Abstract
Currently available antipsychotic drugs (APDs) carry significant, though highly variable, liability to neurologic and metabolic side effects. Pharmacogenetics approaches offer the possibility of identifying patient-specific biomarkers for predicting risk of these side effects. To date, a few single nucleotide polymorphisms (SNPs) in a handful of genes have received convergent support across multiple studies. The primary focus has been on SNPs in dopamine and serotonin receptor genes: persuasive meta-analytic evidence exists for an effect of the dopamine D2 and D3 receptor genes (DRD2 and DRD3) in risk for tardr inesia (TD) and for an effect of variation at the receptor gene (HTR2C) for liability to APD-inducec gain. However, effect sizes appear to be modest, and pharmacoeconomic considerations have not been sufficiently studied, thereby limiting clinical applicability at this time. Effects of these genes and others on risk for TD, extrapyramidal side effects, hyperprolactinemia, and weight gain are revieved in this article.
Keywords: antipsychotic drug, pharmacogenetics, side effect, tardive dyskinesia, weight gain, dopamine, serotonin
Abstract
Actualmente los fármacos antipsicóticos (FAP) disponibles conllevan, con una alta y significativa aunque variable probabilidad, efectos secundarios neurológicos y metabólicos. Las aproximaciones farmacogenéticas ofrecen la posibilidad de identificar biomarcadores específicos para el paciente para predecir el riesgo de estos efectos secundarios. A la fecha, multiples estudios ban convergido en dar sustento a unos pocos polimorfismos de nucleotidos simples (SNPs) de un pequeño grupo de genes. El foco primario ha estado en los SNPs de los genes de los receptores de dopamina y serotonina; estudios de meta-análisis han demostrado una evidencia convincente para el efecto de los genes de los receptores de dopamina D2 y D3 (RDD2 y RDD3) en el riesgo de disquinesia tardía (DT) y para un efecto de variación del gen del receptor 5HT2C (R5HT2C) en la probabilidad de aumento de peso inducido por los FAP. Sin embargo, la magnitud del efecto parece ser modesta y las consideraciones farmacoeconómicas no se han estudiado suficientemente, por lo que la aplicación clínica en este momento es limitada. En este artículo se revisan los efectos de estos y otros genes en los riesgos de DT, efectos secundarios extrapiramidales, hiperprolactinemia y aumento de peso.
Abstract
Les médicaments antipsychotiques disponibles actuellement sont significativemeni responsables, bien que de façon très variable, d'effets secondaires métaboliques et neurologiques. La pharmacogénétique permet d'identifier des biomarqueurs spécifiques des patients permettant de prédire le risque de survenue de ces effets indésirables. À ce jour, un petit nombre de polymorphismes de nucleotide simple (single nucleotide polymorphism ou SNP) issus d'une poignée de gènes, a été identifié au cours de plusieurs études. Les SNP des gènes du récepteur à la dopamine et à la sêrotonine ont été les premiers à être étudiés: des metaanalyses convaincantes ont montré une implication des gènes DRD2 et DRD3 (récepteur à la dopamine D2 et D3) dans le risque de dyskinésies tardives (DT) et celle d'une variation du gène du récepteur HT2C (5-HTR2C) dans la prise de poids due aux antipsychotiques. L'importance de ces effets semble néanmoins modeste et, les considérations pharmacoéconomiques étant insuffisamment étudiées, les applications cliniques restent aujourd'hui limitées. Cet article analyse les effets de ces gènes ainsi que d'autres sur le risque de DT, d'effets extrapyramidaux, d'hyperprolactinémie et de prise de poids.
Background
Schizophrenia (SCZ) is a disease with an estimated lifetime morbid risk approaching 1% worldwide,1 and its public health consequences (mortality- and morbidity) are severe. SCZ is associated with an increase of at least 50% in mortality rates compared with the general population,2 including a suicide rate of approximately- 5%,3 resulting in 10-year average lifespan reduction;4 SCZ accounts for nearly 3% of all years lived with disability5; amongst individuals aged 15 to 44, SCZ is the third-leading cause of disability.6
Despite the demonstrated efficacy of antipsychotic drugs (APDs) in short-term placebo-controlled clinical trials, long-term outcomes frequently remain unsatisfactory. The largest NIH-supported clinical trial of antipsychotic agents conducted to date revealed that both first-generation antipsychotics (FGAs) and second -generation antipsychotic (SGA) agents have limited long-term effectiveness, largely due to high rates of discontinuation (-75% discontinuation within 18 months).7 Similar results were obtained in two large-scale European effectiveness trials.8,9 In each of these trials, clinically significant side effects were noted in the majority of patients, and tolerability was the primary cause of at least 20% of all drug discontinuations.
The high likelihood of medication discontinuation has substantial clinical and economic implications, as treatment nonadherence is perhaps the single strongest predictor of relapse and rehospitalization.10 Patients who have discontinued APDs may be as much as five times more likely to relapse as medicated patients.11 Moreover, nearly half of rehospitalization costs in SCZ may be accounted for by medication nonadherence.12 In addition to the effectiveness trials cited above, many observational studies and controlled trials have presented evidence that perceived side-effect burden frequently leads to both poor attitudes towards medications and a tendency towards discontinuation, nonadherence, and partial adherence.13,14 Although side effects are highly prevalent, there is also substantial variability in liability to clinically significant or intolerable adverse events.15 Consequently, understanding and predicting liability to side effects may be an effective strategy- to improve prognosis in schizophrenia.
Antipsychotic-induced side effects
FGAs were most commonly associated with neuromuscular side effects, including the potentially irreversible movement disorder, tardive dyskinesia (TD).16 In large cohort studies, TD has been shown to affect at least one in five, and perhaps as many as one in three, patients treated chronically with FGAs.17 New onset (incidence) of TD is approximately 3% to 5% per year of treatment, and these rates are increased as much as fivefold in elderly patients.18 In addition to physical discomfort and social stigma, presence of TD has been associated with reduced quality of life, increased psychopathology, and increased mortality rates.19 Even at low doses and/or intermittent treatment schedules, the high prevalence and morbidity associated with TD was the primary impetus for the promotion of SGAs as preferred firstline treatment, at least in the United States.15,20 Although use of SGAs is not entirely free from TD risk, incidence and rates are as much as 80% lower for SGAs compared with FGAs.21,22
Though treatable and reversible, extrapyramidal symptoms (EPS) including Parkinsonian motor difficulties as well as akathisia, are highly prevalent with FGAs and are also associated with patient discomfort, dissatisfaction, and discontinuation of treatment.16 Despite the initial optimism that SGAs would greatly reduce EPS burden, most SGAs still demonstrate a clinically relevant tendency to induce these symptoms.23,24 In a large-scale effectiveness trial in chronic SCZ patients, SGAs were indistinguishable from a low-dose FGA (perphenazine) in rates of new onset of akathisia and EPS (5% to 10% each, irrespective of drug assignment).25 However, meta-analytic reviews of the literature demonstrate that overall EPS burden may be reduced by 30% to 50% with SGAs.26 Because the mechanism of action for all currently approved antipsychotic medications remains blockade of dopamine receptors,27 motor and other side effects (eg, prolactin elevation) remain a concern in the treatment of SCZ.
