Abstract
Current treatment of Major Depressive Disorder utilizes a trial-and-error sequential treatment strategy that results in delays in achieving response and remission for a majority of patients. Protracted ineffective treatment prolongs patient suffering and increases health care costs. In addition, long and unsuccessful antidepressant trials may diminish patient expectations, reinforce negative cognitions, and condition patients not to respond during subsequent antidepressant trials, thus contributing to further treatment resistance. For these reasons, it is critical to identify reliable predictors of antidepressant treatment response that can be used to shorten or eliminate lengthy and ineffective trials. Research on possible endophenotypic as well as genomic predictors has not yet yielded reliable predictors. The most reliable predictors identified thus far are symptomatic and physiologic characteristics of patients that emerge early in the course of treatment. We propose here the term “response endophenotypes” (REs) to describe this class of predictors, defined as latent measurable symptomatic or neurobiologie responses of individual patients that emerge early in the course of treatment, and which carry strong predictive power for individual patient outcomes. Use of REs constitutes a new paradigm in which medication treatment trials that are likely to be ineffective could be stopped within 1 to 2 weeks and other medication more likely to be effective could be started. Data presented here suggest that early changes in symptoms, quantitative electroencephalography, and gene expression could be used to construct effective REs. We posit that this new paradigm could lead to earlier recovery from depressive illness and ultimately produce profound health and economic benefits.
Keywords: major depression, antidepressant medication, predicting treatment response, antidepressant treatment response (ATR) index, response endophenotype
Abstract
El tratamiento actual del trastorno depresivo mayor emplea una estrategia terapéutica secuencial de ensayo-error que se traduce en demoras para alcanzar la respuesta y remisíón para la mayoría de los pacientes. El tratamiento ineficaz prolongado alarga el sufrimiento del paciente y aumenta los costos de salud, Además, los ensayos prolongados e ineficaces con antidepresivos pueden disminuir las expectativas del paciente, reforzar las cogniciones negativas y condicionar a los pacientes a no responder durante los siguientes ensayos con antidepresivos, contribuyendo así a una resistencia a posteriores tratamientos, Por estas razones, es fundamental identificar predictores confiables de la respuesta al tratamiento antidepresivo que puedan utilizarse para abreviar o eliminar los ensayos prolongados e ineficaces. La investigación tanto de posibles endofenotipos como de predictores genómicos aun no ha entregado predictores confiables. Los predictores más confiables que se han identificado hasta ahora son ciertas características sintomáticas y fisiológicas de los pacientes, las que aparecen precozmente durante el curso del tratamiento, Aquí se propone el término “respuesta endofenotípica (RE)” para describir esta clase de predictores, definidos como respuestas precoces y latentes tanto sintomáticas como neurobiológicas que se pueden medir en cada paciente y que tienen un alto poder predictor para la evolución clínica individual. El empleo de la RE constituye un nuevo paradigma para los ensayos de tratamientos medicamentosos que tengan una alta probabilidad de ser inefectivos, ya que éstos podrían ser suspendidos dentro de una o dos semanas para dar inicio a otra medicación con mayor probabilidad de ser eficaz. Los datos aquí presentados sugieren que los cambios precoces en los síntomas, en la electroencefalografía cuantitativa y en la expresión génica podrían ser utilizados para construir REs efectivas. Se postula que este nuevo paradigma podría llevar a recuperaciones más precoces de la enfermedad depresiva y a la larga producir marcados beneficios de salud y económicos.
Abstract
Une stratégie thérapeutique séquentielle par essais/erreurs est actuellement utilisée dans le traitement du trouble dépressif majeur entraînant une réponse et une rémission retardées pour la majorité des patients. Un traitement inefficace prolongé allonge la souffrance du patient et augmente les coûts des soins de santé. De plus, des séquences thérapeutiques longues et infructueuses par antidépresseurs contribuent à diminuer les attentes des patients, à renforcer les opinions négatives et conditionnent les patients à ne pas répondre au cours des traitements ultérieurs, contribuant ainsi à une résistance. Il est donc crucial pour ces raisons d'identifier des prédicteurs fiables de la réponse au traitement antidépresseur, pour raccourcir ou éliminer les séquences thérapeutiques très longues et inefficaces. La recherche sur des facteurs prédictifs endophénotypiques ou génomiques possibles n'est pas encore fiable. Les facteurs prédictifs les plus fiables identifiés jusqu'à maintenant sont des caractéristiques sympiomatiques et physiologiques des patients apparaissant précocement au cours du traitement. Nous proposons ici le terme « d'endophénotypes de la réponse » (ER) pour décrire cette classe de prédicteurs, définis comme des réponses précoces latentes sympiomatiques ou neurobiologiques mesurables pour chaque patient, avec un pouvoir prédictif de l'évolution clinique de chacun. L'utilisation des ER constitue un nouveau paradigme dans lequel les séquences thérapeutiques de traitements risquant d'être inefficaces pourraient être arrêtées dans les 1 à 2 semaines, laissant place à d'autres antidépresseurs probablement plus efficaces. Les données présentées suggèrent que les modifications précoces des symptômes, l'électroencêphalographie quantitative et l'expression des gènes pourraient être utilisés pour bâtir des ER efficaces. Nous postulons que ce nouveau paradigme pourrait conduire à une guêrison précoce de la maladie dépressive et finalement apporter des bénéfices économiques et de santé profonds.
The current treatment paradigm for Major Depressive Disorder
Major Depressive Disorder (MDD) is a significant public health problem. The annual costs of depression are estimated at 83.1 billion US dollars.1 Nearly two thirds of this cost comes from impaired productivity and absenteeism from work. Approximately 14.8 million American adults2 (6.7% of the population) suffer from MDD, and cost employers more than $44 billion per year in lost productive time and 387 million days per year of disability.1 While the economic costs are substantial, the personal costs of prolonged suffering are incalculable.
