Abstract
Historically, Kraepelin speculated that dementia praecox resulted from damage to the cerebral cortex, most notably the frontal and temporal cortices. It is only recently, however, that tools have been available to test this hypothesis. Now, more than a century later, we know that schizophrenia is a brain disorder. This knowledge comes from critical advances in imaging technology- including computerized axial tomography, magnetic resonance imaging, and diffusion imaging - all of which provide an unprecedented view of neuroanatomical structures, in vivo. Here, we review evidence for structural neuroimaging abnormalities, beginning with evidence for focal brain abnormalities, primarily in gray matter, and proceeding to the quest to identify abnormalities in brain systems and circuits by focusing on damage to white matter connections in the brain. We then review future prospects that need to be explored and pursued in order to translate our current knowledge into an understanding of the neurobiology of schizophrenia, which can then be translated into novel treatments.
Keywords: schizophrenia, structural neuroimaging, magnetic resonance imaging, MRI, diffusion tensor imaging, DTI, gray matter, white matter, white matter tractography, fractional anisotropy
Abstract
Desde un punto de vista histórico Kraepelin formuló la hipótesis que la demencia precoz se producía por un daño en la corteza cerebral, más específicamente en las cortezas frontal y temporal. Sin embargo, sólo recientemente se ha podido disponer de herramientas para evaluar esta hipótesis. Desde hace más de un siglo que ya se sabe que la esquizofrenia es un trastorno cerebral. Este conocimiento procede de importantes avances en la tecnología de imágenes -que incluyen la tomografía axial computarizada, las imágenes de resonancia magnética y las imágenes de difusión- todos los cuales aportan una visión, in vivo, sin precedentes de las estructuras neuroanatómicas. En este artículo se revisan las evidencias de las anormalidades estructurales de las neuroimágenes, comenzando con evidencias de alteraciones cerebrales focales, principalmente en la sustancia gris y continuando con las investigaciones para identificar anormalidades en los sistemas y circuitos cerebrales, focalizándose en el daño en las conexiones con la sustancia blanca del cerebro. Luego se revisan futuras líneas de investigación que requieren ser exploradas y desarrolladas para traducir el conocimiento actual en una comprensión de la neurobiología de la esquizofrenia, que puede traducirse en nuevos tratamientos.
Abstract
Historiquement, Kraepelin a émis l'hypothèse que la démence précoce résultait d'une lésion du cortex cérébral, plus particulièrement du cortex frontal et du cortex temporal. Ce n'est cependant que récemment seulement que des outils ont permis de tester cette hypothèse. Maintenant, plus d'un siècle plus tard, nous savons que la schizophrénie est un trouble cérébral grâce à des avancées décisives en imagerie, comprenant la tomographie axiale numérisée, l'imagerie par résonance magnétique et l'imagerie de diffusion, toutes fournissant un aperçu sans précédent des structures neuroanatomiques in vivo. Nous présentons ici les données scientifiques en faveur de l'existence d'anomalies identifiées par la neuro-imagerie structurelle, en commençant par les anomalies cérébrales focales, tout d'abord dans la substance grise et en poursuivant par l'identification des anomalies des circuits et des systèmes cérébraux en s'attachant en particulier aux lésions des connexions de la substance blanche cérébrale. Puis nous abordons les futures perspectives à explorer afin de transformer notre connaissance actuelle en une compréhension de la neurobiologie de la schizophrénie, afin de développer par la suite de nouveaux traitements.
Historical perspective
Early postmortem studies
Schizophrenia is a heterogeneous disorder with variations in expression and, likely, pathophysiology. There is no one symptom that is the sine qua non of schizophrenia, and variations in symptoms occur even in the same patient. Motivation, cognition, memory, executive functioning, affect, and social communication are all altered in schizophrenia. Moreover, this disorder has been described for more than 100 years, and yet the neuropathology of schizophrenia is still unknown, and this is despite the fact that both Kraepelin1 and Bleuler,2 who first described “dementia praecox” and “the schizophrenias,” believed that brain abnormalities would ultimately be linked to the etiology of schizophrenia (see review in ref 3). These early speculations by Kraepelin and Bleuler were further fueled by important inroads that were being made around this same time period into Huntington's chorea, Pick's disease, epilepsy, tertiary syphilis, and Alzheimer's disease.4-8 Of note here, Alzheimer was among the first to study the neuropathology of schizophrenia, although he went on to investigate what he thought of as more tractable brain disorders. In addition, findings from postmortem studies, also conducted during this same time period, were disappointing as they frequently led to conflicting findings largely due to the crude measurement tools that were available, and to the high expectations that researchers had of finding large abnormalities, similar to what had been observed for diseases such as Pick's disease and tertiary syphilis.3,4-8 As it turns out, such abnormalities have been shown to be far smaller and more subtle than researchers had originally believed would be the case.3 This state of affairs led Plum,9 in 1972, to state that “schizophrenia is the graveyard of neuropathologists.”