While SGAs have moderately reduced EPS and substantially reduced TD liability relative to FGAs, these newer antipsychotics are most notable for their propensity to induce weight gain,28 as well as related metabolic disturbances such as hypertriglyceridemia and hyperglycemia.29 Clozapine and olanzapine are the APDs most frequently- associated with weight gain, but all APDs, even first-generation agents, seem to share these effects as a group to varying degrees.30 For example, a largescale effectiveness trial in antipsychotic naïve patients demonstrated clinically significant weight gain (≥7% of baseline) in more than half of patients treated with haloperidol.9 Obesity has serious implications for overall health and survival due to an increased risk for cardiovascular and malignant disorders31; these risks may be of particular importance in patients with SZ who often have limited access to health care and decreased motivation for weight reduction secondary to negative symptomatology.13 Unfortunately, APD-induced weight gain is very difficult to reverse, even with sophisticated behavioral, dietary, and pharmacological interventions.32
Pharmacogenetic studies of antipsychotic-induced side effects
While the side effect profile of APDs is extremely burdensome in the aggregate, there is substantial interindividual variation in the degree of any particular motor or metabolic effect for a given patient.15 Despite extensive research over the last two decades, data on clinical or biological predictors of antipsychotic side effects are limited. A few generalizations can be made, but these are not sufficient for individual-level prognosis: i) both the very old and the very young appear to be more susceptible to most APD-induced adverse events18,32; ii) patients experiencing extrapyramidal symptoms are twice as likely to develop TD as patients who do not exhibit EPS33; iii) olanzapine and clozapine have greater liability for metabolic effects and reduced incidence of motoric side effects compared with most other agents7,9,26-30; iv) APD dose may be correlated with some of these effects, but the relationship is weak and even low doses may carry substantial risk.16,17,32 A priori identification of the patients who will be at a higher risk for development of adverse side effects could help clinicians avoid lengthy ineffective APD trials and limit patients' exposure to drug side effects.
Since the mid-1990s, the field of pharmacogenetics has offered the potential for providing readily accessible, immutable biomarkers - DNA sequence variants - that might be predictive of an individual's propensity for both positive and adverse effects of drugs. However, to date, the promise of personalized medicine has remained unfulfilled. Because academic pharmacogenetic research is often limited to small and clinically heterogeneous samples, individual studies have been unable to provide compelling results. Additionally, the modest effect sizes which are common in complex genetics present an obstacle in the quest for valid biomarkers, which require high sensitivity and specificity for individual clinical prediction. Moreover, examination of disparate polymorphisms across a wide variety of candidate genes has created an impression of scattered, unreplicated findings. Recently, however, a series of findings across multiple laboratories have begun to converge for a few genes related to serotonin and dopamine, the most prominent neurotransmitters targeted by APDs. In the subsequent sections, we will focus on the converging evidence implicating the most wellstudied candidates for pharmacogenetic predictors of antipsychotic-induced side effects. Particular emphasis will be placed on single nucleotide polymorphisms (SNPs) that have a sufficient evidence base to have permitted published meta-analytic studies.
Tardive dyskinesia
Tardive dyskinesia is the most extensively studied APDinduced side effect in the pharmacogenetics literature to date. These studies have typically been cross-sectional in nature, with ascertainment based on retrospective identification of cases with varying treatment histories and duration. The ability to study prevalence, rather than incidence in the context of a clinical trial, has permitted cumulative sample sizes in the thousands. It is important to note, however, that this ascertainment strategy may suffer from false negatives (patients with mild or reversible TD) and false positives (patients with acute motoric abnormalities that do not persist). Within this literature, variants within the genes encoding dopamine D2 and D3 receptors have been the primary focus, as detailed below.
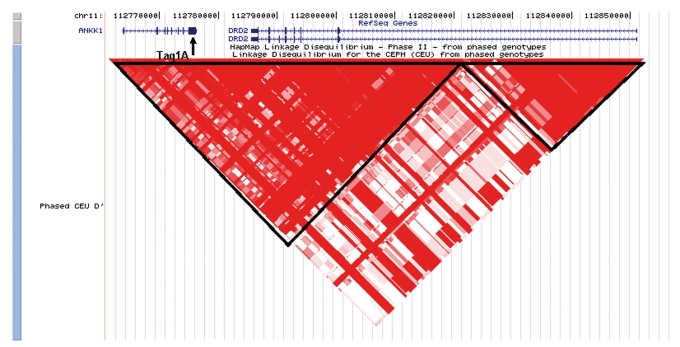
Dopamine D2 receptor blockade is a property of all known antipsychotics, as demonstrated in vitro and in vivo,34 yet a predictive relationship between variation in the DRD2 gene (located on chromosome 11q22) and APD-induced side effects has only been examined in a handful of studies. Most pharmacogenetic studies to date have examined the 3' Taq1A polymorphism (rs1800497), which more recently has been determined to be a nonsynonymous coding SNP in a neighboring ankyrin repeat gene (ANKK1 Glu713Lys).35 Possibly due to linkage disequilibrium with another site (or sites) within DRD2 (Figure 1), the minor (T) allele (also called the Al allele) at rsl800497 has been associated with a 40% reduction in striatal D2 receptor density based on both in vitro assays36 and in vivo imaging studies.37 This allele appears to be protective against TD. As shown in Table I, two recent meta-analyses (based on overlapping sets of studies) have persuasively demonstrated increased rates of TD in A2 (C) allele carriers.38,39 The odds ratio (OR) of 1.30 indicates a 30% increase in risk for TD per allele, so that A2/A2 homozygotes are nearly 80% more likely to develop TD as A1/A1 homozygotes. Alternately, it can be said that AI/AI homozygotes have nearly half the rate of TD compared with A2/A2 homozygotes. However, it is important to note that the A2 allele is the common allele at this SNP, and A1/A1 homozygotes represent <10% of the Caucasian population (Al allele frequencies are much higher in non-white populations).
Figure 1. Location of the Taq1A polymorphism in the context of ANKK1 and DRD2 at chromosome 11q22. Red triangles represent areas of high linkage equilibrium (D').
