Abstract
Major depressive disorder (MDD) is one of the most common psychiatric illnesses in the adult population. It is often associated with an increased risk of cardiovascular disease. Osteoporosis is also a major public health threat. Multiple studies have reported an association between depression and low bone mineral density, but a causal link between these two conditions is disputed. Here the most important findings of the POWER (Premenopausal, Osteoporosis Women, Alendronate, Depression) Study, a large prospective study of bone turnover in premenopausal women with major depression, are summarized. The endocrine and immune alterations secondary to depression that might affect bone mass, and the possible role of poor lifestyle in the etiology of osteoporosis in subjects with depression, are also reviewed, as is the potential effect of antidepressants on bone loss. It is proposed that depression induces bone loss and osteoporotic fractures, primarily via specific immune and endocrine mechanisms, with poor lifestyle habits as potential contributory factors.
Keywords: chronic stress, woman, hormone, pain, immune system, genetic polymorphism
Abstract
El trastorno depresivo mayor (TDM) es una de las enfermedades psiquiátricas más comunes en la población adulta. A menudo está asociada con un aumento del riesgo de enfermedad cardiovascular. La osteoporosis también constituye una importante amenaza para la salud pública. Hay múltiples estudios que han dado cuenta de una asociación entre depresión y baja en la densidad mineral ósea, pero aun se discute una relación causal entre estas dos condiciones. En este artículo se resumen los hallazgos más importantes del estudio POWER (premenopausia, osteoporosis de la mujer, alendronato, depresión), un extenso estudio sobre el recambio óseo en la mujer premenopáusica con depresión mayor. Además se revisan las alteraciones endocrinas e inmunes secundarias a la depresión que pueden afectar la masa ósea y el posible papel de un pobre estilo de vida en la etiología de la osteoporosis en sujetos con depresión, como también el potencial efecto de los antidepresivos en la pérdida ósea. Se postula que la depresión induce pérdida ósea y fracturas osteoporóticas, lo que ocurriría primariamente a través de mecanismos inmunes y endocrinos específicos; en cambio los pobres hábitos de estilo de vida serían potenciales factores contribuyentes.
Abstract
Le trouble dépressif majeur (TDM) est l'une des maladies psychiatriques les plus courantes dans la population adulte, souvent associée à un risque augmenté de maladie cardiovasculaire. L'ostéoporose représente également un problème majeur de santé publique. De nombreuses études ont montré une association entre la dépression et une faible densité minérale osseuse mais le lien causal entre les deux affections fait l'objet de discussions. Cet article résume les résultats les plus importants de l'étude POWER (Premenopausal, Osteoporosis Women, Alendronate, Depression), vaste étude prospective sur le renouvellement osseux des femmes préménopausées atteintes de dépression majeure. Sont aussi étudiés les modifications immunitaires et endocriniennes secondaires à la dépression pouvant influer sur la masse osseuse, le rôle possible d'un style de vie inadapté dans l'étiologie de l'ostéoporose chez les personnes déprimées ainsi que l'effet potentiel des antidépresseurs sur la perte osseuse. Il est suggéré que la dépression induise une perte osseuse et des fractures ostéoporotiques principalement par le biais de mécanismes spécifiques immuns et endocriniens, les mauvaises habitudes de vie y contribuant éventuellement.
Major depressive disorder (MDD), one of the most common psychiatric illnesses in the adult population, is a major cause of disability1 and is associated with a twofold increase in nonsuicidal mortality in women.2 In addition to poor medical compliance and lifestyle factors, endocrine, immune, and autonomic dysregulations may play a causative role in producing medical illnesses in patients with MDD.3,4
The goal of this article is to describe some of the most clinically relevant medical consequences of major depression by summarizing here the findings of the POWER (Premenopausal, Osteoporosis Women, Alendronate, Depression) Study. The medical consequences of depression, as observed in the POWER Study, included osteoporosis, endocrine and immune alterations, subclinical inflammation and alterations in coagulability, chronic pain, and decreased quality of life. Some of the novel pathogenetic mechanisms unraveled will be discussed. This review will conclude by listing some implications for clinical practice and future research.
Medical consequences of major depression in premenopausal mildly depressed women mostly in remission
The POWER study
Women with MDD were characterized with a matched group of healthy women from an immune, endocrine, and inflammatory point of view and prospectively followed for 36 months, with evaluations at baseline, 6, 12, 24, and 36 months.5 In the POWER Study, the presence of a control group prevented artifacts secondary to a nonspecific “study effect” on mood and provided a benchmark for the research measurements taken.
Study design
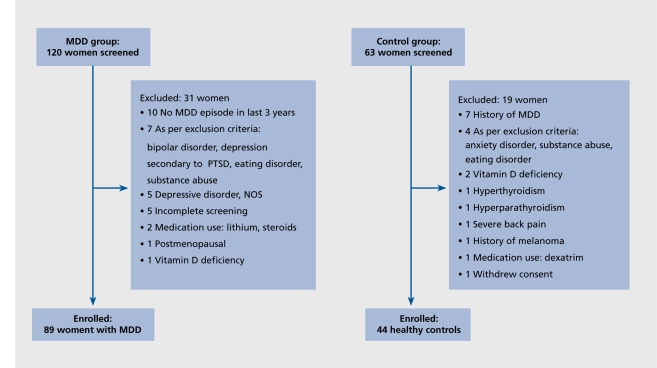
The POWER Study was a three-year prospective investigation of bone turnover and other measurements conducted at the NIH Clinical Center.5 Recruitment was conducted from July 2001 to February 2003 in the Washington, DC, metropolitan area by advertising in newspapers, radio, flyers and on the Internet. We enrolled 89 communitydwelling 21- to 45-year-old premenopausal women with current or recent MDD and 44 healthy control women. Women were enrolled if they met DSM-IV criteria for MDD, and had experienced a depressive episode in the preceding three years. Exclusion criteria were: depression with suicidal risk, schizophrenia, schizoaffective disorders, eating disorders, bipolar illness, hyperthyroidism, vitamin D deficiency, or other conditions or treatments that might affect bone turnover, and menopause, defined as the absence of spontaneous menses during the preceding 6 months. Patients treated for depression were allowed to continue their usual treatment. Patients with anxiety disorders or a history of alcohol or drug dependence in remission for 5 years were eligible. Controls were also excluded if they had a history of any DSM-IV diagnosis other than past alcohol abuse. Control women were individually matched with a subset of 44 patients with MDD based on age (+3 years) and body mass index (BMI) (+2.0). Except for two pairs, all other pairs were also matched by selfdefined race. The Institutional Review Board of the National Institute of Mental Health approved this study. Written informed consent was obtained from each participant. The trial was registered in ClinicalTrials.gov, NCT 00006180. Figure 1 depicts the number of individuals screened and the reasons for exclusion.
Figure 1. Study flow diagram. The number of subjects screened, reasons for exclusion, and number of subjects enrolled in the study are listed. Reproduced from ref 5: Eskandari F, Martinez PE, Torvik S, et al. Premenopausal, Osteoporosis Women, Alendronate, Depression (POWER) Study Group Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329-2336. Copyright © American Medical Association 2007.
Study sample
The study sample was composed of mostly Caucasian, college-educated women in their mid-30s. Smoking, intake of calcium, caffeine and alcohol, and physical fitness were similar between groups.
