Abstract
Genetic epidemiologic studies indicate that all ten personality disorders (PDs) classified on the DSM-IV axis II are modestly to moderately heritable. Shared environmental and nonadditive genetic factors are of minor or no importance. No sex differences have been identified. Multivariate studies suggest that the extensive comorbidity between the PDs can be explained by three common genetic and environmental risk factors. The genetic factors do not reflect the DSM-IV cluster structure, but rather: i) broad vulnerability to PD pathology or negative emotionality; ii) high impulsivity/low agreeableness; and iii) introversion. Common genetic and environmental liability factors contribute to comorbidity between pairs or clusters of axis I and axis II disorders. Molecular genetic studies of PDs, mostly candidate gene association studies, indicate that genes linked to neurotransmitter pathways, especially in the serotonergic and dopaminergic systems, are involved. Future studies, using newer methods like genome-wide association, might take advantage of the use of endophenotypes.
Keywords: personality disorder, axis I disorder, genetics, twin study, molecular genetic study, candidate gene
Abstract
Los estudios de epidemiología genética señalan que los diez trastornos de personalidad (TP) clasificados en el eje II del DSM-IV tienen una herencia leve a moderada. Los factores ambientales compartidos y genéticos no aditivos son de importancia menor o carecen de ésta. No se han identificado diferencias por sexo. Los estudios multivariados sugieren que la amplia comorbilidad entre los TP se puede explicar por tres factores de riesgo ambientales y genéticos comunes. Los factores genéticos no reflejan la estructura de grupos del DSM-IV, pero sí: 1) la alta vulnerabilidad para la patología de los TP o para la emocionalidad negativa, 2) la alta impulsividad/baja afabilidad y 3) la introversión. Los factores de riesgo genéticos y ambientales comunes contribuyen a la comorbilidad entre parejas o grupos de trastornos de los ejes I y II. Los estudios de genética molecular de los TP, principalmente los estudios de asociación de genes candidatos, señalan que están involucrados los genes vinculados a los sistemas de neurotransmisión, principalmente serotoninérgicos y dopaminérgicos. Estudios a futuro, que utilicen métodos más nuevos como la asociación del genoma completo, pueden aprovechar el empleo de endofenotipos.
Abstract
Des études d'épidémiologie génétique montrent que les 10 troubles de la personnalité (TP) classés sur l'axe II du DSM-IV sont légèrement à modérément transmissibles. Les facteurs génétiques non additifs et les facteurs environnementaux partagés sont de peu ou sans importance et il n'y a pas de différences selon le sexe. Des études multivariées suggèrent que trois facteurs de risque génétiques et environnementaux courants peuvent expliquer la comorbidité importante entre les TP. Les facteurs génétiques ne reflètent pas la structure en cluster du DSM-IV mais plutôt: 1) une grande vulnérabilité aux TP ou à une émotivité négative ; 2) une impulsivité importante/peu d'amabilité ; 3) une introversion. Des facteurs de susceptibilité génétiques et environnementaux communs participent à la comorbidité entre les paires ou les groupes des troubles de l'axe I et de l'axe II. Des études de génétique moléculaire des TP, pour la plupart des études d'association de gène candidat, montrent que sont impliqués les gènes liés aux voies des neurotransmetteurs, surtout dans les systèmes sérotoninergiques et dopaminergiques. Des études futures, utilisant la méthodologie de recherche d'associations sur génome entiers pourraient bénéficier de l'utilisation d' endophénotypes.
The introduction of personality disorders (PDs) as diagnostic categories on a separate axis (Axis II) in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) in 19801 had a dramatic effect on the level of interest in these disorders among researchers, and the number of published articles increased substantially. However, the number of genetic epidemiologic studies of the DSM PDs has remained limited compared with studies on both clinical disorders like schizophrenia, depression, and anxiety disorders (which are classified on Axis I in DSM), and on normal personality traits.2-4
The understanding of the role of genetic factors in the etiology of disorders and traits is inseparably linked to classification, since a precise definition of the phenotype is a prerequisite for all successful genetic studies. In this review we will focus on PDs as they are classified in the DSM; a system that serves many purposes, and is not specifically designed for genetic studies. This is a problem not only for the genetics of PDs, and the search for better phenotypes for genetic studies of mental disorders is especially well illustrated in the literature on schizophrenia (eg, refs 5, 6).
The goal of psychiatric genetic epidemiology is to understand the role of genetic and environmental factors in the etiology of mental disorders.7 In this paper we will focus mainly on the genetic factors. After a brief outline of the current DSM axis II PD classification, we will evaluate the evidence for genetic influences on PDs and examine quantitative genetic studies that explore the specificity of the genetic effects, ie, to what extent genetic risk factors are shared between PDs, or between PDs and axis I disorders. Molecular genetic studies that aim to identify gene variants associated with PDs will then be reviewed. It is likely that PDs, like most other psychiatric disorders, are etiologically complex, ie, that they are influenced by a number of genetic and environmental risk factors. Studies examining the interplay between genes and the environment will be addressed both in relation to quantitative and molecular methods. Finally, future directions will be discussed.
The classification of personality disorders
A PD is defined by DSM-IV as an enduring pattern ofinner experience and behavior that deviates markedlyfrom the expectations of the individual's culture, is per-vasive and inflexible, has an onset in adolescence orearly adulthood, is stable over time, and leads to distressor impairment.8 The DSM-IV classification includes 10 categorical PD diagnoses grouped into three clusters: A or the “odd-eccentric,” B or the “dramatic-emotional,” and C or the “anxious-fearful.”8 Cluster A includes para-noid, schizoid, and schizotypal PD, and Cluster B anti-social, borderline, histrionic, and narcissistic PD, whilecluster C includes avoidant, dependent, and obsessive-compulsive PD. Appendix B includes two additional dis-orders: depressive and passive-aggressive PDs.
Although the classification of PDs in DSM-IV is moreempirically based than in former versions, there are several controversial issues that are unresolved. Substantialco-occurrence between the DSM PDs has consistentlybeen found in both clinical9and community samples.10,10The majority of individuals with a PD receive more thanone PD diagnosis, and this high degree of overlap seri-ously challenges the descriptive validity of the PD classification. Comorbidity with Axis I disorders is alsoextensive, and results from both clinical and population-based studies indicate that the key features in the DSM-IV definition (stability over time and early age of onset)do not distinguish PDs from axis I disorders.12 Theunderlying validity of the DSM axis I - axis II divisionhas therefore been questioned (eg, refs 12-14). Thehigher order clustering system has serious limitations,and has not been consistently validated,8 and factor analytic studies often do not find support for this three-factor structure.15 One of the most controversial and longstanding issues in the field of PD classification is, however, whether PDs should be conceptualized dimensionally or as discrete categories. There seems to be a general agreement that PDs are best classified dimensionally,16-18 and several alternative systems are discussed for DSM-V (see ref 19).