The costs of MDD are high, in part because it takes so long for patients with MDD to recover from the illness. Even after 1 year of treatment with enhanced resources under a structured algorithm, only 11% of patients achieved remission.3 This low recovery rate is not simply a matter of needing more or better medications. There are more than 20 treatments for MDD approved as effective by the Food and Drug Administration (FDA). The challenge is choosing the best treatment for each patient. The current treatment guidelines for MDD of the American Psychiatric Association4 support a “watchful waiting” approach to determine if a particular medication will be useful for an individual patient. In order to determine whether a medication will lead to response (≥50% reduction in depressive symptoms) or remission (nearly complete resolution of symptoms), it is recommended that a physician wait to see if it will be effective,4 On average, at least 4 weeks are needed to attain response and 6 weeks to attain remission during treatment with an initial selective serotonin reuptake inhibitor (SSRI) antidepressant; in a number of cases, however, remission can take 12 weeks or longer to attain.5 In practice, physicians commonly wait 6 to 8 weeks to determine if a patient will recover with whichever medication is chosen.6,7
It is not surprising that, under the current treatment paradigm, most patients face a long and frustrating course of treatment. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, the largest study of MDD conducted in the United States, showed that even with enriched resources devoted to treatment, recovery with the first selected SSRI occurred only about 30% of the time.8 More than 20% of those who failed to improve with the first treatment simply stopped taking medication, primarily within the first 2 weeks.9 Although medication may take up to 12 weeks to be effective, 42% of patients discontinue medication within the first 30 days.10 A high proportion of the patients who prematurely stop treatment are from ethnic minority groups,10 and this may contribute to the significantly poorer clinical outcomes observed among ethnic minority patients.11
Failure to respond to treatment at any one step is commonly followed by “sequential treatment” in which a subsequent treatment is utilized either alone in combination,12-14 followed by another period of watchful waiting. In most studies, only about 15% of patients will ultimately fail to benefit from sequential medication treatment, but it may take 1 to 2 years to identify the treatment that will get a patient well - and many discontinue treatment before they can recover.15 For those individuals who leave treatment prematurely, suffering, disability, impaired productivity, and absenteeism from work may continue indefinitely. For those who remain in treatment, the delay in recovery from MDD increases health care costs. While they are depressed, patients with MDD have at least a 50% increase in total health care costs for general medical conditions.16
The current paradigm of watchful waiting is seriouslyflawed. Lengthy medication trials determine with a high degree of certainty whether a particular medication will be effective, Because only a minority of patients will recover with any one medication, however, this paradigm prolongs the length of depressive episodes for most patients, increases health care costs, and increases the likelihood that many patients will drop out and never receive adequate treatment. The approach of lengthy medication trials essentially sacrifices the health of the majority of patients for the certainty of knowing whether a particular antidepressant will be effective.
Limitations of the current treatment paradigm
In sequential treatment, subsequent antidepressant medications commonly are selected based upon their putative mechanism of action (MOA), with medications that have a different MOA usually given preference.17 It has never been shown, however, that MOA is related to effectiveness in switching or combining medications.18 The results from level II treatment in STAR*D suggested that patients respond or remit to different antidepressants at similar rates, regardless of the MOA.19-20 The sole reliable predictor of improvement in sequential treatment is that improvement at one step is associated with further improvement at the next step, whereas failure to improve indicates a poor prognosis for improvement during future treatments.19,21 The STAR*D study demonstrated that each subsequent medication trial was less and less likely to be effective for patients with unsatisfactory response at the previous level.13,19,21-23
The development of increasing resistance over the course of antidepressant treatment is well established but not well understood. It largely has been interpreted as representing the fact that those who fail to benefit from adequate trials of earlier treatments are simply predisposed not to respond to multiple treatments, sometimes because of comorbid conditions.24 This hypothesized process through which successive treatment failures identify and isolate an increasingly treatmentresistant population may account, at least in part, for the escalation in failure rates with successive trials. This “distillation” hypothesis, however, is unlikely to account fully for increasing treatment resistance with multiple antidepressant trials. Even within a trial of a single antidepressant medication, there is a great deal of heterogeneity in onset of improvement that is not easily explained by commonly measured clinical features. Half of patients require more than 6 weeks to enter remission and a significant number of patients still enter remission up to 12 weeks, yet these later remitters eventually may attain a degree of improvement comparable to those who enter remission rapidly.5
A number of factors are likely to affect speed and completeness of medication responsiveness. Whereas some of these factors may reflect heritable or constant biological factors, others may be more dynamic and represent the state of the individual at the specific time that he or she enters treatment.25-27 Many such intraindividual factors are psychological, including patient expectations, cognitions, or conditioned responses. Data from subjects enrolled in clinical trials has shown that patients with high expectations of the effectiveness of their treatment are more likely to benefit from their treatment,28,29 and to respond more rapidly.29 Patients who are uncertain about the benefit of their antidepressant treatment may even discontinue medication before it has had time to work.30 These findings are consistent with the fact that in the setting of a placebo controlled trial, patients* certainty that they will be receiving the active medication as compared with placebo is directly related to their likelihood of response. Patients who are informed that they have a 50% likelihood of receiving active medication are significantly more likely to respond than those who are informed that their probability of receiving medication is only 20%. 31 It is reasonable to postulate that anything in the treatment setting that alters patients' expectations of improvement is likely to alter their likelihood of benefiting from a medication. Insofar as prolonged prior administration of an ineffective antidepressant may diminish expectations of improvement, this practice may contribute to the failure of subsequent trials.
Cognitive theories of depression suggest that, in the context of dysfunctional attitudes that subserve depression, failed treatment attempts would perpetuate negative thoughts and contribute to future failures. Beck's cognitive theory postulates that dysfunctional attitudes develop in response to specific stressors in the midst of an episode of depression.32 The poorer treatment outcomes of some depressive subtypes is partly explained by the patients' level of negative or dysfunctional cognitions.33 Depressed patients' interpretation of negative events also may increase the likelihood of maintaining depression and of poor response to medication.34,35 In the midst of an episode of MDD, ineffective treatment trials may constitute a specific stressor that, interpreted in a negative context, could combine with dysfunctional attitudes to result in increasingly resistant depression in some patients.
Classical conditioning also may play a role in antidepressant resistance during successive trials. Animal models have shown that pharmacologic responses to a number of different therapeutic agents can be classically conditioned,36,37 including responses to antidepressant agents.38 Similarly, pharmacologic nonresponse can also be conditioned to a reuptake inhibitor drug.39 A related concept in the classical conditioning paradigm is the process of latent inhibition, in which frequent administration of a cue (in this case, antidepressant pill-taking) that is not associated with a significant outcome prevents future conditioning to that cue,40 There is evidence to suggest that patients' physiologic responses to antidepressant medications are in part conditioned responses. A number of brain imaging studies have shown that effective antidepressant treatment is associated with decreases in metabolism or brain electrical activity in the prefrontal cortex.41,42 While these changes in function appear to be associated with antidepressant treatment, brain imaging during a placebo lead-in showed that the changes thought to be associated with successful antidepressant treatment actually preceded administration of the medication.25 These findings suggest that a psychological process such as conditioning plays a role in eliciting brain functional changes. Whether nonresponse to pharmacotherapeutic agents can be conditioned in the clinical setting by prolonged nonresponse to antidepressants has not been established.