Table I. List of meta-analytic studies of single nucleotide polymorphisms (SNPs) from candidate genes for tardive dyskinesia (TD), with the associated allele and odds ratio (OR) of the association.
| Gene | SNP | Allele | No of studies | N patients (with /without TD) | OR | Reference |
| DRD2 | Taq1A (rs1800497) | A2 (C) | 6 | 1256(507/749) | 1.30 | Zai et ai 200738 |
| DRD2 | Taq1A (rs1800497) | A2 (C) | 4 | 764(297/467) | 1.30 | Bakker et al 200839 |
| DRD3 | Ser9Gly (rs6280) | Gly(C) | 8 | 780 (317/463) | 1.33 | Lerer et ai 200240 |
| DRD3 | SerSGly (rs6280) | Gly(C) | 11 | 1610(695/915) | 1.17 | Bakker et al 200641 |
| DRD3 | Ser9Gly(rs6280) | Gly(C) | 13 | 2026(928/1098) | 1.16 | Tsai etal 200942 |
| COMT | Val158IMet(rs4680) | Val(G) | 5 | 1089(382/707) | 1.19 | Bakker et al 200839 |
| HTR2A | T102C(rs6313) | C | 6 | 635(256/379) | 1.64 | Lerer et al 200543 |
| CYP2D6 | Loss of function alleles | 8 | 569(220/349) | 1.43 | atsopoulos 200544 | |
| SOD2 | Ala9Val (rs4880) | Ala(T) | 4 | 680(134/546) | 2.04 | Bakker et al 200839 |
Like the D2 receptor, the dopamine D3 receptor is also selectively expressed in the basal ganglia and is considered to be a target of antipsychotic action45; consequently, several pharmacogenetic studies in schizophrenia have examined the DRD3 gene, located on chromosome 3q13.3. To date, only one functional SNP (rs6280), a missense variant resulting in a Ser to Gly substitution at amino acid position 9, has been validated for DRD3.46 The Gly variant has about a 35% allele frequency in non- African populations, and is actually the ancestral allele. The Gly variant has been associated with 4-fold greater dopamine binding affinity in vitro,47 resulting in increased dopamine -mediated cAMP response and prolonged mitogen-associated protein kinase (MAPK) signal.48 Several studies49-52 (but not all)53,54 have indicated that subjects carrying the Gly variant exhibit enhanced symptom response to treatment with clozapine or risperidone.
Concordant with the finding of heightened dopaminergic sensitivity for the Gly allele, multiple studies have demonstrated a significant increase in risk for tardive dyskinesia (TD) amongst Gly carriers. Despite several negative studies in the literature, three recent meta-analytic studies40-42 indicate that this effect is detectable across a large pooled sample including patients of multiple ethnicities (Table I). Intriguingly, a recent studyindicates a strong association of the Gly allele with familial essential tremor, the most common inherited movement disorder.48 However, the effect size for TD risk is modest (OR=1.16 in the largest meta-analysis), with diminishing effects in the more recent studies of this SNP. This pattern of diminishing effect size estimates over time, termed “the winner's curse,” is common in genetics studies and can ultimately- result in rejection of the initial finding as a false positive.55 It is notable that this phenomenon was observed in the context of 13 published studies DRD3 Ser9Gly. Moreover, a very recent study in the large CATIE cohort (n=207 cases with TD vs 503 cases without TD), which was not included in any meta-analysis, demonstrated essentially no effects of either DRD3 Ser9Gly or DRD2 Taq1A.56 Therefore, caution is warranted in the interpretation of other relationships reported across much smaller study sets.
A third dopamine-related gene that has been investigated in multiple pharmacogenetic studies of TD is Catechol Omethyltransferase (COMT). While subcortical dopamine activity is primarily terminated by reuptake mediated by the dopamine transporter, a secondary mechanism for dopamine clearance is metabolic degradation via COMT.57 Additionally, COMT is the predominant mechanism of dopamine clearance in frontal cortex. The COMT gene contains a functional polymorphism that codes for a substitution of methionine (met) for valine (val) at codon 158. The met allele, which has 36% to 48% allele frequency across various ethnicities, results in a thermolabile protein that has one fourth the enzymatic activity of the val carrying protein.58 (In other words, the val allele results in reduced synaptic dopamine due to more rapid clearance). Across five studies meta-analyzed by Bakker and colleagues,39 the val allele was associated with modestly increased risk for TD (OR=1.19; Table I). It is unknown whether the protective effect of the met allele is a direct result of subcortical COMT activity, or is secondary to alterations (eg, upregulation) in frontostriatal circuitry. In addition to dopamine antagonism, one of the common features of many antipsychotics is near-saturation binding of serotonin (5-HT)2 receptors, which has been confirmed in vivo using PET imaging.59,60 While 5-HT binding is often considered a hallmark of SGAs, it is important to note that serotonergic binding properties are observed for several FGAs as well.61,62 The 5-HT2A receptor gene (HTR2A) has been examined in several pharmacogenetic studies of TD; in particular, a promoter region SNP (rs6313), which has been previously- associated with response to antipsychotics (as well as antidepressants), has been extensively studied in relation to TD. While these studies generally converge to indicate a modestly reduced effect of the C allele on symptom response,63 this same allele has been associated with significantly increased risk for tardive dyskinesia.43 As shown in Table I, a recent meta-analysis reported an odds ratio of about 1.6 for C allele carriers across 6 studies; effects were strongest in older patients (age >47 years), and were specifically associated with limb-truncal (but not orofacial) TD43 Notably, this SNP is a perfect proxy for another promoter region SNP,rs6311 (also referred to as -1438G/A), which appears to affect transcription of the receptor.64 Specifically, the G allele (a perfect proxy for the C allele at rs6313) tends to be associated with reduced expression of the receptor. It can therefore be inferred that reduced availability of the 5-HT2A receptor is a risk factor for tardive dyskinesia. Notably, 5-HT2A receptors are strongly expressed in the caudate and putamen,65 and recent evidence obtained from dopamine-depleted rodents suggests a complex interplay of subcortical dopamine and 5-HT in the regulation of motor behavior.66
Two genes outside of the dopamine and 5-HT systems have received sufficient attention in the pharmacogenetics of TD to merit meta-analysis (Table I). Many commonly prescribed APDs, including FGAs (haloperidol, perphenazine, thioridazine), as well as SGAs (risperidone and aripiprazole), are metabolized in the liver by CYP2D6 (debrisoquine hydroxylase).67 The CYP2D6 gene is highly polymorphic, with over 70 known variants (for a current classification, view the allele nomenclature at http://www.imm.ki.se/CYPalleles/). Homozygosity for null alleles gives rise to the “poor metabolizer” phenotype characterized by no enzyme activity while null allele heterozygosity gives rise to an intermediate debrisoquine hydroxylase metabolic phenotype characterized by impaired - but not absent - enzyme activity.68 Reduced CYP2D6 activity can be expected to result in higher effect dose as measured by blood levels of active drug, with potential for increased dose-dependent side effects. Consistent with this pharmacokinetic prediction, a metaanalysis of 8 studies demonstrated a moderate effect of (any) loss of function alleles on risk for TD (OR=1.43), while homozygotes (poor metabolizers) had 1.64-fold greater odds of suffering tardive dyskinesia.44 A recent small study further confirms these results.69 A similar effect has been studied for SOD2, the gene encoding manganese superoxide dismutase, a mitochondrial enzyme involved in oxidative metabolism. A functional SNP (Ala9Val), affecting efficiency of MnSOD transport, has been associated with TD risk; counterintuitively, the less efficient val allele is protective.39 Homozygotes for the Ala (T) allele are about twice as likely to develop TD compared with val carriers (Table I).
