Abstract
Major Depressive Disorder (MDD) is a prevalent illness that is frequently associated with significant disability, morbidity and mortality. Despite the development and availability of numerous treatment options for MDD, studies have shown that antidepressant monotherapy yields only modest rates of response and remission. Clearly, there is an urgent need to develop more effective treatment strategies for patients with MDD, One possible approach towards the development of novel pharmacotherapeuiic strategies for MDD involves identifying subpopulations of depressed patients who are more likely to experience the benefits of a given (existing) treatment versus placebo, or versus a second treatment. Attempts have been made to identify such “subpopulations, ” specifically by testing whether a given biological or clinical marker also serves as a moderator, mediator (correlate), or predictor of clinical improvement following the treatment of MDD with standard, first-line antidepressants. In the following article, we will attempt to summarize the literature focusing on several major areas (“leads”) where preliminary evidence exists regarding clinical and biologic moderators, mediators, and predictors of symptom improvement in MDD, Such clinical leads will include the presence of hopelessness, anxious symptoms, or medical comorbidity. Biologic leads will include gene polymorphisms, brain metabolism, quantitative electroencephalography, loudness dependence of auditory evoked potentials, and functional brain asymmetry
Keywords: depression, predict, antidepressant, outcome, response, remission
Abstract
El trastorno depresivo mayor (TDM) es una enfermedad prevalente que está asociada frecuentemente con incapacidad, morbilidad y mortalidad significativas. A pesar del desarrollo y de la disponibilidad de numerosas opciones terapéuticas para el TDM, los estudios han mostrado que la monoterapia antidepresiva sólo produce bajas frecuencias de respuesta y remisión. Es claro que hay una urgente necesidad de desarrollar estrategias terapéuticas más efectivas para los pacientes con TDM, Una posible aproximación para el desarrollo de novedosas estrategias farmacoterapéuticas para el TDM implica identificar subpoblaciones de pacientes depresivos que con mayor probabilidad experimenten los beneficios de un tratamiento dado (existente) versus placebo, o versus un segundo tratamiento. Se han realizado intentas para identificar tales “subpoblaciones”, especificamente analizando si un determinado marcador biológico o clinico también sirve como un moderador, mediador (correlato) o predictor de la mejorfa clinica en el tratamiento del TDM con antidepresivos estándar de primera linea. En este articulo se intentará resumir la literatura focalizada en algunas áreas principales (“pistas”) donde existe evidencia preliminar relacionada con moderadores, mediadores y predictores clinicosy biológicos de mejorfa de sintomas en el TDM, Las pistas clinicas incluirán la presencia de desesperanza, sintomas ansiosos o comorbilidad médica. Las pistas biológicas incluirán polimorfismo genético, metabolismo cerebral, electroencefalografia cuantitativa, dependencia a la intensidad del volumen de los potenciales avocados auditives y asimetria cerebral funcional.
Abstract
Le trouble dépressif majeur (TDM) est une pathologie prêvalente fréquemment associée à une invalidité, une morbidité et une mortalité significatives. Malgré le développement et l'existence de nombreux traitements pour le TDM, des études ont montré que les monothérapies antidépressives ne donnaient que de modestes taux de réponse et de rémission. Il devient vraiment urgent de développer des stratégies thérapeutiques efficaces pour les patients atteints de TDM, Une approche éventuelle pour un tel développement serait d'identifier des sous-populations de patients déprimés plus susceptibles de bénéficier d'un traitement donné (existant) versus placebo ou versus un second traitement. Des tentatives ont été menées afin d'identifier de telles « sous-populations », en vérifiant en particulier si un marqueur biologique ou clinique donné pouvaitt aussi servir de modérateur, médiateur (corrélat) ou prédicieur de l'amélioration clinique consécutive au traitement du TDM avec des antidépresseurs standard de première intention. Dans cet article, nous allons essayer de résumer la littérature dirigée vers plusieurs axes importants (« directeurs ») et pour lesquels il existe des arguments préliminaires en ce qui concerne les prédicteurs, médiateurs et modérateurs cliniques et biologiques de l'amélioration des symptômes du TDM, Ces symptômes cliniques « directeurs » incluront les symptômes de désespoir, d'anxiété ou de comorbidité médicale. Le polymorphisme génétique, le métabolisme cérébral, l'éleciroencéphalographie quantitative, la dépendance à l'intensité du son des potentiels évoqués auditifs et l'asymétrie cérébrale fonctionnelle feront partie des critères biologiques directeurs exposés.
Definitions
Major Depressive Disorder (MDD) is a prevalent illness that is frequently associated with significant disability, morbidity, and mortality. Results from the 2003 National Comorbidity Replication study found that the lifetime prevalence of MDD among American adults is 16.2%, ranking it among the most common and costly medical illnesses.1 Despite the development and availability of numerous treatment options for MDD, studies have shown that antidepressant monotherapy yields only modest rates of response and remission. For example, a metaanalysis2 of all double-blind placebo-controlled studies of antidepressants published since 1980 revealed response rates of 53% for antidepressants and 36% for placebo (absolute difference in response rate of 16.8%). Similarly, Petersen et al3 report remission rates as low as 20% to 23% following each successive treatment among patients with MDD enrolled in one of two academically affiliated, depression-specialty clinics. In fact, only about. 50% of all patients enrolled ultimately achieved full remission of their depression. Similarly, only about one in three patients with MDD experienced a remission of their depression following treatment with the selective serotonin reuptake inhibitor (SSRI) citalopram during the first level of the large, multicenter, Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.4 Clearly, there is an urgent need to develop safer, better-tolerated, and more effective treatments for MDD.
There are three major “paths” towards the development of novel pharmacotherapeutic strategies for MDD (Table I).5 The first, approach involves developing new antidepressants to be used as monotherapy. A second approach involves combining pharmacologic agents, including established treatments (ie, established antidepressants), existing but not established agents, and new or novel agents. Finally, a third approach involves identifying subpopulations of depressed patients who are more likely to experience the benefits of a given (existing) treatment versus placebo, or versus a second treatment. Attempts have been made to identify such “subpopulations,” specifically by testing whether a given biological clinical marker also serves as a moderator, mediator (correlate), or predictor of clinical improvement following the treatment of MDD with standard, first-line antidepressants. A predictor of treatment (efficacy) outcome can involve factors (whether clinical or biologic), the presence or magnitude of which influences the likelihood of a particular outcome occurring during treatment. Efficacy outcomes in MDD commonly include either the resolution of depressive symptoms during treatment (the magnitude of reduction in depressive symptoms), the rapidity of response (the time course of symptom reduction), the attainment of a treatment response, or the attainment of symptom remission.
Table I. Common pathways towards the development of more effective pharmacologic strategies for Major Depressive Disorder (MDD).
| • Develop new agents as monotherapy |
• Combine two or more pharmacologic treatments
|
• Identify subpopulations of MDD patients who are more likely to experience the benefits of a given treatment
|
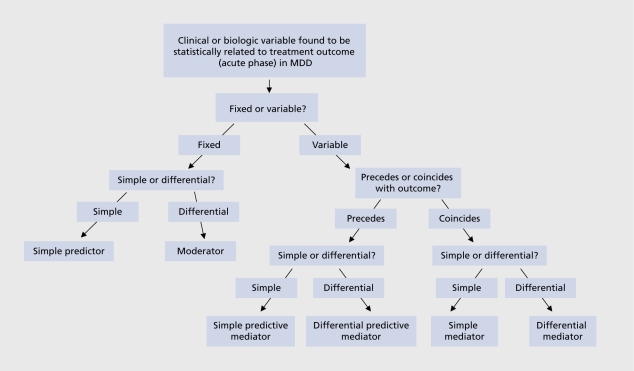
Differential predictors or moderators of efficacy outcome are a special subcategory of outcome predictors. Moderators of outcome involve factors (clinical or biologic), the presence or magnitude of which at baseline (immediately before treatment is initiated) influences the relative likelihood of a particular outcome occurring following treatment with one versus another agent. Thus, moderators of response can help predict differential efficacy between two or more treatments for MDD (for example, patients who present with a given moderator are more likely to respond to treatment with one antidepressant versus another than patients who do not present with that, given moderator).