CT and MRI are introduced
Research interest in investigating brain abnormalities in schizophrenia thus waned until 1976, when the first computed tomography (CT) study showed enlarged lateral ventricles in schizophrenia.10 Following this study, a large number of CT and magnetic resonance imaging (MRI) studies followed, with the first MRI study of schizophrenia conducted in 1984 by Smith and coworkers.11 The first quantitative MRI study of schizophrenia was subsequently conducted by Andreasen and coworkers in 1986,12 and the first quantitative MRI study that included contiguous slices of the entire brain and correlations with specific clinical symptoms was conducted by Shenton and coworkers in 1992.13
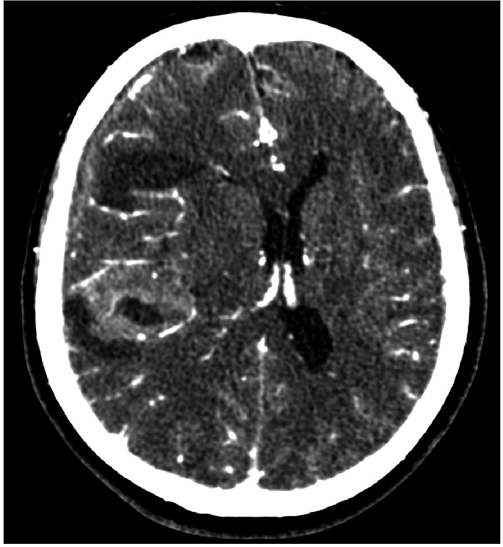
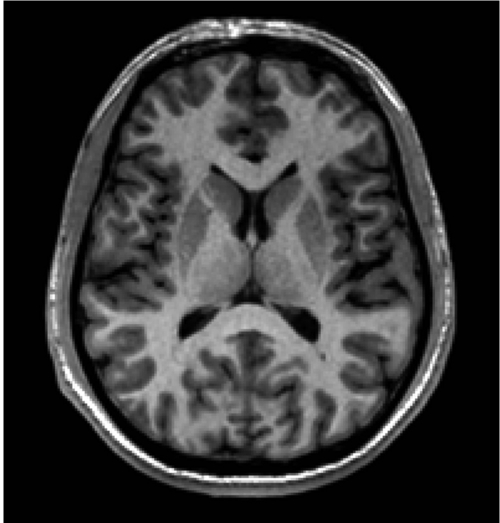
An example of a CT scan is depicted in Figure 1 , which shows a clear differentiation between bone, brain, and cerebrospinal fluid (CSF). In contrast, the differentiation between gray matter and white matter (ie, soft tissue) is not very clear using CT. The ability to differentiate between gray and white matter tissue had to await the introduction of magnetic resonance imaging (Figure 2). Parenthetically, and of historical interest here, following the introduction of CT, nuclear magnetic resonance (NMR), as it was called then, was developed in the early 1970s based on the work of Peter Mansfield14 and Paul Lauterbur.15 These individuals went on to share the Nobel Prize in Medicine in 2003 for their independent contributions to the seminal ideas that led to MRI and its application, in vivo, to humans. Importantly, MRI has no known adverse effects (ie, no radiation as in X-rays and CT), and it is a far more powerful tool than CT for visualizing soft tissue contrast in the brain and body.
Figure 1. This CT scan shows separation of brain from fluid, including CSF and blood. This is a CT scan, post-contrast, of a patient with a bleed from an aneurysm (black color is blood and CSF). CT, computed tomography; CSF, cerebrospinal fluid.