Basic quantitative studies
In quantitative genetics, which include family, twin, and adoption studies, the degree to which individual liability to a disorder results from familial effects (in family studies) or genetic and environmental factors (in twin and adoption studies) is estimated. Twin studies have been most commonly used to examine the effects of genetic risk factors on mental disorders, including PDs, and sophisticated analytical models and statistical tools have been developed.20,21 The proportion of phenotypic differences between individuals (or proportion of variance) in a particular population that can be attributed to genetic differences is called heritability. In the classical twin model the total variance in a phenotype is partitioned into three variance components, each accounted for by three latent variables: additive genetic, shared environment, and individual-specific environment. This implies that the genetic and environmental effects are not directly measured, ie, we do not know which specific genes or environmental factors influencing the phenotype. Genetic effects are usually additive, meaning that the independent effects of different alleles or loci act in an additive way to increase risk for the disorder or trait, but they can also be nonadditive, which means that different alleles or loci interact with other alleles or loci (epistasis) or different alleles in the same locus (dominance). Shared environment includes all environmental exposures that contribute to making twins similar, and individual-specific or unique environment includes all environmental exposures that make them different, plus measurement error.
Modern twin studies are based on the liability-threshold model,22 which assumes that a large number of genetic and environmental risk factors with small individual effects are involved, resulting in a distribution of liability or risk in the population that approximates normality. A dichotomous disorder will appear when a certain threshold is exceeded. Twin studies can be used regardless of whether PDs are defined categorically or dimensionally, but the statistical power is higher if the phenotype is ordinal or continuous.23
Normal and abnormal personality traits
Normal personality traits have repeatedly been shown tobe influenced by genetic factors with heritability estimatesranging from approximately 30% to 60%.24,25 The genetic effects are mainly additive, but nonadditive contributionsof a smaller magnitude have been identified in studies with sufficient statistical power.24 Shared environmentalfactors are usually found to be of minor on no impor-tance.24 Similar heritability estimates have been found fora dimensional classification of personality disorders basedon self-report.26 Numerous studies have shown relativelyhigh correlations between DSM PDs and normal personality traits of the five-factor model, which includes fivebroad bipolar domains of extraversion (vs introversion), agreeableness (vs antagonism) conscientiousness (vsimpulsivity), neuroticism (vs emotional stability), andopenness (vs closedness to experience),27 but the extent towhich this is due to genetic factors is not known.
DSM personality disorders
Cluster A
Prior studies have suggested that familial/genetic factors contribute to the etiology of the three PDs making up the DSM Cluster A.28 A series of twin studies that examine various measures of schizoid, schizotypal, and paranoidlike traits using self-report questionnaires have nearly uniformly found significant heritability for these traits and failed to find shared environmental effects (eg, refs 29-33). Heritabilities are typically in the range of 35% to 60%. In a twin study using structured interview data, but based on a clinical sample, Torgersen et al34 found lower heritability estimates for paranoid PD (28%) and schizoid PD (29%), but much higher heritability for schizotypal PD (61%). The method of ascertainment and the relatively low number of participants make the estimates from this study uncertain. In a more recent population-based study of dimensional representations of the DSM-IV cluster A PDs based on structured interviews, Kendler et al35 estimated heritability to be 21% for paranoid, 28% for schizotypal, and 26% for schizoid PD. No shared environmental effects or sex differences were found.
In twin studies unreliability of measurement will decrease the heritability estimates. Although the inter-rater reliability in Kendler et al's abovementioned study was excellent, the test-retest reliability or stability of measurement for PDs has been shown to be imperfect.36 It is also likely that genetic and environmental risk factors assessed by self-report questionnaires vs interviews are different. A second study from the same sample was therefore undertaken.37 Data from a previous self-report questionnaire study were used in addition to the abovementioned interview data to account for unreliability of measurement by using two measures differing in both time and mode of assessment. The estimated heritabilities were substantially higher than in the first study: 66% for paranoid, 55% to 59% for schizoid, and 72% for schizotypal PD.
Cluster B
Antisocial PD-like measures have been extensively studied using genetic epidemiological methods. In a metaanalysis of 51 twin and adoption studies on antisocial behavior based largely on records, self-report, and family report, Rhee & Waldman38 found that the variance could most parsimoniously be explained by additive genetic factors (32%), nonadditive genetic factors (9%), shared environmental factors (16%) and individual-specific environmental factors (43%). There were no significant differences in the magnitude of genetic and environmental influences for males and females.
In a review of family studies on borderline PD, White et al39 found the disorder to aggregate in families. However, significant methodological problems made the results uncertain. Distel et al estimated that additive genetic factors explained 42% of the variance in borderline PD features assessed by self -report questionnaire, using data from three countries.40 Non-shared environment accounted for the rest. In a subsequent extended twin-family study by the same group the heritability of borderline PD features was found to be 45%, but the genetic effects were both additive (21%) and dominant (24%).41 Nonadditive effects are difficult to detect using the classical twin model due to lack of statistical power.23 However, such effects have been found for normal personality traits in twin-sibling studies with large samples.42
Results from a twin study based on structured interviews in a clinical sample suggest that heritability estimates for borderline, histrionic, and narcissistic PD were high, 69%, 63%, and 77% respectively.34 More recently, however, Torgersen et al43 conducted a population-based twin study of dimensional representations of the DSM-IV cluster B PDs. Heritability was estimated to be 38% for antisocial PD, 31% for histrionic PD, 24% for narcissistic PD and 35% for borderline PD. No shared environmental influences or sex or effects were found.
Cluster C
A family study of the anxious-fearful cluster indicated significant familiality for DSM-III avoidant and dependent PD,44 and in a clinically based twin study, heritability estimates for avoidant, dependent, and obsessive -compulsive PD were found to be 28%, 57%, and 77%, respectively34 Results from a population-based study of dimensional representations of DSM-IV Cluster C PDs,45 however, indicated that heritability estimates were similar for avoidant PD (35%), but lower for dependent (31%) and for obsessive-compulsive PD (27%), again illustrating the importance of method of ascertainment. This discrepancy is probably in part due to difference in methods of ascertainment. No shared environmental effects or sex differences have been found for cluster C PDs.
Disorders in Appendix B
In a population-based twin study of depressive PD, Ørstavik et al46 found that liability could best be explained by additive genetic and unique environmental factors alone, with heritability estimates of 49% in females and 25% in males. Unlike the results for the other DSM-IV PDs, both quantitative and qualitative sex-differences were found corresponding to findings from studies on major depression.47 Significant familial aggregation has also been found for DSM-IV passive aggressive PD.48
Multivariate studies
If heritability has been established, several more complex models can be employed to explore the nature and mode of action of the genetic risk factors.7 Multivariate analyses, which comprise models where several phenotypes are included and different structures of the latent factors can be specified,20 can be used to estimate to what extent genetic and environmental risk factors are
specific to a given PD or shared in common with other PDs or axis I disorders, and thus to investigate sources of comorbididity.49,50 By including measures of the same phenotypes on different points in time, they can also be used to determine if genetic effects differ over time in a developmental perspective.