Clinical features of the participants
Seventeen percent of women with MDD were currently depressed (defined as a depressive episode during the preceding 4 weeks). On average, women with MDD exhibited mild symptoms of depression and anxiety and had a good level of functioning. However, the cumulative history of depression averaged approximately 5 years and four episodes of depression. Age of onset of depression was in the late teens, and approximately one half of the patients with MDD also suffered or had previously suffered from anxiety disorders. More than 80% of the women with MDD were taking antidepressants.
Low bone mass
Bone mineral density (BMD) was approximately 2% lower in MDD subjects versus controls at the AP spine and at the femoral neck, and tended to be lower at the radius; T-score (a comparison of a patient's BMD to that of a healthy 30-year-old of the same sex and ethnicity) was significantly lower at the femoral neck and the radius. The prevalence of low BMD was greater in women with MDD vs controls (28% vs 11%, P=0.04); greater at femoral neck (17% vs 2%, P=0.02) and total hip (15% vs 2% P=0.03), and tended to be greater at the lumbar spine (20% vs 9%; P=0.14).
Twenty-five women with MDD had a T-score lower than -1 SD at the spine or hip, and two of these women had osteoporosis, defined as a T-score at the AP spine or hip lower than -2.5 SD. These 25 women had significantly lower BMI and weight (P<0.001), and were less physically fit (P<0.001) than did women with MDD and normal BMD and higher T-scores. Of 73 women with MDD who were taking an antidepressant medication at the time of enrollment, 54 were taking one form of selective serotonin reuptake inhibitors.
Main findings
One in five premenopausal women with MDD exhibited low BMD. Decreased bone mass was especially common at the hip: hip fractures are the most serious consequence of osteoporosis. The bone mass deficits observed were of clinical significance. The 2% difference observed at the hip is similar to or greater in magnitude than that of recognized osteoporosis risk factors such as cigarette smoking, lack of exercise, and absence of calcium supplementation.6-8 Because the relationship between BMD and fracture risk is exponential, small deficits in BMD translate into larger increases in fracture rates. Given a prevalence of MDD in the 134 million US women between the ages of 21 and 45 of approximately 16%,9 we estimate that nearly 4 million women with MDD may have undetected deficits in BMD of similar magnitude.
How do the bone findings relate to the rest of the literature? Depression and osteoporosis: a research synthesis with meta-analysis
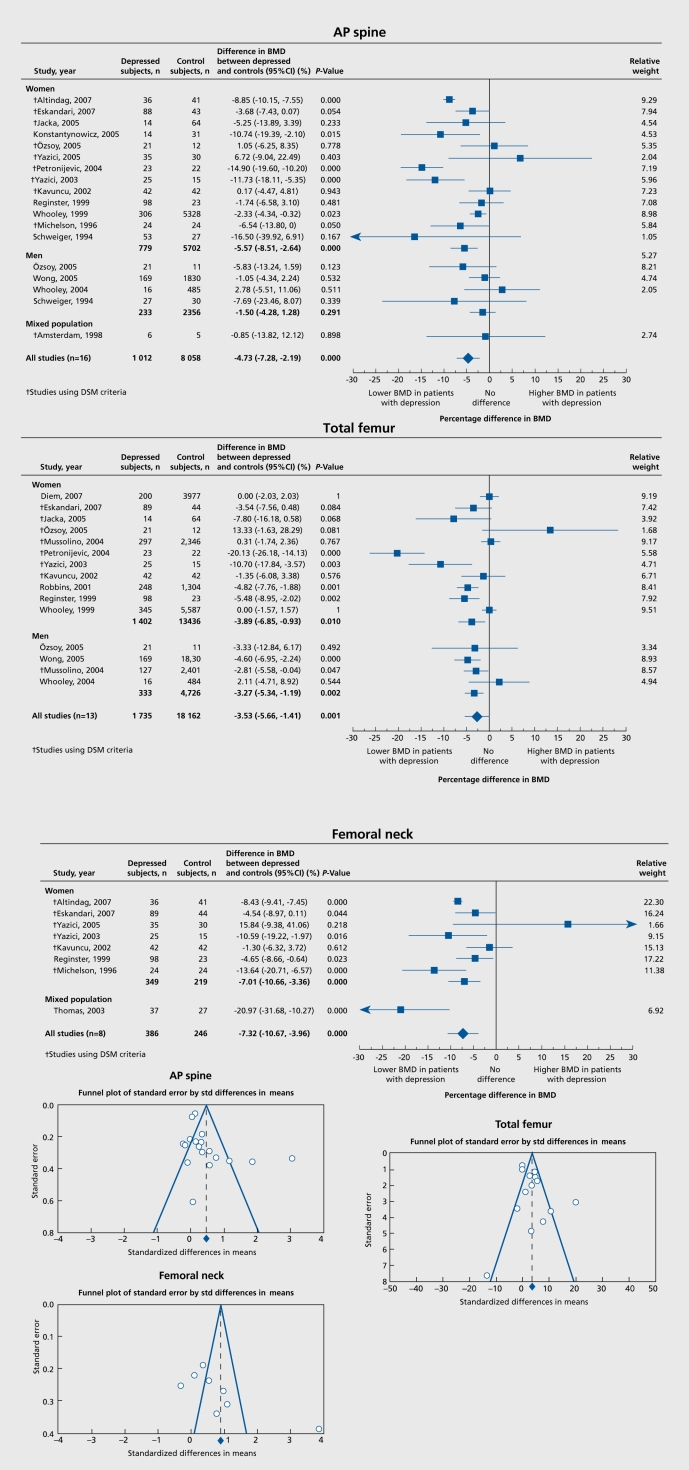
In spite of the evidence reported here and many other published reports since 1994,10 neither depressive symptoms nor MDD currently appear among the risk factors for osteoporosis in official statements. In the last NIH consensus conference on osteoporosis, depression was not listed as a risk factor for osteoporosis. Furthermore, a 2006 issue of Endocrine News, an official publication of the US Endocrine Society, contained an exhaustive table listing 19 causes of secondary osteoporosis, including bone loss secondary to space flight in astronauts, but failed to mention depression for secondary osteoporosis!11 The sustained need to change this incorrect, longstanding perception prompted us to perform a metaanalysis of the 20 original articles on bone mass in subjects with depression.12 At each site, bone mass was lower in subjects with depression as compared with controls: AP spine bone mineral density was 4.73% lower, total femur bone mineral density was 3.53% lower, and femoral neck bone mineral density was 7.32% lower (Figure 2) The deficits in bone mineral density in subjects with depression are of clinical significance and likely to increase fracture risk over the lifetime of these subjects.
Figure 2. Meta-analysis forest plots for AP spine bone mineral density (BMD), total femur BMD and femoral neck BMD. (A) AP spine BMD: Most studies show lower AP spine BMD in depressed subjects, with 3 studies indicating that BMD was higher in controls. (B) Total femur: Most studies show lower total femur BMD in depressed subjects, with 3 studies indicating that BMD was higher in controls, and 2 studies showing no difference. (C) Femoral neck BMD: Most studies show lower femoral neck BMD in depressed subjects, with three studies indicating that BMD was higher in controls. For each one of these three graphs under the first column are listed in chronological order the studies included and the year of publication; these studies are subdivided in studies conducted only in women, only in men, and studies conducted in men and women. Studies that utilized DSM criteria to characterize depression are indicated with a dagger. In the last column the relative weight of each individual study is reported. Reproduced from ref 12: Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G. Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res. 2010;42:467-482. Copyright ©Thieme 2010.
