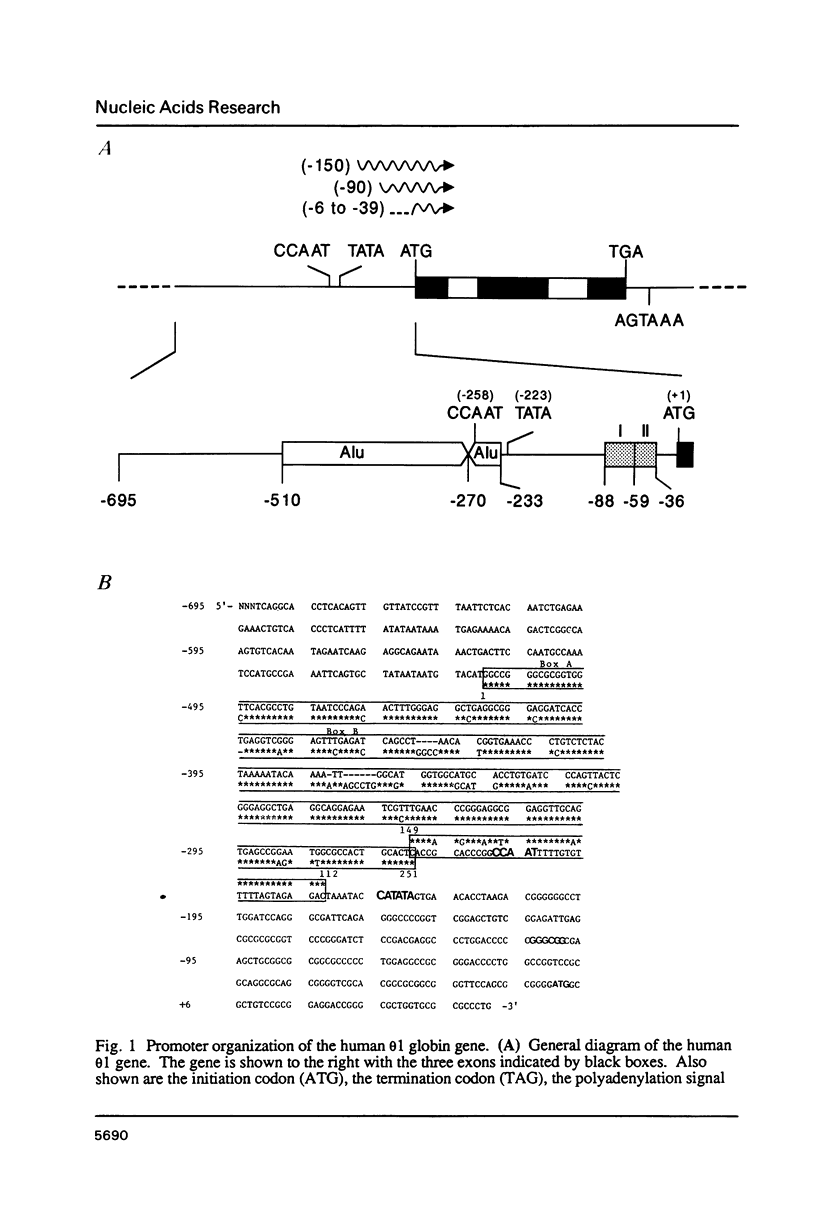
- Hsu S. L., Marks J., Shaw J. P., Tam M., Higgs D. R., Shen C. C., Shen C. K. Structure and expression of the human theta 1 globin gene. Nature. 1988 Jan 7;331(6151):94–96. doi: 10.1038/331094a0. [DOI] [PubMed] [Google Scholar]
- Hwu H. R., Roberts J. W., Davidson E. H., Britten R. J. Insertion and/or deletion of many repeated DNA sequences in human and higher ape evolution. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3875–3879. doi: 10.1073/pnas.83.11.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Jurka J., Smith T. A fundamental division in the Alu family of repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4775–4778. doi: 10.1073/pnas.85.13.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Sekeris C. E., Grosveld F. G. Alpha-amanitin insensitive transcription of the human epsilon-globin gene. Nucleic Acids Res. 1985 Nov 25;13(22):7993–8005. doi: 10.1093/nar/13.22.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Brown D. D. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978 Dec;15(4):1145–1156. doi: 10.1016/0092-8674(78)90042-9. [DOI] [PubMed] [Google Scholar]
- Krol A., Carbon P., Ebel J. P., Appel B. Xenopus tropicalis U6 snRNA genes transcribed by Pol III contain the upstream promoter elements used by Pol II dependent U snRNA genes. Nucleic Acids Res. 1987 Mar 25;15(6):2463–2478. doi: 10.1093/nar/15.6.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Proudfoot N. J., Whitelaw E. The gene for theta-globin is transcribed in human fetal erythroid tissues. Nature. 1987 Oct 8;329(6139):551–554. doi: 10.1038/329551a0. [DOI] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Shaw J. P., Shen C. K. Sequence organization and genomic complexity of primate theta 1 globin gene, a novel alpha-globin-like gene. Nature. 1986 Jun 19;321(6072):785–788. doi: 10.1038/321785a0. [DOI] [PubMed] [Google Scholar]
- Marks J., Shaw J. P., Shen C. K. The orangutan adult alpha-globin gene locus: duplicated functional genes and a newly detected member of the primate alpha-globin gene family. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1413–1417. doi: 10.1073/pnas.83.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., McEwan C., McKie A. B., Reid A. M. Expression of the mouse HPRT gene: deletional analysis of the promoter region of an X-chromosome linked housekeeping gene. Cell. 1986 Jan 31;44(2):319–328. doi: 10.1016/0092-8674(86)90766-x. