Abstract
Technological advances in the field of human genetics have resulted in a wave of discoveries of common DNA sequence variants that are associated with a risk of common complex diseases, such as heart attack, that account for a substantial proportion of morbidity, mortality, and health care costs in most contemporary populations. The overall predictive power of these sequence variants can be considerable, due to the high incidence of these diseases and the sheer number of associations that have been discovered. Health care providers have been slow to utilize this knowledge for preventative medicine. However, several companies have taken on a translational role by offering genetic tests based on these discoveries direct to consumers. In this paper, we review the current state and future prospects of such genetic tests, as scientists involved both in the discovery of disease associations and the development of genetic tests.
Keywords: genetic test, consumer, genome-wide association study, disease risk, ancestry
Abstract
Los avances tecnológicos en el campo de la genética humana han producido una ola de descubrimientos de variantes de la secuencia común del ADN que están asociadas con un riesgo de enfermedades comunes y complejas, como el ataque cardíaco, que dan cuenta de un porcentaje significativo de morbilidad, mortal idad y costos de salud en la mayoría de las poblaciones actuales. El poder predictor en conjunto de estas variantes de la secuencia puede ser considerable, debido a la alta incidencia de estas enfermedades y al reducido número de asociaciones que se han descubierto. Los proveedores de atención de salud han utilizado lentamente este conocimiento para la medicina preventiva. Sin embargo, algunas empresas han asumido un papel translacional al ofrecer pruebas genéticas basadas en estos descubrimientos dirigidas a los usuarios. En este artículo se revisa tanto el estado actual y las perspectivas futuras de tales pruebas genéticas, como a los científicos involucrados en el descubrimiento de las asociaciones de enfermedades y en el desarrollo de las pruebas genéticas.
Abstract
Les avancées technologiques en génétique humaine ont permis une série de découvertes de variants fréquents de séquence d'ADN associés à un risque de maladies complexes courantes, comme la crise cardiaque, responsables d'une morbi-mortalité et de coûts liés à la santé très importants chez la plupart de nos contemporains. La puissance prédictive globale de ces variants génétiques peut être considérable en raison de la fréquence élevée de ces maladies et du seul nombre d'associations découvertes. Les acteurs du système de santé ont mis du temps à utiliser ces données en médecine préventive. Plusieurs laboratoires ont néanmoins accepté de servir d'intermédiaire en proposant directement au grand public des tests génétiques basés sur ces découvertes. En tant que scientifiques impliqués à la fois dans la découverte d'associations de maladies et dans le développement de tests génétiques, nous analysons dans cet article l'état actuel et le devenir de tels tests.
A surge of discoveries in the genetics of disease
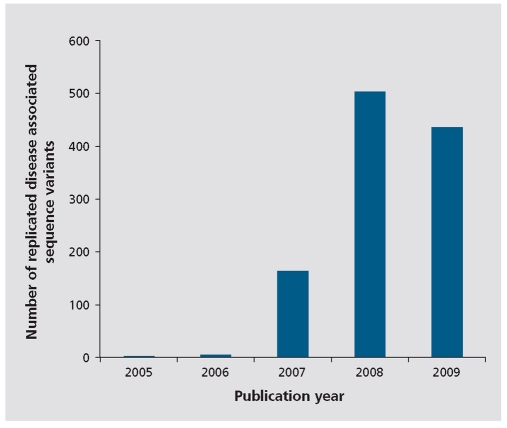
The past 4 years have yielded an unparalleled number of discoveries in the field of the genetics of human disease. In particular, huge strides have been made in the discovery of DNA sequence variants that are associated with risk of common complex diseases, such as type 2 diabetes, myocardial infarction, Crohn's disease, breast cancer, and prostate cancer. Prior to 2006, in spite of huge efforts by dedicated researchers, only a few sequence variants had been found to be associated with these diseases that had been adequately verified by replication in well-powered studies. By October of 2009, according to the Catalog of Genome-Wide Association Studies,1,2 the number of replicated associations of sequence variants with these diseases was 44, 10, 59, 14, and 28, respectively (obtained by counting unique variants reported as statistically significant in more than one study, or in at least two population samples from the same study). Figure 1 shows the total count of replicated disease associations by year of publication, based on the same criteria, as reported by this database. These numbers will almost certainly continue to rise at a rapid rate. This sudden progress is due to the advent of genomewide association studies (GWAS), made possible by a combination of at least four key developments. The first is the wealth of knowledge that has been produced about sequence variation in the human genome, in large part due to the Human Genome Project6 and the ensuing HapMap project.7 The second is the development of high-throughput microarray genotyping technologies, that now enable researchers to