Abstract
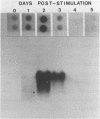
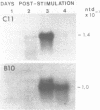
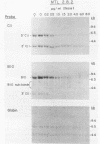
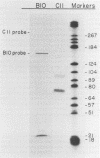
The expression of two serine proteases is induced by antigenic stimulation in cytotoxic T lymphocytes. Using nuclear run-on analysis the increase in steady state mRNA level has been shown to correspond to transcriptional activation. However, the two genes appear to be sequentially rather than coordinately induced. Both genes were shown to be more sensitive to DNase I digestion than a beta-globin gene in cytotoxic T cells. In addition, for the cytotoxic cell protease 1 gene the 5' region of the gene was more sensitive than the 3' end. Two DNaseI hypersensitive sites were seen in the 5' flanking sequences of both genes. The DNA sequences of the upstream regions of both genes were determined and compared. Although the two flanking sequences are overall quite dissimilar, there are short regions which are shared between the two CTL-protease genes. A number of these have been implicated in regulating the expression of other T cell genes.
Full text
PDF







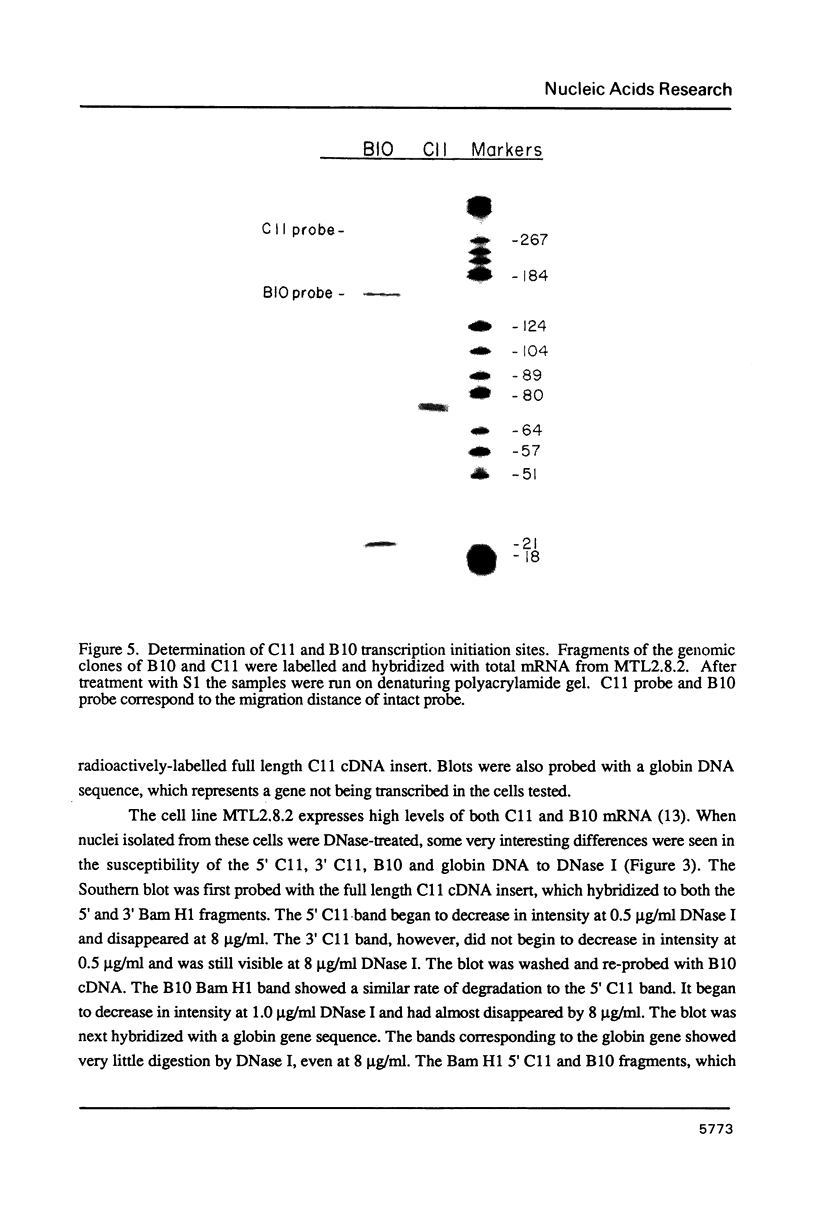


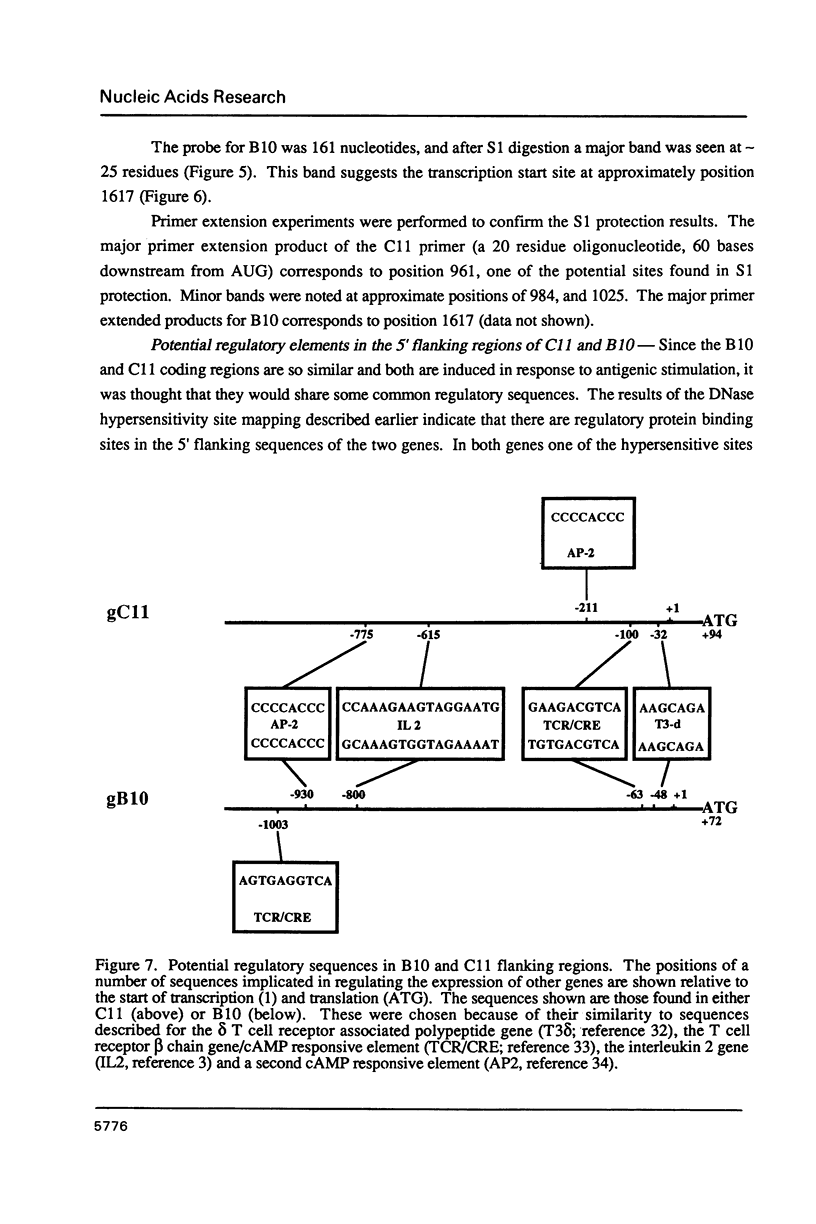



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Chou H. S., Loh D. Y. A conserved sequence in the T-cell receptor beta-chain promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3551–3554. doi: 10.1073/pnas.85.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleackley R. C., Havele C., Paetkau V. Cellular and molecular properties of an antigen-specific cytotoxic T lymphocyte line. J Immunol. 1982 Feb;128(2):758–767. [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Durand D. B., Bush M. R., Morgan J. G., Weiss A., Crabtree G. R. A 275 basepair fragment at the 5' end of the interleukin 2 gene enhances expression from a heterologous promoter in response to signals from the T cell antigen receptor. J Exp Med. 1987 Feb 1;165(2):395–407. doi: 10.1084/jem.165.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. Chromatin structure, DNA structure. Nature. 