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Fischel-Ghodsian N., Higgs D. R. Recombination at the human alpha-globin gene cluster: sequence features and topological constraints. Cell. 1987 May 8;49(3):369–378. doi: 10.1016/0092-8674(87)90289-3. [DOI] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Paolella G., Lucero M. A., Murphy M. H., Baralle F. E. The Alu family repeat promoter has a tRNA-like bipartite structure. EMBO J. 1983;2(5):691–696. doi: 10.1002/j.1460-2075.1983.tb01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable C., Ayres T. M., Shen C. K. Distinctive sequence organization and functional programming of an Alu repeat promoter. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5291–5295. doi: 10.1073/pnas.81.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable C., Shen C. K. Competitive and cooperative functioning of the anterior and posterior promoter elements of an Alu family repeat. Mol Cell Biol. 1986 Jun;6(6):2041–2052. doi: 10.1128/mcb.6.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Page D. C., Weissenbach J. A sex chromosome rearrangement in a human XX male caused by Alu-Alu recombination. Cell. 1987 Nov 6;51(3):417–425. doi: 10.1016/0092-8674(87)90637-4. [DOI] [PubMed] [Google Scholar]
- Sawada I., Schmid C. W. Primate evolution of the alpha-globin gene cluster and its Alu-like repeats. J Mol Biol. 1986 Dec 20;192(4):693–709. doi: 10.1016/0022-2836(86)90022-7. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Marks J., Shen C. K. Evidence that the recently discovered theta 1-globin gene is functional in higher primates. Nature. 1987 Apr 16;326(6114):717–720. doi: 10.1038/326717a0. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. The organization, structure, and in vitro transcription of Alu family RNA polymerase III transcription units in the human alpha-like globin gene cluster: precipitation of in vitro transcripts by lupus anti-La antibodies. J Mol Appl Genet. 1982;1(4):343–360. [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Willard C., Nguyen H. T., Schmid C. W. Existence of at least three distinct Alu subfamilies. J Mol Evol. 1987;26(3):180–186. doi: 10.1007/BF02099850. [DOI] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]