simultaneously and relatively cheaply assess genotypes at hundreds of thousands of single-nucleotide polymorphisms (SNPs) across the genome. The third is the availability of collections of DNA samples from large numbers of individuals who suffer from the diseases of interest and controls from the same populations. Another factor that has contributed to the success of GWAS is the close and fruitful cooperation between research groups and journals in defining conservative and robust standards for the verification of disease association signals obtained using this approach. The recent discoveries of sequence variants associated with the risk of complex diseases represent an important step in the task of understanding their biology, of which we are still remarkably ignorant. While some of the newly discovered associations were found in genes already suspected of playing a role in etiology, most are in, or close to, genes with no prior connection to the disease in question. These latter discoveries, in particular, represent important new points of departure for more focused research into the biology and etiology of these diseases.
Figure 1. The number of replicated sequence variants associated with diseases and medically relevant traits by publication year of first report in genome-wide association studies according to the Catalog of Genome-Wide Association Studies on October 20th 2009. Counts were obtained by counting unique variants reported as statistically significant (at the level of P< 1 .0 x 1 0-5) in more than one study or in at least two population samples from the same study. They represent a total of 1 1 08 replicated associations to sequence variants based on 1 32 diseases and medically relevant traits. Note that in some cases the Catalog of Genome-Wide Association Studies reports multiple correlated single-nucleotide polymorphisms (SNPs) from the same genomic region, and thus the numbers shown are likely to be overestimates. However, the 1 108 associated SNPs belong to 871 different genomic regions, and this latter number is likely to be an underestimate of the overall number of associations discovered This is because some regions are known to contain multiple independent associations to the same trait - for example, region 8q24 and prostate cancer.3 Also, not all bona fide disease-associated sequence variants are included in the catalog, for example several that have been reported for age-related macular degeneration.4,5 .
While the discovery rate of new disease-associated variants shows no signs of decline, there is good reason to believe that much of the lowest-hanging fruit has already been picked. These are the common sequence variants that have an easily detected impact on disease risk, given the existing sample sizes of cases and controls (ie, with an odds ratio of more than 1.1) and that are covered by the existing microarray genotyping platforms. Some researchers argue for continuation of the GWAS approach, with larger sample sizes to detect more common variants with small effect.8 Others argue for a change of strategy, pointing out that the combined effects of variants that are likely to be found with more GWAS only account for a part of the overall heritability of the diseases concerned.9 Proposals have been made to pay greater attention to rare variants, copy number variants, epigenetic factors, or epistatic effects between unlinked sequence variants. At least some of these aims will be achieved in the near future, as further technological developments make full genome sequencing and more comprehensive microarray genotyping platforms realistic options for large-scale disease studies.
Translation of disease association findings for public use
Clearly, there is more to be found, and it seems obvious to us that all of the aforementioned lines of research should be pursued. However, at the same time as geneticists continue their hunt for new disease-associated sequence variants and attempt to determine the functional relevance of the variants they have already discovered, they must address an equally pressing issue of practical concern in relation to existing knowledge. To date, more than 1000 sequence variants have been discovered with robustly verified disease associations to tens of major complex diseases.1 These diseases account for a substantial proportion of morbidity, mortality, and health care costs in most contemporary populations. Even though the functional impact of most of these variants is not yet understood, their association with disease imbues them with intrinsic value for both the general public and health care practitioners. This is because the strength of their association in a population reflects the degree to which they can be used to improve predictions of disease risk for individuals.