Mediators of efficacy outcome (sometimes also referred to as correlates) are measurable changes (usually biologic) that occur during treatment and correlate with treatment outcome. These changes can either precede (in which case they may also predict outcome - “predictive mediators”), or temporally coincide with treatment outcome (“simple mediators”). Differential mediators of outcome are also possible (changes that predict or correlate with an event, following treatment with one agent but not another). Figure 1 provides an overview regarding the combinations pertaining to mediators, moderators, and predictors of efficacy outcome in MDD. Table II outlines potential clinical, scientific, and treatment-development implications that may derive by identifying mediators, moderators, and predictors of efficacy outcome in MDD.
Table II. Potential clinical, scientific and treatment development applications of predictors, moderators and mediators of treatment outcome in Major Depressive Disorder.
| • Identification of factors which are simple predictors of treatment outcome would allow for the stratification of potlonrs according to risk for treatment-resistance, which, in turn, could lead to the development of tailored aprroaches that would improve overall treatment outcome (ie, choosing a more “aggressive” treatment a priori. |
| • Identification of moderators (ie, differential predictors) of treatment outcome may load to the development of tailored treatment approaches (algorithms) for a given subgroup of MDD patients that would improve treatment outcome (ie, matching treatment with MDD subtype). |
| • Prédictive or nonpredictive mediators (correlates) of treatment outcome may provide mechanistic insights into the underlying pathophysiology of MDD, thereby helping identify new molecular targets for drug development or for defining clinically relevant subgroups. |
| • Prédictive or nonpredictive mediators (correlates) of treatment outcome may be used in screening for potential new antidepressants (for example, selecting pharmacologic agents that also result in similar changes in clinical or preclinical models). |
In the following paragraphs, we will attempt to summarize the literature focusing on several major areas (“leads”) where preliminary evidence exists regarding clinical and biologic moderators, mediators, and predictors of symptom improvement in MDD. In the first section, we will focus on clinical variables while, in the second section, on biological variables.
Clinical factors
To date, the overwhelming majority of published studies focusing on identifying predictors of response during the acute-phase of treatment of M'DD involve the SSRIs. These studies focus on examining the role of illness characteristics (ie, depressive subtype) or comorbidity (psychiatric (ie, axis I), characterologic (axis II), and medical (axis III), and will be reviewed according to antidepressant class.
SSRI treatment
In general, the presence and/or extent of factors associated with personality or temperament, including the presence of a Diagnostic and Statistical Manual of Mental Disorders (DSM)-defined personality disorder,6-9 neuroticism,10 hypochondriacal concerns,11 dysfunctional attitudes,12 or temperamental style13 do not appear to predict response to the SSRIs.
Figure 1. Schematic depiction of definitions. MDD, major depressive disorder.
In contrast, the presence and or degree of general14 as well as specific medical comorbidity, including hypercholesterolemia,15 greater body weight,16 other risk factors for vascular disease,17,18 hypofolatcmia,18-20 and magnetic resonance imaging (MRI) white-matter hyperintensities18,21,22 consistently appear to predict poorer outcome during the acute phase of treatment of MDD with the SSRIs, although other factors, such as the presence of mild hypothyroidism23 and anemia,24 do not.
The presence and severity of several symptoms of depression have also been linked to poorer prognosis, including hopelessness,25 cognitive symptoms of depression including executive dysfunction,26 physical symptoms of depression (somatic symptoms including pain, fatigue, physical symptoms of anxiety, and gastrointestinal symptoms),27-30 and psychomotor retardation.27 Early improvement in depressive symptoms appears to also predict better outcome during the acute phase of treatment of MDD with fluoxetine, and vice versa.31,32
Illness features including greater chronicity,7,8 atypical depression,7 depression with anger attacks,7 or depression with comorbid attention deficity-hyperactivity disorder,33 or insomnia8,34,35 do not appear to confer a worse prognosis. However, greater MDD severity was found to predict a greater likelihood of attaining remission of depression following treatment with the SSRI escitalopram than several older SSRIs (fluoxetine, sertraline, paroxetine, citalopram) in MDD (moderator).36
The presence of an anxious MDD subtype (defined using the “syndromal” approach as MDD presenting with at least one comorbid DSM anxiety disorder) was found to result, in poorer outcome during the acute phase of treatment of MDD with fluoxetine7 but not sertraline.8 Until recently, however, several relatively small studies9,37-40 defining anxious MDD using the “dimensional” approach (most commonly defined as a score of 7 or more on the anxiety-somatization subscale (HDRS-AS)41 of the Hamilton Depression Rating Scale (HDRS),42 and have not confirmed earlier findings by Fava et al.7 The HDRSAS subscale is comprised of the following HDRS items: psychic anxiety, somatic anxiety, somatic symptoms-gastrointestinal, somatic symptoms-general, hypochondriasis, and insight. Other studies37,43,44 which employ a scale different than the HDRS-AS to define anxious MDD (dimensional approach) have also not confirmed the findings of the earlier work by Fava et al.7 However, recently, evidence stemming from Levels 1 and 2 of STAR*D do suggest significantly lower remission rates following the treatment of MDD with either first-line (citalopram) or second-line treatment strategies (switching to antidepressants versus augmentation or combination strategies).45
Most of the studies described above examining the potential role of several factors as possible predictors of outcome following the acute phase of treatment of MDD with an SSRI share two major limitations: (i) most involve a relatively small sample size, resulting in limited statistical power to detect an effect of a factor on treatment, outcome; and (ii) most involve analyses conducted in either univariate or bivariate fashion (ie, simply controlling for overall depression severity at baseline). More recently, Trivedi et al4 conducted multivariate analyses in STAR*D, examining potential predictors of response to open-label citalopram (up to 60 mg, up to 14 weeks of treatment) in MDD utilizing a datasct of unprecedented statistical power (n=2876). Variables examined as potential predictors of outcome included several demographic (ie, age, gender, race, sociodemographic variables) and clinical (age of onset of MDD, duration of episode, the presence of psychiatric and medical comorbidity) factors. Participants who were Caucasian, female, employed, or had higher levels of education or income had higher chances of success. Longer depressive episodes, more concurrent psychiatric disorders (especially anxiety disorders and or drug abuse) and general medical disorders, and lower baseline psychosocial functioning and quality of life were associated with poorer chances of success.
Treatment with older agents (TCAs and MAOIs)
In general, results of these studies parallel those focusing on the use of SSRIs in MDD.
While the results of two studies suggest, that the presence of a comorbid personality disorder confers an increased risk of poor outcome during the treatment, of MDD with the tricyclic antidepressants (TCAs),46,47 the majority of studies do not. support this relationship.8,48-55 However, two studies do report poorer outcome among MDD patients with than without a comorbid cluster C personality disorder during TCA treatment.53-56
Several studies do not report the presence of neuroticism to predict antidepressant response following TCA treatment in MDD.50-52,55 The interactions of certain elements of temperament, (novelty seeking, harm avoidance, and reward dependence) were found to help predict response to TCAs in one,50 but not a subsequent, study.57
Symptom chronicity was found to result in poor outcome during treatment of MDD with the TCAs in one,52 but not. a second study.8 Finally, specific symptoms including insomnia8,35 and suicidal ideation58 do not appear to predict response to TCA treatment. However, the presence of somatic symptoms of depression,59 elevated cholesterol levels,60 but. not the presence and/or extent of medical comorbidity61 have been linked to lower chances of responding to the TCA nortriptyline in MDD.
Although earlier studies had suggested that patients with anxious MDD may respond more poorly to treatment with the TCAs and/or monamine oxidase inhibitors (MAOIs),62-64 a number of studies did not find a significant relationship between the presence of an anxious MDD subtype and poorer outcome following treatment with an MAOI65-70 or TCA.9,38,40,48,65-70 .Finally, the presence of atypical MDD has been shown to predict a greater likelihood of clinical response to treatment with the M AOI phenelzine than the TCA imipramine.69,71
Treatment with newer agents
Only a handful of studies specifically focus on identifying predictors of acute-phase outcome (efficacy) during the treatment of MDD with newer agents. Nelson and Cloninger72 reported the interaction of several temperamental factors, including reward dependence and harm avoidance, to predict response to the serotonin (5HT2-receptor antagonist ncfazodone in MDD (n=18). This was confirmed shortly thereafter using a larger database (n=1119).73 However, the predictive power of neuroticism in the latter study accounted for a trivial 1.1% of the total variance in outcome, raising questions regarding the clinical relevance of this finding.