Increased abdominal fat and increased serum levels of prothrombotic factor
Both central adiposity13,14 and increased plasminogen activator inhibitor (PAI) -1 concentration15,16 are associated with increased risk of cardiovascular diseases. PAI1 is an important pathophysiological link between visceral obesity, insulin resistance, and cardiovascular diseases.17,18 Subjects with MDD are prone to increased central fat distribution.19,20 Although the exact mechanisms are not known, alterations of the hypothalamicpituitary-adrenal (HPA) axis secondary to depression, such as increased 24-hour plasma cortisol concentration,21,22 could contribute to central obesity23,24 Augmented coagulability due to increased concentration or activity of coagulation factors25,26 and PAI-127,28 has in fact been reported in other hypercortisolemic states, such as Cushing's syndrome, and in patients treated with glucocorticoids.
We tested whether MDD was associated with changes in the prothrombotic factors, PAI-1 and fVIII, as well as with altered body fat distribution, which may lead to hypercoagulability, and subsequent cardiovascular diseases.29 We also assessed whether these factors correlate with the severity of depression and cortisol concentration.
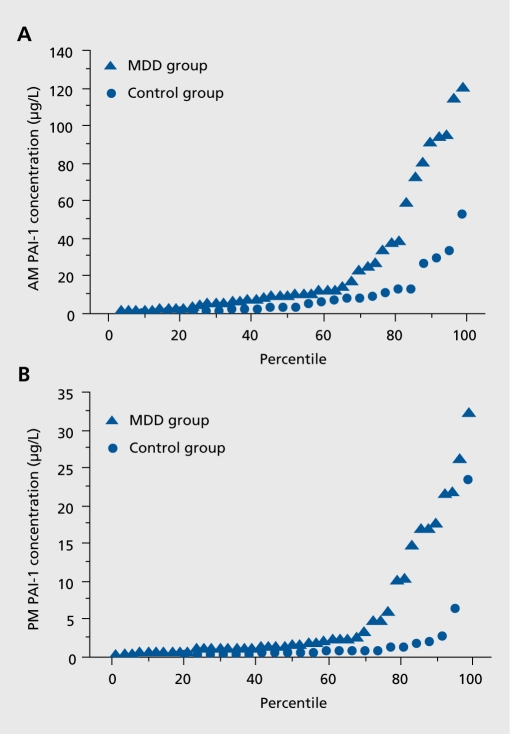
PAI-1 concentration (Figure 3) and fVIII activity were significantly higher at 0800 h than 2000 h in both the MDD and control groups, confirming the existence of circadian rhythmicity. Both PAI-1 and fVIII were significantly higher at 2000 h in women with MDD than in controls.
Figure 3. Plasminogen activator-1 (PAI1). PAI-1 concentrations exhibit an exponential distribution both in subjects with MDD and controls. Panel A: 0800 h PAI-1 concentration. Panel B: 2000 h PAI-1 concentration. Reproduced from ref 29: Eskandari F, Mistry S, Martinez PE, et al; POWER (Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression) Study Group. Younger, premenopausa women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: implications for greater cardiovascular risk. Metabolism. 2005;54:918-924. Copyright © W. B. Saunders 2005.
Women with MDD had higher PAI-1 concentration and fVIII activity and more abdominal fat than healthy controls. Increased central body fat in association with symptoms of depression and anxiety has already been reported in large epidemiological studies of men and women.19,20 The increase in prothrombotic factors in young women with MDD, reported for the first time in the POWER Study, is likely to be of clinical importance. These abnormalities persisted after correction for body weight, and were even more evident in the subset of subjects individually matched for age and BMI, suggesting that the association between depression and these factors was specific.
PAI-1 concentrations were similar to those reported in the subjects who later developed diabetes mellitus in a large prospective study30 Similarly, increased risk of diabetes mellitus has been reported in subjects with increased fVIII activity.31
Abnormal plasma C-reactive protein levels
C-reactive protein (CRP), a nonspecific marker of inflammation, is regarded as a risk factor for cardiovascular events. Recently, it has been proposed to include CRP as a clinical criterion for the metabolic syndrome as well.32 CRP is being proposed as a marker clinically useful for following prospectively subjects; however, only limited information on its variability over time exists. The reported variability over a week is approximately 30% to 50%, underlining the importance of performing serial sampling, especially if the values are in a high range.33 Of note, a diurnal variation in CRP levels with a peak at 15:00, as well as a small seasonal variation with highest values at winter-time were recently noted in a 45-year old population of a large cohort study of healthy men and women.34
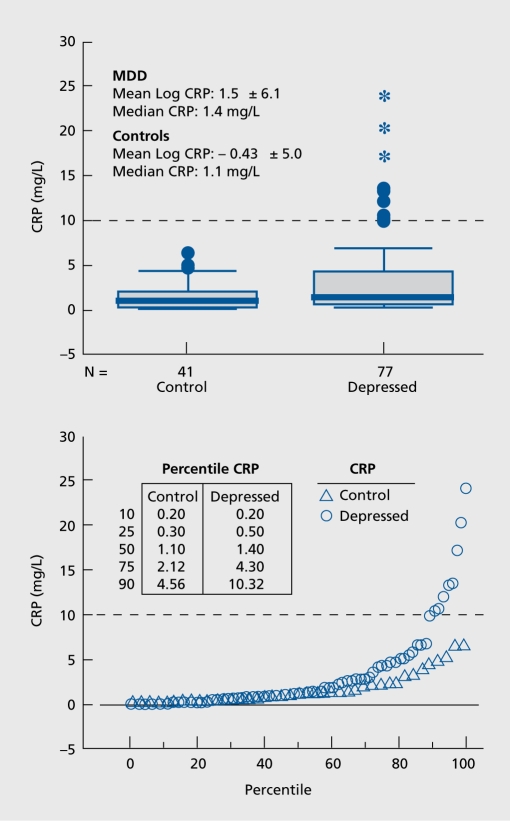
Women with MDD tended to have consistently higher CRP levels than controls over 12 months (P=0.07).35 BMI was positively related to log [CRP] in women with MDD only. Nine women out of 77 women with MDD had CRP levels greater than 10 mg/L, a value associated with a very high cardiovascular risk (Figure 4). This subset was obese and had significantly higher triglycerides, total cholesterol, LDL-cholesterol, fasting insulin, and Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) than the rest of women with MDD. The variations in CRP levels over time were high (intra- and interindividual coefficients of variations of ~30-50% and ~70-140%, respectively). No control had CRP levels greater than 10 mg/L.