Disease risk stratification (by age, sex, weight, and other biological markers) forms the cornerstone of effective screening programs. It also serves an important role in increasing the health awareness of individuals, thereby promoting the adoption of preventative measures and leading to earlier diagnosis. The overall result is not only a healthier population, but a reduction in the burgeoning cost of health care, much of which stems from late treatment of preventable diseases. It follows that considerable additional health benefit and cost-effectiveness could be achieved through the addition of recently discovered genetic factors. Although such genetic tests are available, they have yet to be routinely adopted by major health care providers. This may be partly due to a lack of familiarity with such tests, or with their scientific basis, or due to an inherent resistance that stems from financial or organizational concerns. However, genetic tests are also available directly to the public through the Internet, where they have been positively received through a combination of health concerns, curiosity, and recreational interest in genetics and ancestry.
The authors of this article are members of a research team at deCODE Genetics that has contributed to the ongoing wave of discoveries in the genetics of complex disease and intends to remain at the forefront of this field in the coming years. However, from 2007 deCODE Genetics has also been a leader in using this knowledge to develop tests to evaluate genetic risk, both in the form of diagnostic products aimed at health care providers, and a personal genome scan, under the name of deCODEme, which is sold directly to consumers via the Internet. In what follows, we will outline the nature of the genetic tests provided by deCODE Genetics and others, and the value we believe they can bring by informing individuals about their health prospects and motivating them to take preventative measures where possible or to seek early diagnosis. We will also discuss some of the concerns raised by such genetic tests.
The nature of the genome and possibilities of genetic testing
Before moving on to a description of the kinds of genetic tests that are currently available to health care providers and the general public, it may be helpful to consider the nature of the genome. This can help us to understand what kind of information the genome can offer and thereby what kind of genetic tests are possible. The genome constitutes an extremely complex set of instructions for the assembly and maintenance of an organism, within some normal range of environmental conditions. For humans these instructions are almost exclusively encoded in the sequence of the roughly 3 billion nucleotides that make up the genome.
We may consider a human being as a vast collection of phenotypic traits, ranging from, for example, height and skin pigmentation to less perceptible features such as blood insulin levels or the build-up of amyloid plaques in brain tissue. All such traits are the outcome of an interaction between instructions from one or more parts of the genome and some set of environmental factors. Most phenotypic traits exhibit some variation among individuals that reflects underlying differences in DNA sequence and differences in exposure to environmental conditions. In some cases, differences between individuals exposed to normal environmental conditions are solely due to DNA sequence variants from a single gene. An example of a trait that is fully determined by sequence variants, and is inherited in accordance with simple Mendelian rules of inheritance, is the capacity to metabolize the amino acid phenylalanine, that when lacking, results in the disease phenylketonuria. More often, however, trait variation among individuals can be traced to many DNA sequence variants and environmental factors.
The power to correctly predict traits such as the development of disease for individuals using a genetic test (that is, the clinical validity of the test) depends on the nature of the relationship between genotype and phenotype. Many of the key human diseases, the so-called common complex diseases, are substantially affected by environmental factors. This means that the predictive power of genetic tests for these diseases will be less than for “simple” traits such as phenylketonuria (although the validity for such tests could be boosted by including known environmental risk factors). Nonetheless, the potential health and economic rewards gained from improving risk predictions for diseases that affect large numbers of individuals in a population are substantial. No matter how many sequence variants and environmental factors contribute to a given phenotypic trait, all other things being equal, the accuracy of prediction is always increased by the inclusion of just one truly associated sequence variant.