It is difficult to demonstrate the role of expectations, cognitions, or conditioned responses in the failure to respond to successive antidepressant medication trials in humans. It is known that administration of an antidepressant is less effective after the patient has received no benefit from either a first antidepressant21 or a placebo,43 but multiple crossover trials would be necessary to determine the mechanism for this loss of effectiveness. There is clearer evidence from human pain studies, however, that ineffective medication trials directly contribute to decreases in the effectiveness of subsequent analgesic medications. The effectiveness of an analgesic medication is degraded when administered after an ineffective dose of medication or placebo; furthermore, the more doses of the ineffective compound that are given, the less likely that the analgesic will have a therapeutic effect.44,45 Blinded administration of effective analgesics also diminishes their effectiveness.46 Expectations, conditioning, and cognitive factors all have been shown to be involved in mediating these effects.46,47
In summary, unsuccessful antidepressant trials maydiminish patient expectations, reinforce negative cognitions, and condition patients not to respond during subsequent antidepressant trials. Regardless of the psychological mechanism, the above theories and data suggest that ineffective medication trials may, in and of themselves, predispose patients to experience diminished medication effectiveness in future trials.
The state of endophenotypic and genomic predictors
There are several strategies that could be employed to overcome the shortcomings of the current paradigm for prescribing antidepressant medications. One of these would be to identify, prior to treatment, the medication that has the highest likelihood of benefitting the patient. Research has sought to indentify “endophenotypes” that could predict response or remission to specific antidepressants for individual patients. As defined by Gottesman and Gould,48 an endophenotype must meet five criteria:
The endophenotype is associated with illness in the population.
The endophenotype is heritable.
The endophenotype is primarily state-independent (manifests in an individual whether or not illness is active).
Within families, endophenotype and illness cosegregate.
The endophenotype found in affected family members is found in nonaffected family members at a higher rate than in the general population.
Endophenotypes thus are measureable characteristics or physiologic indices that fill “the gap between available descriptors and between the gene and the elusive disease process.”49 Exhaustive studies of clinical features, family history, as well as sleep patterns and neuroendocrine correlates, have identified general prognostic indicators for treatment outcome for depression.50,51 In some cases, the predictors may be useful for groups of patients with certain subtypes of depression (ie, psychotic depression).52 While some symptomatic and physiologic features in MDD patients demonstrate promise as putative endophenotypes, many do not fulfill the actual criteria for an endophenotype or meet the goal of providing greater prognostic specificity than the definition of the illness itself.53 Some brain imaging findings also have demonstrated prognostic significance54-57 and may fulfill the criteria for an endophenotype.58 Part of the challenge in identifying true endophenotypes in MDD is that the physiologic and genetic underpinnings of MDD are complex and poorly understood. As a result, imaging findings may reflect confounds such as interindividual heterogeneity in brain structure or function unrelated to illness, or the effects of previous or concomitant medication treatment.58 No clinically meaningful endophenotypes predictive of response to specific medications in individual patients prior to the start of treatment yet have been identified.59,60
An alternative to the endophenotypic approach has been to examine genetic polymorphisms as possible outcome predictors. Recent studies have suggested that common genetic variations may be associated with response to specific antidepressant medications.61-63 For example, some common polymorphisms in serotonin system genes have been shown to influence the outcome of SSRI treatment.62,64 Many of these results have not consistently replicated or do not allow the estimation of prediction accuracy in a clinical population.65 The relative lack of reproducibility in pharmacogenetic studies may reflect the fact that the contributions of individual polymorphisms may be small and, therefore, large populations may be needed to detect the effect.66 Given the complexity of influences “downstream” from genotype,64 genotype alone may be insufficient to capture the state of those systems that subserve antidepressant action in an individual patient. To date, research on possible genomic factors has not yet yielded reliable predictors.
Response endophenotypes
The most reliable treatment response predictors identified thus far are symptomatic and physiologic characteristics of patients that emerge early in the course of treatment. We propose here the term “response endophenotypes” to describe this class of predictors. Specifically, we define response endophenotypes (REs) as latent measurable symptomatic or neurobiologie responses of individual patients that emerge early in a course of treatment and which carry strong predictive power for individual patient outcomes. In some diseases, endophenotypic characteristics are elicited by a physiologic challenge (ie, glucose tolerance tests, stress electrocardiography).53,67 The distinction of the term response endophenotype is that it describes a class of markers that are exclusively observed in response to specific treatment challenges. Although there is evidence that response to medication is at least in part genetically mediated, it is not firmly established that the REs presented below necessarily are heritable. It is therefore appropriate to consider REs as putative endophenotypes, pending research to establish heritability and fulfillment of the other characteristics of an endophenotype.48
In the prediction of treatment response in MDD, there are significant advantages to composing endophenotypes exclusively from measureable changes in an individual in response to a specific treatment. First, the fact that these characteristics are measured “within subjects” likely enhances stability, statistical reliability, and therefore predictive accuracy of the measures. Preliminary data presented below suggest that use of REs may facilitate prompt and accurate matching of patients with the medication most likely to benefit them. Second, the fact that RE components are measured in response to newly administered treatments may overcome some of the confounding factors inherent in the development of conventional endophenotypes in MDD. It is problematic to derive prognostic significance from static, cross-sectional measures in MDD patients; such measurements are inevitably affected by the number and severity of prior episodes, the current phase of illness, and the extent and types of prior and current treatment.58 Examination of dynamic measures specific to the current treatment may detect features that are common across individuals who will respond to the treatment, irrespective of confounding factors. There are three broad classes of measures that may change within the first 48 hours to 2 weeks of treatment that have been identified thus far as potential predictors of treatment response or remission, and therefore may be useful as components of an RE. Each of these is discussed separately below.
Early changes in depressive symptoms
The average time to response in treatment with a prototypical SSRI is 1 month, and to remission is 6 weeks.5 While some patients continue to enter remission up to 12 weeks or even longer after the initiation of treatment, the time to symptomatic improvement is much shorter. Many patients, particularly those with milder symptoms, show improvement (defined by at least a 20% decrease in depressive symptoms) within the first 2 weeks of treatment.68-71 Although some have suggested that early response is likely to represent a placebo response,72,73 early response is in fact twice as likely with medication as with placebo.71
The largest meta-analytic study of this topic was performed by Szegedi and colleagues,74 who examined 6562 subjects treated primarily with mirtazepine, but also with SSRIs, tricyclic antidepressants (TCAs), and venlafaxine. These investigators found that more than 50% of patients had at least a 20% improvement in depression rating scores by the end of 2 weeks of treatment. Of those who did not show early improvement, only 11% and 4.1% showed eventual response and remission, respectively. Early improvement was a highly sensitive predictor of stable response (81% to 98%) or stable remission (87% to 100%), and so was a positive prognostic sign. However, the usefulness of early symptom improvement was limited by the poor specificity for stable response (43% to 60%) or remission (19% to 28%). The results of all of these studies are difficult to evaluate because they come from placebo-controlled treatment trials of selected study populations. It is clear that early symptom improvement is a positive prognostic sign, and the absence of early improvement is a negative prognostic sign. The poor specificity of the finding, however, makes it difficult to make treatment decisions based solely upon early symptom improvement; absence of early improvement by itself is insufficiently powerful evidence to prompt a change in treatment. It is possible that early symptom changes could form part of the basis for REs to reliably predict response and remission to the specific medication that the patient receives within the first 2 weeks of treatment.