Rush et al43,41,74 did not find the presence of pretreatment anxiety or insomnia to confer a better or poorer prognosis during treatment with the noradrenaline-dopamine reuptake inhibitor (NDRI) bupropion. However, a more recent, analysis involving 10 randomized, double-blind clinical trials comparing bupropion with an SSRI for MDD did reveal a greater likelihood of clinical response following treatment, with an SSRI than bupropion among patients with anxious MDD (moderator).75
Sir et al39 and Davidson et al76 did not find that, the presence of an anxious subtype of MDD or anxious symptoms in MDD had influenced the likelihood of responding to venlafaxine in MDD, although Silverstone and Salinas77 found a slower onset of antidepressant effects among venlafaxinc-trcated patients with MDD and comorbid generalized anxiety disorder (GAD) than those without, comorbid GAD, and patients with anxious depression, as defined by elevated scores on the HDRSAS scale, were significantly less likely to remit, following venlafaxine treatment in Level 2 of STAR*D.45 However, postmenopausal women with MDD who were not on hormone-replacement therapy were found to be much more likely to attain remission of MDD following treatment with the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine than an SSRI than either premenopausal women or postmenopausal women on hormone replacement therapy in one study.78
Kornstein et al79 did not find either age nor gender to influence efficacy outcome following treatment with the SNRI duloxetine. Mallinckrodt et al80 did not find the presence of a melancholic subtype to influence efficacy outcome following treatment with duloxetine. However, greater MDD severity was found to predict a greater likelihood of attaining remission of depression following treatment with the SNRI duloxetine than the SSRIs fluoxetine and paroxetine in MDD (moderator).81
Biologic factors
To date, numerous studies have explored several potential genetic markers of outcome during the acute phase of treatment of MDD. The majority of these studies stem from one of two fields: genetics and neurophysiology. Due to the paucity of reports focusing on non-SSRI agents, biologic factors will be reviewed according to field (ie, genetics versus neurophysiology) rather than class (ie, SSRI versus non-SSRI treatment).
Genetic markers
A number of reports explore various genetic markers as predictors of clinical response to antidepressants in MDD. The vast majority of these focus on genes coding for proteins directly involved in the monoaminergic system, including tryptophan hydroxylase (TPH - the rate -limiting step in serotonin synthesis), the serotonin transporter (5-HTT),the serotonin 5-HT-2 receptors, the monoamine oxidase enzyme (MAO), and the catechol-O-methyltransf erase enzyme (COMT). The overwhelming majority of these studies involve treatment with the SSRIs.
SSRIs
Three studies suggest that patients with a specific polymorphism (A218C) in the gene coding for the TPH enzyme may respond more poorly to SSRIs than those without such a polymorphism,82-84 although this was not confirmed in three other studies.85-88 Early on, the results of some88-98 but not all99,103 studies also suggested that depressed patients with a certain (insertion/deletion) polymorphism located in the promoter region of the gene coding for the serotonin transporter (5 HTTPR) have a relatively poorer response to the SSRIs than those without. Several pooled analyses and meta-analyses have subsequently confirmed a predictive role for 5HTTPR genotype with regards to SSRI response in MDD, more so for Caucasian than Asian patients.104-106 More recently, however, Kraft, et al107 and, subsequently, Hu et al108 did not find an association between response to the SSRI citalopram and 5 HTTPR genotype among 1914 subjects who participated in the first level of the STAR*D trial. This report, provides the strongest, evidence to date against a role for variation at this gene as a factor predicting clinical response to the SSRIs.
Similarly, there have been conflicting reports regarding the role of 5-HT2-receptor genotype as a predictor of SSRI response. Specifically, two studies have identified a specific single nucleotide polymorphism (SNP) in the promoter region of the 5-HT2 receptor (A1438G) that appears to predict response to the SSRIs in MDD.91,109 However, this finding was not confirmed in a third report.110 More recently, however, McMahon et al111 conducted an analysis of numerous candidate genes as potential predictors of response to open-label citalopram in MDD utilizing the STAR*D level-1 dataset (n=1953). Of 68 candidate genes investigated, only genetic variation at the locus coding for the 5-HT7 receptor gene was found to consistently predict clinical outcome,111 with differences in genotype (comparison of two homozygous groups) accounting for an 18% difference in the absolute risk of having no response to treatment.
Relatively fewer studies have focused on genes coding for proteins not directly related to the monoaminergic system. Using a STAR*D-based dataset, Pcrlis et al112 demonstrated a relationship between the presence of a variant (KCNK2) in a gene (TREK1) coding for a potassium channel and the likelihood of experiencing symptom improvement, following treatment of MDD with the SSRI citalopram. In a separate study, Paddock et al113 reported that genetic variation in a kainic acid-type glutamate receptor was associated with response to the antidepressant citalopram (marker (rsl954787) in the GRIK4 gene, which codes for the kainic acid-type glutamate receptor KA1). There is also a STAR*D-based report, suggesting a relationship between the likelihood of achieving remission of symptoms during treatment with the SSRI citalopram and genotype at. one of the markers (rs4713916) in the FKBP5 gene, a protein of the hypothalamic-pituitary adrenal (HPA) system modulating the glucocorticoid receptor.114
Other agents
Studies looking at genetic markers as predictors of response to other antidepressants are few. The results of one study report 5HTTPR genotype to influence the likelihood of responding to the tricyclic antidepressant (TCA) nortriptyline in MDD115 although this could not be replicated in a separate study.99 Two separate studies report. 5HTTPR genotype to predict response to the SNRI venlafaxine,116 and the 5-HT2 alpha-2 adrenergic receptor inhibitor mirtazapine.117 Finally, there is also a single study examining the role of MAO-A genotype as a predictor of clinical response to the MAOI moclobemide; no relationship was found.118
Reports from studies comparing agents of different classes
Reports examining for genetic predictors of response from randomized, double-blind clinical trials comparing two antidepressants of different classes are few Although preliminary, such studies can be useful in genetic markers that may serve as moderators of treatment, efficacy. Joyce et al119 studied 169 MDD patients randomized to treatment with either fluoxetine or nortriptyline, and examined whether 5HTTPR or G-protein beta3-subunit (C825T) genotype influenced symptom improvement, following treatment with either of these two agents. For patients younger than 25 years of age, the T allele of the G protein beta3 subunit, was associated with a poorer response to nortriptyline. There was no relationship between 5HTTPR genotype and response to treatment with either antidepressant among this age group, nor was there any relationship between G protein beta3 subunit genotype status and response to paroxetine. Among patients 25 years of age or older, however, 5HTTPR genotype predicted response to both fluoxetine and nortriptyline. Findings stemming from this report have yet to be replicated. Similarly, Szegcdi et al120 studied the relationship between the COMT (vall58met) polymorphism status and antidepressant response following treatment with paroxetine versus mirtazapine (5-HT2-alpha-2 adrenergic receptor antagonist) in MDD. Patients homozygous for COMT-met showed a poorer response to mirtazapine than patients with other genotypes. A similar finding was not observed during paroxetine treatment. Preliminary findings from these two trials have yet to be prospectively confirmed.