Diseases may be defined as the fraction of variation in physiological function that lies outside the normal range, such that either the quality of life is impaired, or the probability of untimely death is raised to an unacceptable level. It is no coincidence that diseases are the focus of most existing genetic tests, because they have been the primary focus to date of research into genotype-phenotype associations in humans. However, once reliable information has been gathered about an association between any phenotypic trait and a set of sequence variants, it becomes possible to develop a genetic test to estimate genetic propensity for that trait. In fact, there are already several genetic tests on the market that include nondisease traits such as eye color, male-pattern baldness, bitter taste perception, and drug metabolism. Such traits are often ignored by commentators on genetic testing, but they are likely to play a larger role in this field as our understanding of the functional impact of sequence variants on normal human phenotypic variation advances. In addition to enabling the assessment of genetic predispositions to particular phenotypic traits, the genome holds a record of ancestry and genealogical relationships between all people. This information is inscribed into the genome as it is replicated and transmitted from parents to offspring with a small number of changes in the form of mutations and recombination events. In the world of consumer genetic tests, ancestry has been a leading area of interest, with considerable sales of tests based on the uniparentally inherited genetic material from mitochondria and Y chromosomes.10
The use of genetic markers to verify the existence of close family relationships between individuals is a relatively trivial task that is routinely performed in forensic laboratories and in tests that are already available to the public. The introduction of microarray genotyping platforms with hundreds of thousands of SNPs is likely to facilitate the development of more powerful algorithms to explicitly test for more distant genealogical relationships between individuals. Such data are already used in genetic tests in conjunction with recently developed statistical methods from population genetics to provide detailed assessments of ancestry and admixture. The results from these analyses are in effect summary analyses of the genealogical relationship of an individual to different populations from around the world. As the magnitude of comparative data from such populations grows and the number of sequence variants assessed increases (for example, through full genome sequencing), there will be considerable improvements in the detail and accuracy of ancestry assessments and genealogical testing.
Genetic tests of ancestry are typically defined as recreational by commentators, and somehow qualitatively different from tests that evaluate disease risk.11,12 However, it is important to bear in mind that in the genome, information about function and ancestry is inexorably intertwined. Sequence variants that are used in an ancestry test today, on the basis of having no known function, may well be found to be associated with a disease or medically relevant traits tomorrow. Moreover, to the extent that disease risks vary between populations due to differing frequencies of the underlying associated sequence variants, it follows that tests of ancestry are in effect tests, albeit low-powered tests, of genetic risk of disease.
The past and present of genetic testing
At present, a wide range of genetic tests are available either through health care providers or by companies direct to the consumer (DTC). Indeed, there are now so many that it is impossible to provide a comprehensive overview of them all. To begin with, most tests were performed by university or hospital laboratories within the scope of health care provision. They were based on rare and highly penetrant sequence variants that are strongly associated with a particular disease. Included in this category are tests used for prenatal and newborn screening, diagnostic testing for chromosome abnormalities, carrier testing, and predictive testing for particular conditions such as Huntington's disease and hemochromatosis. In time, companies offering such tests emerged, in some cases established by people from the aforementioned universities or hospitals. An early example is the company Myriad Genetics, that patented and marketed predictive tests for breast cancer based on variants in the BRCA genes (that confer a roughly fivefold risk of developing this disease).
