Abstract
The concept that estrogens exert important neuroprotective actions has gained considerable attention during the past decade. Numerous studies have provided a deep understanding of the seemingly contradictory actions of estrogens. We realize more than ever that the effects of estrogens (with and without simultaneous or sequential progestins) are diverse and sometimes opposite, depending on the use of different estrogenic and progestinic compounds, on different delivery routes, on different concentrations, on treatment sequence, and on the age and health status of the women who receive hormone therapy. During the past few years, we have gained an increasing appreciation of the impact of estrogens on the immune system and on inflammation. In addition, we have learned that estrogens cannot only protect against cell death, but can also stimulate the birth of new neurons. Here we posit the concept that estrogen's modulation of the immune status may be the basic mechanism that underlies its ability to protect against neurodegeneration and its powerful neuroregenerative actions. We hope that this update will encourage even richer dialogues between basic and clinical scientists to ensure that future clinical studies fully consider the information that can be derived from basic science studies. Only then will we have a better understanding of the impact of hormones on the menopausal and postmenopausal period in a woman's life.
Keywords: estradiol, stroke, estrogen therapy, menopause, brain injury, cell death, neuroprotection, neurogenesis, inflammation, immune system, animal model
Abstract
Durante la última década el concepto de que los estrógenos ejercen importantes acciones neuroprotectoras ha ganado considerable atención. Hay numerosos estudios que ban proporcionado una comprensión profunda de las acciones aparentemente contradiciorias de los estrógenos. Actualmente se comprende más que nunca que los efectos de los estrógenos (con y sin progestinas simultáneas o en secuencia) son diversos y algunas veces opuestos. Estos efectos dependen del uso de diferentes compuestos estrogénicos y progestínicos, de las variadas vías de administración, de las diversas concentraciones, de la secuencia de tratamiento y de la edad y esiado de salud de la mujer que recibe la ierapia hormonal. Durante los últimos años, se ha alcanzado una mayor comprensión acerca del impacio de los estrógenos en el sisiema inmune y en la inflamación. Además, se sabe que los estrógenos no sólo pueden proteger contra la muerte celular, sino que también pueden estimular el nacimiento de nuevas neuronas. Se propone que el concepto de la modulación que tienen los estrógenos sobre el sistema inmune puede ser el mecanismo básico que subyace a su capacidad de protección contra la neurodegeneración y sus poderosas acciones neurorregenerativas. Se espera que esta actualización fomente los enriquecedores diálogos entre los cientistas básicos y los clínicos para asegurar que los futuros estudios clínicos consideren muy bien la información que pueda derivasse de estudios de ciencia básica. Sólo entonces se tendrá una mejor comprensión del impacto de las hormonas en el período menopáusico y posimenopáusico en la vida de la mujer.
Abstract
Ces 10 dernières années, l'idée d'une action neuroprotectrice importante des oestrogènes a retenu particulièrement l'attention. Les actions apparemment contradictoires de ces hormones sont nettement mieux comprises grâce aux nombreuses études cliniques. Nous réalisons plus que jamais que leurs effets (avec ou sans progestatif associé de façon simultanée ou séquentielle) sont variés et parfois opposés, dépendant de l'utilisation des différents composés oestrogéniques ou progestatifs, des différents modes d'administration et concentrations, de la chronologie des traitements, et enfin de l'âge et de l'état de santé des femmes qui reçoivent le traitement hormonal. Ces dernières années, l'appréciation de l'impact des oestrogènes sur le système immunitaire et l'inflammation s'est considérablement étendue. Nous avons appris non seulement qu'ils protégeaient les cellules de l'apopiose, mais qu'ils stimulaient également la production de nouveaux neurones. Nous postulons dans cet article que la modulation oestrogénique de l'état immunitaire pourrait être le mécanisme de base qui sous-tend sa capacité protectrice contre la neurodégénération et sa puissante activité neurorégénératrice. Nous espérons que cette mise à jour encouragera un dialogue plus riche entre des scientifiques cliniciens et fondamentalistes pour s'assurer que les études cliniques futures prendront complètement en compte l'information provenant de la recherche fondamentale. C'est seulement alors que nous comprendrons l'impact des hormones sur la période de ménopause et de post-ménopause dans la vie d'une femme.
In 2002 we contributed an article to Dialogues in Clinical Neuroscience which discussed the neuroprotective actions of estrogens.1 In this review we build on the understanding we had at that point, and will discuss the importance of the accumulating data that point to the complexities of estrogen action. Clearly, many factors, including the type of estrogen used, the dose and route of administration, and the age and previous hormonal and health status of the women being treated, must be taken into consideration when designing clinical studies and when interpreting results.