Extrapyramidal symptoms
Compared with the relative plethora of studies on tardive dyskinesia, pharmacogenetic studies of EPS are lacking. However, a few studies have reported allelic effects on acute side effects that are consistent with those reported for TD. For example, Eichammer et al70 reported increased incidence of akathisia amongst DRD3 Gly carriers; however, two studies of extrapyramidal symptoms have been negative.71,72 One additional study identified another DRD3 SNP (rs167771) which was associated with EPS in a study of 270 risperidonetreated patients,73 but this result awaits replication. One small study has demonstrated an effect on EPS risk for the C allele of rs6313 in HTR2A that parallels its effect on TD.71 Although not previously examined in TD studies, a SNP in RGS2 (rs4606) has been associated with extrapyramidal symptoms in two studies.74,75 Although a third study was negative, this regulator of intracellular dopamine signaling merits additional research.76
Prolactin elevation
While prolactin elevation has also not been widely studied across most of the genes listed in Table I, there have been seven published studies examining DRD2 TaqlA.77-83 As displayed in Table II, these studies have yielded mixed results across a variety of APDs. Notably, the three positive studies all reported that the Al allele was associated with increased risk for hyperprolactinemia, and a fourth study demonstrated the same effect in females only. This is the opposite allele that was associated with TD, which may reflect the fact that prolactin response is mediated via the tuberoinfundibular pathway (hypothalamus and pituitary).84
Table II. List of studies of the Taq1A polymorphism (rs1800497) from the ANKK1/DRD2 locus in association with antipsychotic drug-related prolactin levels.
| Reference | Drug | N patients | Allele | Significant? |
| Calarge et al 200977 | Risperidone | 107 | A1 (T) | Yes |
| Kwon et al 200878 | Aripiprazole | 90 | No | |
| Yasui-Furukori et al 200879 | Risperidone | 174 | No | |
| Aklillu et al 200780 | Perphenazine | 22 | A1(T) | Yes |
| Anderson et al 200781 | Rispendone | 101 | No | |
| Young et al 200482 | Various | 144 | A1(T) | Yes |
| Mihara et ai 200083 | Nemonapride | 25 | A1(T) | Females only |
Weight gain
It has been suggested that increased 5-HT binding profiles may account for the increased liability to weight gain observed in the second-generation antipsychotics.85 A survey of the literature of the regulation of feeding behavior points to a major role for 5-HT, with both animal and human investigations showing, in general, that increasing 5-HT results in decreased feeding, with the reverse also true.86 Pharmacologic agonists of 5-HT2C lead to decreased feeding in animals87 it is logical to speculate that 5-HT2C antagonists, including most secondgeneration antipsychotics, might lead to increased food intake.
Perhaps the best evidence for a specific role of serotonin-related genetic factors in antipsychotic-induced weight gain is provided by studies of the promoter region polymorphism, -759 T/C (rs3813929), in the HTR2C gene (on the X chromosome). Reynolds and colleagues88 studied 123 adult drug-naïve Han Chinese SCZ patients treated primarily with risperidone or chlorpromazine. Subjects with the T allele at this locus gained significantly less weight than subjects with the C allele in short-term (6- and 10-week) treatment; none of the 27 subjects with the T allele met criteria for severe (>7%) weight gain after 6 weeks, as compared with 28% of the 96 subjects without the T allele. Two studies89,90 also reported an association of the T allele to reduced weight gain in a small samples of clozapinetreated patients, although this effect was only significant in males in one of these. Ellingrod and colleagues91 reported that the T allele is associated with less weight gain in Caucasian patients treated with olanzapine, and Templeman et al92 reported the same for weight gain associated with a mixed group of antipsychotics in a small Spanish first-episode cohort. Recently, Lane et al93 extended these findings to include risperidone (in 123 Han Chinese inpatients), and Ryu et al94 demonstrated the same effect for the T allele in 84 Korean inpatients treated on various antipsychotic monotherapies. A few studies, however, have not detected significant associations between-759 T/C and clozapineinduced weight gain95-97 which may reflect the winner's curse, but it should be noted that these studies were restricted to chronic patients with extensive prior treatment. A meta-analysis of 8 studies demonstrated a greater than 2-fold increase in risk for clinically- significant (7% to 10% or greater) weight gain from baseline associated with the C allele at this SNP.98
Analogous to the aforementioned role of RGS2 in EPS, one gene involved in intracellular signaling has been repeatedly with respect to APD-induced weight gain. GNB3 encodes a subunit of a heterotrimeric guanine nucleotide-binding protein (G protein), which integrates signals between receptors and effector proteins.99 An SNP polymorphism (C825T) in this gene has been associated with essential hypertension and obesity; this SNP is also associated with relative prevalence of a high-activity splice variant of GNB3.100 According to a recent meta-analysis, five studies have examined effects of this SNP on APDinduced weight gain; the T allele was marginally associated with increased weight gain.101 However, this effect was consistent with its effect on BMI and other metabolic variables in the general population, so the mechanism in the context of APD treatment remains unclear.
Conclusions and future directions
As summarized in the preceding sections, pharmacogenetic studies have begun to converge on a few genetic variants that are replicably associated with the common APD-induced motor and metabolic side effects. However, three factors limit the ability of the field to deliver on the promise of personalized medicine at this time, and point to critical issues for the next generation of pharmacogenetic studies. First, a treating psychiatrist would be unable to use this information to offer a validated alternative, due to the lack of pharmacogenetic head-to-head comparisons of treatment with differing mechanisms. Second, even fairly consistent single-gene results, such as those observed for DRD3 and TD, fail to provide large enough effect sizes to make confident clinical decisions. In order to provide a clinically useful test, with sufficient sensitivity and specificity to make confident individual predictions, a combination of SNPs across different loci will be required. Third, the economics of conducting pharmacogenetic tests on a large clinical scale will need to be justified to payers, including the insurance companies and the federal government. In order to do so, pharmacogenetics researchers will need to quantify the beneficial economic impact of tailored prescription practices.102
Of course, any personalized clinical decision-making process will optimally include validated predictors of symptom response as well as adverse effects. The variability in symptom response ranges from patients who experience rapid symptom remission to a subset of patients often described as “treatment-refractory.”15 Even when fully adherent with medication, as many as 40% of patients fail to demonstrate adequate response on the hallmark positive symptoms of hallucinations and delusions.103 Unfortunately, the literature on pharmacogenetics of response is more difficult to summarize than for side effects; due to wide differences in trial methodology and definition of dependent measures, no metaanalytic studies have been published in the last decade. (One early meta-analysis of clozapine response identified an effect of HTR2C T102C, as described earlier.61) Finally, it should be noted that candidate gene approaches to pharmacogenetics run a dual risk of either an overly restrictive search space, or a potentially overwhelming number of candidates. While initial pharmacogenetic studies have primarily focused on dopamine and serotonin genes, the slow pace of individual candidate gene investigations has resulted in many additional scattered and isolated studies across investigators. On the other hand, the advent of genome-wide association studies (GWAS) provides a hypothesis-free method of generating candidate genes for novel complex phenotypes. Unfortunately, this method carries its own statistical concerns, most notably limitations in statistical power (due to correction for multiple comparisons) in necessarily limited clinical trial samples.