Figure 4. Analysis of baseline C-reactive protein (CRP) levels in women with major depressive disorder (MDD) and healthy control women Upper panel: Box plots showing median, quartiles, and extreme values. The box represents the values between the 25th and 75th percentiles. The horizontal bar across each box represents the median value. Asterisks represent extreme values (values more than 3 box lengths from the upper edge of box). Open circles represent outliers (values between 1.5 and 3 box lengths from upper edge of box). One depressed subject with high CRP levels (29.3 mg/L) had reported recovery from an acute infection at the time of visit and was therefore excluded from the analyses. Lower panel: Percentile distribution of CRP values. CRP values for select percentiles are shown in the inset table. At the uppermost percentile (75th), women with MDD have CRP levels over twice as high as control women. The dashed line marks the CRP level of 10 mg/L above which there are only MDD subjects. Reproduced from ref 35: Cizza G, Eskandari F, Coyle M, et al; P.O.W.E.R (Premenopausal, Osteoporosis Women, Alendronate, Depression) Study Group. Plasma CRP levels in premenopausal women with major depression: a 12-month controlled study. Horrn Metab Res. 2009;41:641-648 Copyright © Thieme 2009.
Approximately 16% of the 134 million US women between the ages of 21 and 45 are or have been suffering from MDD. Hence, millions of women currently may have abnormal CRP levels, especially when associated with obesity.
Elevated neuroimmune biomarkers in plasma and sweat
Elevated cytokine levels have been reported in plasma of subjects with MDD, with inconsistent results.36,37 HPA axis abnormalities, alterations in autonomic and pain mediators, including vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), substance P (SP), and calcitoningene-related peptide (CGRP), have also been reported with inconsistent results and could contribute to immune dysregulation.38,39 Elevated cytokines in MDD have been linked to osteoporosis, diabetes, cardiovascular disease, sleep disturbances, and decreased pain threshold.
At first, we measured cytokine concentration in plasma hourly for 25 hours, using recycling immunoaffinity chromatography (RIC) coupled with laser-induced fluorescence detection, a highly sensitive and specific methodology, to measure multiple analytes in minute volumes.5 Compared with the 14 controlled women, the 17 women with MDD had much higher concentrations of the proinflammatory cytokines interleukin (IL)-13, IL-2, IL6 and tumor necrosis factor (TNF)-ct, similar levels of the anti-inflammatory cytokine IL-10, and lower levels of anti-inflammatory IL-13. Twenty-four-hour blood drawing allows for a more comprehensive assessment of cytokine levels than single-time measurements. As many of these cytokines were secreted in a circadian fashion, performing measurements around the clock provides a window on biorhythms and their potential disruption as a consequence of depression. This method is however invasive and impractical, as it can only be performed in an inpatient setting.
Skin patch, a novel method for collection of cytokines in sweat
We therefore developed and validated a skin patch, a novel method to measure cytokines in the sweat. The skin patch coupled with RIC, previously validated in healthy controls40 allowed identification of a specific pattern of neuroimmune dysregulation not previously detected in mildly depressed women. Women with MDD exhibited in sweat several fold elevations of proinflammatory cytokines, sympathetic (NPY) and sensory (SP and CGRP) neuropeptides, and diminished parasympathetic- associated neuropeptide, VIP.41 Cytokine levels in sweat closely related to the levels in plasma. This methodology avoids confounds to biomarker measurements associated with previous methods of sweat collection (exercise,42 sauna heat,43 and blood drawing.37 An elevation in proinflammatory cytokines of this magnitude substantially increases medical morbidity including osteoporosis, cardiovascular disease, and metabolic disorders. Cytokines also regulate neurotransmitters, hormones, and neuropeptides44 and modulate many behaviors, including mood, pain, and sickness behavior which are dysregulated in patients with depression. The elevated sympathetic (NPY) and sensory (SP and CGRP)-associated neuropeptides in both sweat patch eluates and plasma are consistent with their role in depression, This pattern of higher levels of proinflammatory cytokines, lower VIP (parasympathetic activity), and higher NPY (sympathetic activity) in patients with MDD, could be associated with increased cardiovascular risk in patients with MDD.
The elevated levels of SP and CGRP reported here confirm previous reports of the role of these peptides in pain perception, and of painful somatic symptoms correlating with depression severity in up to two thirds of patients with MDD.39 The lower VIP levels we observed are consistent with reduced parasympathetic tone that has been reported in depression, and with the effectiveness of parasympathetic vagal stimulation in treatment of refractory depression.45
Novel endocrine alterations in women with MDD: low 24-hour adiponectin and high nocturnal leptin concentration
MDD is associated with endocrine and immune system dysfunction and quite indirectly disruption of multiple circadian systems. White adipose tissue, an organ with endocrine functions, secretes the adipocytokines, leptin and adiponectin. Leptin signals to the central nervous system (CNS) the amount of energy stores to regulate food intake and energy expenditure.46 If adequate body fat is present, energy can be expended for costly processes like reproduction and growth. Leptin modulates several endocrine axes, including the HPA axis by negative feedback at the hypothalamus, and elevated leptin has been associated with osteopenia (reviewed in ref 47). Leptin controls appetite, food intake, sexual maturation, and reproductive functions, and immune functions, all of which are disrupted in depression. This hormone is secreted in an exquisitely pulsatile fashion; its secretion is inversely related to those of ACTH and cortisol. Reports of serum leptin levels in depressed subjects are conflicting, with studies finding either no differences, lower levels in depressed men, elevated levels in depressed men and women, or elevated levels only in depressed women.
Adiponectin was first reported as an adipocyte secretory protein in 1995, but only recently has its physiology been investigated.48 Plasma adiponectin concentrations are about two to three times greater than those of most other hormones, and its concentrations, unlike those of other adipocytokines, are inversely related to adiposity. Adiponectin receptors (AdipoR1 and R2) have been identified in the periphery and CNS. AdipoR1 is abundant in skeletal muscle and AdipoR2 exists primarily in the liver.46 AdipoR1 and AdipoR2 are also present in the paraventricular nucleus of the hypothalamus, amygdala, area postrema and, diffusely, in the periventricular areas and cortex.
While leptin's rhythmicity is well described, adiponectin's 24-hour secretory profile is not well known. Adiponectin exhibits diurnal and ultradian rhythms in normal-weight men.49 Circulating concentrations of adiponectin have been reported in depressed patients, but only at single time points. In some such studies, adiponectin was lower in newly diagnosed and drug-naïve MDD subjects, and was inversely related to depression severity.50 However, in others, there was no significant relationship between single adiponectin measurements and depressive symptoms.51 To date, 24-hour secretory profiles of adiponectin have not been described in MDD patients. Because MDD subjects have a higher CVD prevalence, and reduced adiponectin is associated with negative health consequences, adiponectin rhythmicity in patients with depression is of interest. The relationship of adiponectin to the HPA axis and leptin also remains unknown in MDD subjects. In a satellite study47 we aimed to establish: (i) whether women with MDD have decreased circulating concentrations of adiponectin and/or disruption of adiponectin secretory rhythmicity; (ii) the relationship of adiponectin and leptin secretion with depression; (iii) the temporal correlations among circadian concentrations of adiponectin, leptin, ACTH, and cortisol.