Early changes in brain electrical activity
One biomarker that has shown promise as a predictor of treatment response is quantitative electroencephalography (QEEG). Prefrontal QEEG power75-77 may identify patients who are most likely to respond to all major antidepressant medication classes. Research has shown that QEEG changes in the prefrontal region may reliably identify antidepressant medication responders within the first 48 hours to 1 week of treatment.42,78 These findings are consistent with the fact that rhythmic midline prefrontal EEG activity has been shown to reflect the activity of anterior cingulate and midline prefrontal cortex,79 brain areas implicated in mood regulation and the pathogenesis of depression.
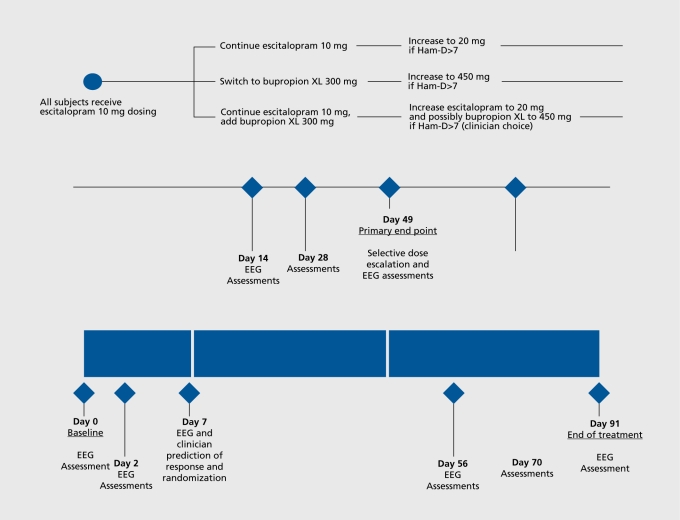
Based upon these previous results, a multisite study was designed to test the usefulness of QEEG as a predictor. The BRITE-MD study (“Biomarkers for Rapid Identification of Treatment Effectiveness in Major Depression,” NCT00289523), examined for the first time the usefulness of a new putative neurophysiologic biomarker for medication response and remission, the Antidepressant Treatment Response (ATR) index.80 ATR is based upon QEEG data collected on two occasions, at pretreatment baseline (immediately before medication is started) and at the end of 1 week of treatment with medication. ATR is based upon alpha and theta band features of frontal brain electrical activity integrated and scaled from 0 (low probability of response or remission to the medication) to 100 (high probability). BRITE-MD is the largest single study of any type of neurophysiology biomarker in MDD undertaken to date (N =375). All subjects were treated with an initial 1 week of escitalopram 10 mg, during which time ATR was calculated. Subjects then were randomized either to continue escitalopram, switch to bupropion, or receive a combination of the two medications (Figure 1).
Figure 1. Study flow chart. Ham-D, Hamilton Depression Rating Scale; SSRI, selective serotonin reuptake inhibitor; EEG, electroencephalography.
The outcome measure was the Hamilton Depression Rating Scale (Ham-D17) score at week 7, with response defined as a 50% decrease from the baseline score and remission defined as a final score ≤7. Other putative predictors examined in BRITE included other biomarkers (serum drug levels, as well as serotonin transporter [5-HTTLPR] and postsynaptic serotonin receptor [5-HT2a] genetic polymorphisms), early changes in symptoms (measured with the Ham-D17 at 1 week), and clinician prediction of the likelihood of response (using a clinical global impression measure at 1 week).
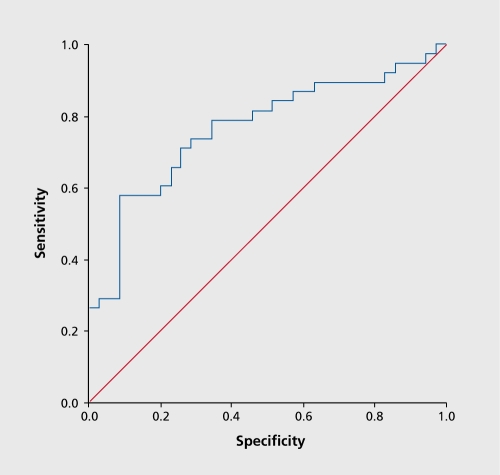
The Receiver Operating Characteristic (ROC) curve for predictive accuracy of ATR with escitalopram is shown in Figure 2. An optimal threshold was chosen on this curve (58.6) to maximize accuracy in predicting response, with values above this threshold designated as a “positive” ATR and those below the threshold as “negative.”
Figure 2. Receiver Operating Characteristic curve for ATR prediction of response to escitalopram treatment. ATR, Antidepressant Treatment Response index.