Women usually undergo the menopausal transition when they are about 51 years of age. This dramatic physiological change, which may be abrupt or may occur over a period of a few years, is marked by a decline in ovarian hormone secretion and cessation of reproductive fertility. The postmenopausal period is often associated with vaginal dryness, urinary symptoms, osteoporosis, and a panel of neurological manifestations such as hot flushes, and greater instances of sleep disturbance, emotional instability, cerebrovascular stroke, and Alzheimer's disease. Over the past century the average life expectancy in the United States has increased to over 80 years, while the age of menopause has remained fixed at age 51. Thus, today, more than ever before, a greater number of women and a larger proportion of women are destined to spend over three decades of their lives in a hypoestrogenic state. This makes it imperative that we understand the intricacies of estrogen action, when it is protective, and when it increases risk.
Estrogens and progestins
Estradiol and progesterone belong to a family of steroid hormones with complex actions. Estradiol-17β (E2), the predominant and most biologically active estrogen, is an 18 carbon (C-18) steroid with an aromatic A-ring. It is synthesized mainly by the ovary; however, other organs and tissues, including adipose tissue, the brain (neurons, astrocytes, and microglia), cells of the immune system, and bone, are thought to produce it as well. Progesterone is a C-21 steroid hormone, which is not only an active hormone in and of itself, but is also a precursor to estrogens. In addition to the estrogens and progestins produced in human tissues, a wide variety of estrogenic and progestogenic compounds are synthesized in other species or are pharmacologically manufactured through pathways that have been developed by researchers and have been used widely by the pharmaceutical industry. When used in hormone therapy for postmenopausal women, the actions of these different compounds are diverse, complex, and sometimes contradictory. Their effects depend upon the concentration, whether they are given simultaneously or sequentially, the route of delivery, and on the age and health status of the women who receive hormone therapy. Turgeon et aP have provided a detailed review of our current understanding of estrogens, progestogens, their related compounds, agonists, and antagonists.
Estrogens and stroke
Stroke is the third leading cause of death for middleaged and older women and a major health problem that affects 500 000 Americans each year.3 Every year approximately 40 000 more women than men are affected by stroke.4 Initially, this gender difference was thought to be explained by a combination of both the longer life expectancy of women and the protective roles of estrogen, since the incidence of stroke increases after menopause and the risk continues to rise with age.5 However, this interpretation has been questioned since recent clinical trials including the Women's Health initiative (WHI) reported negative impact of estrogen therapy (ET)4,6-8 and some studies in animal models also suggest that estrogens are not universally protective and can be deleterious under some circumstances.9 In an attempt to reconcile these seemingly contradictory data, our lab has used animal models to explore the mechanisms of estrogen's neuroprotective and neuroregenerative actions.
Estrogens and stroke: use of animal models to decipher mechanisms of action
Even the best, well-designed clinical studies cannot benefit from the experimental advantages of many basic science studies, since studies performed with experimental animal models allow replication with adequate numbers of animals, controls with equivalent genetic backgrounds and previous exposure to similar environments, wellcontrolled environments during the entire study, and lack of selection or recall bias. Thus, investigators have developed several animal models to investigate the pathophysiology and potential treatments for stroke. Since most cerebrovascular strokes (>70%) in aging human populations are ischemic, and not hemorrhagic, we adopted an animal model that reproduces ischemic infarcts. We have utilized permanent middle cerebral artery occlusion (MCAO) as a model of permanent occlusion of the middle cerebral artery, which vascularizes the cerebral cortex, the striatum, and the hippocampus, to examine the effects of estrogen in neurodegeneration. Blockade of this artery at its base results in about a 50% decrease in blood flow and causes severe metabolic impairment in a core region, called the “ischemic core” and many neurons in these regions die by necrosis within hours following injury. In contrast, regions that surround the ischemic core, the ischemic penumbra, undergo more moderate metabolic impairment and are potentially salvageable by effective therapeutic agents. We have previously demonstrated that E2 exerts powerful neuroprotective action in ischemic penumbra where E2 protects neurons from delayed programmed cell death or apoptosis.10-12
Studies in the early 1990s suggested that estradiol is a neuroprotective factor that profoundly attenuates the degree of ischemic brain injury: i) female gerbils demonstrate less neuronal pathology than males after ischemia induced by unilateral carotid artery occlusion; ii) female rats sustain over 50% less infarction than males and ovariectomized female rats following ischemia induced by transient occlusion of the middle cerebral artery (MCA); iii) both ovariectomized females and castrated males that are treated with estradiol suffer less MCAO-induced injury than vehicle-treated gonadecetomized controls.1 Our work has contributed significantly to the understanding of the neuroprotective actions of physiological levels of E2. We have shown that low, physiological levels of E2, exert profound neuroprotective actions after MCAO.10-13 We have also demonstrated that E2 effectively reduces the infarct volume in middle-aged animals, suggesting that a constellation of factors responsible for mediating E2's protective actions is preserved during the initial stages of aging.14 Further, we have found that E2 must be administered prior to the onset of injury, since acute administration of E, at the time of injury does not reduce the extent of infarction.15 Collectively, the results of these studies suggest that postmenopausal women who are estrogenreplaced may suffer a decreased degree of brain injury following a stroke, compared with their hypoestrogenic counterparts. However, we must be careful in extrapolating from rodents to humans until the appropriate clinical studies are performed.