1982 Dec 2;300(5891):402–403. doi: 10.1038/300402a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Enver T., Brewer A. C., Patient R. K. Simian virus 40-mediated cis induction of the Xenopus beta-globin DNase I hypersensitive site. Nature. 1985 Dec 19;318(6047):680–683. doi: 10.1038/318680a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujii M., Sugamura K., Sano K., Nakai M., Sugita K., Hinuma Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J Exp Med. 1986 Mar 1;163(3):550–562. doi: 10.1084/jem.163.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Mills G. B., Cheung R. K., Lee J. W., Grinstein S. Transmembrane ion fluxes during activation of human T lymphocytes: role of Ca2+, Na+/H+ exchange and phospholipid turnover. Immunol Rev. 1987 Feb;95:59–87. doi: 10.1111/j.1600-065x.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., Shewmaker C. K., Jat P., Flavell R. A. Localization of DNA sequences necessary for transcription of the rabbit beta-globin gene in vitro. Cell. 1981 Jul;25(1):215–226. doi: 10.1016/0092-8674(81)90246-4. [DOI] [PubMed] [Google Scholar]
- Isakov N., Mally M. I., Scholz W., Altman A. T-lymphocyte activation: the role of protein kinase C and the bifurcating inositol phospholipid signal transduction pathway. Immunol Rev. 1987 Feb;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Kozumbo W. J., Harris D. T., Gromkowski S., Cerottini J. C., Cerutti P. A. Molecular mechanisms involved in T cell activation. II. The phosphatidylinositol signal-transducing mechanism mediates antigen-induced lymphokine production but not interleukin 2-induced proliferation in cloned cytotoxic T lymphocytes. J Immunol. 1987 Jan 15;138(2):606–612. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Science. 1986 May 16;232(4752):858–861. doi: 10.1126/science.3518058. [DOI] [PubMed] [Google Scholar]
- Lobe C. G., Havele C., Bleackley R. C. Cloning of two genes that are specifically expressed in activated cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1448–1452. doi: 10.1073/pnas.83.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe C. G., Upton C., Duggan B., Ehrman N., Letellier M., Bell J., McFadden G., Bleackley R. C. Organization of two genes encoding cytotoxic T lymphocyte-specific serine proteases CCPI and CCPII. Biochemistry. 