A positive ATR biomarker predicted response and remission to treatment in the escitalopram arm with high accuracy. ATR values predicted response with 74% overall accuracy, 58% sensitivity, 91 % specificity, 88% positive predictive accuracy, and 67% negative predictive accuracy ATR also predicted remission with 74% overall accuracy, 61% sensitivity, 82% specificity, 68% positive predictive accuracy, and 77% negative predictive accuracy. Neither serum drug level not genetic polymorphisms were significant predictors of response or remission with escitalopram. Responders at week 7 had significantly larger decreases than nonresponders in Ham-D17 scores at day 7 (P=0.005), although remitters did not. Clinician prediction based upon global impression of improvement at day 7 did not predict final outcome. Logistic regression showed that ATR and early Ham-D17 changes were additive predictors of response, but ATR was the sole significant predictor of remission.80
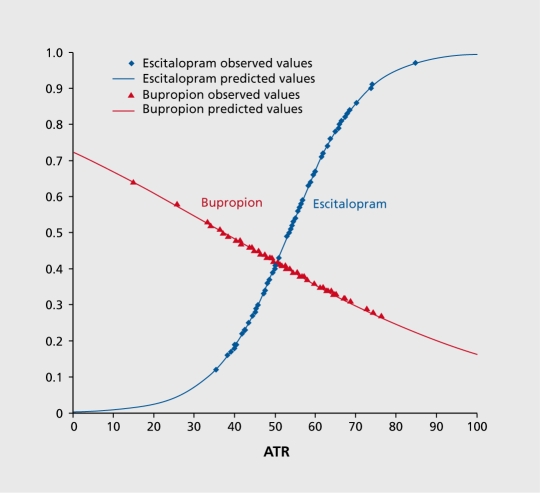
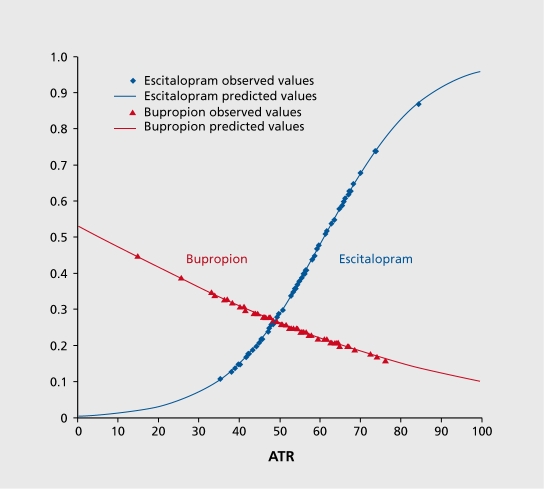
Another goal of BRITE was to examine the prognostic significance of a negative biomarker. The overall response rate to escitalopram in the study was 52%, but in those with a positive ATR biomarker, the response rate was 61%. Conversely, in those with a negative ATR biomarker, the response rate to escitalopram was only 28%. Analyses showed that a low ATR value predicted not only nonresponse to escitalopram, but also subsequent response to treatment among those subjects who were randomly assigned to receive the antidepressant bupropion. Subjects with ATR values above the threshold were more than 2.4 times as likely to respond to escitalopram as those with low ATR values (68% vs 28%, P=.001). Subjects with ATR values below the threshold who were switched to bupropion treatment were 1.9 times as likely to respond to bupropion alone than those who remained on escitalopram treatment (53% vs 28%, P=.034, Figure 3 and Figure 4).81
Figure 3. Logistic regression models of escitalopram and bupropion responders stratified by ATR values. ATR values of subjects randomly assigned to each treatment and who responded to escitalopram or bupropion treatment. Subjects who responded to escitalopram (blue) tended to have higher ATR values, and those who responded to bupropion (red) tended to have lower ATR values. Markers represent observed values and lines represent modeled values. ATR, Antidepressant Treatment Response index Adapted from ref 81: Leuchter AF, Cook IA, Gilmer WS, et ai. Effectiveness of a quantitative electroencephalographs biomarker for predicting differential response or remission with escitalopram and bupropion in Major Depressive Disorder. Psychiatry Res. 2009:169:124131. Copyright© Elsevier, 2009 .
Figure 4. Logistic regression models of escitalopram and bupropion remitters stratified by ATR values. ATR values of subjects randomly assigned to each treatment and who remitted with escitalopram or bupropion treatment. Subjects who remitted with escitalopram (blue) tended to have higher ATR values, and those who remitted with bupropion (red) tended to have lower ATR values. Markers represent observed values and lines represent modeled values. ATR, Antidepressant Treatment Response index Adapted from ref 81: Leuchter AF, Cook IA, Gilmer WS, et al. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in Major Depressive Disorder. Psychiatry Res. 2009:169:124131. Copyright © Elsevier, 2009 .
These differences were statistically significant. One measure of the potential impact of the use of the ATR biomarker is the “number needed to treat” (NNT), namely the number of patients to whom such a test would need to be applied in order to realize one improved patient outcome.82,83 These results equate to a NNT of 10 to 11, which is in the range that has been considered to be clinically significant.84 These results must be interpreted with the caveat that treatment was not assigned prospectively on the basis of ATR values.
These results are encouraging, and suggest that ATR may be useful as a component of a RE for predicting early in the course of treatment which medication will be most helpful to an individual patient with MDD. The fact that ATR data appear to be complementary to earlychanges in depression rating scores suggests that a RE model that integrates symptom and neurophysiology measures may be the most useful.
Gene expression markers
Some of the more intriguing putative biomarkers for antidepressant treatment response are early changes in gene expression. Animal and cell culture research, as well as study of postmortem human brains, indicates that regulation of gene expression represents a major component of the mechanism of action of available antidepressants. The expression of a host of gene families are altered by antidepressant treatment, including those for trophic factors that promote cell proliferation, growth, and resiliency (BDNF, FGF, and VEGF), cell signaling pathways, and pathways for neurotransmitter transport and metabolism, among others.85,86
Because direct examination of gene expression in patients' brains is impractical, recent research has examined gene expression in peripheral leukocytes, which share identical genetic material and may exhibit similarly altered expression in response to antidepressant medications. There have been limited small previous studies of gene expression through leukocyte mRNA in response to antidepressant or lithium treatment in patients with MDD or bipolar disorder.87-93 These studies have confirmed and extended research from animals, showing significant differences prior to treatment between bipolar or MDD subjects and normal controls in expression of trophic and transcriptional factors, as well as cell signaling proteins. In some small studies, antidepressant treatment tended to normalize gene expression patterns and the degree of normalization was proportional to the degree of symptom improvement.90,92 No study has utilized microarray-based screening of large numbers of expressed genes to predict treatment response in MDD, but one study has performed such screening in a small number of subjects with juvenile epilepsy and identified patterns of change in expression that accurately differentiated subjects who were seizure-free on valproate from those who were not.94
Because of limited research in this area, the gene expression approach is highly speculative. Furthermore, the biological basis through which gene expression changes measured in peripheral blood reflect the central effectiveness of medications admittedly is not fully clear. There are several possible mechanisms including: i) parallel expression changes in the brain and peripheral blood; ii) leukocyte responses to change in the brain; iii) responses of the leukocytes to a change in the physiological state of the subject; and/or iv) changes in the composition of the leukocyte population. Regardless of the mechanism, sufficient data exist to support the plausibility of testing the use of gene expression in peripheral leukocytes to predict clinical responsiveness to antidepressants. Expression profiles could potentially be applied in the clinic to aid in the treatment of MDD, and because the fundamental measure is the change in gene expression within a patient between two time points, each patient acts as his or her own control, greatly reducing the artifacts that could arise from directly comparing gene expression across unmatched subjects, such as subject-to-subject expression differences due to extraneous factors such as ethnicity, gender, age, or environment factors.