One way to enhance sample size and statistical power in the short run is to utilize a strategy that permits crosssectionally defined phenotypes. In a proof of principle study, we have recently utilized the Affymetrix 500K microarray in a sample of our retrospectively-characterized patients with schizophrenia. (Initial case-control analyses were SCZ diagnosis were published for data obtained from the first 322 Caucasian subjects.104 All subjects self-identified as Caucasian non-Hispanic; testing of 210 ancestry informative markers (AIMs) revealed no evidence of population stratification). In this same sample, we have performed a preliminary analysis examining treatment responsiveness, using clozapine assignment as a proxy for poor response. Detailed chart reviews permitted classification of 97% of the sample. Approximately 35% of patients were assigned clozapine due to treatment nonresponsiveness, and groups were matched on key demographic variables including age, duration of illness, sex, and family history. Despite the small sample for this interim analysis, one SNP nearlyobtained genome -wide significance (P=4.3*10-7).This SNP neighbors CNTN4 (contactin-4), a neuronal membrane protein that functions as a cell adhesion molecule, and is thought to be critical for the formation of axon connections in the developing nervous system105; CNTN4 has also recently been implicated in autism.106
In the longer term, much larger prospective studies will be required to achieve to: i) obtain clear estimates for risk parameters; and ii) determine whether application of a pharmacogenetic risk profile is clinically and economically advantageous. Optimally, such studies mayfocus on the first episode of SCZ, which typically occurs in late adolescence or early adulthood107 and may be the most critical period in the life of an individual with SCZ. Successful treatment of the initial psychotic episode is crucial for minimizing the cascading effects of social and vocational deterioration.108,109 From a methodological perspective, studies of first-episode patients minimize potential confounds associated with chronic illness and variable history of prior treatment; first-episode cohorts are also marked by reduced duration of psychotic symptoms, substance abuse, and functional/social disabilities.110 Bycontrast, studies of chronic SCZ may systematically overrepresent patients who are not fully responsive to treatment or are nonadherent to treatment (or both), and underestimate APD response. First-episode samples maybe less biased on these factors and therefore may be more informative about the spectrum of outcomes with APD treatments. While large-scale prospective trials involving first-episode cohorts are logistically challenging, such studies would hold substantial promise for advancing the field in the next decade.
Selected abbreviations and acronyms
- 5-HT
serotonin
- APD
antipsychotic drug
- EPS
extrapyramidal symptom
- FGA
first-generation antipsychotic
- SCZ
schizophrenia
- SGA
second-generation antipsychotic
- SNP
single-nucleotide polymorphism
- TD
tardive dyskinesia
Contributor Information
Todd Lencz, Center for Translational Psychiatry, Feinstein Institute for Medical Research, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA; Division of Psychiatry Research, The Zucker Hillside Hospital, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA; Department of Psychiatry and Behavioral Science, Albert Einstein College of Medicine, Bronx, NY, USA.
Anil K. Malhotra, Center for Translational Psychiatry, Feinstein Institute for Medical Research, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA; Division of Psychiatry Research, The Zucker Hillside Hospital, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA; Department of Psychiatry and Behavioral Science, Albert Einstein College of Medicine, Bronx, NY, USA.
REFERENCES
- 1.Saha S., Chant D., Welham J., McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goff DC., Gather C., Evins AE., Henderson DC., et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–194. doi: 10.4088/jcp.v66n0205. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BW., Heaton RK., Gladsjo JA., et al. Heterogeneity in functional status among older outpatients with schizophrenia: employment history, living situation, and driving. Schizophr Res. 2002;55:205–215. doi: 10.1016/s0920-9964(01)00218-3. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. World Health Report 2001: new understanding, new hope. Geneva: World Health Organization, 2001 [Google Scholar]
- 5.Murray CJL., Lopez AD. The Global Burden of Disease: a Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and projected to 2020. Cambridge, MA: Harvard University Press; 1996 [Google Scholar]
- 6.World Health Organization, international Classification of Functioning, Disability and Health- ICF. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 7.Lieberman JA., Stroup TS., McEvoy JP., et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 8.Jones PB., Barnes TR., Davies L., et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 9.Kahn RS., Fleischhacker WW., Boter H., et al. EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 10.Kane JM. Treatment adherence and long-term outcomes. CNS Spectr. 2007;12(10suppl 17):21–26. doi: 10.1017/s1092852900026304. [DOI] [PubMed] [Google Scholar]
- 11.Robinson D., Woerner MG., Alvir JMJ., et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 12.Weiden PJ., Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21:419–429. doi: 10.1093/schbul/21.3.419. [DOI] [PubMed] [Google Scholar]
- 13.Correll CU. Balancing efficacy and safety in treatment with antipsychotics. CNS Spectr. 2007;12(10 suppl 17):12–20. doi: 10.1017/s1092852900026298. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DG., Woerner MG., Alvir JM., Bilder RM., Hinrichsen GA., Lieberman JA. Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophr Res. 2002;57:209–219. doi: 10.1016/s0920-9964(01)00312-7. [DOI] [PubMed] [Google Scholar]
- 15.Tandon R., Belmaker RH., Gattaz WF., et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res. 2008;100:20–38. doi: 10.1016/j.schres.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Casey DE. Neuroleptic drug-induced extrapyramidal syndromes and tardive dyskinesia. Schizophr Res. 1991;4:109–120. doi: 10.1016/0920-9964(91)90029-q. [DOI] [PubMed] [Google Scholar]
- 17.Kane JM., Woerner M., Lieberman J. Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol. 1988;8(4 suppl):52S–56S. [PubMed] [Google Scholar]
- 18.Jeste DV., Caligiuri MP. Tardive dyskinesia. Schizophr Bull. 1993;19:303–315. doi: 10.1093/schbul/19.2.303. [DOI] [PubMed] [Google Scholar]
- 19.Ascher-Svanum H., Zhu B., Faries D., Peng X., Kinon BJ., Tohen M. Tardive dyskinesia and the 3-year course of schizophrenia: results from a large, prospective, naturalistic study. J Clin Psychiatry. 2008;69:1580–1588. doi: 10.4088/jcp.v69n1008. [DOI] [PubMed] [Google Scholar]