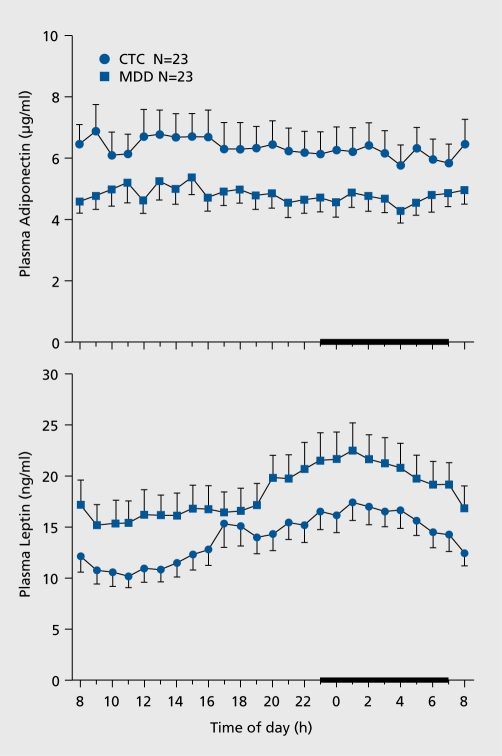
From the whole POWER sample, we individually matched 23 consecutively studied women with MDD with 23 control subjects, based on age ±3.0 years and BMI ±2.0 kg/m2. In control subjects, diurnal fluctuation in adiponectin was about 30% (Figure 5, upper panel). Adiponectin was higher during the day, with a zenith around 1430 h, an initial fall around 1600 h, a further decline after 2300 h and then another increase at about 0300 h. Women with MDD exhibited similar adiponectin rhythmicity. Mean adiponectin concentrations were about 25% lower at all 24-h time points in women in the MDD versus control group. The diagnosis of MDD accounted for approximately 25% to 30% of the variability in adiponectin (0800 h adiponectin R2 0.285; P=0.004; 24-h mean adiponectin R2 0.267; P=0.001).
Figure 5. Mean 24-hour plasma adiponectin and leptin concentrations in women with major depressive disorder (MDD, N=23) and Controls (N=23). Upper panel adiponectin concentrations: In both groups, adiponectin exhibited a circadian variation characterized by slightly higher values during early afternoon with a peak around 1400 h. Adiponectin remained lower in women with MDD over 24 h. 0800 h and 24 h mean adiponectin was significantly lower in women with MDD compared with controls (0800 h P adjusted for weight =0.0053; mean 24-h P adjusted for weight =0.042; analysis of covariance). Error bars represent SEM. Lower panel leptin levels. In both groups, leptin exhibited a circadian variation characterized by a nocturnal zenith around 0200 h and a nadir around 1 000 h. Leptin remained higher in women with MDD over 24 h 0800 h and 24 h mean leptin was significantly higher in women with MDD compared with controls (0800 h P adjusted for weight =0.010; mean 24-h P adjusted for weight =0.021; analysis of covariance). Mean nocturnal (2000 h-0400 h) leptin, was greater in women with MDD than controls (21.05 + 0.91 ng/mL vs 16.10 + 0.98 ng/mL, respectively, P<0.001). Error bars represent SEM Reproduced from ref 47: Cizza G, Nguyen VT, Eskandari F, et al; POWER Study Group. Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry 2010;71:1079-1087. Copyright © Physicians Postgraduate Press 2010.
Adiponectin was inversely related to the cumulative duration of depression (R=-0.51; P=0.03) and tended to be inversely related to the duration and severity of depression (R=-0.41; P=0.09). Adiponectin accounted for approximately 20% of the waist-hip ratio variability (0800 h adiponectin R2 0.194; P=0.002; 24-h mean adiponectin R2 0.230; P=0.007).
Leptin was higher in women with MDD at all 24-h time points. Leptin's rhythmicity was similar between groups. In control subjects, leptin exhibited its typical diurnal variation, with higher concentrations during the night, and a zenith around 0200 h. The lowest concentrations were observed during the day, with a nadir around 1100 h. Leptin was higher in women with MDD at all 24-h time points. Leptin's rhythmicity was similar between groups.
Adrenocorticotropic hormone (ACTH) and cortisol showed typical diurnal variations, with higher values in the morning. The HPA axis was only marginally altered in this sample of women with MDD. There were no differences in circadian secretion between groups; however, 0800 h ACTH was somewhat higher in women with MDD than in control subjects (P=0.05).
In summary, women with MDD exhibited lower circadian adiponectin plasma concentrations than did closely matched control subjects. As reduced adiponectin has been shown to predict type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), premenopausal women with MDD may be at increased risk for both conditions. Women with MDD also had increased nocturnal leptin, elevated morning ACTH, and decreased nocturnal ACTH and cortisol. ACTH and cortisol were more strongly related in women with MDD than in control subjects, suggesting mild HPA-axis activation in women with MDD.
A 25% decrease in adiponectin has been reported in prospective studies to increase the risk of T2DM and CVD49-51 In addition, to our knowledge, this was the first report describing the circadian rhythm of adiponectin in women with MDD. It is possible that short sleep and/or sleep disruption decreased adiponectin secretion. Sleep disturbances are one of the components of the depressive syndrome, according to DSM-IV criteria, and approximately 60% of depressed patients have insomnia.
Women with MDD had approximately 25% higher concentrations of leptin; because MDD is a state of increased sympathetic tone,52 higher leptin may have been secondary to activation of the sympathetic nervous system, which is known to stimulate leptin secretion. Leptin has been shown in a mouse model to centrally inhibit bone formation via the sympathetic system. Apposition of new bone takes place mostly at night, as indicated by circadian measurements of markers of bone turnover53; therefore higher nocturnal leptin levels may contribute to the development of osteoporosis in women with MDD.
Glucocorticoid receptor gene polymorphisms in women with MDD: relevance to central obesity
Glucocorticoid receptor (GR) gene polymorphisms are associated with glucocorticoid hypersensitivity and visceral obesity. Structural alterations in GR gene are known to affect target tissue responsiveness to glucocorticoids. Two polymorphisms, Bcl1 and N363S, have been associated with central obesity and altered glucocorticoid sensitivity54 Furthermore, Bcl1 polymorphism has been linked to visceral obesity in homozygous (GG) carriers,55 higher sensitivity to dexamethasone,54 higher salivary cortisol,56 and hyperinsulinemia.57 An association between major depressive disorder (MDD) and Bcl1 polymorphism was noted recently.58,59
We examined the relative distribution of specific polymorphisms of GR (Bcl1, N363S, rs33388, rs33389) in women with MDD compared with healthy controls.60 Both the rs33888 and rs33889 polymorphisms of the GR gene were included in the study to explore a potential role between altered glucocorticoid sensitivity and MDD.61
We also explored whether GR polymorphisms were associated with abdominal obesity and insulin resistance. For Bcl1 SNP homozygous GG polymorphism was significantly more frequent (P=0.03) in women with MDD than in controls. In the total sample the genotype frequencies were 41.9% for CC, 43.2% for CG, and 14.81% for GG genotypes, respectively. GG homozygotes had slightly higher waist-to-hip ratio (WHR) than non GG carriers (GG: 0.9±0.07, non GG: 0.8±0.05; P<0.02), although BMI was similar in both groups.