Estrogen receptor α mediates estrogen's neuroprotective actions
To date, two forms of nuclear estrogen receptor (ER) have been cloned. Since the discovery of the second form of ER (ERβ) in 1996, researchers have investigated the similarities and differences in the distribution and actions of these two forms of ER. Recently, we found that ischemic injury upregulates ERα expression in the cortex of ovariectomized animals without influencing ERβ expression.16 Consequently, we hypothesized that the dramatic re-expression of ERα after stroke injury mediates E2's profound neuroprotection against ischemia.17,18 Using ERα knockout mice, we found that the presence of this receptor subtype is a prerequisite for the ability of E2 to exert protective action against ischemic injury.18 Taken together, the injured brain seems to provide signals conveying the need for the reappearance of ERα, which may mediate the ability of E2 to protect against neuronal apoptosis and possibly reinitiate differentiation of the injured brain.
Anti-inflammatory actions of estrogens
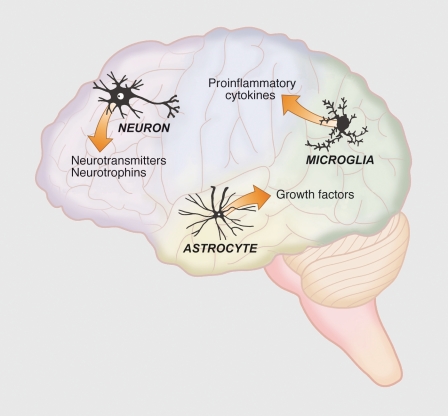
More recently we have begun to appreciate the importance of inflammation in neurodegeneration and the role of E2 acting as an anti-inflammatory factor. Many neurological disorders such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, and cerebrovascular stroke include inflammation processes in the underlying mechanisms of the disorder.19 In vivo and in vitro models of brain injury and neurodegenerative diseases have provided substantial evidence that physiological levels of E2 suppress inflammation through ERα and ERβ (reviewed in refs 19-22). These studies demonstrate that estrogens act not only on neurons and astrocytes, but also on microglia, the resident macrophages of the brain (Figure 1). These studies also highlight the tremendous importance of understanding the crosstalk between the nervous, endocrine, and immune systems to fully appreciate the protective role of E2 during neurological diseases and injury.19,23
Figure 1. Overview of the brain cell types and neuromodulators influenced by estrogens. The ability of estrogens to exert trophic and protective actions depends upon their ability to alter the birth and death of neurons, synaptogenesis, and neuritogenesis. Estradiol influences neurons, astrocytes, and microglia through altering the expression of a broad profile of neurotransmitters and neuropeptides and their receptors, pro- and anti-inflammatory agents, and factors which influence, birth, survival, growth, and maturation of neurons.