Neurophysiology
Brain functioning and metabolism
A number of studies have examined the potential relationship between functional changes, including changes in regional blood glucose metabolism as measured by positron emission tomography (PPT), and clinical response following the treatment of MDD with standard antidepressants. Mayberg,121,122 for instance, studied the relationship between regional metabolic changes in the central nervous system (CNS) and clinical response following a 6-week trial of the SSRI fluoxetine for MDD. The results of her work suggest, that metabolism in certain brain areas, as measured by PET, may serve as a mediator of response to the SSRIs. Specifically, she found an increase in brain stem and dorsal cortical metabolism (prefrontal, parietal, anterior cingulatc, and posterior cingulate), and a decrease in limbic and striatum metabolism (subgenual cingulate, hippocampus, insula, and palladium) from week 1 to week 6 of treatment among fluoxetine responders. Fluoxetine nonrcsponders did not demonstrate changes in these areas during the same treatment period (weeks 1-6). Similarly, Tosifescu et al123 established a relationship between normalization in measures of brain biocnergetic metabolism among patients with SSRI-resistant MDD who experienced symptom improvement (clinical response) following T3 augmentation of their SSRI treatment, regimen.
In a recent work, Mayberg et al121 reviewed earlier studies examining the relationship between regional metabolic changes and symptom improvement during the treatment of MDD with antidepressants, and concluded that a significant correlation between normalization of frontal hypometabolism and clinical improvement was the best-replicated finding. However, a similar relationship (ie, between an increase in frontal metabolism and symptom improvement) was also reported during placebo treatment.121 The results of the latter study suggest that such changes, at least as detected by the technology available at the time, appear to be related to nonspecific (placebo) rather than specific (drug) treatment effects and, therefore, may not serve as robust differential treatment mediators. Little et al,124 for instance, examined whether there are differences in the relationship between brain metabolism at baseline (predictor or moderator) and symptom improvement between two antidepressants of different class (the NDRI bupropion versus the SNRI venlafaxine). For the most part, similar findings predicted symptom improvement for both agents (frontal and left temporal hypometabolism), although some differences emerged (compared with control subjects, bupropion responders (n = 6) also had cerebellar hypermetabolism, whereas venlafaxine responders showed bilateral temporal and basal ganglia hypometabolism). This study has yet to be replicated, either with regards to baseline brain metabolism (ie, moderator of response), or changes in baseline brain metabolism (ie, mediator of response).
Quantitative EEG
Quantitative electroencephalography (QEEG) involves the use of computer software analysis to deconstruct, electroencephalographic (EEG) tracings and quantify parameters including frequency and amplitudes (traditional EEG involves manual readings). A relevant measurement, generated by the software traditionally employed by QEEG is called cordance, which involves a combination of absolute power (the power of a frequency band) and relative power (the percentage of power in a frequency band compared with the total power across all frequency bands).125,126 Cordance of frontal EEG measurements in the theta band (4 to 8Hz) has consistently been found to correlate with antidepressant response in M'DD. Specifically, the result of several studies suggest a decrease in theta cordance from prefrontal EEG leads during the first, week of treatment, with either an SSRI, an SNRI, or a variety of antidepressants, to predict, greater symptom improvement, following 4 to 10 weeks of treatment.127-129 In contrast, an increase in prefrontal theta cordance during the first week of treatment was demonstrated among placeboresponders, suggesting that prefrontal theta cordance may serve as a differential (predictive) mediator of response to antidepressants versus placebo.130 Interestingly enough, a report by Hunter et al131 suggests that the decrease in prefrontal EEG theta cordance during the week immediately preceding the initiation of treatment of MDD with antidepressants (fluoxetine, venlafaxine) or placebo (placebo lead-in period) is related to the likelihood of responding to antidepressants but not placebo following 9 weeks of treatment (moderator of response). Thus, the sum of the evidence reviewed above suggests a potential role for the change in prefrontal theta EEG cordance during the first week of treatment in MDD as a mediator and predictor of response to antidepressants but not placebo (differential mediator). Although the exact physiologic relevance of this probable treatment mediator is, at present, unclear, several lines of evidence suggest it may serve as a proxy for changes in underlying prefrontal cortex metabolism (see ref 127 for further details).
Loudness dependence of auditory evoked potentials
Much less is known regarding the potential predictive ability of other EEG-related biomarkers. Loudness dependence of auditory evoked potentials (LDAEP) is one such measurement, derived from EEG recordings thought to correspond to the primary auditory cortex following the administration of an auditory stimulus.125 A “strong” LDAEP suggests that the characteristics of evoked potentials following an auditory stimulus are highly dependent on the intensity (loudness) of the auditory stimulus.134 In contrast, a “weak” LDAEP suggests that evoked potentials following an auditory stimulus do not vary much as a function of how loud the sound is.132 To date, a variety of clinical studies have demonstrated that patients with “strong” LDAEP at baseline are more likely to respond to treatment with SSRIs than those with “weak” LDEAP.133-137 However, in a small (n=35) randomized, open-label trial comparing the SSRI citalopram with the norepinephrine reuptake inhibitor (NRI) reboxetine for MDD, patients with ”strong“ LDAEP were more likely to respond to citalopram than reboxetine while patients with ”weak“ LDAEP were more likely to response to reboxetine than citalopram138 (differential predictor or moderator of response). Doubleblind, randomized clinical trials involving treatment with antidepressants of different class (ie, SSRI versus NRI) which are specifically designed to examine any potential moderating effects of LDAEP (ie, randomization based on LDAEP status would also need to occur) have yet to be conducted.
Brain functional asymmetry (dichotic listening)
Dichotic listening tasks involve auditory stimuli being presented to both the left and the right ear. Potential differences in perception (perceptual asymmetry) are then used as a proxy for brain functional asymmetry. Brader et al140 first studied the relationship between the presence of perceptual asymmetry following dichotic listening tasks at baseline and symptom improvement following treatment with the TCAs. A left-car (right hemisphere) advantage was significantly more common among nonresponders than responders. This was replicated for fluoxetine (SSRI) treatment in two different studies140,141 and bupropion (NDRI) treatment in a separate study.142
Conclusion
A number of potential clinical predictors of symptom improvement, during the pharmacologic treatment of MDD have been identified to date, mostly from studies focusing on the acute phase of treatment of MDD with the SSRIs. These include the presence of a greater number of concurrent psychiatric disorders (especially anxiety disorders), or general medical disorders (ie, cardiovascular illness, hypofolatemia).The presence of or more of these factors should alert clinicians to alter their treatment approach in order to help optimize the chances of patients recovering from depression. For instance, clinicians may chose to initiate therapy with two treatments, ie, pharmacotherapy and psychotherapy, schedule more frequent follow-up visits, increase the dose sooner in treatment nonresponders, or resort to various switching, augmentation, or combination strategies sooner for patients who do not experience a sufficient improvement in symptoms. Several potential clinical mediators of response have also been identified including the presence of severe MDD (escitalopram and duloxetine versus “older” SSRIs), anxious M..DD (bupropion versus SSRIs), atypical MDD (MAOIs versus TCAs), and hormonal status among women (venlafaxine versus “older” SSRIs). However, at the present time, such “leads” are preliminary and have not been prospectively confirmed in randomized, double-blind clinical trials. Finally, preliminary studies have identified a number of putative “biomarkers,” relating to genetic or neurophysiologic (particularly quantitative EEG (QEEG)-based measurements as well as measures of prefrontal cortical metabolism), which appear to correlate with symptom improvement during the treatment of MDD with standard antidepressants (mediators of response). Conducting further studies designed to establish reliable, replicable, and robust biological factors which function as predictors, mediators, or moderators of clinical improvement in MDD could benefit, the field in several ways, from enhancing our ability to develop more effective treatments to improving our ability to choose an individualized pharmacotherapeutic regimen for patients with MDD which would result in a more rapid and robust resolution of depressive symptoms.