DSM-IV personality disorders
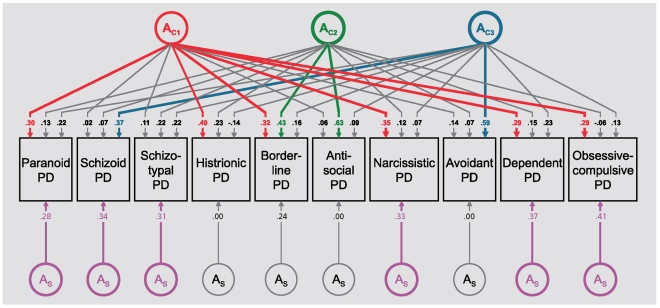
Cluster A PDs have been found to aggregate in families of probands with schizophrenia (see below). Familial coaggregation has also been found for borderline PD and antisocial PD39 and for borderline PD and all the other cluster B PDs,51 as well as for the DSM-III cluster C PDs.44 A population-based twin study including all PDs within cluster B indicated that borderline PD and antisocial PD appeared to share genetic risk factors above and beyond those shared in common with the other cluster B disorders,43 and a twin study of cluster C PDs suggested that genetic factors influencing obsessive-compulsive PD appeared to be relative specific to this disorder.45 Kendler et al, in the only population-based multivariate twin study including all 10 DSM-IV PDs that has been published,52 found that the best-fitting model included three genetic and three environmental factors in addition to disorder-specific factors. The structure of the genetic factors is shown in Figure 1. The first genetic factor (AC1) had high loadings on PDs from all 3 clusters including paranoid, histrionic, borderline, narcissistic, dependent, and obsessive-compulsive PD. This factor probably reflects a broad vulnerability to PD pathology and/or negative emotionality, and is related to genetic liability to the normal personality trait neuroticism. The second genetic factor (AC2)was quite specific with substantial loadings only on borderline and antisocial PD. This is consistent with the results from the abovementioned family studies,39 and suggests genetic liability to a broad phenotype for impulsive/aggressive behavior. The third factor identified (AC3) had high loadings only on schizoid and avoidant PD. This can be interpreted in several ways. It might in part reflect genetic risk for schizophrenia spectrum pathology (see below). From the perspective of the five-factor model of normal personality it reflects genetic liability for introversion.53 Finally, it is noteworthy that obsessive-compulsive PD had the highest disorder-specific genetic loading, which parallels prior findings that this PD shares little genetic and environmental liability with the other cluster C PDs.
Figure 1. Genetic parameter estimates from best fitting model for ten DSM-IV personality disorders. Path estimates are standardized regression coefficients, so they must be squared to equal the proportion of variance accounted for in the dependent variable. A stands for additive genetic effects. The subscripts C and S stand, respectively, for common factor and disorder-specific effects. The first, second and third genetic common factors are indicated by the subscripts C1' C2 and C3'. Paths with values ≥+0.28 (which account for ≥8% of phenotypic variance) are colored with the first, second, and third common factor indicated by, respectively, red, green, and blue and the disorder-specific factors by magenta. Paths not exceeding the + 0.28 cutoff are depicted in gray. From ref 52: Kendler KS, Aggen SH, Czajkowski N, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders a multivariate twin study. Arch Gen Psychiatry. 2008;65:1438-1446. Copyright © American Medical Association 2008.
The results are also to a large extent consistent with a prior multivariate twin study of the dimensional classification system of personality disorder trait mentioned above26 in which Livesley et al identified four genetic factors loading on four phenotypic dimensions called “emotional dysregulation,” “dissocial behavior,” “inhibition,” and “compulsivity.”
Taken together these results indicate that genetic risk factors for DSM-IV PDs do not reflect the cluster A, B, and C typology. However, this is well reflected in the structure of the environmental risk factors, suggesting that the comorbidity of PDs within clusters is due to environmental experiences.
Personality disorders and Axis I disorders
Several lines of evidence indicate specific axis I/axis II relationships,54,55 suggesting that common genetic or environmental liability factors might predispose to several disorders within clusters that transcend the axis I/axis II division.13,49,56
Schizophrenia
A number of family and adoption studies have examined the risk for paranoid, schizoid, and schizotypal PDs in relatives of schizophrenic and control probands. While a few studies can be found where all three cluster A PDs are at increased risk in relatives of schizophrenic probands,57,58 more common are studies that find that only schizotypal PD59-63 or schizotypal PD and paranoid PD64 have a significant familial relationship with schizophrenia. Taken together, these results suggest that schizotypal PD has the closest familial relationship to schizophrenia, followed by paranoid and schizoid PD, and are consistent with the hypothesis that a common genetic risk factor for cluster A PDs reflects - in the general population - the liability to schizophrenia.35 The extended phenotype believed to reflect this genetic liability to schizophrenia is often described by the term schizophrenia spectrum. Schizotypal PD has been suggested to be the prototypical disorder in this spectrum.65 In a recent family study, Fogelson et al66 showed that avoidant PD, currently classified in DSM cluster C, also occurred more frequently in relatives of probands with schizophrenia even after controlling for schizotypal and paranoid PD. This replicates findings from earlier studies,58,67 and suggest that avoidant PD should also be included in this spectrum. It is also in part in accordance with the results from the multivariate study by Kendler et al described above,52 where avoidant and schizoid PD share genetic liability.
Internalizing disorders
Mood and anxiety disorders (often called internalizing disorders) share genetic and environmental liability factors with each other,68 and with the normal personality trait neuroticism,69 which correlates strongly with several PDs, especially in cluster B and C.53
Family studies indicate that borderline PD and major depression share familial risk factors.51,70 In a populationbased multivariate twin study of major depression and DSM-IV PDs, Reichborn-Kjennerud et al71 found that dimensional representations of borderline PD from cluster B, avoidant PD from cluster C, and paranoid PD from cluster A were all independently and significantly associated with increased risk for major depression. Multivariate twin modeling indicated that one latent factor accounted for the genetic covariance between major depression and the three PDs. The genetic correlations between major depression and borderline, avoidant, and paranoid PD were respectively +0.56, +0.22, and +0.40. No sex differences or shared environmental effects were found. These results indicate that vulnerability to general PD pathology and major depression are closely related. In a bivariate twin study, Ørstavik et al72 found that a substantial part of the covariation between major depressive disorder and depressive PD was accounted for by genetic factors with a genetic correlation of 0.56. Results from another population-based twin study, investigating the sources of cooccurrence between social phobia and of avoidant PD in females, indicated that social phobia and avoidant PD were influenced by identical genetic factors, whereas the environmental factors influencing the two disorders were uncorrelated.73 This suggests that an individual with high genetic liability will develop avoidant PD versus social phobia entirely as a result of environmental risk factors unique to each disorder, which is in accordance with the hypothesis of underlying psychobiological dimensions cutting across the axis I/ axis II classification system.