Women with MDD were more likely to be carriers of a specific polymorphism (GG) of Bcl1in the GR gene with a genotype frequency of 15%. The relationship between Bcl1 polymorphism and MDD may be explained at least in part by GR hypersensitivity to glucocorticoids, as demonstrated by the increased response to ACTH and cortisol suppression with low-dose dexamethasone in subjects with Bcl1 polymorphism. Women with MDD had also higher BMI and abdominal adiposity than controls: in particular, women with MDD and Bcl1 GG genotype of Bcl1 had higher WHR as compared with their non GG counterparts. This suggests that GG genotype confers suceptibilty to increased abdominal fat independent of total body adiposity. Glucocorticoids promote intra-abdominal fat accumulation through various mechanisms. Omental fat has a higher glucocorticoid binding capacity than subcutaneous fat, more transcriptional activity of GR and greater sensitivity of glucocorticoids on lipoprotein lipase activity.62
In summary, premenopausal women with MDD had higher BMI, WHR, total body fat, and abdominal fat percent compared with controls. Homozygous Bcl1 GG genotype was more frequent in these subjects, as was a higher WHR without higher BMI.
Does all the above matter? Chronic pain: the role of substance P and calcitonin-gene-related peptide
Whereas it is established that organic pain may induce depression, it is unclear whether pain is more common in healthy subjects with depression. Patients with MDD may present with somatic symptoms, including aches and pain. The prevalence of pain in patients presenting with MDD, in whom pain was not the primary complaint, has not been well studied. We established the prevalence of pain and associated symptoms and determined whether there is a relationship between pain intensity and the clinical features of depression.39 We also administered two scales of everyday stressors, the Hassles and Uplifts Scales and a widely used quality-of-life instrument, the SF-36.
Pain and associated symptoms
Pain was much more common in depressed subjects and, within subjects with depression, in depressed subjects with atypical or melancholic episode subtypes. Pain location was distinct; the head and neck were the most common sites. The intensity of pain was mild. Women with depression reported average values of approximately 2 (range 0-10) in all seven (general activity, mood, walking ability, normal work, relations with others, sleep, and enjoyment of life) interferences scales. Therefore, in women with depression, pain interfered with the activities inquired, but only to a mild extent. Fatigue, anxiety, and concentration and memory problems were more prevalent in subjects with depression. A greater proportion of subjects with depression than controls (57% vs 25%; P=0.01) experienced four or more symptoms commonly associated with pain. The vast majority of depressed subjects reported having at least one symptom, while nearly half of controls were symptom-free.
Relationship between pain intensity and clinical features of depression
Pain intensity was significantly related with the current severity of depression (r2=0.076; P=0.04), and tended to be related with the current severity of anxiety (r2=0.065; P=0.07), and the number of episodes of depression (r2= 0.072; P=0.09).
Cytokine and neuropeptide measurements
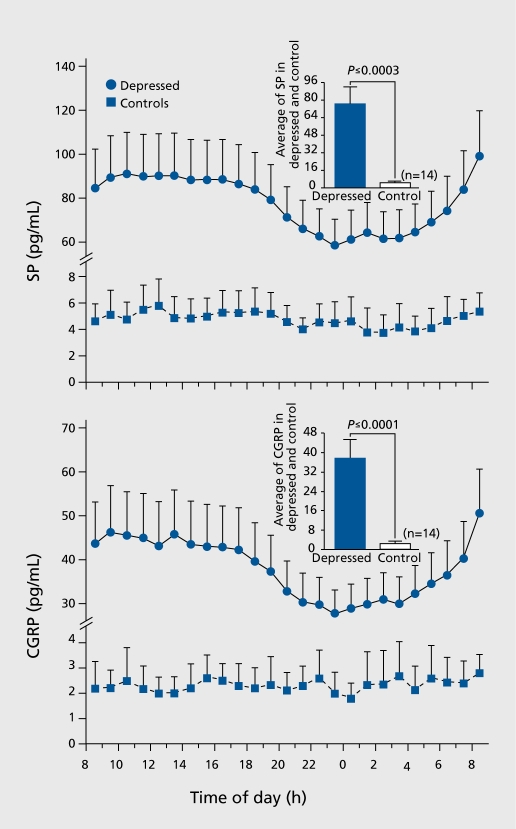
Women with depression had higher mean circulating levels of SP and CGRP than controls (Figure 6). Both Substance P (SP) and calcitonin-gene-related-peptide (CGRP), two neuropeptides which are known mediators of pain, exhibited a 24-h single cosinor rhythm in women with depression which was remarkably similar to controls; the zenith occurred at 12:24 and 12:15 respectively, and the nadir at 00:24 and 00:15, respectively. SP (zenith: 13:50, nadir: 01:50) exhibited a significant rhythm in controls whereas no significant rhythm in CGRP was observed in controls.
Figure 6. Plasma levels of substance P (SP) and calcitonin-gene-relatedpeptide (CGRP). Mean 24-h levels of SP (upper panel) and CGRP (lower panel) were lower in women with depression compared with controls. Reproduced from ref 39: Hartman JM, Berger A, Baker K, et al. Quality of life and pain in premenopausal women with major depressive disorder: The POWER Study. Health Qual Life Outcomes. 2006;4:2. Copyright © Biomed Central 2006.
Quality of life
Women with depression reported a lower quality of life. They scored lower than controls in the emotional and social well-being domain of the SF-36, as well as in one dimension of the physical well-being domain, general health. Thus, they described themselves as being less vital, affected emotionally, and impaired in their social life. The impact of their depression on quality of life was comparable to that reported in subjects with breast cancer or morbid obesity.63,64
Daily hassles and uplift scale
Daily hassles occurred more frequently and more severely in women with depression. These included: worries about physical appearance, misplacing things, and not having enough energy. In contrast, both groups experienced daily uplifts to a similar extent.
Summary
Women with depression had a higher prevalence of pain than generally reported in the literature. SP and CGRP, two pain-related neuropeptides, were higher around the clock in depressed subjects compared with controls.
Implications for practice and future research
Bone loss
The usefulness of antidepressants for bone loss in MDD should be evaluated. Prospective studies should establish whether women with MDD experience a more sustained bone loss during the peri- or postmenopausal period than nondepressed women. Exploratory studies of bone mass should be conducted in conditions associated with an activation of the sympathetic nervous system, such as post-traumatic stress disorders. The possibility that subjects with depression may fail to reach peak bone mass should be investigated.
Prothrombotic factors
Increased levels of prothrombotic factors may explain some of the mechanisms leading to augmented risk of cardiovascular disease in depression. The clinical significance of our observations should be further validated in large prospective studies.
CRP
This should be measured in women with depression, especially if overweight. Since dieting is effective in lowering CRP levels,65 weight loss might be recommended even in moderately overweight women with depression, especially if they have higher CRP levels and/or other cardiovascular risk factors. Given its large day-to-day variability, clinical decisions should be based on at least two CRP serial measurements taken several days apart. CRP is a wellaccepted marker of inflammation; however, it is not clear whether CRP is itself a risk factor for cardiovascular disease. Therefore, large-scale use of CRP measurements should await the proof that it is involved in the pathogenesis of cardiovascular disease. CRP should be measured by the high sensitivity assay and because of its skewed distribution it should be classified based on cutpoints established in prospective clinical trials and clinically interpreted in conjuction with the lipid values.
Cytokines and sweat patch
Given their circadian variability66 they should be measured by frequent sampling in inpatient setting or in the sweat collected for several hours as a valid and practical alternative in ambulatory settings. RIC and other ultrasensitive methods should be used.