Selected abbreviations and acronyms
- 5-HT
serotonin
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EEG
electroencephalogram
- HDRS
Hamilton Depression Rating Scale
- LDAEP
loudness dependence of auditory evoked potentials
- MAOI
monoamine oxidase inhibitor
- MDD
Major Depressive Disorder
- SSRI
selective serotonin reuptake inhibitor
- STAR*D
Sequenced Alternatives to Relieve Depression
- TCA
tricyclic antidepressant
Contributor Information
George I. Papakostas, Depression Clinical Research Program, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Maurizio Fava, Depression Clinical Research Program, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
REFERENCES
- 1.Kessler RC., Berglund P., Dernier O., et al The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Papakostas GI., Fava M. Does the probability of receiving placebo influence the likelihood of responding to placebo or clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Neuropsychopharmacology. 2006;31(s1):s158. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Petersen T., Papakostas GI., Posternak MA., et al Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacoi. 2005;25:336–341. doi: 10.1097/01.jcp.0000169036.40755.16. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH., Rush AJ., Wisniewski SR., et al, STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Papakostas Gl. Augmentation strategies in the treatment of MDD: examining the evidence involving the use of atypical antipsychotic agents. CNSSpectr. 2007;12(12 suppl 22):10–12. doi: 10.1017/s1092852900016023. [DOI] [PubMed] [Google Scholar]
- 6.Fava M., Bouffides E., Pava JA., McCarthy MK., Steingard RJ., Rosenbaum JF. Personality disorder comorbidity with major depression and response to fluoxetine treatment. Psychother Psychosom. 1994;62:160–167. doi: 10.1159/000288918. [DOI] [PubMed] [Google Scholar]
- 7.Fava M., Uebelacker LA., Alpert JE., Nierenberg AA., Pava JA., Rosenbaum JF. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- 8.Hirschfeld RM., Russell JM., Delgado PL., et al Predictors of response to acute treatment of chronic and double depression with sertraline or imipramine. J Clin Psychiatry. 1998;59:669–675. doi: 10.4088/jcp.v59n1205. [DOI] [PubMed] [Google Scholar]
- 9.Russell JM., Koran LM., Rush J., et al Effect of concurrent anxiety on response to sertraline and imipramine in patients with chronic depression. Depress Anxiety. 2001;13:18–27. doi: 10.1002/1520-6394(2001)13:1<18::aid-da3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Petersen T., Papakostas Gl., Bottonari K., et al NEO-FFI factor scores as predictors of response to fluoxetine treatment in depressed outpatients. Psychiatry Res. 2002;109:9–16. doi: 10.1016/s0165-1781(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 11.Demopulos C., Fava M., McLean NE., Alpert JE., Nierenberg AA., Rosenbaum JF. Hypochondriacal concerns in depressed outpatients. PsychosomatMed. 1996;58:314–320. doi: 10.1097/00006842-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Fava M., Bless E., Otto MW., Pava JA., Rosenbaum JF. Dysfunctional attitudes in major depression. Changes with pharmacotherapy. J Nerv Ment Dis. 1994;182:45–49. [PubMed] [Google Scholar]
- 13.Newman JR., Ewing SE., McColl RD., et al Tridimensional personality questionnaire and treatment response in major depressive disorder: a negative study. J Affect Disord. 2000;57:241–247. doi: 10.1016/s0165-0327(99)00046-4. [DOI] [PubMed] [Google Scholar]
- 14.losifescu DV., Nierenberg AA., Alpert JE., et al The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry. 2003;160:2122–2127. doi: 10.1176/appi.ajp.160.12.2122. [DOI] [PubMed] [Google Scholar]
- 15.Sonawalla S., Papakostas Gl., Petersen T., et al Elevated cholesterol levels in major depressive disorder associated with non-response to fluoxetine treatment. Psychosomatics. 2002;43:310–316. doi: 10.1176/appi.psy.43.4.310. [DOI] [PubMed] [Google Scholar]
- 16.Papakostas Gl., Petersen T., losifescu DV., et al Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol. 2004;7:1–5. doi: 10.1017/S1461145704004602. [DOI] [PubMed] [Google Scholar]
- 17.losifescu DV., Clementi-Craven N., Fraguas R., et al Cardiovascular risk factors may moderate pharmacological treatment effects in major depressive disorder. PsychosomatMed. 2005;67:703–706. doi: 10.1097/01.psy.0000170338.75346.d0. [DOI] [PubMed] [Google Scholar]
- 18.Papakostas Gl., losifescu DV., Renshaw PF., et al Brain MRI white matter hyperintensities, cardiovascular risk factors, and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder (Part II) Psychiatry Res: Neuroimag. 2005;140:301–307. doi: 10.1016/j.pscychresns.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Fava M., Borus JS., Alpert JE., Nierenberg AA., Rosenbaum JF., Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 20.Papakostas Gl., Petersen T., Lebowitz BD., et al. The relationship between serum folate, vitamin B12 and homocysteine levels in major depressive disorder and the timing of clinical improvement to fluoxetine. Int J Neuropsychopharmacol. 2005;8:523–528. doi: 10.1017/S1461145705005195. [DOI] [PubMed] [Google Scholar]
- 21.losifescu DV., Renshaw PF., Lyoo IK., et al Brain MRI white matter hyperintensities, cardiovascular risk factors, and treatment outcome in major depressive disorder. Br J Psychiatry. 2006;188:180–185. doi: 10.1192/bjp.188.2.180. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulos GS., Murphy CF., Gunning-Dixon FM., et al Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 23.Fava M., Labbate LA., Abraham ME., Rosenbaum JF. Hypothyroidism and hyperthyroidism in major depression revisited. J Clin Psychiatry. 1995;56:186–192. [PubMed] [Google Scholar]
- 24.Mischoulon D., Burger JK., Spillmann MK., Worthington JJ., Fava M., Alpert JE. Anemia and macrocytosis in the prediction of serum folate and vitamin B12 status, and treatment outcome in major depression. J Psychosomat Res. 2000;49:183–187. doi: 10.1016/s0022-3999(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas Gl., Petersen T., Pava J., et al Hopelessness as a predictor of non-response to fluoxetine in major depressive disorder. Ann Clin Psychiatry. 2007;19:5–8. doi: 10.1080/10401230601163451. [DOI] [PubMed] [Google Scholar]
- 26.Alexopoulos GS., Kiosses DN., Heo M., Murphy CF., Shanmugham B., Gunning-Dixon, F Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Burns RA., Lock T., Edwards DR., et al Predictors of response to aminespecific antidepressants. J Affect Disord. 1995;35:97–106. doi: 10.1016/0165-0327(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 28.Denninger JW., Papakostas Gl., Mahal Y., et al Somatic symptoms in outpatients with major depressive disorder treated with fluoxetine. Psychosomatics. 2006;47:348–352. doi: 10.1176/appi.psy.47.4.348. [DOI] [PubMed] [Google Scholar]
- 29.Papakostas Gl., Petersen T., losifescu DV., Alpert JE., Nierenberg AA., Fava M. Somatic symptoms as predictors of time to onset of response to fluoxetine in major depressive disorder. J Clin Psychiatry. 2004;5:543–546. doi: 10.4088/jcp.v65n0414. [DOI] [PubMed] [Google Scholar]
- 30.Papakostas Gl., McGrath PJ., Stewart J., et al Psychic and somatic anxiety symptoms as predictors of response to fluoxetine in major depressive disorder. Psychiatry Res. 2008;161:116–120. doi: 10.1016/j.psychres.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Nierenberg AA., McLean NE., Alpert JE., Worthington JJ., Rosenbaum JF., Fava M. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152:1500–1503. doi: 10.1176/ajp.152.10.1500. [DOI] [PubMed] [Google Scholar]
- 32.Nierenberg AA., Farabaugh AH., Alpert JE., et al Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry. 2000;157:1423–1428. doi: 10.1176/appi.ajp.157.9.1423. [DOI] [PubMed] [Google Scholar]
- 33.Alpert JE., Maddocks A., Nierenberg AA., O'Sullivan R., et al Attention deficit hyperactivity disorder in childhood among adults with major depression. Psychiatry Res. 1996;62:213–219. doi: 10.1016/0165-1781(96)02912-5. [DOI] [PubMed] [Google Scholar]