- 20.Lehman AF., Lieberman JA., Dixon LB., et al. American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2suppl):1–56. [PubMed] [Google Scholar]
- 21.Kane JM. Tardive dyskinesia rates with atypical antipsychotics in adults: prevalence and incidence. J Clin Psychiatry. 2004;65 suppl 9:16–20. Review. [PubMed] [Google Scholar]
- 22.Correll CU., Leucht S., Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- 23.Kane JM., Fleischhacker WW., Hansen L., Perlis R., Pikalov A 3rd., Assunçâo-Talbott S. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70:627–643. doi: 10.4088/JCP.08r04210. [DOI] [PubMed] [Google Scholar]
- 24.Leucht S., Wahlbeck K., Hamann J., Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361:1581–1589. doi: 10.1016/S0140-6736(03)13306-5. [DOI] [PubMed] [Google Scholar]
- 25.Miller del D., Caroff SN., Davis SM., et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008;193:279–288. doi: 10.1192/bjp.bp.108.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leucht S., Corves C., Arbter D., Engel RR., Li C., Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a metaanalysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 27.Kapur S., Matno D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Parsons B., Allison DB., Loebel A., Williams K., Giller E., Romano S., Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Kane JM., Barrett EJ., Casey DE., Correll CU., Gelenberg AJ., Klein S., Newcomer JW. Metabolic effects of treatment with atypical antipsychotics. J Clin Psychiatry. 2004;65:1447–1455. doi: 10.4088/jcp.v65n1102. [DOI] [PubMed] [Google Scholar]
- 30.Allison DB., Mentore JL., Heo M., Chandler LP., Cappelleri JC., Infante MC., Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1 999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 31.Allison DB., Newcomer JW., Dunn AL., et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am JPrevMed. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Correll CU. Monitoring and management of antipsychotic-related metabolic and endocrine adverse events in pediatric patients. Int Rev Psychiatry. 2008;20:195–201. doi: 10.1080/09540260801889179. [DOI] [PubMed] [Google Scholar]
- 33.Tenback DE., van Harten PN., Slooff CJ., van Os J. Evidence that early extrapyramidal symptoms predict later tardive dyskinesia: a prospective analysis of 10,000 patients in the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Am J Psychiatry. 2006;163:1438–1440. doi: 10.1176/ajp.2006.163.8.1438. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S., Barlow K., VanderSpek SC., Javanmard M., Nobrega JN. Druginduced receptor occupancy: substantial differences in measurements made in vivo vs ex vivo. Psychopharmacology (Berl). 2001;157:168–171. doi: 10.1007/s002130100790. [DOI] [PubMed] [Google Scholar]
- 35.Neville MJ., Johnstone EC., Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 36.Thompson J., Thomas N., Singleton A., et al. D2 dopamine receptor gene (DRD2) TaqIA polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Pohjalainen T., Rinne JO., Nâgren K., et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 38.Zai CC., De Luca V., Hwang RW., et al. Meta-analysis of two dopamine D2 receptor gene polymorphisms with tardive dyskinesia in schizophrenia patients. Mol Psychiatry. 2007;12:794–795. doi: 10.1038/sj.mp.4002023. [DOI] [PubMed] [Google Scholar]
- 39.Bakker PR., van Harten PN., van Os J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1 A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry. 2008;13:544–556. doi: 10.1038/sj.mp.4002142. [DOI] [PubMed] [Google Scholar]
- 40.Lerer B., Segman RH., Fangerau H., et al. Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology. 2002;27:105–119. doi: 10.1016/S0893-133X(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 41.Bakker PR., van Harten PN., van Os J. Antipsychotic-induced tardive dyskinesia and the Ser9Gly polymorphism in the DRD3 gene: a meta analysis. Schizophr Res. 2006;83:185–192. doi: 10.1016/j.schres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Tsai HT., North KE., West SL., Poole C. The DRD3 rs6280 polymorphism and prevalence of tardive dyskinesia: A meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 8. [Epub ahead of print] doi: 10.1002/ajmg.b.30946. [DOI] [PubMed] [Google Scholar]
- 43.Lerer B., Segman RH., Tan EC., et al. Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol. 2005;8:411–425. doi: 10.1017/S1461145705005389. [DOI] [PubMed] [Google Scholar]
- 44.Patsopoulos NA., Ntzani EE., Zintzaras E., loannidis JP. CYP2D6 polymorphisms and the risk of tardive dyskinesia in schizophrenia: a meta-analysis. Pharmacogenet Genomics. 2005;15:151–158. doi: 10.1097/01213011-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz JC., Diaz J., Pilon C., Sokoloff P. Possible implications of the dopamine D receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31:277–287. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JC., Levesque D., Martres MP., Sokoloff P. Dopamine D3 receptor: basic and clinical aspects. Clin Neuropharmacol. 1993;16:295–314. doi: 10.1097/00002826-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Lundstrom K., Turpin MP. Proposed schizophrenia-related gene polymorphism: expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki forest virus system. Biochem Biophys Res Commun. 1996;23:225:1068–1072. doi: 10.1006/bbrc.1996.1296. [DOI] [PubMed] [Google Scholar]
- 48.Jeanneteau F., Funalot B., Jankovic J., et al. Afunctional variant of the dopamine D3 receptor is associated with risk and age-at- onset of essential tremor. Proc Natl Acad Sci USA. 2006:11;103:10753–10758. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaikh S., Collier DA., Sham PC., et al. Allelic association between a Ser9-Gly polymorphism in the dopamine D3 receptor gene and schizophrenia. Hum Genet. 1996;97:714–719. doi: 10.1007/BF02346178. [DOI] [PubMed] [Google Scholar]
- 50.Scharfetter J., Chaudhry HR., Hornik K., et al. Dopamine D3 receptor gene polymorphism and response to clozapine in schizophrenic Pakistani patients. Eur Neuropsychopharmacol. 1999;10:17–20. doi: 10.1016/s0924-977x(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 51.Szekeres G., Kéri S., Juhâsz A., et al. Role of dopamine D3 receptor (DRD3) and dopamine transporter (DAT) polymorphism in cognitive dysfunctions and therapeutic response to atypical antipsychotics in patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;124:1–5. doi: 10.1002/ajmg.b.20045. [DOI] [PubMed] [Google Scholar]
- 52.Lane HY., Hsu SK., Liu YC., Chang YC., Huang CH., Chang WH. Dopamine D3 receptor Ser9Gly polymorphism and risperidone response. J Clin Psychopharmacol. 2005;25:6–11. doi: 10.1097/01.jcp.0000150226.84371.76. [DOI] [PubMed] [Google Scholar]