Conclusion
The use of REs for predicting antidepressant treatment response and remission has the potential to overturn a flawed biomedical paradigm that forms the basis for clinical research and treatment in MDD, namely, the long empiric medication trial. Fewer than half of patients respond to treatment under this paradigm, and fewer than one third recover. This paradigm leads to prolonged suffering and increased health care costs. If we were successful in identifying response endophenotypes for patients with MDD, medications would be prescribed under an entirely new paradigm that relied upon an early response profile of each patient. The concept of the response endophenotype shifts from the examination of endophenotypes and genotypes, which have not proved highly productive, to the study of dynamic treatmentemergent characteristics. In this paper we have suggested early changes in symptoms, brain neurophysiology, and patterns of changes in gene expression as potential REs. The RE concept need not be limited, however, to these few measures. Any early treatment-emergent measures that could be examined within the individual patient could be incorporated in this paradigm. We posit that this paradigm could optimize response and remission rates with medication and prove superior to the current approach, leading to earlier symptom improvement, recovery from the illness, and ultimately profound health and economic benefits in terms.
Acknowledgments
Financial support of this project was provided by a grant from the National Institute on Mental Health (R01 MH 085925) and by an endowment in depression research from Joanne and George Miller and Family (Dr Cook), and by NIH Training Grant T32 MH017140 (Mr Korb).
Andrew Leuchter, MD, has provided scientific consultation or served on advisory boards for Aspect Medical Systems, Eli Lilly and Company, Novartis Pharmaceuticals, MEDACorp, AstraZeneca, Takeda Pharmaceuticals, and Merck & Co. He has served on a speaker's bureau for Eli Lilly and Company and Wyeth-Ayerst Pharmaceuticals. He has received research/grant support from the National Institute of Mental Health, the National Center for Complementary and Alternative Medicine, Aspect Medical Systems, Eli Lilly and Company, Novartis Pharmaceuticals, Wyeth-Ayerst Pharmaceuticals, Merck & Co, Pfizer, Vivometrics, and MedAvante.
Ian A. Cook, MD, has served as an advisor and consultant for Ascend Media, Bristol-Meyers Squibb, Cyberonics Inc, and Janssen. He has served on the Speaker's Bureau for Bristol-Meyers Squibb, Medical Education Speakers Network, Pfizer Pharmaceuticals Inc, and Wyeth Pharmaceuticals. Dr Cook receives Research Support from Aspect Medical Systems, Cyberonics Inc, Eli Lilly & Company, Novartis Pharmaceuticals, Pfizer, Inc, and Sepracor.
Dr Hunter and Mr Korb have nothing to disclose.
Contributor Information
Andrew F. Leuchter, Laboratory of Brain Behavior, and Pharmacology, and the Depression Research and Clinical Program, Semel Institute for Neuroscience and Human Behavior at UCLA, and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Ian A. Cook, Laboratory of Brain Behavior, and Pharmacology, and the Depression Research and Clinical Program, Semel Institute for Neuroscience and Human Behavior at UCLA, and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Aimee M. Hunter, Laboratory of Brain Behavior, and Pharmacology, and the Depression Research and Clinical Program, Semel Institute for Neuroscience and Human Behavior at UCLA, and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Alexander S. Korb, Laboratory of Brain Behavior, and Pharmacology, and the Depression Research and Clinical Program, Semel Institute for Neuroscience and Human Behavior at UCLA, and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
REFERENCES
- 1.Greenberg P., Kessler R., Birnbaum H. The economic burden of depression in the United States: how did it change between 1990 and 2000? . J Clin Psych. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R., Chiu W., Dernier O. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). . Arch Gen Psych. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ., Trivedi MH., Carmody TJ., et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. . Biol Psychiatry. 2004;56:6–53. doi: 10.1016/j.biopsych.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Fochtmann LJ., Gelenberg AJ. 2nd ed. Washington, DC: American Psychiatric Association; Guideline Watch: Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 2005 [Google Scholar]
- 5.Trivedi MH., Rush AJ., Wisniewski SR., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. . Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Quitkin FM., Petkova E., McGrath PJ., et al. When should a trial of fluoxetine for major depression be declared failed? . Am J Psychiatry. 2003;160:734–740. doi: 10.1176/appi.ajp.160.4.734. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M., Bschor T., Pfennig A., et al. WFSBP Task force on unipolar depressive disorders. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders in Primary Care. . World J Biol Psychiatry . 2007;8:67–104. doi: 10.1080/15622970701227829. [DOI] [PubMed] [Google Scholar]
- 8.Rush AJ. STAR*D: what have we learned? . Am J Psych. 2007;164:201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- 9.Warden D, Trivedi MH, Wisniewski SR., et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. . Am J Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 10.Olfson M., Marcus S., Tedeschi M. Continuity of antidepressant treatment for adults with depression in the United States. . Am J Psych. 2006;163:101–108. doi: 10.1176/appi.ajp.163.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Lesser IM., Castro DB., Gaynes BN., et al. Ethnicity/race and outcome in the treatment of depression: results from STAR*D. . Med Care. 2007;45:1043–1051 . doi: 10.1097/MLR.0b013e3181271462. [DOI] [PubMed] [Google Scholar]
- 12.Fava GA., Ruini C., Belaise C. The concept of recovery in major depression. . Psychol Med. 2007;37:307–317. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- 13.Fava M., Rush AJ., Wisniewski SR., et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. . Am J Psychiatry. 2006;163:1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi MH., Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. . Drug Alcohol Depend. 2007;88(suppl 2):S61–S71. doi: 10.1016/j.drugalcdep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keitner Gl., Ryan CE., Miller IW., Norman WH. Recovery and major depression: factors associated with twelve-month outcome. . Am J Psychiatry. 1992;149:1127–1128. doi: 10.1176/ajp.149.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Simon GE., Von Korff M., Barlow W. Health care costs of primary care patients with recognized depression. . Arch Gen Psychiatry. 1995;52:850–856. doi: 10.1001/archpsyc.1995.03950220060012. [DOI] [PubMed] [Google Scholar]
- 17.Stahl SM., Grady MM. Differences in mechanism of action between current and future antidepressants. . J Clin Psych. 2003;64:13–17. [PubMed] [Google Scholar]
- 18.Thase ME, Blomgren SL., Birkett MA., Apter JT., Tepner RG. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. . J Clin Psychiatry. 1997;58:16–21 . doi: 10.4088/jcp.v58n0103. [DOI] [PubMed] [Google Scholar]