- 34.Fava M., Hoog SL., Judge RA., Kopp JB., Nilsson ME., Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. J Clin Psychopharmacoi. 2002;22:137–147. doi: 10.1097/00004714-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Simon GE., Heiligenstein JH., Grothaus L., Katon W., Revicki D. Should anxiety and insomnia influence antidepressant selection: a randomized comparison of fluoxetine and imipramine. J Clin Psychiatry. 1998;59:49–55. doi: 10.4088/jcp.v59n0202. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy SH., Andersen HF., Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31:122–131 . [PMC free article] [PubMed] [Google Scholar]
- 37.Feiger AD., Flament MF., Boyer P., Gillespie JA. Sertraline versus fluoxetine in the treatment of major depression: a combined analysis of five double-blind comparator studies. Int Clin Psychopharmacoi. 2003;18:203–210. doi: 10.1097/00004850-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Lenze EJ., Mulsant BH., Dew MA., et al Good treatment outcomes in late-life depression with comorbid anxiety. J Affect Disord. 2003;77:247–254. doi: 10.1016/s0165-0327(02)00177-5. [DOI] [PubMed] [Google Scholar]
- 39.Sir A., D'Souza RF., Uguz S., et al Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. J Clin Psychiatry. 2005;66:1312–1320. doi: 10.4088/jcp.v66n1015. [DOI] [PubMed] [Google Scholar]
- 40.Tollefson GD., Holman SL., Sayler ME., Potvin JH. Fluoxetine, placebo, and tricyclic antidepressants in major depression with and without anxious features. J Clin Psychiatry. 1994;55:50–59. [PubMed] [Google Scholar]
- 41.Cleary P., Guy W. Factor analysis of the Hamilton Depression Scale. Drugs Exp CliniRes. 1977;1:115–120. [Google Scholar]
- 42.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rush AJ., Trivedi MH., Carmody TJ., et al Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology, 2001;25:131–138. doi: 10.1016/S0893-133X(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 44.Rush AJ., Batey SR., Donahue RMJ., Ascher JA., Carmody TJ., Metz A. Does pretreatment anxiety predict response to either bupropion SR or sertraline? J Affect Disord. 2001; 64:81–87. doi: 10.1016/s0165-0327(00)00250-0. [DOI] [PubMed] [Google Scholar]
- 45.Fava M., Rush AJ., Alpert JE., et al Do Outpatients with anxious vs. nonanxious major depressive disorder have different treatment outcomes? A STAR*D Report. Am J Psychiatry. 2008;165:342–351 . doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 46.Patience DA., McGuire RJ., Scott AI., Freeman CP. The Edinburgh Primary Care Depression Study: personality disorder and outcome. Br J Psychiatry. 1995;167:324–330. doi: 10.1192/bjp.167.3.324. [DOI] [PubMed] [Google Scholar]
- 47.Reich JH. Effect of DSM-III personality disorders on outcome of tricyclic antidepressant-treated nonpsychotic outpatients with major or minor depressive disorder. Psychiatry Res. 1990;32:175–181. doi: 10.1016/0165-1781(90)90084-i. [DOI] [PubMed] [Google Scholar]
- 48.Friedman, RA, Parides, M, Baff, R, Moran, M, Kocsis, JH Predictors of response to desiprarnine in dysthymia. J Clin Psychopharmacoi. 1995;15:280–283. doi: 10.1097/00004714-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Joffe RT., Regan JJ. Personality and response to tricyclic antidepressants in depressed patients. J Nerv Ment Dis. 1989;177:745–749. doi: 10.1097/00005053-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Joyce PR., Mulder RT., Cloninger CR. Temperament predicts clomipramine and desiprarnine response in major depression. J Affect Disord. 1994;30:35–46. doi: 10.1016/0165-0327(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 51.Kocsis JH., Mason BJ., Frances AJ., Sweeney J., Mann JJ., Marin D. Prediction of response of chronic depression to imipramine. J Affect Disord. 1989;17:255–260. doi: 10.1016/0165-0327(89)90008-6. [DOI] [PubMed] [Google Scholar]
- 52.Mynors-Wallis L., Gath D. Predictors of treatment outcome for major depression in primary care. Psychol Med. 1997;27:731–736. doi: 10.1017/s0033291796004126. [DOI] [PubMed] [Google Scholar]
- 53.Papakostas Gl., Petersen T., Farabaugh A., et al Psychiatric comorbidity among responders and nonresponders to nortriptyline in treatment-resistant major depressive disorder. J Clin Psychiatry. 2003;64:1357–1361. doi: 10.4088/jcp.v64n1112. [DOI] [PubMed] [Google Scholar]
- 54.Shea MT., Pilkonis PA., Beckham E., et al Personality disorders and treatment outcome in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry. 1990;147:711–718. doi: 10.1176/ajp.147.6.711. [DOI] [PubMed] [Google Scholar]
- 55.Zuckerman DM., Prusoff BA., Weissman MM., Padian NS. Personality as a predictor of psychotherapy and pharmacotherapy outcome for depressed outpatients. J Consult Clin Psychol. 1980;48:730–735. doi: 10.1037//0022-006x.48.6.730. [DOI] [PubMed] [Google Scholar]
- 56.Peselow ED., Fieve RR., DiFiglia C. Personality traits and response to desiprarnine. J Affect Disord. 1992;24:209–216. doi: 10.1016/0165-0327(92)90105-f. [DOI] [PubMed] [Google Scholar]
- 57.Sato T., Hirano S., Narita T., et al. Temperament and character inventory dimensions as a predictor of response to antidepressant treatment in major depression. J Affect Disord. 1999;56:153–161. doi: 10.1016/s0165-0327(99)00047-6. [DOI] [PubMed] [Google Scholar]
- 58.Papakostas Gl., Petersen T., Pava J., et al Hopelessness and suicide in treatment-resistant depression: prevalence and impact on treatment outcome. J Nerv Ment Dis. 2003;191:444–449. doi: 10.1097/01.NMD.0000081591.46444.97. [DOI] [PubMed] [Google Scholar]
- 59.Papakostas Gl., Petersen T., Denninger J., et al Somatic symptoms in treatment-resistant depression. Psychiatry Res. 2003;118:39–45. doi: 10.1016/s0165-1781(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 60.Papakostas Gl., Petersen T., Sonawalla S., et al Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47:146–151. doi: 10.1159/000070584. [DOI] [PubMed] [Google Scholar]
- 61.Papakostas Gl., Petersen T., losifescu DV., et al Axis III co-morbidity in treatment-resistant depression. Psychiatry Res. 2003;118:183–188. doi: 10.1016/s0165-1781(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 62.Flint AJ., Rifat SL. Anxious depression in elderly patients. Response to antidepressant treatment. Am J Geriatr Psychiatry, 1997;5:107–115. [PubMed] [Google Scholar]
- 63.Grunhaus L., Rabin D., Greden JF. Simultaneous panic and depressive disorder: response to antidepressant treatments. J Clin Psychiatry. 1986;47:4–7. [PubMed] [Google Scholar]
- 64.Grunhaus L., Harel Y., Krugler T., Pande AC., Haskett RF. Major depressive disorder and panic disorder. Effects of comorbidity on treatment outcome with antidepressant medications. Clin Neuropharmacol. 1988;11:454–461. doi: 10.1097/00002826-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Angst J., Scheidegger P., Stabl M. Efficacy of moclobemide in different patient groups. Results of newsubscales of the Hamilton Depression Rating Scale. Clin Neuropharmacol. 1993;16 (suppl)2:S55–S62. [PubMed] [Google Scholar]
- 66.Delini-Stula A., Mikkelsen H., Angst J. Therapeutic efficacy of antidepressants in agitated anxious depression - a meta-analysis of moclobemide studies. J Affect Disord. 1995;35:21–30. doi: 10.1016/0165-0327(95)00034-k. [DOI] [PubMed] [Google Scholar]
- 67.Liebowitz MR., Quitkin FM., Stewart JW., et al Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988;45:129–137. doi: 10.1001/archpsyc.1988.01800260037004. [DOI] [PubMed] [Google Scholar]
- 68.Quitkin FM., Stewart JW., McGrath PJ., et al Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145:306–311. doi: 10.1176/ajp.145.3.306. [DOI] [PubMed] [Google Scholar]
- 69.Quitkin FM., McGrath PJ., Stewart JW., et al Atypical depression, panic attacks, and response to imipramine and phenelzine. A replication. Arch Gen Psychiatry. 1990;47:935–941. doi: 10.1001/archpsyc.1990.01810220051006. [DOI] [PubMed] [Google Scholar]
- 70.Robinson DS., Kayser A., Corcella J., Laux D., Yingling K., Howard D. Panic attacks in outpatients with depression: response to antidepressant treatment. Psychopharmacoi Bull. 1985;21:562–567. [PubMed] [Google Scholar]