One thing the tests available through health care providers have in common is that the variants tested are rare and highly penetrant (ie, their clinical validity is high). Consequently, very few individuals from the general population would be expected to receive positive results from such tests - as is the case, for example, in population screening for phenylketonuria mutations. In many cases, however, such tests are provided on the basis of clinical diagnosis or familial risk, which increases the fraction of positive results from the tests. For individuals who receive positive results, the implications tend to be a very high probability of disease. Thus, testtakers often meet a genetic counselor prior to tests and after in light of a positive result (with treatment if applicable). The use of tests for rare and highly penetrant sequence variants is widespread among health care providers in most countries. Many have also adopted predictive tests for breast cancer (using variants in the BRCA gene), and Alzheimer's disease, based on more common variants in the APOE gene that confer a fourfold risk. However, they have been slow or reluctant to take advantage of the recent wave of robustly replicated GWAS discoveries of variants associated with increased disease risk ranging from 1.05-fold to 7-fold. In spite of the reluctance of health care providers to adopt genetic tests for common diseases, a growing number of companies have been harnessing findings from GWAS and other genetic studies to design tests that are sold DTC, mainly through the Internet. Most currently available DTC tests are based on a handful of sequence variants and focus on a specific application, such as ancestry, family relationships, or the testing of genetic risk for particular diseases. Often a particular company will offer several such small tests covering one or more of these areas. On top of this, a few companies are now offering DTC personal genome scans. These products are mostly based on large-scale and cost-effective microarray genotyping platforms that test 500 000 to 1 million SNPs from across the entire genome, but in a few cases on more expensive full-genome sequencing. The four best-known providers of DTC personal genome scans are, in order of appearance on the market, deCODEme (the product the authors are involved with), 23 and me, Navigenics, and Knome. Such products typically provide consumers with estimates of risk or predispositions to many diseases or traits, in addition to a range of tests of ancestry and family relationships through a secure personal Web site account. Aside from the breadth of tests that such a large number of SNPs can offer, one advantage of the personal genome scans for consumers is that they can (at least in principle) obtain updated estimates for all tests as new discoveries are made. Thus, the initial purchase of the test may be viewed as an investment that yields interest in the form of accumulating knowledge from new discoveries. To date, the companies providing DTC personal genome scans have been fairly active in updating the tests offered on their Web sites. In addition, customers can download their genotypes onto their own computers and analyze the data for themselves (for example, by uploading genotypes on Web sites such as SNPedia or seeking advice on layman Web sites such as DNA-forums.org).
Addressing the concerns of some scientists about DTC genetic tests
Many of the recent commentaries about DTC genetic testing in the scientific literature have focused on the personal genome scans11-17 and in particular on concerns about the disease risk estimates they provide to customers. These concerns may be divided into two main themes.
First, there are questions about the validity of the tests. The most common criticism here is that because the risk conferred by each variant used in these tests tends to be low (odds ratio <2), then the accuracy in predicting risk of disease for individuals (ie, their clinical validity) will also be low.11-13,15,18 Such comments either explicitly or implicitly compare the new tests with older tests that are already in use by health care providers in a way that is highly misleading. If we take a multifactorial disease such as heart attack, then it is self-evident that one cannot design a genetic test with predictive power as great as that for Huntington's disease, which is rarer, solely caused by mutations in one gene, and is negligibly affected by environmental factors. However, it is wrong to think that the predictive power of genetic tests based on GWAS findings is insignificant and not clinically relevant. Take, for example, the test for heart attack in the deCODEme personal genome scan, which includes eight independent SNPs with strong evidence for association to this disease19 -23 at the time of writing. This test alone can allow for the identification of 10% of people of European decent who have at least 1.4 times greater risk of developing a heart attack than the average person in the population. The average relative risk in this group is 1.6. Since the lifetime risk of suffering a heart attack is 42% for men over the age of 40,24 it follows that this test can identify men who have, on average, a lifetime risk of 67% of developing the most lethal disease of man.25 There is a dramatic difference between 42% and 67% lifetime risk. For example, individuals who are in the top quintile of the concentration of LDL cholesterol have only 1.3 times the population average risk of having a heart attack. Hence, a genetic test based on recently discovered sequence variants can identify people with added risk of heart attack that is twice that of those who are at the top of the cholesterol curve. In this context, it is important to keep in mind that the ability to assess risk of the heart attack by measuring serum cholesterol has transformed cardiology into the most important field of preventive medicine. Given the rather high lifetime risk of heart attack in most industrialized populations, then it follows that even a small increase in predictive power for each individual can be valuable.