Substance-use disorders
Numerous family, adoption and twin studies have demonstrated that antisocial PD, conduct disorder, and substance-use disorders (often called externalizing disorders) share a common genetic liability (eg, refs 68,74). In a family-twin study, Hicks et al75 found that a highly heritable (80%) general vulnerability to all the externalizing disorders accounted for most of the familial resemblance. Disorder-specific vulnerabilities were detected for conduct disorder, alcohol dependence, and drug dependence, but not for antisocial PD. The same group also reported an association between externalizing disorders and reduced amplitude of the P3 component of the brain event-related potential, suggesting that this could be a common biological marker for the biological vulnerability to these disorders.76
Longitudinal studies
Most of the genetic studies that have investigated changes in genetic influences on PDs over time have used measures related to antisocial PD. The following examples illustrate the potential of longitudinal quantitative genetic methods. In a twin study, Lyons et al77 demonstrated that the genetic influence on symptoms of DSM-III-R antisocial PD was much more prominent in adulthood than in adolescence. Silberg et al78 studying twins between 10 and 17 years of age found a single genetic factor that influenced antisocial behavior beginning at age 10 through young adulthood, a shared environmental effect beginning in adolescence, a transient genetic effect at puberty and genetic influences specific to adult antisocial behavior. In another recent twin study of externalizing disorders, biometric analyses revealed increasing genetic variation and heritability for men but a trend toward decreasing genetic variation and increasing environmental effects for women.79
Gene-environment interplay
In the traditional models of disease etiology in psychiatric epidemiology the causal pathway is conceptualized as moving from the environment to the organism. However, since genes influence behavior, genetic factors can indirectly influence or control exposure to the environment,20 called gene-environment correlation.20,80,81 Genetic factors can also control an individual's sensitivity to the environment, ie, genetic factors influence or alter an organism's response to environmental stressors.20,80,81 This is usually called gene-environment interaction. In quantitative studies of gene-environment interplay, genetic factors are either inferred (eg, disorder in biological parent in adoption studies) or modeled as a latent variable.80,82
Twin and adoption studies have provided much of the evidence for gene-environment correlations by demonstrating genetic influences for a number of measures of the environment.80 Overall, the evidence from twin and adoption studies suggests that gene-environment correlations are mediated by heritable personality traits and possibly PDs.81,83,84
The initial indications that gene-environment interaction was likely to be operating came from adoption and twin studies.85 Gene-environment interaction was demonstrated in an adoption study as early as in 1974, when Crowe86 found that early institutional care was a risk factor for later antisocial behavior only when a genetic risk factor was present. In another adoption study, Cadoret et al87 found significant gene-environment interaction by showing that there was a negligible risk for antisocial behavior from a genetic risk alone (antisocial behavior in the biological parent), no effect of an adverse adoptive family environment alone, but a substantial effect when both were present. The finding was replicated in a later study with a larger number of adoptees,88 Jaffe et al,89 using a twin design, found significant gene-environment interaction with respect to childhood maltreatment and the development of antisocial behavior, and in a twin study Tuvblad et al90 demonstrated a significant gene-environment interaction by showing that the heritability for adolescent antisocial behavior is higher in socioeconomic advantaged environments. Using an advanced family design, Feinberg et al91 recently found an interaction of genotype and both parental negativity and low warmth predicting antisocial behavior. Significant gene-environment interaction has also been demonstrated in schizophrenia spectrum disorders. In an adoption study Tienari et al92 showed that there was a significant association between disordered rearing and the diagnosis of schizophrenia spectrum disorder in the offspring of mothers with but not in offspring of mothers without the diagnoses. In a community based twin study, Hicks et al demonstrated a significant gene-environment interaction with a number of environmental risk factors showing that greater environmental adversity was associated with increased genetic risk for antisocial PD and substance use disorders.93 Significant gene-environment interaction has also been demonstrated in quantitative studies of anxiety and mood disorders.81
Molecular genetic studies
Traditionally, linkage and association studies have been most commonly used for mapping disease loci.94 Most of the molecular genetic studies of PDs has been done using hypothesis-driven candidate gene association studies95 focusing on particular genes related to the neurotransmitter pathways, especially in the serotonergic and dopaminergic systems. Although the number of genetic association studies are increasing exponentially, only a very small fraction of positive results are replicated.96,97 Until further replications are published the results reviewed below must therefore be considered tentative.
Cluster A
Consistent with the hypothesis that schizophrenia and related PDs are linked to dopaminergic dysfunction, Rosmond et al98 found that Cluster A PDs were associated with a polymorphism in the gene coding for the dopamine 2 receptor (DRD2). Building on results from quantitative genetic studies indicating that common genetic risk factors exist for schizotypal PD and schizophrenia, Stefanis et al99 examined the potential impact of SNPs within the four most prominent candidate genes for schizophrenia. Dysbindin (DTNBP1) and D-aminoacid oxidase (DAAO) both showed associations with symptoms of schizotypy. Similarly, Fanous et al100 using a linkage approach, found that a subset of schizophrenia susceptibility genes also affect schizotypy in nonpsychotic relatives. Significant associations with schizotypal personality traits have also been found in several studies with polymorphisms in the gene coding for catecholO-methyltransferase (COMT)100,102,103 an enzyme involved in the degradation of catecholamines, and linked to the etiology of schizophrenia.104
Cluster B
Multiple lines of evidence suggest that dysfunction in the serotonin (5-HT) system is associated with impulsivity, aggression, affective lability, and suicide. Genes linked to the function of this neurotransmitter can therefore be considered possible candidate genes for borderline and antisocial PD. Kennedy and coworkers found that borderline PD was associated with polymorphisms in the serotonin transporter gene (5-HTTLPR),105 and polymorphisms in the gene coding for the catabolic enzyme monoamine oxidase A (MAOA), involved in the regulation of biogenic amines like serotonin, norepinephrine, and dopamine,106 but not polymorphisms in the gene coding for the serotonin 5-HT2A receptor.107 Recently the group has conducted a gene-gene interaction study with a number of polymorphisms in seven serotonin genes (including the three mentioned above), concluding that “serotonin genes and their interaction may play a role in the susceptibility to borderline PD._108 Other groups have reported similar findings. A main effect of the 5-HTTLPR polymorphism on borderline PD has been found in bulimic women,109 and Lyons-Ruth et al found a significant relationship between the short 5HTTLPR allele and both borderline and antisocial PD,110 but other studies have failed to find an association between this polymorphism and cluster B PDs.111 Polymorphisms in the MAOA gene have been found to be associated with cluster B PDs,112 and antisocial traits.113 Tryptophan hydroxylase is the rate-limiting enzyme in the serotonin metabolic pathway. Two genes related to this enzyme, the tryptophan hydroxylase 1 and 2 genes (TPH1and TPH2), have been associated with borderline PD114 and personality traits related to emotional instability, as well as to cluster B and cluster C PDs.115 Taken together, these findings suggest that borderline and antisocial PD and possibly also the other cluster B PDs, are influenced by genes regulating the serotonergic system. They are also consistent with the finding of shared genetic influence on borderline PD and antisocial PD, and on borderline PD and the other cluster B PDs found in multivariate twin studies.43,52