The inflammatory response associated with stroke is complex, but an accumulating body of evidence clearly shows that estrogens may directly or indirectly regulate three components of the inflammatory response: i) microglial activation; ii) activation of the enzyme; inducible nitric oxide synthase (iNOS); and iii) the activation of cytokines/chemokines. These components of inflammation may interact with each other and are not mutually exclusive. Microglia become activated in response to injury, proliferate, migrate to the site of injury, and change in both morphology and cell surface markers. E2 suppresses microglial activation, and this response is regulated by both estrogen receptors. Microglia, peripheral infiltrating macrophages, and astrocytes are the primary source of the iNOS enzyme during stroke. Activation of iNOS during stroke produces high, concentrated levels of nitric oxide that promote neuronal cell death. Many studies have shown that E2 suppresses iNOS in animal models of neuroinflammation, stroke, and Alzheimer's disease, and this response is also regulated by both estrogen receptors.19,24,25
Cytokines are secreted proteins that appear to play a critical role in the pathophysiology of human cerebral ischemia. There is a positive correlation between high levels of proinflammatory cytokines in serum or cerebrospinal fluid greater stroke severity. These cytokines include: interleukin 6 (IL-6), IL-1β, tumor necrosis factor alpha (TNF-α), and macrophage chemoattractant protein-1 (MCP-1). Conversely, increased levels of antiinflammatory cytokines (eg, IL-10) correlate with diminished stroke severity and an improved outcome. Cytokines in the brain perform pleiotropic functions in inflammation and are synthesized primarily by microglia and astrocytes, but also can be produced by neurons.26,27 In several different brain injury paradigms, subcutaneous E2 generally suppresses proinflammatory cytokines, and enhances the production anti-inflammatory cytokines.16-27 Studies from our laboratory have shown that immediate treatment with E2 following MCAO suppresses brain levels of the proinflammatory cytokines IL-6 and MCP1.16
Estradiol and neurogenesis
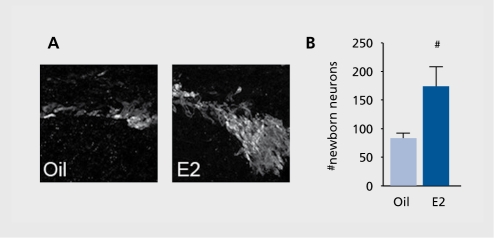
One of the most remarkable discoveries in modern neuroscience is that the adult brain continues to generate new neurons under both normal and neurodegenerative conditions. We have explored whether E2 stimulates generation of newborn neurons after stroke. We have found that low, physiological levels of E2 increase the number of newborn neurons (Figure 2). Interestingly, both ERa and ERp play essential functional roles, and the presence of both receptor forms is the prerequisite for E2 to enhance neurogenesis. Although precise roles for each ER form are yet to be determined, our study clearly demonstrates that the presence of both receptors is important in expansion of neuronal populations in the subventricular zone after ischemic injury.28
Figure 2. Estradiol influences the number of newborn neurons. Panel A shows confocal micrographs of newborn neurons dual-labeled with bromodeoxyuridine and doublecortin in vehicle and estradiol-treated mice following stroke injury. Panel B shows the mean of groups of 4 to 6 animals in each experimental group and shows that the differences are statistically significant.
So far, we have not been able to determine whether these newborn neurons actually differentiate into mature neurons at this time point, whether they migrate to the site of injury, and whether they undergo synapse formation with neighboring neurons. These future studies will allow us to determine whether this elevated level of neurogenesis is critical to the recovery of function.
Importance of timing of estrogen therapy
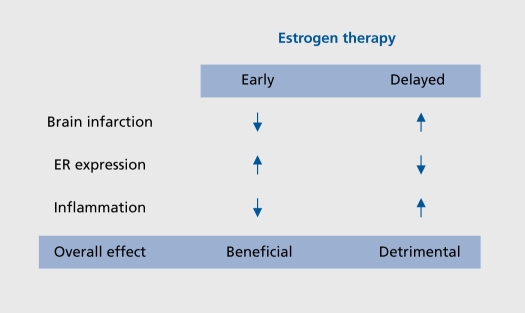
Recent studies describing the seemingly contradictory actions of estrogens in stroke injury have led us to believe that the timing of estrogen therapy relative to the time of the menopause may be an important factor to consider. It is important to remember that in the WHI, the mean age of the subjects was 63 years, and thus, the majority of subjects were 12 years past the perimenopausal transition prior to the initiation of any hormone treatment.5-29 We tested the hypothesis that an extended period of hypoestrogenicity both prevents E2 from protecting the brain against ischemia, and simultaneously suppresses its anti-inflammatory actions. We found that E2 exerts profound neuroprotective action when administered immediately upon ovariectomy, but not when administered after 10 weeks of hypoestrogenicity. This dichotomous action is due to differential actions that estradiol has when administered immediately versus when treatment is initiated after a delay (Figure 3). Consistently, E2 treatment given immediately at the time of ovariectomy attenuated central and peripheral production of proinflammatory cytokines after ischemic stroke. In contrast, E2 did not suppress production of proinflammatory molecules when it was administered 10 weeks postovariectomy.16 These results demonstrate that a prolonged period of hypoestrogenicity disrupts both neuroprotective and anti-inflammatory actions of E2. Our findings may help to explain the results of the WHI that reported no beneficial effect of ET against stroke because the majority of the subjects initiated ET after an extended period of hypoestrogenicity.