Comparable estimates of average relative risk in the top 10% of people for other diseases included in the deCODEme genome scan are, for example, 5.2 for agerelated macular degeneration, 1.8 for type 2 diabetes and 3.0 for Crohn's disease. There is good reason to believe that the predictive power of these genetic tests will increase in the near future. On the one hand, we expect additional associated sequence variants to be discovered. On the other hand, because risk estimates in current tests are typically only stratified by sex and ethnicity, advances can be made through the inclusion of other relevant background variables (for example, the waist-tohip ratio and smoking history in the case of heart attack). In both cases, further epidemiological research is needed. However, contrary to the views of some commentators,11,13 these are not grounds for delay. The value of the discoveries so far, as reflected in the aforementioned example of heart attack, unequivocally warrants their use in genetic tests.
The second main theme of concern raised by commentators relates to the capacity of consumers to understand and cope with disease risk estimates from tests.8,9,12-14 Underlying these concerns is a somewhat paternalistic and patronizing view that information about disease risk is dangerous to the general public unless mediated in person by medical experts. Among the alleged dangers to the public are anxieties from overinterpreting risk estimates, which could lead to increased demands on health care providers and unnecessary medical procedures. We are not aware of any evidence that has been reported in support of this view, but there is at least some indirect evidence against it.26 However, even if such information were to provoke anxiety, the right of regulators or medical experts to prevent access to it is questionable. Consider the following analogy of a hypothetical company that provides individuals with reliable estimates of their risk of being mugged, murdered, run over, or burgled based on their age, sex, and address. There is no question that someone living in a rough inner-city area with limited economic means would have considerably greater risk on all counts than people living in more affluent areas. Would this be a legitimate reason for preventing individuals from seeking such potentially anxiety-provoking information? Another issue raised by commentators is that of clinical utility,10,13 that is, the extent to which knowledge of increased risk can reduce the burden of a disease through prevention or treatment. Although frequently raised in discussions of DTC genetic tests, this issue is really only relevant within the scope of health care provision (for example in the case of Huntington's disease). Thus, for DTC genetic tests, clinical utility is a secondary issue when balanced against peoples' right to seek information about themselves at their own cost. Given that such tests are in accordance with the accepted scientific literature and adhere to consumer laws (ie, that they deliver what their providers promise), then it is hard to see how regulators could prevent the public from buying them.
The challenge for providers of DTC genetic tests
In our opinion, the key to the success of DTC genetic tests for consumers, the companies that provide them, and regulators, is clarity and transparency. Whether tests report disease risk estimates, ancestry analyses, or evaluation of genealogical relationships, the information used to motivate consumers to buy tests and then explain the results should be as clear and accurate as possible. In particular, the probabilistic rather than deterministic nature of disease risk estimation must be unambiguous and comprehensible to the layman (and to medical experts). A key task is also to use the scientific literature in an accurate and responsible manner, for example by including only sequence variants with associations to disease that have been robustly replicated. One way to uphold such standards is through transparency, ie, by providing information about all the sequence variants used and the parameter values for risk models and their sources in the scientific literature. Most of the current providers of DTC personal genome scans have followed this approach, to a greater or lesser extent.
If such basic ground rules are adhered to, we believe that DTC genetic tests can provide considerable value to the general public, in particular while tests based on diseaseassociated variants discovered through GWAS are not available through health care providers. From a public health perspective, there is real preventative value to be gained from making people aware of their health and the risks posed to it. It is true that many of the lifestyle changes recommended for prevention through the application of DTC genetic tests could benefit many individuals, regardless of whether they take such tests. However, as most health care providers know, people are generally reluctant to change their lifestyle, even in the face of stern advice from medical experts. We would argue that when genetic risk factors are added on to conventional lifestyle risk factors in motivating people to take preventative measures, the outcome provides a greater impetus to act. Of course, from the perspective of personal autonomy, even if people choose to disregard advice about disease prevention, their right to seek information about genetic risk should prevail.