Cluster C
It has previously been suggested that the 5-HTTLPR polymorphism was associated with anxiety-related traits,116 but later studies have yielded conflicting results (see ref 117). Patients diagnosed with cluster C PDs, have not been found to be significantly higher in the frequency of the short form allele of the 5HTTLPR.111 Recent results, on the other hand, indicate that variations in the COMT gene contribute to genetic risk shared across a range of anxiety-related phenotypes.118,119 Joyce120 found an association between avoidant and obsessive-compulsive PD symptoms and the dopamine D3 receptor (DRD3) polymorphism. In a later study and a meta-analysis, the finding for obsessivecompulsive symptoms were replicated, leading the authors to conclude that DRD3 may contribute to the development of obsessive-compulsive PD.121
Gene-environment interplay
Few studies of gene-environment correlation using measured genes and measured environments have been published. Dick et al121 found that individuals who had a polymorphism in a gene (GABRA2) associated with alcohol dependence were less likely to be married, in part because they were at higher risk for antisocial PD and were less likely to be motivated by a desire to please others. Other results confirm the existence of gene-environment correlation with measured genes in both the dopaminergic and serotonergic system, and provide preliminary support for the finding that correlations are mediated by behavioral and personality characteristics.84
Gene-environment interaction studies using identified susceptibility genes rather than unmeasured latent genetic factors can provide more secure estimates.84 Based on results from quantitative genetic studies showing gene-environment interaction for antisocial behavior, Caspi et al123 studied the association between childhood maltreatment, and a functional polymorphism in the promoter region of the MAOA gene on antisocial behavior assessed through a range of categorical and dimensional measures using questionnaire and interview data plus official records. The results showed no main effect of the gene, a main effect for maltreatment and a substantial and significant interaction between the gene and adversity. The maltreated children whose genotype conferred low levels of MAOA expression more often developed conduct disorder and antisocial personality than children with a high activity MAOA genotype. Foley et al124 replicated this finding and extended the initial analysis by showing that the gene-environment interaction could not be accounted for by gene-environment correlation. Other studies have failed to replicate the gene-environment interaction effect (eg, ref 125). In a recent meta-analysis, however, the original finding was replicated. In addition the findings was extended to include childhood (closer in time to the maltreatment), and the possibility of a spurious finding was ruled out by accounting for gene-environment correlation.126 The interaction between MAOA and childhood maltreatment in the etiology of antisocial PD appear to be one of the few replicated findings in the molecular genetics of PDs.
Future directions
Information from genetic epidemiologic studies can contribute to improvement in the validity of diagnoses of mental disorders, and thereby a more empirically based classification system.49,56,127 Several lines of evidence, including multivariate twin studies, have shown that common axis I disorders can be divided into two main groups (internalizing and externalizing) based on shared etiological factors.49,68 Currently an alternative classification system are being considered for DSM-V based on the hypothesis that, in addition to phenotypic similarity, spectra or clusters of disorder can be identified based on shared liability or risk factors.56 Such clusters transcend the axis I-axis II division. Multivariate twin studies, including a comprehensive number of axis I and axis II disorders, could provide new important insights relevant to this proposal and further clarify the etiology of mental disorders by identifying genetic and environmental risk factors shared in common between groups of disorders.
Methods like genome-wide association studies,128 analyses of copy-number variation,129 studies of rare genetic variants,130 epigenetic methods,131 and deep sequencing of genomic regions132 have not yet been applied to PDs, and will hopefully contribute to our understanding of the genetic etiology of these disorders in the future. One problem is, however, that the current phenotypes might be inadequate.128 It is highly unlikely that the new DSM-V classification of PDs will provide a solution. A strategy that has been proposed to increase the rate of success for molecular genetics in psychiatry is the use of endophenotypes, defined as a heritable characteristic that is along the pathway between a disorder and genotype.5 Although the strategy has not yet proven to be successful,133 it has been suggested that this approach should be applied to the study of PDs by using clinical dimensions like for example affective instability, impulsivity, and aggression instead of diagnoses.134
REFERENCES
- 1.American Psychiatric Association. . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association, 1980 [Google Scholar]
- 2.McGuffin P, Moffitt T, Thapar A. Personality disorders, In: McGuffin P, Owen MJ, Gottesman II, eds. . Psychiatric Genetics and Genomics. Oxford, UK: Oxford University Press, 2002:183–210. [Google Scholar]
- 3.Livesley WJ, Jang KL. The Behavioral genetics of personality disorder. . Ann Rev Clin Psychol. 2008:4247–274. doi: 10.1146/annurev.clinpsy.4.022007.141203. [DOI] [PubMed] [Google Scholar]
- 4.Reichborn-Kjennerud T. Genetics of personality disorders. . Psychiatr Clin N Am. 2008;31:421–440. doi: 10.1016/j.psc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. . Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. . Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendler KS. Psychiatric genetics: a methodologic critique. . Am J Psychiatry. 2005;162:3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association 1994 [Google Scholar]
- 9.Oldham JM, Skodol AE, Kellman HD, Hyler SE, Rosnick L, Davies M. Diagnosis of DSM-III-R personality disorders by two structured interviews: patterns of comorbidity. . Am J Psychiatry. 1992;149:213–220. doi: 10.1176/ajp.149.2.213. [DOI] [PubMed] [Google Scholar]
- 10.Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. . Br J Psychiatry. 2006;188:423–431. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- 11.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the national comorbidity survey replication. . Biol Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krueger RF. Continuity of axes I and II, toward a unified model of personality, personality disorders, and clinical disorders. . J Personality Disord. 2005;19:233–261. doi: 10.1521/pedi.2005.19.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. . Am J Psychiatry. 1991;148:1647–1658. doi: 10.1176/ajp.148.12.1647. [DOI] [PubMed] [Google Scholar]