Depression, leptin, adiponectin, and risk of diabetes and obesity
Future studies should establish whether a causal link exists between MDD and decreased daily production of adiponectin. These studies should be large enough and of sufficient duration to detect clinical events such as T2DM or CVD. The effects of antidepressants on adiponectin should be investigated, as should the mechanisms that lead to decreased adiponectin and increased leptin secretion, respectively.
Glucocorticoid receptor gene polymorphism
Future research should focus on establishing the role of the Bcl1 GG genotype of GR gene in metabolic syndrome within larger studies of women with active MDD.
Depression, chronic pain, and quality of life
Women with depression should be clinically screened for pain and patients complaining of pain, especially if not clearly attributable to an identifiable organic process should be evaluated for depression. Quality of life is substantially affected even in mild depression. Future research should establish the role of MDD in the natural history of pain, attempt to characterize the biological mechanisms predisposing to pain, and determine whether men with depression also have increased prevalence of pain symptoms.
Conclusions
We propose that the sample of women with MDD studied in the POWER Study is representative of a distinct emerging clinical phenotype characterized by osteopenia, an increased risk for insulin resistance and cardiovascular disease, a chronic state of subclinical inflammation, increased diathesis to coagulopathy states, major endocrine, immune, and neuropeptide alterations, and increased propensity to chronic pain (Figure 7). The collective body of evidence generated in the POWER Study and summarized here has established the novel concept that the medical consequences of depression previously thought to be limited to severely depressed, unmedicated subjects are definitely present also in the milder clinical forms. Of note, these women were mildly depressed or in either spontaneous or medication-induced clinical remission, and highly functional in these conditions. As these clinical forms represent epidemiologically the majority of subjects with depression, and bearing in mind the high lifetime prevalence of major depression, a chronic and recurrent condition, this phenotype has major clinical and public health implications.67
Acknowledgments
This research was supported in part by the Intramural Research Programs of the National Institute of Mental Health, the National Institute of Diabetes, Digestive and Kidney Diseases, the National Center for Complementary and Alternative Medicine, and the Warren Magnuson Clinical Center of the National Institutes of Health in Bethesda, MD. The following individuals were investigators of the POWER Protocol (Premenopausal Osteoporosis Women Alendronate Depression): Giovanni Cizza (Principal Investigator) and, alphabetically, Anne Berger, Marc R. Blackman, Karim A. Calis, Gyorgy Csako, Bart Drinkard, Farideh Eskandari, Philip W. Gold, McDonald Horne, Christine Kotila, Pedro Martinez, Kate Musallam, Terry M. Phillips, James. C. Reynolds, Nancy G. Sebring, Esther Sternberg, and Sara Torvik (Associate Investigators).
I wish to thank: all the subjects participating in this study; Ms Kate Musallam, nurse manager, and all the other NIMH nurses who supported these studies. The informatics support for this study was provided by Mr Frank Pierce from ®Esprit Health.
Selected abbreviations and acronyms
- BMD
Bone mineral density
- CRP
C-reactive protein
- HPA
hypothalamic-pituitary-adrenal
- MDD
major depressive disorder
- PAI-1
plasminogen activator inhibitor
REFERENCES
- 1.Evans DL., Charney DS., Lewis L., et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Zheng D., Macera CA., Croft JB., et al. Major depression and all-cause mortality among white adults in the United States. Ann Epidemiol. 1997;7:213–218. doi: 10.1016/s1047-2797(97)00014-8. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP., Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 4.Brown ES., Varghese FP., McEwen BS. Association of depression with medical illness: does cortisol play a role? . Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 5.Eskandari F., Martinez PE., Torvik S., et al. Premenopausal, Osteoporosis Women, Alendronate, Depression (POWER) Study Group. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 6.Ward KD., Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259–270. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaiuti D., Shea B., Iovine R., et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;3:CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 8.Shea B., Wells G., Cranney A., et al. Calcium supplementation on bone loss in postmenopausal women. Cochrane Database Syst Rev. 2004;1:CD004526. doi: 10.1002/14651858.CD004526.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC., Berglund P., Demler O., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 10.Schweiger U., Deuschle M., Korner A., et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;151:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen C. Catching secondary causes of osteoporosis. Endocrine News. 2006:1. [Google Scholar]
- 12.Cizza G., Primma S., Coyle M., Gourgiotis L., Csako G. Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res. 2010;42:467–482. doi: 10.1055/s-0030-1252020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prineas RJ., Folsom AR., Kaye SA. Central adiposity and increased risk of coronary artery disease mortality in older women. Ann Epidemiol. 1993;3:35–41. doi: 10.1016/1047-2797(93)90007-q. [DOI] [PubMed] [Google Scholar]
- 14.Cox BD., Whichelow MJ., Prevost AT. The development of cardiovascular disease in relation to anthropometric indices and hypertension in British adults. Int J Obes Relat Metab Disord. 1998;22:966–973. doi: 10.1038/sj.ijo.0800705. [DOI] [PubMed] [Google Scholar]
- 15.Thogersen AM., Jansson JH., Boman K., et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 16.Scarabin PY., Aillaud MF., Amouyel P., et al. Associations of fibrinogen, factor VII and PAI-1 with baseline findings among 10,500 male participants in a prospective study of myocardial infarction--the PRIME Study. Prospective Epidemiological Study of Myocardial Infarction. Thromb Haemost. 1998;80:749–756. [PubMed] [Google Scholar]
- 17.Landin K., Stigendal L., Eriksson E., et al. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator inhibitor-1. Metabolism. 1990;39:1044–1048. doi: 10.1016/0026-0495(90)90164-8. [DOI] [PubMed] [Google Scholar]
- 18.Alessi MC., Peiretti F., Morange P., et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 19.Rosmond R., Bjorntorp P. Psychiatric ill-health of women and its relationship to obesity and body fat distribution. Obes Res. 1998;6:338–345. doi: 10.1002/j.1550-8528.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahlberg AC., Ljung T., Rosmond R., et al. Depression and anxiety symptomsin relation to anthropometry and metabolism in men. Psychiatry Res. 2002;112:101–110. doi: 10.1016/s0165-1781(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 21.Linkowski P., Mendlewicz J., Leclercq R., et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61:429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 22.Gold PW., Calabrese JR., Kling MA., et al. Abnormal ACTH and cortisol responses to ovine corticotropin releasing factor in patients with primary affective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:57–65. doi: 10.1016/0278-5846(86)90044-8. [DOI] [PubMed] [Google Scholar]
- 23.Pasquali R., Cantobelli S., Casimirri F., et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 24.Weber-Hamann B., Hentschel F., Kniest A., et al. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–277. doi: 10.1097/00006842-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Patrassi GM., Dal Bo Zanon R., Boscaro M., et al. Further studies on the hypercoagulable state of patients with Cushing's syndrome. Thromb Haemost. 1985;54:518–520. [PubMed] [Google Scholar]