- 71.Quitkin FM., Harrison W., Stewart JW., et al Response to phenelzine and imipramine in placebo nonresponders with atypical depression. A new application of the crossover design. Arch Gen Psychiatry. 1991;48:319–323. doi: 10.1001/archpsyc.1991.01810280035005. [DOI] [PubMed] [Google Scholar]
- 72.Nelson EC., Cloninger CR. The tridimensional personality questionnaire as a predictor of response to nefazodone treatment of depression. J Affect Disord. 1995;35:51–57. doi: 10.1016/0165-0327(95)00038-o. [DOI] [PubMed] [Google Scholar]
- 73.Nelson, E Cloninger., CR Exploring the TPQ as a possible predictor of antidepressant response to nefazodone in a large multi-site study. J Affect Disord. 1997;44:197–200. doi: 10.1016/s0165-0327(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 74.Rush AJ., Carmody TJ., Haight BR., Rockett CB., Zisook S. Does pretreatment insomnia or anxiety predict acute response to bupropion SR? Ann Clin Psychiatry. 2005;17:1–9. doi: 10.1080/10401230590905263. [DOI] [PubMed] [Google Scholar]
- 75.Papakostas Gl., Stahl SM., Krishen A., et al Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression) J Clin Psychiatry. 2008;69:1287–1292. doi: 10.4088/jcp.v69n0812. [DOI] [PubMed] [Google Scholar]
- 76.Davidson JR. Meoni P, Haudiquet V, Cantillon M, Hackett D. Achieving remission with venlafaxine and fluoxetine in major depression: its relationship to anxiety symptoms. Depress Anxiety. 2002;16:4–13. doi: 10.1002/da.10045. [DOI] [PubMed] [Google Scholar]
- 77.Silverstone PH., Salinas E. Efficacy of venlafaxine extended release in patients with major depressive disorder and comorbid generalized anxiety disorder. J Clin Psychiatry. 2001;62:523–529. doi: 10.4088/jcp.v62n07a04. [DOI] [PubMed] [Google Scholar]
- 78.Thase ME Entsuah R., Cantillon M Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health. 2005;14:609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- 79.Kornstein SG., Wohlreich MM., Mallinckrodt CH., Watkin JG., Stewart DE. Duloxetine efficacy for major depressive disorder in male vs. female patients: data from 7 randomized, double-blind, placebo-controlled trials. J Clin Psychiatry. 2006;67:761–770. doi: 10.4088/jcp.v67n0510. [DOI] [PubMed] [Google Scholar]
- 80.Mallinckrodt CH., Watkin JG., Liu C., Wohlreich MM., Raskin J. Duloxetine in the treatment of Major Depressive Disorder: a comparison of efficacy in patients with and without melancholic features. BioMed Central Psychiatry, 2005;5:1. doi: 10.1186/1471-244X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thase ME., Pritchett YL., Ossanna MJ., Swindle RW., Xu J., Detke MJ. Efficacy of duloxetine and selective serotonin reuptake inhibitors: comparisons as assessed by remission rates in patients with major depressive disorder. J Clin Psychopharmacoi. 2007;27:672–676. doi: 10.1097/jcp.0b013e31815a4412. [DOI] [PubMed] [Google Scholar]
- 82.Ham BJ., Lee BC., Paik JW., et al Association between the tryptophan hydroxylase-1 gene A218C polymorphism and citalopram antidepressant response in a Korean population. Progr Neuropsychopharmacol Biol Psychiatry. 2007;31:104–107. doi: 10.1016/j.pnpbp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Serretti A., Zanardi R., Rossini D., Cusin C., Lilli R., Smeraldi E. Influence of tryptophan hydroxylase and serotonin transporter genes on fluvoxamine antidepressant activity. Mol Psychiatry. 2001;6:586–592. doi: 10.1038/sj.mp.4000876. [DOI] [PubMed] [Google Scholar]
- 84.Serretti A., Zanardi R., Cusin C., Rossini D., Lorenzi C., Smeraldi E. Tryptophan hydroxylase gene associated with paroxetine antidepressant activity. Europ Neuropsychopharmacology, 2001;11:375–380. doi: 10.1016/s0924-977x(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 85.Hong CJ., Chen TJ., Yu YW., Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- 86.Kato M., Fukuda T., Wakeno M., et al Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology. 2006;53:186–195. doi: 10.1159/000094727. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida K., Naito S., Takahashi H., et al Monoamine oxidase: A gene polymorphism, tryptophan hydroxylase gene polymorphism and antidepressant response to fluvoxamine in Japanese patients with major depressive disorder. Progr Neuropsychopharmacol Biol Psychiatry. 2002;26:1279–1283. doi: 10.1016/s0278-5846(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 88.Arias B., Catalan R., Gasto C., Gutierrez B., Fananas L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacoi. 2003;23:563–567. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- 89.Durham LK., Webb SM., Milos PM., Clary CM., Seymour AB. The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology. 2004;174:525–529. doi: 10.1007/s00213-003-1562-3. [DOI] [PubMed] [Google Scholar]
- 90.Bozina N., Peles AM., Sagud M., Bilusic H., Jakovljevic M. Association study of paroxetine therapeutic response with SERT gene polymorphisms in patients with major depressive disorder. World J Biol Psychiatry. 2008;9:190–197. doi: 10.1080/15622970701308397. [DOI] [PubMed] [Google Scholar]
- 91.Kato M., Wakeno M., Okugawa G., Fukuda T., Azuma J., Kinoshita T., Serretti A. No association of TPH1 218A/C polymorphism with treatment response and intolerance to SSRIs in Japanese patients with major depression. Neuropsychobiology. 2007;56:167–171. doi: 10.1159/000119734. [DOI] [PubMed] [Google Scholar]
- 92.Kronenberg S., Apter A., Brent D. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolescent Psychopharmacoi. 2007;17:741–750. doi: 10.1089/cap.2006.0144. [DOI] [PubMed] [Google Scholar]
- 93.Ng CH., Easteal S., Tan S., Schweitzer I., Ho BK., Aziz S. Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Progr Neuropsychopharmacol Biol Psychiatry. 2006;30:953–957. doi: 10.1016/j.pnpbp.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 94.Rausch JL., Hobby HM., Shendarkar N., Johnson ME., Li J. Fluvoxamine treatment of mixed anxiety and depression: evidence for serotonergically mediated anxiolysis. Clin Psychopharmacoi. 2001;21:139–142. doi: 10.1097/00004714-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Smeraldi E., Zanardi R., Benedetti F., Di Bella D., Perez J., Catalane M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 96.Yu YW., Tsai SJ., Chen TJ., Lin CH., Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- 97.Zanardi R., Benedetti F., Di Bella D., Catalano M., Smeraldi E. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacoi. 2000;20:105–107. doi: 10.1097/00004714-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 98.Zanardi R., Serretti A., Rossini D., et al. Factors affecting fluvoxamine antidepressant activity: influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry. 2001;50:323–330. doi: 10.1016/s0006-3223(01)01118-0. [DOI] [PubMed] [Google Scholar]
- 99.Dmitrzak-Weglarz M., Rajewska-Rager A., Gattner K., et al Association studies of 5HT2Â and 5HTTLPR polymorphisms and drug response in depression patients. Eur Neuropsychopharmacol. 2007;17(s4):S238. [Google Scholar]
- 100.Kim D.K., Lim S.W., Lee S., et al Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000;11:215–219. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- 101.Kraft JB., Slager SL., McGrath PJ., Hamilton SP. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry. 2005;58:374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 102.Minov C., Baghai TC., Schule C., et al Serotonin-2A-receptor and -transporter polymorphisms: lack of association in patients with major depression. Neurosci Lett. 2001;303:119–122. doi: 10.1016/s0304-3940(01)01704-9. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida K., Ito K., Sato K., et al Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Progr Neuropsychopharmacol Biol Psychiatry. 2002;26:383–386. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 104.Serretti A., Cusin C., Rausch JL., Bondy B., Smeraldi E. Pooling pharmacogenetic studies on the serotonin transporter: a mega-analysis. Psychiatry Res. 2006;145:61–65. doi: 10.1016/j.psychres.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 105.Serretti A., Kato M., De Ronchi D., Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 106.Smlts KM., Smits LJ., Schouten JS., Stelrna FF., Nelemans P., Prins MH. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: a systematic review. Mol Psychiatry, 2004;9:433–441. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- 107.Kraft JB., Peters EJ., Slager SL., et al Analysis of association between the serotonin transporter and antidepressant response in a large clinical sample. Biol Psychiatry. 2007;61:734–742. doi: 10.1016/j.biopsych.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 108.Hu XZ., Rush AJ., Charney D., et al Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 109.Choi MJ., Kang RH., Ham BJ., Jeong HY., Lee MS. Serotonin receptor 2A gene polymorphism (-1438A/G) and short-term treatment response to citalopram. Neuropsychobiology. 2005;52:155–162. doi: 10.1159/000087847. [DOI] [PubMed] [Google Scholar]