- 14.Widiger TA. Personality disorder and Axis I psychopathology: the problematic boundary of Axis I and axis II. . J Personality Disord. 2003;17:90–108. doi: 10.1521/pedi.17.2.90.23987. [DOI] [PubMed] [Google Scholar]
- 15.Sheets E, Craighead WE. Toward an empirically based classification of personality pathology. . Clinl Psychol Sci Pract. 2007;14:77–93. [Google Scholar]
- 16.Oldham JM, Skodol AE. Charting the future of axis II. . J Personal Disord. 2000;14:17–29. doi: 10.1521/pedi.2000.14.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and Statistical Manual of Mental Disorders - Fifth Edition. . J Abnorm Psychol. 2005;114:494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- 18.Widiger TA, Trull TJ. Plate tectonics in the classification of personality disorder - shifting to a dimensional model. . Am Psychologist. 2007;62:71–83. doi: 10.1037/0003-066X.62.2.71. [DOI] [PubMed] [Google Scholar]
- 19.Krueger RF, Skodol AE, Livesley WJ, Shrout PE, Huang YQ. Synthesizing dimensional and categorical approaches to personality disorders, refining the research agenda for DSM-V Axis II. . Int J Methods Psych Res. 2007;16:S65–S73. doi: 10.1002/mpr.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendler KS. Twin studies of psychiatric illness: an update. . Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- 21.Neale MC, Boker SM, Xie G, Maes HH. Mx Statistical Modeling, 5th, 1999, Box 710 MCV, Richmond, VA 23298: Medical College of VA of VA Commonwealth Univ, Ref Type: Computer Program. [Google Scholar]
- 22.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. . Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 23.Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. . Behav Genet. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard TJ, Loehlin JC. Genes, evolution, and personality. . Behav Genet. 2001;31:243–273. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- 25.Ando J, Suzuki A, Yamagata S, et al. Genetic and environmental structure of Cloninger's temperament and character dimensions. . J Personal Disord. 2004;18:379–393. doi: 10.1521/pedi.18.4.379.40345. [DOI] [PubMed] [Google Scholar]
- 26.Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. . Arch Gen Psychiatry. 1998;55:941–948. doi: 10.1001/archpsyc.55.10.941. [DOI] [PubMed] [Google Scholar]
- 27.Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: a meta-analytic review. . Clin Psychol Rev. 2004;23:1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Parnas J, Licht D, Bovet P. Cluster A personality disorders: a review. In: Maj M, Akiskal H, Mezzich JE, Okasha A, eds. . Personality Disorders. New York, NY: John Wiley & Sons Ltd. 2005:1–124. [Google Scholar]
- 29.Kendler KS, Heath A, Martin NG. A genetic epidemiologic study of self-report suspiciousness. . Comp Psychiatry. 1987;28:187–196. doi: 10.1016/0010-440x(87)90026-5. [DOI] [PubMed] [Google Scholar]
- 30.Claridge G, Hewitt JK. A Biometrical study of schizotypy in a normal population. . Person Individ Diff. 1987;8:303–312. [Google Scholar]
- 31.Kendler KS, Hewitt JK. The structure of self-report schizotypy in twins. . J Personal Disord. 1992:61–17. [Google Scholar]
- 32.Linney YM, Murray RM, Peters ER, Macdonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. . Psychol Med. 2003;33:803–816. doi: 10.1017/s0033291703007906. [DOI] [PubMed] [Google Scholar]
- 33.Jang KL, Woodward TS, Lang D, Honer WG, Livesley WJ. The genetic and environmental basis of the relationship between schizotypy and personality - a twin study. . J Nerv Ment Dis. 2005;193:153–159. doi: 10.1097/01.nmd.0000154842.26600.bd. [DOI] [PubMed] [Google Scholar]
- 34.Torgersen S, Lygren S, Oien PA, et al. A twin study of personality disorders. . Comp Psychiatry. 2000;41:416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- 35.Kendler KS, Czajkowski N, Tambs K, et al. Dimensional representations of DSM-IV Cluster A personality disorders in a population-based sample of Norwegian twins: a multivariate study. . Psychol Med. 2006;36:1583–1591. doi: 10.1017/S0033291706008609. [DOI] [PubMed] [Google Scholar]
- 36.McGlashan TH, Grilo CM, Sanislow CA, et al. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: toward a hybrid model of axis II disorders. . Am J Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendler KS, Myers J, Torgersen S, Neale MC, Reichborn-Kjennerud T. The heritability of cluster A personality disorders assessed by both personal interview and questionnaire. . Psychol Med. 2007;37:655–665. doi: 10.1017/S0033291706009755. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. . Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 39.White CN, Gunderson JG, Zanarini MC, Hudson JI. Family studies of borderline personality disorder: A review. . Harv Rev Psychiatry. 2003;11:8–19. doi: 10.1080/10673220303937. [DOI] [PubMed] [Google Scholar]
- 40.Distel MA, Trull TJ, Derom CA, et al. Heritability of borderline personality disorder features is similar across three countries. . Psychol Med. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- 41.Distel MA, Rebollo-Mesa I, Willemsen G, et al. Familial resemblance of borderline personality disorder features: genetic or cultural transmission?. . Plos One. 2009;4:e5334. doi: 10.1371/journal.pone.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller MC, Coventry WL, Heath AC, Martin NG. Widespread evidence for non-additive genetic variation in Cloninger's and Eysenck's personality dimensions using a twin plus sibling design. . Behav Genet. 2005;35:707–721. doi: 10.1007/s10519-005-6041-7. [DOI] [PubMed] [Google Scholar]
- 43.Torgersen S, Czajkowski N, Jacobson K, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. . Psychol Med. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- 44.Reich JH. Familiality of DSM-III dramatic and anxious personality clusters. . J Nerv Ment Dis. 1989;177:96–100. doi: 10.1097/00005053-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Reichborn-Kjennerud T, Czajkowski N, Neale MC, et al. Genetic and environmental influences on dimensional representations of DSM-IV cluster C personality disorders: a population-based multivariate twin study. . Psychol Med. 2007;37:645–653. doi: 10.1017/S0033291706009548. [DOI] [PubMed] [Google Scholar]
- 46.Orstavik RE, Kendler KS, Czajkowski N, Tambs K, Reichborn-Kjennerud T. Genetic and environmental contributions to depressive personality disorder in a population-based sample of Norwegian Twins. . J Affect Dis. 2007;99:181–189. doi: 10.1016/j.jad.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. . Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 48.Czajkowski N, Kendler KS, Jacobson KC, Tambs K, Roysamb E, Reichborn-Kjennerud T. Passive-aggressive (negativistic) personality disorder: a population-based twin study. . J Personal Disord. 2008;22:109–122. doi: 10.1521/pedi.2008.22.1.109. [DOI] [PubMed] [Google Scholar]
- 49.Krueger RF, Markon KE. Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. . Ann Rev Clin Psychol. 2006:2111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. . Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 51.Zanarini MC, Barison LK, Frankenburg FR, Reich B, Hudson Jl. Family history study of the familial coaggregation of borderline personality disorder with axis I and nonborderline dramatic cluster axis II disorders. . J Personal Disord. 2009;23:357–369. doi: 10.1521/pedi.2009.23.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendler KS, Aggen SH, Czajkowski N, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. . Arch Gen Psychiatry. 2008;65:1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: A meta-analytic review. . Clin Psychol Rev. 2004;23:1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Tyrer P, Gunderson J, Lyons M, Tohen M. Extent of comorbidity between mental state and personality disorders. . J Personal Disord. 1997;11:242–259. doi: 10.1521/pedi.1997.11.3.242. [DOI] [PubMed] [Google Scholar]