It is also important to highlight the educational nature of the Web sites of many companies that offer DTC genetic tests. They usually contain detailed information on hundreds of Web pages about diseases, ancestry, and genetic discoveries and methods that are used to provide results. This information is typically available to anyone through various front-end Web pages, where potential buyers can explore the kind of information they would receive as customers. Anyone can therefore learn a great deal about diseases, ancestry, and genetics without paying for a test. Whether the decision to buy a test is motivated by health concerns, recreational curiosity, or vanity, the consumer is almost certain to gain not only an increased understanding of genetics in general, but also what the recent wave of discoveries in the human genetics of disease and ancestry mean for them personally.
Conclusion
We believe that DTC genetic tests play a key translational role for the science of genetics, democratizing and disseminating privileged knowledge to the public. No matter how clichéd it sounds, knowledge is power. While some medical experts may complain about patients armed with results from DTC genetic tests or information about disease symptoms from the internet,13 we believe that a knowledgeable public is an empowered public
Contributor Information
Agnar Helgason, deCODE Genetics, Reykjavik, Iceland; Department of Anthropology, University of Iceland, Reykjavik, Iceland.
Kári Stefánsson, deCODE Genetics, Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Reykjavik, Iceland.
REFERENCES
- 1.Hindorff LA, Junkins HA, Mehta JP, Manolio TA. A Catalog of Published Genome-Wide Association Studies. Available at: www.genome.gov/gwastudies. Accessed November 2009 [Google Scholar]
- 2.Hindorff LA, Sethupathy P, Junkins HA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudmundsson J, Sulem P, Manolescu A, et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39:631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 4.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39:1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 6.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 7.Altshuler D, Brooks LD, Chakravarti A, Collins FS, Daly MJ, Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschhorn JN. Genomewide association studies--illuminating biologic pathways. . N Engl J Med. 2009;360:1699–1701. doi: 10.1056/NEJMp0808934. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696– 1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- 10.Bolnick DA, Fullwiley D, Duster T, et al. Genetics. The science and business of genetic ancestry testing. Science. 2007;318:399–400. doi: 10.1126/science.1150098. [DOI] [PubMed] [Google Scholar]
- 11.Evans JP, Green RC. Direct to consumer genetic testing: avoiding a culture war. Genet Med. 2009;11:568–569. doi: 10.1097/GIM.0b013e3181afbaed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farkas DH, Holland CA. Direct-to-consumer genetic testing: two sides of the coin. J Mol Diagn. 2009;11:263–265. doi: 10.2353/jmoldx.2009.090034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle--will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 14.Kaye J. The regulation of direct-to-consumer genetic tests. Hum Mol Genet. 2008;17(R2):R180–R183. doi: 10.1093/hmg/ddn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenzer J, Brownlee S. Knowing me, knowing you. BMJ. 2008;336:858–860. doi: 10.1136/bmj.39534.451458.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–495. doi: 10.1038/nrg2606. [DOI] [PubMed] [Google Scholar]
- 17.Henrikson NB, Bowen D, Burke W. Does genomic risk information motivate people to change their behavior? Genome Med. 2009;1:37. doi: 10.1186/gm37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft P, Hunter DJ. Genetic risk prediction--are we there yet? N Engl J Med. 2009;360:1701–1703. doi: 10.1056/NEJMp0810107. [DOI] [PubMed] [Google Scholar]
- 19.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction . Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 20.Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of earlyonset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdmann J, Grosshennig A, Braund PS, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 25.Geneva, Switzerland World Health Organization. The Global Burden of Disease - 2004 Update. 2008 [Google Scholar]
- 26.Cassidy MR, Roberts JS, Bird TD, et al. Comparing test-specific distress of susceptibility versus deterministic genetic testing for Alzheimer's disease. Alzheimers Dement. 2008;4:406–413. doi: 10.1016/j.jalz.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]