- 19.Rush AJ, Trivedi MH., Wisniewski SR., et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. . N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 20.Rush AJ., Wisniewski SR., Warden D., et al. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. . Arch Gen Psychiatry. 2008;65:870–880. doi: 10.1001/archpsyc.65.8.870. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi MH., Fava M., Wisniewski SR., et al. Medication augmentation after the failure of SSRIs for depression. . N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 22.Nierenberg AA., Fava M., Trivedi MH., et al. A comparison of lithium and T augmentation following two failed medication treatments for depression: a STAR*D report. . Am J Psychiatry. 2006;163:1519–1530. doi: 10.1176/ajp.2006.163.9.1519. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ., Stewart JW., Fava M., et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. . Am J Psychiatry. 2006;163:1531–1541 . doi: 10.1176/ajp.2006.163.9.1531. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ., Thase ME., Dubé S. Research issues in the study of difficultto-treat depression. . Biol Psychiatry. 2003;53:743–753. doi: 10.1016/s0006-3223(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 25.Hunter AM., Leuchter AF., Morgan ML., Cook IA. Changes in brain function (quantitative EEG cordance) during placebo lead-in and treatment outcomes in clinical trials for major depression. . Am J Psychiatry. 2006;163:1426–1432. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- 26.Hunter AM., Ravikumar S., Cook IA., Leuchter AF. Changes in brain function (QEEG cordance) during placebo lead-in and SCL-90-R symptom dimension changes in MDD during randomized treatment with antidepressant or placebo. . Acta Psychiatrics Scandinavica. 2009;119:266–273. doi: 10.1111/j.1600-0447.2008.01305.x. [DOI] [PubMed] [Google Scholar]
- 27.Price DD., Finniss DG., Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. . Ann Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 28.Rutherford BR., Rose SA., Sneed JR., Roose SP. Study design affects participant expectations: a survey. . J Clin Psychopharmacol. 2009;29:179–181. doi: 10.1097/JCP.0b013e31819a9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krell HV., Leuchter AF., Morgan M., et al. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. . J Clin Psychiatry. 2004;65:1174–1179. doi: 10.4088/jcp.v65n0904. [DOI] [PubMed] [Google Scholar]
- 30.Aikens JE., Nease DE Jr., Nau DP., et al. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. . Ann Earn Med. 2005;3:23–30. doi: 10.1370/afm.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papakostas Gl., Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. . Eur Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT., Rush AJ., Shaw BF., Emery G. New York, NY: Guilford Publications; Cognitive Therapy of Depression. 1979 [Google Scholar]
- 33.Gaudiano BA., Miller IW. Dysfunctional cognitions in hospitalized patients with psychotic versus nonpsychotic major depression. . Compr Psychiatry. 2007;48:357–365. doi: 10.1016/j.comppsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Abramson LY., Metalsky Gl., Alloy LB. Hopelessness depression: a theory based subtype of depression. . Psychol Rev. 1989;96:358–372. [Google Scholar]
- 35.Candrian M., Farabaugh A., Pizzagalli DA., et al. Perceived stress and cognitive vulnerability mediate the effects of personality disorder comorbidity on treatment outcome in major depressive disorder: a path analysis study. . J Nerv Ment Dis. 2007;195:729–737. doi: 10.1097/NMD.0b013e318142cbd5. [DOI] [PubMed] [Google Scholar]
- 36.Ader R., Felten DL., Cohen N. 2nd ed. San Diego, CA: Academic Press; Psychoneuroimmunology. 1991 [Google Scholar]
- 37.Ader R., Cohen N. Psychoneuroimmunology: conditioning and stress. . Ann Rev Psychology. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- 38.Ginsburg BC., Lamb RJ. Reinforcement magnitude modulation of ratedependent effects of fluvoxamine and desipramine in the rat. . Behav Pharmacol. 2008;19:829–835. doi: 10.1097/FBP.0b013e32831d9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broadbear JH., Winger G., Cicero TJ., Woods JH. Effects of response contingent and noncontingent cocaine injection on hypothalamic-pituitaryadrenal activity in rhesus monkeys. . J Pharmacol Exp Ther. 1999;290:393–402. [PubMed] [Google Scholar]
- 40.Tarpy RM. New York, NY: McGraw-Hill Humanities/Social Sciences/Langua; Contemporary Learning Theory and Research. 1997 [Google Scholar]
- 41.Brody AL., Saxena S., Silverman DH., et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. . Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 42.Cook IA., Leuchter AF., Morgan M., et al. Early changes in prefrontal activity characterize clinical responders to antidepressants. . Neuropsychopharmacology. 2002;27:120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 43.Khan A., Redding N., Brown WA. The persistence of the placebo response in antidepressant clinical trials. . J Psychiatr Res. 2008;42:791–796. doi: 10.1016/j.jpsychires.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Moertel CG., Taylor WF., Roth A., Tyce FA. Who responds to sugar pills? . Mayo Clin Proc. 1976;51:96–100. [PubMed] [Google Scholar]
- 45.Batterman RC. Methodology of analgesic evaluation: experience with orphenadrine citrate compound. . Curr Ther Res Clin Exp. 1965;7:639–647. [PubMed] [Google Scholar]
- 46.Amanzio M., Polio A., Maggi G., Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. . Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 47.Amanzio M., Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. . J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gottesman II., Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. . Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 49.Hasler G., Drevets WC., Manji HK., Charney DS. Discovering endophenotypes for major depression. . Neuropsychopharmacology. 2004;29:1765–1781 . doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 50.Fava M., Rush AJ., Alpert JE., et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. . Am J Psychiatry. 2008;165:342–351 . doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 51.Nunes EV., Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. . JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 52.Duval F., Lebowitz BD., Mâcher JP. Treatments in depression. . Dialogues Clin Neurosci. 2006;8:191–206. doi: 10.31887/DCNS.2006.8.2/fduval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gould TD., Gottesman II. Psychiatric endophenotypes and the development of valid animal models. . Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 54.Mayberg HS., Brannan SK., Mahurin RK., et al. Cingulate function in depression: a potential predictor of treatment response. . Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 55.Pizzagalli D., Pascual-Marqui RD., Nitschke JB., et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. . Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 56.Canli T., Cooney RE., Goldin P., et al. Amygdala reactivity to emotional faces predicts improvement in major depression. . Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 57.Korb AS., Hunter AM., Cook IA., Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. . Clin Neurophysiol. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savitz JB., Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. . Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz P, Berney P. Clinicians' predictions of patient response to psychotropic medications. . Dialogues Clin Neurosci. 2004; 6:105–111. doi: 10.31887/DCNS.2004.6.1/pschulz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perlis RH., losifescu DV., Renshaw PF. Biological predictors of treatment response in affective illness. . Psychiatr Clin North Am. 2003;26:323–344. doi: 10.1016/s0193-953x(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 61.Kim H., Lim SW., Kim S., et al. Monoamine transporter gene polymorphisms and antidepressant response in Koreans with late-life depression. . JAMA. 2006;296:1609–1618. doi: 10.1001/jama.296.13.1609. [DOI] [PubMed] [Google Scholar]