- 55.Dolan-Sewell RT, Krueger RF, Shea MT. Co-occurrence with syndrome disorders In: Livesley WJ ed. . Handbook of Personality Disorders: Theory, Research and Treatment. New York, NY: The Guilford Press, 2001:84–104. [Google Scholar]
- 56.Parnas J, Cannon TD, Jacobsen B, Schulsinger H, Schulsinger F, Mednick SA. Lifetime DSM-III-R diagnostic outcomes in the offspring of schizophrenic mothers, Results from the Copenhagen High-Risk Study. . Arch Gen Psychiatry. 1993;50:707–714. doi: 10.1001/archpsyc.1993.01820210041005. [DOI] [PubMed] [Google Scholar]
- 57.Andrews G, Goldberg DP, Krueger R, et al. Exploring the feasibility of a meta-structure for DSM-V and ICD-11: could it improve utility and validity?. . Psychol Med. 2009;391:993–2000. doi: 10.1017/S0033291709990250. [DOI] [PubMed] [Google Scholar]
- 58.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study III, Schizophrenia-related personality disorders in relatives. . Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- 59.Kety SS, Wender PH, Jacobsen B, et al. Mental illness in the biological and adoptive relatives of schizophrenic adoptees, Replication of the Copenhagen Study in the rest of Denmark. . Arch Gen Psychiatry. 1994;51:442–455. doi: 10.1001/archpsyc.1994.03950060006001. [DOI] [PubMed] [Google Scholar]
- 60.Onstad S, Skre I, Edvardsen J, Torgersen S, Kringlen E. Mental disorders in first-degree relatives of schizophrenics. . Acta Psychiatr Scand. 1991;83:463–467. doi: 10.1111/j.1600-0447.1991.tb05577.x. [DOI] [PubMed] [Google Scholar]
- 61.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. . Arch Gen Psychiatry. 2001;58:581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 62.Tienari P, Wynne LC, Laksy K, et al. Genetic boundaries of the schizophrenia spectrum: Evidence from the Finnish adoptive family study of schizophrenia. . Am J Psychiatry. 2003;160:1587–1594. doi: 10.1176/appi.ajp.160.9.1587. [DOI] [PubMed] [Google Scholar]
- 63.Torgersen S, Onstad S, Skre I, Edvardsen J, Kringlen E. "True" schizotypal personality disorder: a study of co-twins and relatives of schizophrenic probands. . Am J Psychiatry. 1993;150:1661–1667. doi: 10.1176/ajp.150.11.1661. [DOI] [PubMed] [Google Scholar]
- 64.Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S. A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. . Am J Psychiatry. 1985;142:447–455. doi: 10.1176/ajp.142.4.447. [DOI] [PubMed] [Google Scholar]
- 65.Siever L, Davis KL. The pathopysiology of schizophrenia disorders: perspectives from the spectrum. . Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 66.Fogelson DL, Nuechterlein KH, Asarnow RA, et al. Avoidant personality disorder is a separable schizophrenia-spectrum personality disorder even when controlling for the presence of paranoid and schizotypal personality disorders - the UCLA family study. . Schizophr Res. 2007;91:192–199. doi: 10.1016/j.schres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. . Arch Gen Psychiatry. 2001;58:581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 68.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. . Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 69.Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. . Am J Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- 70.Riso LP, Klein DN, Anderson RL, Ouimette PC. A family study of out-patients with borderline personality disorder and no history of mood disorder. . J Personal Disord. 2000;14:208–217. doi: 10.1521/pedi.2000.14.3.208. [DOI] [PubMed] [Google Scholar]
- 71.Reichborn-Kjennerud T, Czajkowski N, Røysamb E, Orstavik RE, et al. Major depression and dimensional representations of DSM-IV personality disorders: a population-based twin study. . Psychol Med. 2009 Nov 17. Epub ahead of print doi: 10.1017/S0033291709991954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ørstavik RE, Kendler KS, Czajkowski N, Tambs K, Reichborn-Kjennerud T. The relationship between depressive personality disorder and major depressive disorder: a population-based twin study. . Am J Psychiatry. 2007;164:1866–1872. doi: 10.1176/appi.ajp.2007.07010045. [DOI] [PubMed] [Google Scholar]
- 73.Reichborn-Kjennerud T, Czajkowski N, Torgersen S, et al. The relationship between avoidant personality disorder and social phobia: a population-based twin study. . Am J Psychiatry. 2007;164:1722–1728. doi: 10.1176/appi.ajp.2007.06101764. [DOI] [PubMed] [Google Scholar]
- 74.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, lacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. . J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- 75.Hicks BM, Krueger RF, lacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders - a twin-family study. . Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 76.Hicks BM, Bernat E, Malone SM, et al. Genes mediate the association between P3 amplitude and externalizing disorders. . Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyons MJ, True WR, Eisen SA, et al. Differential heritability of adult and juvenile antisocial traits. . Arch Gen Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- 78.Silberg JL, Rutter M, Tracy K, Maes HH, Eaves L. Etiological heterogeneity in the development of antisocial behavior: the Virginia twin study of adolescent behavioral development and the young adult follow-up. . Psychol Med. 2007;37:1193–1202. doi: 10.1017/S0033291707000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hicks BM, Blonigen DM, Kramer MD, et al. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. . J Abnorm Psychol. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology. New York, NY: The Guilford Press, 2006 [Google Scholar]
- 81.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. . J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 82.Purcell S, Sham P. Variance components models for gene-environment interaction in quantitative trait locus linkage analysis. . Twin Res. 2002;5:572–576. doi: 10.1375/136905202762342035. [DOI] [PubMed] [Google Scholar]
- 83.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. . Psychol Med. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 84.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. . Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. . World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- 86.Crowe RR. An adoption study of antisocial personality. . Arch Gen Psychiatry. 1974;31:785–791. doi: 10.1001/archpsyc.1974.01760180027003. [DOI] [PubMed] [Google Scholar]
- 87.Cadoret RJ, Cain CA, Crowe RR. Evidence for gene-environment interaction in the development of adolescent antisocial behavior. . Behav Genet. 1983;13:301–310. doi: 10.1007/BF01071875. [DOI] [PubMed] [Google Scholar]
- 88.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. . Arch Gen Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- 89.Jaffee SR, Caspi A, Moffitt TE, et al. Nature X nurture: genetic vulnerabilities interact with physical maltreatment to promote conduct problems. . Dev Psychopathol. 2005;17:67–84. doi: 10.1017/s0954579405050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. . J Child Psychol Psychiatry. 2006;47:734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 91.Feinberg ME, Button TMM, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and adolescent antisocial behavior and depression - evidence of genotype x parenting environment interaction. . Arch Gen Psychiatry. 2007;64:457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- 92.Tienari P, Wynne LC, Sorri A, et al. Genotype-environment interaction in schizophrenia-spectrum disorder - long-term follow-up study of Finnish adoptees. . Br J Psychiatry. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- 93.Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. . Arch Gen Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sham P, McGuffin P. Linkage and association, In: McGuffin P, Owen MJ, Gottesman II eds. . Psychiatric Genetics & Genomics. Oxford, UK: Oxford University Press, 2002:55–73. [Google Scholar]