- 26.Casonato A., Pontara E., Boscaro M., et al. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing's syndrome. Blood Coagul Fibrinolysis. 1999;10:145–151. doi: 10.1097/00001721-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Patrassi GM., Sartori MT., Viero ML., et al. The fibrinolytic potential in patients with Cushing's disease: a clue to their hypercoagulable state. Blood Coagul Fibrinolysis. 1992;3:789–793. doi: 10.1097/00001721-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosi B., Sartorio A., Pizzocaro A., et al. Evaluation of haemostatic and fibrinolytic markers in patients with Cushing's syndrome and in patients with adrenal incidentaloma. Exp Clin Endocrinol Diabetes. 2000;108:294–298. doi: 10.1055/s-2000-8000. [DOI] [PubMed] [Google Scholar]
- 29.Eskandari F., Mistry S., Martinez PE., et al. POWER (Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression) Study Group. Younger, premenopausal women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: implications for greater cardiovascular risk. Metabolism. 2005;54:918–924. doi: 10.1016/j.metabol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Festa A., D'Agostino R., Jr, Tracy RP., et al. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 31.Duncan BB., Schmidt MI., Offenbacher S., Wu KK., Savage PJ., Heiss G. Factor VIII and other hemostasis variables are related to incident diabetes in adults. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1999;22:767–772. doi: 10.2337/diacare.22.5.767. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM., Wilson PW., Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? . Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 33.Riese H., Vrijkotte TG., Meijer P., Kluft C., de Geus EJ. Diagnostic strategies for C-reactive protein. BMC Cardiovasc Disord. 2002;2:9. doi: 10.1186/1471-2261-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabatine MS., Morrow DA., Jablonski KA., et al. PEACE Investigators. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 35.Cizza G., Eskandari F., Coyle M., et al. P.O.W.E.R. (Premenopausal, Osteoporosis Women, Alendronate, Depression) Study Group. Plasma CRP levels in premenopausal women with major depression: a 12-month controlled study. Horm Metab Res. 2009;41:641–648. doi: 10.1055/s-0029-1220717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raison CL., Capuron L., Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marques-Deak AH., Neto FL., Dominguez WV., et al. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiatr Res. 2007;41:152–159. doi: 10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Marques-Deak A., Cizza G., Sternberg E. Brain-immune interactions and disease susceptibility. Mol Psychiatry. 2005;10:239–250. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- 39.Hartman JM., Berger A., Baker K., et al. Quality of life and pain in premenopausal women with major depressive disorder: The POWER Study. Health Qual Life Outcomes. 2006;4:2. doi: 10.1186/1477-7525-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques-Deak A., Cizza G., Eskandari F., et al. Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study. J Immunol Methods. 2006;315:99–109. doi: 10.1016/j.jim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Cizza G., Marques AH., Eskandari F., et al. POWER Study Group Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol Psychiatry. 2008;64:907–911. doi: 10.1016/j.biopsych.2008.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones AP., Webb LM., Anderson AO., Leonard EJ., Rot A. Normal human sweat contains interleukin-8. J Leukoc Biol. 1995;57:434–437. doi: 10.1002/jlb.57.3.434. [DOI] [PubMed] [Google Scholar]
- 43.Sato K., Sato F. Interleukin-1 alpha in human sweat is functionally active and derived from the eccrine sweat gland. Am J Physiol. 1994;266:R950–R959. doi: 10.1152/ajpregu.1994.266.3.R950. [DOI] [PubMed] [Google Scholar]
- 44.Silverman MN., Pearce BD., Biron CA. Miller AH Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gjerris A., Rafaelsen OJ., Vendsborg P., Fahrenkrug J., Rehfeld JF. Vasoactive intestinal polypeptide decreased in cerebrospinal fluid (CSF) in atypical depression. Vasoactive intestinal polypeptide, cholecystokinin and gastrin in CSF in psychiatric disorders. J Affect Disord. 1984;7:325–337. doi: 10.1016/0165-0327(84)90054-5. [DOI] [PubMed] [Google Scholar]
- 46.Ahima RS., Qi Y., Singhal NS., Jackson MB., Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55:S145–S154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 47.Cizza G., Nguyen VT., Eskandari F., et al. POWER Study Group. Low 24hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71:1079–1087. doi: 10.4088/JCP.09m05314blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scherer PE., Williams S., Fogliano M., Baldini G., Lodish HF. A novel serum-protein similar to C1Q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 49.Lindsay RS., Funahashi T., Hanson RL., et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 50.Snehalatha C., Mukesh B., Simon M., Viswanathan V., Haffner SM., Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26:3226–3229. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 51.Hung YJ., Hsieh CH., Chen YJ., et al. Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clinical Endocrinology. 2007;67:784–789. doi: 10.1111/j.1365-2265.2007.02963.x. [DOI] [PubMed] [Google Scholar]
- 52.Gold PW., Wong ML., Goldstein DS., et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A. 2005;102:8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hassager C., Risteli J., Risteli L., Jensen SB., Christiansen C. Diurnal variation in serum markers of type-1 collagen-synthesis and degradation in healthy premenopausal women. J Bone Miner Res. 1992;7:1307–1311. doi: 10.1002/jbmr.5650071110. [DOI] [PubMed] [Google Scholar]
- 54.van Rossum EF., Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- 55.Rosmond R. Association studies of genetic polymorphisms in central obesity: a critical review. Int J Obes Relat Metab Disord. 2003;27:1141–1151. doi: 10.1038/sj.ijo.0802397. [DOI] [PubMed] [Google Scholar]
- 56.Rosmond R., Chagnon YC., Holm G., Chagnon M., Perusse L., Lindell K., et al. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8:211–218. doi: 10.1038/oby.2000.24. [DOI] [PubMed] [Google Scholar]
- 57.Weaver JU., Hitman GA., Kopelman PG. An association between a Bc1I restriction fragment length polymorphism of the glucocorticoid receptor locus and hyperinsulinaemia in obese women. J Mol Endocrinol. 1992;9:295–300. doi: 10.1677/jme.0.0090295. [DOI] [PubMed] [Google Scholar]
- 58.van Rossum EF., Binder EB., Majer M., Koper JW., Ising M., Modell S., et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 59.van West D., Van Den Eede F., Del-Favero J., et al. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006;31:620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- 60.Krishnamurthy P., Romagni P., Torvik S., et al. P.O.W.E.R. (Premenopausal, Osteoporosis Women, Alendronate, Depression) Study Group. Glucocorticoid receptor gene polymorphisms in premenopausal women with major depression. Horm Metab Res. 2008;40:194–198. doi: 10.1055/s-2007-1004541. [DOI] [PubMed] [Google Scholar]
- 61.DeRijk RH., Schaaf M., de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol. 2002;81:103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 62.Buemann B., Vohl MC., Chagnon M., et al. Abdominal visceral fat is associated with a BclI restriction fragment length polymorphism at the glucocorticoid receptor gene locus. Obes Res. 1997;5:186–192. doi: 10.1002/j.1550-8528.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 63.Casso D., Buist DS., Taplin S. Quality of life of 5-10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25. doi: 10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doll HA., Petersen SE., Stewart-Brown SL. Obesity and physical and emotional well-being: associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res. 2000;8:160–170. doi: 10.1038/oby.2000.17. [DOI] [PubMed] [Google Scholar]
- 65.Selvin E., Paynter NP., Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 66.Vgontzas AN., Bixler EO., Lin HM., Prolo P., Trakada G., Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 67.Cizza G., Primma S., Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20:367–373. doi: 10.1016/j.tem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]