1988 Sep 6;27(18):6941–6946. doi: 10.1021/bi00418a040. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for expression of the interleukin 2 receptor. J Immunol. 1985 Mar;134(3):1640–1643. [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Interleukin 2-induced lymphocyte proliferation is independent of increases in cytosolic-free calcium concentrations. J Immunol. 1985 Apr;134(4):2431–2435. [PubMed] [Google Scholar]
- Mueller C., Gershenfeld H. K., Lobe C. G., Okada C. Y., Bleackley R. C., Weissman I. L. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J Exp Med. 1988 Mar 1;167(3):1124–1136. doi: 10.1084/jem.167.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond M. J., Letellier M., Parker J. M., Lobe C., Havele C., Paetkau V., Bleackley R. C. A serine protease (CCP1) is sequestered in the cytoplasmic granules of cytotoxic T lymphocytes. J Immunol. 1987 Nov 15;139(10):3184–3188. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Shaw J., Meerovitch K., Elliott J. F., Bleackley R. C., Paetkau V. Induction, suppression and superinduction of lymphokine mRNA in T lymphocytes. Mol Immunol. 1987 May;24(5):409–419. doi: 10.1016/0161-5890(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Durand D. B., Bressler P., Holbrook N. J., Norris C. A., Kamoun M., Kant J. A., Crabtree G. R. Promoter region of interleukin-2 gene undergoes chromatin structure changes and confers inducibility on chloramphenicol acetyltransferase gene during activation of T cells. Mol Cell Biol. 1986 Sep;6(9):3042–3049. doi: 10.1128/mcb.6.9.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis R. H., Rubin H. Calcium protects DNase I from proteinase K: a new method for the removal of contaminating RNase from DNase I. Anal Biochem. 1980 Sep 1;107(1):260–264. doi: 10.1016/0003-2697(80)90519-9. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Sims J. E., Rabbitts T. H. T3 delta pre-mRNA is transcribed from a non-TATA promoter and is alternatively spliced in human T cells. EMBO J. 1986 Jun;5(6):1245–1252. doi: 10.1002/j.1460-2075.1986.tb04353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]