- 110.Sato K., Yoshida K., Takahashi H., et al Association between -1438G/A promoter polymorphism in the 5-HT(2A) receptor gene and fluvoxamine response in Japanese patients with major depressive disorder. Neuropsychobiology. 2002;46:136–140. doi: 10.1159/000066394. [DOI] [PubMed] [Google Scholar]
- 111.McMahon FJ., Buervenich S., Charney D., et al Variation in the gene encoding the serotonin 2Â receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perlis RH., Moorjani P., Eagerness J., et al Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D Study. Neuropsychopharmacol. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paddock S., Laje G., Charney D., et al Association of GRIK4 with Outcome of Antidepressant Treatment in the STAR*D Cohort. Am J Psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 114.Lekman M., Laje G., Charney D., et al The FKBP5-gene in depression and treatment response-an association study in the sequenced treatment alternatives to relieve depression (STAR*D) cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsapakis EM., Checkley S., Kerwin RW., Aitchison KJ. Association between the serotonin transporter linked polymorphic region gene (5HTTLPR) and response to tricyclic antidepressants (TCAs) Eur Neuropsychopharmacol. 2003;13:S250. [Google Scholar]
- 116.Choi TK., Koo MS., Lee SH., Seok JH., Kirn SJ. Serotonin transporter gene polymorphism associated with venlafaxine treatment response. Eur Neuropsychopharmacol. 2007;17(s4):S228. [Google Scholar]
- 117.Kang RH., Wong ML., Choi MJ., Paik JW., Lee MS. Association study of the serotonin transporter promoter polymorphism and mirtazapine antidepressant response in major depressive disorder. Progr Neuropsychopharmacol Biol Psychiatry. 2007;31:1317–1321. doi: 10.1016/j.pnpbp.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 118.Muller DJ., Schulze TG., Macciardi F., et al Moclobemide response in depressed patients: association study with a functional polymorphism in the monoamine oxidase A promoter. Pharmacopsychiatry, 2002;35:157–158. doi: 10.1055/s-2002-33199. [DOI] [PubMed] [Google Scholar]
- 119.Joyce PR., Mulder RT., Luty SE., et al Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol. 2003;6:339–346. doi: 10.1017/S1461145703003663. [DOI] [PubMed] [Google Scholar]
- 120.Szegedi A., Rujescu D., Tadic A., et al The catechol-O-methyltransferase Val108/158Met polymorphism affects short-term treatment response to mirtazapine, but not to paroxetine in major depression. Pharmacogenomics J. 2005;5:49–53. doi: 10.1038/sj.tpj.6500289. [DOI] [PubMed] [Google Scholar]
- 121.Mayberg HS., Silva JA., Brannan SK., et al The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 122.Mayberg HS., Brannan SK., Tekell JL., et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 123.losifescu DV., Bob NR., Nierenberg AA., Jensen JE., Fava M., Renshaw, PF Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 124.Little JT., Ketter TA., Kimbrell TA., et al Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol Psychiatry. 2005;57:220–228. doi: 10.1016/j.biopsych.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 125.Hunter AM., Cook IA., Leuchter AF. The promise of the quantitative electroencephalogram as a predictor of antidepressant treatment outcomes in major depressive disorder. Psychiatr Clin N Am. 2007;30:105–124. doi: 10.1016/j.psc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 126.Leuchter AF., Cook IA., Lufkin RB., et al Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neurolmage. 1994;1:208–219. doi: 10.1006/nimg.1994.1006. [DOI] [PubMed] [Google Scholar]
- 127.Bares M., Brunovsky M., Kopecek M., et al Changes in QEEG prefrontal cordance as a predictor of response to antidepressants in patients with treatment resistant depressive disorder: a pilot study. J Psych Res. 2007;41:319–325. doi: 10.1016/j.jpsychires.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 128.Cook IA., Leuchter AF., Morgan M., et al Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacol. 2002;27:120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 129.Cook IA., Leuchter AF., Morgan ML., Stubbeman W., Slegman B., Abrams M. Changes in prefrontal activity characterize clinical response in SSRI nonresponders: a pilot study. J Psych Res. 2005;39:461–466. doi: 10.1016/j.jpsychires.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 130.Leuchter AF., Cook IA., Witte EA., Morgan M., Abrams M. Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 131.Hunter AM., Leuchter AF., Morgan ML., Cook IA. Changes in brain function (quantitative EEG cordance) during placebo lead-in and treatment outcomes in clinical trials for major depression. Am J Psychiatry. 2006;163:1426–1432. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- 132.Hegerl U., Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- 133.Galllnat J., Bottlender R., Juckel G., et al The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl). 2000;148:404–411. doi: 10.1007/s002130050070. [DOI] [PubMed] [Google Scholar]
- 134.Linka T., Millier BW., Bender S., Sartory G. The intensity dependence of the auditory evoked N1 component as a predictor of response to Citalopram treatment in patients with major depression. Neurosci Lett. 2004;367:375–378. doi: 10.1016/j.neulet.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 135.Mulert C., Juckel G., Augustin H., Hegerl U. Comparison between the analysis of the loudness dependency of the auditory N1/P2 component with LORETA and dipole source analysis in the prediction of treatment response to the selective serotonin reuptake inhibitor citalopram in major depression. Clin Neurophysiol. 2002;113:1566–1572. doi: 10.1016/s1388-2457(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 136.Mulert C., Juckel G., Brunnmeier M., et al Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 137.Paige SR., Fitzpatrick DF., Kline JP., Balogh SE., Hendricks SE. Event-related potential amplitude/intensity slopes predict response to antidepressants. Neuropsychobiology. 1994;30:197–201. doi: 10.1159/000119161. [DOI] [PubMed] [Google Scholar]
- 138.Juckel G., Pogarell O., Augustin H., et al Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J Clin Psychiatry. 2007;68:1206–1212. doi: 10.4088/jcp.v68n0806. [DOI] [PubMed] [Google Scholar]
- 139.Bruder GE., Stewart JW., Voglmaier MM., et al Cerebral laterality and depression: relations of perceptual asymmetry to outcome of treatment with tricyclic antidepressants. Neuropsychopharmacology. 1990;3:1–10. [PubMed] [Google Scholar]
- 140.Bruder GE., Otto MW., McGrath PJ., et al Dichotic listening before and after fluoxetine treatment for major depression: relations of laterality to therapeutic response. Neuropsychopharmacology, 1996;15:171–179. doi: 10.1016/0893-133X(95)00180-L. [DOI] [PubMed] [Google Scholar]
- 141.Bruder GE., Stewart JW., McGrath PJ., Deliyannides D., Quitkin FM. Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neuropsychopharmacology, 2004;29:1752–1761. doi: 10.1038/sj.npp.1300519. [DOI] [PubMed] [Google Scholar]
- 142.Brader GE., Stewart JW., Schaller JD., McGrath PJ. Predicting therapeutic response to secondary treatment with bupropion: Dichotic listening tests of functional brain asymmetry. Psychiatry Res. 2007;153:137–143. doi: 10.1016/j.psychres.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]