- 95.Zondervan KT, Cardon LR. Designing candidate gene and genome-wide case-control association studies. . Nat Protocols. 2007;2:2492–2501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ioannidis JPA, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. . Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 97.NCI-NHGRI Working Group on Replication in Association Studies, Replicating genotype-phenotype associations. . Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 98.Rosmond R, Rankinen T, Chagnon M, et al. Polymorphism in exon 6 of the dopamine D-2 receptor gene (DRD2) is associated with elevated blood pressure and personality disorders in men. . J Hum Hypertens. 2001;15:553–558. doi: 10.1038/sj.jhh.1001231. [DOI] [PubMed] [Google Scholar]
- 99.Stefanis NC, Trikalinos TA, Avramopoulos D, et al. Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. . Biol Psychiatry. 2007;62:784–792. doi: 10.1016/j.biopsych.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Fanous AH, Neale MC, Gardner CO, et al. Significant correlation in linkage signals from genome-wide scans of schizophrenia and schizotypy. . Mol Psychiatry. 2007;12:958–965. doi: 10.1038/sj.mp.4001996. [DOI] [PubMed] [Google Scholar]
- 101.Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN. Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. . Mol Psychiatry. 2002;7:706–711. doi: 10.1038/sj.mp.4001070. [DOI] [PubMed] [Google Scholar]
- 102.Stefanis NC, van Os J, Avramopoulos D, et al. Variation in catechol-Omethyltransf erase val met genotype associated with schizotypy but not cognition: A population study in 543 young men. . Biol Psychiatry. 2004;56:510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 103.Schurhoff F, Szoke A, Chevalier F, et al. Schizotypal dimensions: An intermediate phenotype associated with the COMT high activity allele. . Am J Med Genet B-Neuropsych Genet. 2007;144B:64–68. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- 104.Lewandowski KE. Relationship of catechol-o-methyltransferase to schizophrenia and its correlates: Evidence for associations and complex interactions. . Harv Rev Psychiatry. 2007;15:233–244. doi: 10.1080/10673220701650409. [DOI] [PubMed] [Google Scholar]
- 105.Ni XQ, Chan K, Bulgin N, et al. Association between serotonin transporter gene and borderline personality disorder. . J Psych Res. 2006;40:448–453. doi: 10.1016/j.jpsychires.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Ni XQ, Sicard T, Bulgin N, et al. Monoamine oxidase A gene is associated with borderline personality disorder. . Psychiatr Genet. 2007;17:153–157. doi: 10.1097/YPG.0b013e328016831c. [DOI] [PubMed] [Google Scholar]
- 107.Ni XQ, Bismil R, Chan K, et al. Serotonin 2A receptor gene is associated with personality traits, but not to disorder, in patients with borderline personality disorder. . Neurosci Lett. 2006;408:214–219. doi: 10.1016/j.neulet.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Ni X, Chan D, Chan K, McMain S, Kennedy JL. Serotonin genes and gene-gene interactions in borderline personality disorder in a mached casecontrol study. . Prog Neuropharmacol Biol Psychiatry. 2009;33:128–133. doi: 10.1016/j.pnpbp.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 109.Steiger H, Richardson J, Joober R, et al. The 5HTTLPR polymorphism, prior maltreatment and dramatic-erratic personality manifestations in women with bulimic syndromes. . J Psychiatr Neurosci. 2007;32:354–362. [PMC free article] [PubMed] [Google Scholar]
- 110.Lyons-Ruth K, Holmes BM, Sasvari-Szekely M, Ronai Z, Nemoda Z, Pauls D. Serotonin transporter polymorphism and borderline or antisocial traits among low-income young adults. . Psychiatr Genet. 2007;17:339–343. doi: 10.1097/YPG.0b013e3281ac237e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacob CP, Strobel A, Hohenberger K, et al. Association between allelic variation of serotonin transporter function and neuroticism in anxious cluster C personality disorders. . Am J Psychiatry. 2004;161:569–572. doi: 10.1176/appi.ajp.161.3.569. [DOI] [PubMed] [Google Scholar]
- 112.Jacob CP, Muller J, Schmidt M, et al. Cluster B personality disorders are associated with allelic variation of monoamine oxidase a activity. . Neuropsychopharmacology. 2005;30:1711–1718. doi: 10.1038/sj.npp.1300737. [DOI] [PubMed] [Google Scholar]
- 113.Williams LM, Gatt JM, Kuan SA, et al. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. . Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- 114.Wilson ST, Stanley B, Brent DA, Oquendo MA, Huang Y, Mann JJ. The tryptophan hydroxylase-1 A218C polymorphism is associated with diagnosis, but not suicidal behavior, in borderline personality disorder. . Am J Med Genet Part B. 2009;150B:202–208. doi: 10.1002/ajmg.b.30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.utknecht L, Jacob C, Strobel A, et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. . Int J Neuropsychopharmacol. 2007;10:309–320. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- 116.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. . Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 117.Munafo MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. . Mol Psychiatry. 2005;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- 118.Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. . Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- 119.Hettema JM, An SS, Bukszar J, et al. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. . Biol Psychiatry. 2008;64:302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joyce PR, Rogers GR, Miller AL, Mulder RT, Luty SE, Kennedy MA. Polymorphisms of DRD4 and DRD3 and risk of avoidant and obsessive personality traits and disorders. . Psychiatr Res. 2003;119:1–10. doi: 10.1016/s0165-1781(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 121.Light KJ, Joyce PR, Luty SE, et al. Preliminary evidence for an association between a dopamine D3 receptor gene variant and obsessive-compulsive personality disorder in patients with major depression. . Am J Med Genet B-Neuropsych Genet. 2006;141B:409–413. doi: 10.1002/ajmg.b.30308. [DOI] [PubMed] [Google Scholar]
- 122.Dick DM, Agrawal A, Shuckit MA, et al. Marital status, alcohol dependence, and GRBRA2: evidence for gene-environment correlation and interaction. . J Stud Alcohol. 2006;67:185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- 123.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. . Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 124.Foley DL, Eaves LJ, Wormley B, et al. Childhood adversity, monoamine A geneotype, and risk for conduct disorder. . Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 125.Huizinga D, Haberstick BC, Smolen A, et al. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. . Biol Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 126.Kim-Cohen J, Caspi A, Taylor A, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. . Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 127.Kendler KS. Reflections on the relationship between psychiatric genetics and psychiatric nosology. . Am J Psychiatry. 2006;163:1138–1146. doi: 10.1176/ajp.2006.163.7.1138. [DOI] [PubMed] [Google Scholar]
- 128.Cichon S, Craddock N, Daly M, et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. . Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cook EH, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. . Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 130.Goldstein DB. Common genetic variation and human traits. . N Engl J Med. 2009;360:1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- 131.Stuffrein-Roberts S, Joyce PR, Kennedy MA. Role of epigenetics in mental disorders. . Aust N Z J Psychiatry. 2008;42:97–107. doi: 10.1080/00048670701787495. [DOI] [PubMed] [Google Scholar]
- 132.lint J, Munafo MR. The endophenotype concept in psychiatric genetics. . Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. . Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siever LJ. Endophenotypes in the personality disorders. . Dialogues Clin Neurosci. 2005;7:139–151. doi: 10.31887/DCNS.2005.7.2/lsiever. [DOI] [PMC free article] [PubMed] [Google Scholar]