- 62.McMahon FJ., Buervenich S., Charney D., et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. . Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perlis RH., Moorjani P., Fagerness J., et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR*D study. . Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anguelova M., Benkelfat C., Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. . Mol Psychiatry. 2003;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- 65.Perlis RH., Patrick. A., Smoller JW., Wang PS. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. . Neuropsychopharmacology. 2009;34:2227–2236. doi: 10.1038/npp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malhotra AK., Murphy GM Jr. Kennedy JL. Pharmacogenetics of psychotropic drug response. . Am J Psychiatry. 2004;161:780–796. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 67.Gottesman I., Shields J. New York, NY: Academic Press; Schizophrenia and Genetics: a Twin Study Vantage Point. 1972 [Google Scholar]
- 68.Nierenberg AA., McLean NE., Alpert JE., Worthington JJ., Rosenbaum JF., Fava M. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. . Am J Psychiatry. 1995;152:1500–1503. doi: 10.1176/ajp.152.10.1500. [DOI] [PubMed] [Google Scholar]
- 69.Rojo JE., Gibert K., Cobo J., Rodriguez-Cano E., Vallejo J. Onset of antidepressant action: a pharmacological question? . Hum Psychopharmacoi. 2005;20:425–433. doi: 10.1002/hup.708. [DOI] [PubMed] [Google Scholar]
- 70.Taylor MJ., Freemantle N., Geddes JR., Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action. . Arch Gen Psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papakostas Gl., Perlis RH., Scalia MJ., Petersen TJ., Fava M. A meta-analysis of early sustained response rates between antidepressants and placebo for the treatment of major depressive disorder. . J Clin Psychopharmacoi. 2006;26:56–60. doi: 10.1097/01.jcp.0000195042.62724.76. [DOI] [PubMed] [Google Scholar]
- 72.Stewart JW., Quitkin FM., McGrath PJ., et al. Use of pattern analysis to predict differential relapse of remitted patients with major depression during 1 year of treatment with fluoxetine or placebo. . Arch Gen Psychiatry. 1998;55:334–343. doi: 10.1001/archpsyc.55.4.334. [DOI] [PubMed] [Google Scholar]
- 73.Nierenberg AA., Quitkin FM., Kremer C., et al. Placebo-controlled continuation treatment with mirtazapine: acute pattern of response predicts relapse. . Neuropsychopharmacology. 2004;29:1012–8102. doi: 10.1038/sj.npp.1300405. [DOI] [PubMed] [Google Scholar]
- 74.Szegedi A., Jansen WT., van Willigenburg AP., et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. . J Clin Psychiatry. 2009;70:344–353. doi: 10.4088/jcp.07m03780. [DOI] [PubMed] [Google Scholar]
- 75.Knott VJ., Telner Jl., Lapierre YD., et al. Quantitative EEG in the prediction of antidepressant response to imipramine. . J Affect Disord. 1996; 39:175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 76.Ulrich G., Haug H-J., Fahndrich Acute versus chronic EEG effects of maprotiline- and in clomipramine-treated depressive inpatients and the prediction of therapeutic outcome. . J Affect Disord. 1994;32:213–217. doi: 10.1016/0165-0327(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 77.Ulrich G., Haug H., Stieglitz R., Fahndrich E. Are there distinct biochemical subtypes of depression? EEG characteristics of clinically defined on-drug responders and non-responders. . J Affect Disord. 1988;15:181–185. doi: 10.1016/0165-0327(88)90088-2. [DOI] [PubMed] [Google Scholar]
- 78.Leuchter AF., Uijtdehaage SH., Cook IA., et al. Relationship between brain electrical activity and cortical perfusion in normal subjects. . Psychiatry Res. 1999;90:125–140. doi: 10.1016/s0925-4927(99)00006-2. [DOI] [PubMed] [Google Scholar]
- 79.Asada H., Fukuda Y., Tsunoda S., et al. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. . Neurosci Lett. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- 80.Leuchter AF., Cook IA., Marangell LB., et al. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE-MD study. . Psychiatry Res. 2009;169:124–131. doi: 10.1016/j.psychres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Leuchter AF., Cook IA., Gilmer WS., et al. Effectiveness of a quantitative electroencephalographs biomarker for predicting differential response or remission with escitalopram and bupropion in Major Depressive Disorder. . Psychiatry Res. 2009;169:132–138. doi: 10.1016/j.psychres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Cook RJ., Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. . BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinclair JC., Cook RJ., Guyatt GH., et al. When should an effective treatment be used? Derivation of the threshold number needed to treat and the minimum event rate for treatment. . J Clin Epidemiol. 2001;54:253–262. doi: 10.1016/s0895-4356(01)00347-x. [DOI] [PubMed] [Google Scholar]
- 84.Papakostas, Gl, Thase, ME, Fava, M, et al. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. . Biol Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 85.Altar CA., Vawter MP., Ginsberg SD. Target identification for CNS diseases by transcriptional profiling. . Neuropsychopharmacology. 2009;34:18–54. doi: 10.1038/npp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanis KQ., Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. . Ann Med. 2007;39:531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- 87.Begemann M., Sargin D., Rossner MJ., et al. Episode-specific differential gene expression of peripheral blood mononuclear cells in rapid cycling supports novel treatment approaches. . Mol Med. 2008;14:546–552. doi: 10.2119/2008-00053.Begemann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iga J., Ueno S., Yamauchi K., et al. Serotonin transporter mRNA expression in peripheral leukocytes of patients with major depression before and after treatment with paroxetine. . Neurosci Lett. 2005;389:12–16. doi: 10.1016/j.neulet.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 89.Iga J., Ueno S., Yamauchi K., Numata S., et al. Gene expression and association analysis of LIM (PDLIM5) in major depression. . Neurosci Lett. 2006;400:203–207. doi: 10.1016/j.neulet.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 90.Iga J., Ueno S., Yamauchi K., et al. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. . Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Iga J., Ueno S., Yamauchi K., et al. Altered HDAC5 and CREB mRNA expressions in the peripheral leukocytes of major depression. . Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:628–632. doi: 10.1016/j.pnpbp.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 92.Iga J., Ueno S., Ohmori T. Molecular assessment of depression from mRNAs in the peripheral leukocytes. . Ann Med. 2008;40:336–342. doi: 10.1080/07853890802082088. [DOI] [PubMed] [Google Scholar]
- 93.Sun X., Young LT., Wang JF., et al. Identification of lithium-regulated genes in cultured lymphoblasts of lithium responsive subjects with bipolar disorder. . Neuropsychopharmacology. 2004;29:799–804. doi: 10.1038/sj.npp.1300383. [DOI] [PubMed] [Google Scholar]
- 94.Tang Y., Glauser TA., Gilbert DL., et al. Valproic acid blood genomic expression patterns in children with epilepsy - a pilot study. . Acta Neurol Scand. 2004;109:159–168. doi: 10.1046/j.1600-0404.2003.00253.x. [DOI] [PubMed] [Google Scholar]