- 53.Malhotra AK., Goldman D., Buchanan RW., et al. The dopamine D3 receptor (DRD3) Ser9Gly polymorphism and schizophrenia: a haplotype relative risk study and association with clozapine response. Mol Psychiatry. 1998;3:72–75. doi: 10.1038/sj.mp.4000288. [DOI] [PubMed] [Google Scholar]
- 54.Xuan J., Zhao X., He G., Yu L., et al. Effects of the dopamine D Receptor (DRD3) gene polymorphisms on risperidone response: a pharmacogenetic study. Neuropsychopharmacoiogy. 2008;33:305–311. doi: 10.1038/sj.npp.1301418. [DOI] [PubMed] [Google Scholar]
- 55.Xiao R., Boehnke M. Quantifying and correcting for the winner's curse in genetic association studies. Genet Epidemiol. 2009;33:453–462. doi: 10.1002/gepi.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai HT., Caroff SN., Miller DD., et al. A candidate gene study of tardive dyskinesia in the CATIE schizophrenia trial. Am J Med Genet B Neuropsychiatr Genet. 2009 May 27. [Epub ahead of print] doi: 10.1002/ajmg.b.30981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mànnistô PT., Ulmanen I., Lundstrôm K., et al. Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res. 1992;39:291–350. doi: 10.1007/978-3-0348-7144-0_9. [DOI] [PubMed] [Google Scholar]
- 58.Lachrnan HM., Papolos DF., Saito T., Yu YM., Szumlanski CL., Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatrie disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Farde L., Nyberg S., Oxenstierna G., Nakashima Y., Halldin C., Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidonetreated schizophrenic patients. J Clin Psychopharmacol. 1995;15:19S–23S. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 60.Kapur S., Zipursky RB., Remington G., et al. 5- HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–928. doi: 10.1176/ajp.155.7.921. [DOI] [PubMed] [Google Scholar]
- 61.Rasmussen K., Aghajanian GK. Potency of antipsychotics in reversing the effects of a hallucinogenic drug on locus coeruleus neurons correlates with 5-HT2 binding affinity. Neuropsychopharmacoiogy. 1988;1:101–107. doi: 10.1016/0893-133x(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 62.Trichard C., Paillère-Martinot ML., Attar-Levy D., Recassens C., Monnet F., Martinet JL. Binding of antipsychotic drugs to cortical 5-HT2A receptors: a PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatry. 1998;155:505–508. doi: 10.1176/ajp.155.4.505. [DOI] [PubMed] [Google Scholar]
- 63.Arranz MJ., Munro J., Sham P., et al. Meta-analysis of studies on genetic variation in 5-HT2A receptors and clozapine response. Schizophr Res. 1998;32:93–99. doi: 10.1016/s0920-9964(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 64.Myers RL., Airey DC., Manier DH., Shelton RC., Sanders-Bush E. Polymorphisms in the regulatory region of the human serotonin 5-HT2A receptor gene (HTR2A) influence gene expression. Biol Psychiatry. 2007;61:167–173. doi: 10.1016/j.biopsych.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Waeber C., Palacios JM. Binding sites for 5-hydroxytryptamine-2 receptor agonists are predominantly located in striosomes in the human basal ganglia. Brain Res Mol Brain Res. 1994;24:199–209. doi: 10.1016/0169-328x(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 66.Bishop C., Tessmer JL., Ullrich T., Rice KC., Walker PD. Serotonin 5-HT2A receptors underlie increased motor behaviors induced in dopaminedepleted rats by intrastriatal 5-HT2A/2C agonism. J Pharmacol Exp Ther. 2004;310:687–694. doi: 10.1124/jpet.104.066365. [DOI] [PubMed] [Google Scholar]
- 67.Caccia S. New antipsychotic agents for schizophrenia: pharmacokinetics and metabolism update. Curr Opin Investig Drugs. 2002;3:1073–1080. [PubMed] [Google Scholar]
- 68.Bertilsson L., Dahl ML., Dalén P., Al-Shurbaji A. Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53:111–122. doi: 10.1046/j.0306-5251.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobylecki CJ., Jakobsen KD., Hansen T., Jakobsen IV., Rasmussen HB., Werge T. CYP2D6 genotype predicts antipsychotic side effects in schizophrenia inpatients: a retrospective matched case-control study. Neuropsychobiology. 2009;59:222–226. doi: 10.1159/000223734. [DOI] [PubMed] [Google Scholar]
- 70.Eichhammer P., Albus M., Borrmann-Hassenbach M., et al. Association of dopamine D3-receptor gene variants with neuroleptic induced akathisia in schizophrenic patients: a generalization of Steen's study on DRD3 and tardive dyskinesia. Am J Med Genet. 2000;96:187–119. doi: 10.1002/(sici)1096-8628(20000403)96:2<187::aid-ajmg13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 71.Gunes A., Scordo MG., Jaanson P., Dahl ML. Serotonin and dopamine receptor gene polymorphisms and the risk of extrapyramidal side effects in perphenazine-treated schizophrenic patients. Psychopharmacology (Berl). 2007;190:479–484. doi: 10.1007/s00213-006-0622-x. [DOI] [PubMed] [Google Scholar]
- 72.Guzey C., Scordo MG., Spina E., Landsem VM., Spigset O. Antipsychoticinduced extrapyramidal symptoms in patients with schizophrenia: associations with dopamine and serotonin receptor and transporter polymorphisms. Eur J Clin Pharmacol. 2007;63:233–241. doi: 10.1007/s00228-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 73.Gassô P., Mas S., Bernardo M., Alvarez S., Parellada E., Lafuente A. A common variant in DRD3 gene is associated with risperidone-induced extrapyramidal symptoms. Pharmacogenomics J. 2009;9:404–410. doi: 10.1038/tpj.2009.26. [DOI] [PubMed] [Google Scholar]
- 74.Greenbaum L., Strous RD., Kanyas K., et al. Association of the RGS2 gene with extrapyramidal symptoms induced by treatment with antipsychotic medication. Pharmacogenet Genomics. 2007;17:519–528. doi: 10.1097/FPC.0b013e32800ffbb4. [DOI] [PubMed] [Google Scholar]
- 75.Greenbaum L., Smith RC., Rigbi A., et al. Further evidence for association of the RGS2 gene with antipsychotic-induced parkinsonism: protective role of a functional polymorphism in the 3'-untranslated region. Pharmacogenomics J. 2009;9:103–110. doi: 10.1038/tpj.2008.6. [DOI] [PubMed] [Google Scholar]
- 76.Al Hadithy AF., Wilffert B., Bruggeman R., et al. Lack of association between antipsychotic-induced Parkinsonism or its subsyrnptorns and rs4606 SNP of RGS2 gene in African-Caribbeans and the possible role of the medication: the Curacao extrapyramidal syndromes study X. Hum Psychopharmacol. 2009;24:123–128. doi: 10.1002/hup.997. [DOI] [PubMed] [Google Scholar]
- 77.Calarge CA., Ellingrod VL., Acion L., et al. Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics. 2009;19:373–382. doi: 10.1097/FPC.0b013e328329a60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon JS., Kim E., Kang DH., Choi JS., Yu KS., Jang IJ., Shin SG. APLUS study group. TaqlA polymorphism in the dopamine D2 receptor gene as a predictor of clinical response to aripiprazole. fur. Neuropsychopharmacol. 2008;18:897–907. doi: 10.1016/j.euroneuro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Yasui-Furukori N., Saito M., Tsuchimine S., et al. Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1491–1495. doi: 10.1016/j.pnpbp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Aklillu E., Kalow W., Endrenyi L., Harper P., Miura J., Ozdemir V. CYP2D6 and DRD2 genes differentially impact pharmacodynamic sensitivity and time course of prolactin response to perphenazine. Pharmacogenet Genomics. 2007;17:989–993. doi: 10.1097/FPC.0b013e3282f01aa3. [DOI] [PubMed] [Google Scholar]