Figure 3. Estradiol protects the brain only if treatment is initiated immediately after hypoestrogenicity is induced. Estradiol decreases the size of the infarct, induces estrogen receptor (ER) and suppresses inflammation only if it is administered immediately after ovariectomy. We have used ovariectomy to mimic the menopause. These findings strongly suggest that, if estrogen therapy (ET) is initiated after several years of postmenopause, as was the case in the Womens' Health Initiative, that ET will not be effective in protecting the brain against neurodegeneration.
Summary
We have summarized recent studies that have increased our understanding of the complex actions of estrogens on the brain. These basic science and clinical studies give us a new appreciation of the breadth of estrogen actions in the adult brain to maintain function after injury or during disease. Much more work is necessary before we fully understand the many ways through which estrogens exert beneficial actions, but it is clear that estradiol protects the brain from injury and enhances neurogenesis by acting to both enhance survival of neurons and stimulate the birth of new neurons, respectively. Estradiol's anti-inflammatory actions may underpin both the protective and reparative effects. We hope that our growing knowledge of the pleiotropic actions of this hormone will lead to preventative and restorative therapies for neurodegenerative conditions, which will, in turn improve the lives of our aging population.
Acknowledgments
This work was supported by the NIH: AG02224 (PMW) and NRSAAG27614 (CMB).
Contributor Information
Phyllis M. Wise, Departments of Physiology and Biophysics ; Biology ; and Obstetrics and Gynecology, University of Washington, Seattle, Washington, USA.
Shotaro Suzuki, Departments of Physiology and Biophysics.
Candice M. Brown, Departments of Physiology and Biophysics.
REFERENCES
- 1.Dubal DB., Wise PM. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues Clin Neurosci. 2002;4:149–161. doi: 10.31887/DCNS.2002.4.2/ddubal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turgeon JL., Carr MC., Maki PM., Mendelsohn ME., Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 3.Paganini-Hill A. Estrogen replacement therapy and stroke. Prog Cardiovasc Dis. 1995;38:223–242. doi: 10.1016/s0033-0620(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell CD. Hormone replacement therapy and stroke: the current state of knowledge and directions for future research. Semin Neurol. 2006;26:123–130. doi: 10.1055/s-2006-933316. [DOI] [PubMed] [Google Scholar]
- 5.Mitka M. Studies explore stroke's gender gap. JAMA. 2006;295:1755–1756. doi: 10.1001/jama.295.15.1755. [DOI] [PubMed] [Google Scholar]
- 6.Nordell VL., Scarborough MM., Buchanan AK., Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 7.Sohrabji F., Bake S. Age-related changes in neuroprotection: is estrogen pro-inflammatory for the reproductive senescent brain? Endocrine. 2006;29:191–197. doi: 10.1385/ENDO:29:2:191. [DOI] [PubMed] [Google Scholar]
- 8.Viscoli CM., Brass LM., Kernan WN., Sarrel PM., Suissa S., Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 9.Bushnell CD., Hum P., Colton C., et al. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke. 2006;37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 10.Prewitt AK., Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Res. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solum DT., Handa RJ. Localization of estrogen receptor alpha (ER[alpha]) in pyramidal neurons of the developing rat hippocampus. Dev Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 12.Shughrue P., Stumpf W., Maclusky N., Zielinski N., Hochberg R. Developmental changes in estrogen receptors in mouse cerebral cortex between birth and postweaning: studied by autoradiography with 11 beta-methoxy-16 alpha-[125l]iodoestradiol. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME., Liu Y., Wise PM. Estradiol enhances Akt activation in cortical expiant cultures following neuronal injury. Mol Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- 14.Bryant D., Shedahl L., Marriott L., Shapiro R., Dorsa D. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 15.Dubai DB., Kashon M., Pettigrew L., et al. Estradiol protects against ischem ic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S., Brown CM., Dela Cruz CD., Yang E., Bridwell DA., Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubal DB., Shughrue PJ., Wilson ME., Merchenthaler I., Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubal DB., Zhu H., Yu J., et al. Estrogen receptor alpha, not beta is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad SciUSA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vegeto E., Benedusi V., Maggi A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 21.Morissette M., Le Saux M., D'Astous M., et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Dhandapani KM., Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Butts CL., Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cell Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vegeto E., Belcredito S., Etteri S., et al. Estrogen receptor-a mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vegeto E., Belcredito S., Ghisletti S., Meda C., Etteri S., Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147:2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 26.Kadhim HJ., Duchateau J., Sebire G. Cytokines and brain injury: invited review. J intensive Care Med. 2008;23:236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- 27.Czlonkowska A., Ciesielska A., Gromadzka G., Kurkowska-Jastrzebska I. Estrogen and cytokines production - the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11:1017–1030. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S., Gerhold LM., Bottner M., et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 29.McCullough LD., Hum PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]