- 81.Anderson GM., Scahill L., McCracken JT., et al. Effects of short- and longterm risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Young RM., Lawford BR., Barnes M., et al. Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2*A1 allele. Br J Psychiatry. 2004;185:147–151. doi: 10.1192/bjp.185.2.147. [DOI] [PubMed] [Google Scholar]
- 83.Mihara K., Kondo T., Suzuki A., et al. Prolactin response to nemonapride, a selective antagonist for D2 like dopamine receptors, in schizophrenic patients in relation to TaqlA polymorphism of DRD2 gene. Psychopharmacology (Berl). 2000;149:246–250. doi: 10.1007/s002139900364. [DOI] [PubMed] [Google Scholar]
- 84.Compton MT., Miller AH. Antipsychotic-induced hyperprolactinemia and sexual dysfunction. Psychopharmacol Bull . 2002;36:143–164. [PubMed] [Google Scholar]
- 85.Reynolds GP. Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. J Psychopharmacol. 2004;18:340–345. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- 86.Adan RA., Vanderschuren LJ., la Fleur SE. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci. 2008;29:208–217. doi: 10.1016/j.tips.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Davis R., Faulds D. Dexfenf luramine. An updated review of its therapeutic use in the management of obesity. Drugs. 1996;52:696–724. doi: 10.2165/00003495-199652050-00007. [DOI] [PubMed] [Google Scholar]
- 88.Reynolds GP., Zhang ZJ., Zhang XB. Association of antipsychotic druginduced weight gain with a 5-HT2C receptor gene polymorphism. Lancet. 2002;359:2086–2087. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- 89.Miller DD., Ellingrod VL., Holman TL., Buckley PF., Arndt S. Clozapineinduced weight gain associated with the 5HT2C receptor -759C/T polymorphism. Am J Med Genet. 2005;133:97–100. doi: 10.1002/ajmg.b.30115. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds GP., Zhang Z., Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Ami J Psychiatry. 2003;160:677–679. doi: 10.1176/appi.ajp.160.4.677. [DOI] [PubMed] [Google Scholar]
- 91.Ellingrod VL., Perry PJ., Ringold JC., et al. Weight gain associated with the -759C/T polymorphism of the 5HT2C receptor and olanzapine. Am J Med Genet. B Neuropsychiatr Genet. 2005;34:76–78. doi: 10.1002/ajmg.b.20169. [DOI] [PubMed] [Google Scholar]
- 92.Templeman LA., Reynolds GP., Arranz B., San L. Polymorphisms of the 5HT2C receptor and leptin genes are associated with antipsychotic druginduced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15:195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 93.Lane HY., Liu YC., Huang CL., et al. Risperidone-related weight gain: genetic and nongenetic predictors. J Clin Psychopharmacol. 2006;26:128–134. doi: 10.1097/01.jcp.0000203196.65710.2b. [DOI] [PubMed] [Google Scholar]
- 94.Ryu S., Cho EY., Park T. -759 QT polymorphism of 5-HT2C receptor gene and early phase weight gain associated with antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:673–677. doi: 10.1016/j.pnpbp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 95.Tsai SJ., Hong CJ., Yu YW., Lin CH. -759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet. 2002;360:1790. doi: 10.1016/S0140-6736(02)11705-3. [DOI] [PubMed] [Google Scholar]
- 96.Basile VS., Masellis M., De Luca V., Meltzer HY., Kennedy JL. 759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet. 2002;360:1790–1791. doi: 10.1016/s0140-6736(02)11706-5. [DOI] [PubMed] [Google Scholar]
- 97.Theisen FM., Hinney A., Brômel T., et al. Lack of association between the -759C/T polymorphism of the 5-HT2C receptor gene and clozapine-induced weight gain among German schizophrenic individuals. Psychiatr Genet. 2004;14:139–142. doi: 10.1097/00041444-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 98.Reynolds GP., Templeman LA., Zhang ZJ. The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1021–1028. doi: 10.1016/j.pnpbp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 99.Rosskopf D., Busch S., Manthey I., Siffert W. G protein beta 3 gene: structure, promoter, and additional polymorphisms. Hypertension. 2000;36:33–41. doi: 10.1161/01.hyp.36.1.33. [DOI] [PubMed] [Google Scholar]
- 100.Siffert W. G-protein beta3 subunit 825T allele and hypertension. Curr Hypertens Rep. 2003;5:47–53. doi: 10.1007/s11906-003-0010-4. [DOI] [PubMed] [Google Scholar]
- 101.Souza RP., De Luca V., Muscettola G., et al. Association of antipsychotic induced weight gain and body mass index with GNB3 gene: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1848–1853. doi: 10.1016/j.pnpbp.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 102.erlis RH., Ganz DA., Avorn J. Pharmacogenetic testing in the clinical management of schizophrenia: a decision-analytic model. Clin Psychopharmacol. 2005;25:427–434. doi: 10.1097/01.jcp.0000177553.59455.24. [DOI] [PubMed] [Google Scholar]
- 103.Conley RR., Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 104.Lencz T., Morgan TV., Athanasiou M., et al. Converging evidence for a pseudoautosotnal cytokine receptor gene locus in schizophrenia. Mol Psychiatry. 2007;12:572–580. doi: 10.1038/sj.mp.4001983. [DOI] [PubMed] [Google Scholar]
- 105.Osterfield M., Egelund R., Young LM., Flanagan JG. Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development. 2008;135:1189–1199. doi: 10.1242/dev.007401. [DOI] [PubMed] [Google Scholar]
- 106.Roohi J., Montagna C., Tegay DH., et al. Disruption of contactin 4 in three subjects with autism spectrum disorder. J Med Genet. 2009;46:176–182. doi: 10.1136/jmg.2008.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.icchioni MM., Murray RM. Schizophrenia. BMJ. 2007;335:91–95. doi: 10.1136/bmj.39227.616447.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larsen TK., Friis S., Haahr U., et al. Early detection and intervention in first-episode schizophrenia: a critical review. Acta Psychiatr Scand. 2001;103:323–334. doi: 10.1034/j.1600-0447.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 109.Melle I., Larsen TK., Haahr U., et al. Prevention of negative symptom psychopathologies in first-episode schizophrenia: two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry. 2008;65:634–640. doi: 10.1001/archpsyc.65.6.634. [DOI] [PubMed] [Google Scholar]
- 110.Lencz T., Robinson DG., Xu K., et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in firstepisode schizophrenia patients. Am J Psychiatry. 2006;163:529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]