Figure 2. This MR scan shows one coronal slice through the superior aspects of the lateral ventricles. Note the clear differentiation between gray and white matter. Gray matter appears gray and can be seen in the ribbon around the cortex, as well as in subcortical brain regions. White matter appears white, and the lateral ventricles are black. MR, magnetic resonance.
The first MR scanners were built in the 1980s, with, as noted above, the first MRI of a patient with schizophrenia performed in 1984. These early MR images of the brain were quite poor in resolution, with slices as thick as 1 cm, and they did not cover the entire brain. This is in contrast to major advances in technology today, where 1-mm slice thickness is common, and it is relatively easy to image the entire brain in a relatively short period of time. Today, in fact, MR scanners are used routinely to measure tissue properties in the brain and body at submillimeter spatial resolution, based on the NMR of hydrogen in the magnetic field.
Put even more into perspective, the ability to examine the inner workings of the human body was limited, historically, to the study of cadavers. Advances in medical imaging technology, however, have provided researchers with a new window into the living human body. Such advances have revolutionized nearly every area of medicine, with psychiatry in the forefront of this revolution. These advances include both dramatic improvements in image resolution and the development of novel imaging techniques, from CT to positron emission tomography (PET), to single-photon emission computed tomography (SPECT), to MRI, including functional MRI (fMRI) and diffusion tensor imaging (DTI), to magnetic resonance spectroscopy (MRS), and ultrasound - all of which provide an unprecedented view, in exquisite detail, of anatomical structures and/or functions in the living human.
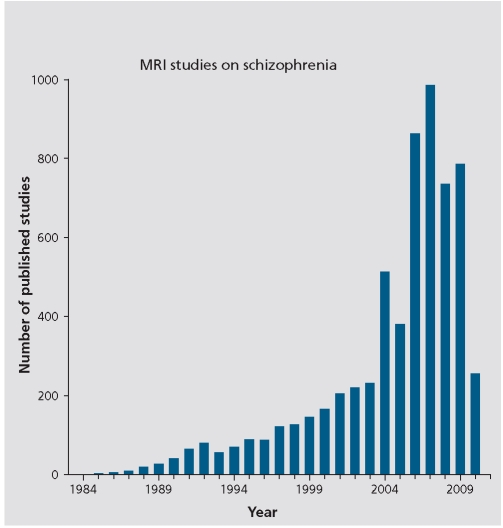
To return to the discussion of MRI, it is noteworthy that between 1984 and the present there has been a burgeoning of MRI studies in schizophrenia. Further, during this 26-year time period there are more definitive findings with respect to brain abnormalities in schizophrenia than have been documented in any previous time period in the history of schizophrenia research. Figure 3 shows the number of MRI studies conducted, each year, from 1984 to 2010, based on a PubMed search using the terms: schizophrenia, MRI, magnetic resonance imaging, and neuroimaging. What is evident here is the increase over time in the number of MRI studies: from 1 in 1984, to 71 in 1994, to 514 in 2004, to 786 in 2009 (Total =6305). These studies have led to a wealth of knowledge about the brain and schizophrenia - knowledge that would not have been possible without the revolutionary advances in neuroimaging technology.
Figure 3. Graph of MRI studies in schizophrenia between 1984 and April 2010. MRI, magnetic resonance imaging.
Acquisition and post-processing techniques have also continued to advance and it is now possible to segment the brain, automatically, into gray matter, white matter, and CSF, as well as to delineate small brain regions of interest. The segmentation of brain into tissue classes seems a trivial task today, but it required more than 15 years of computer vision research to make this a reality. We tend to take for granted many of the postprocessing tools that we have available today, including software packages that enable registration, segmentation, tractography, and region of interest delineations, eg, Slicer (http://www.slicer.org), Brain Voyager (http://www.brainvoyager.com/), Brains2 (http://www.psychiatry.uiowa.edu/mhcrc/IPLpages/BRAI NS.htm), SPM (http://www.fil.ion.ucl.ac.uk/spm/), and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). These tools, in fact, were many years in the making and have significantly improved our ability to segment the brain and to investigate more precisely brain abnormalities in schizophrenia and other disorders, compared with healthy controls. Furthermore, the fact that these tools are more automated means that a larger number of subjects can be evaluated in a shorter amount of time than was possible using only manually driven methods to characterize brain regions of interest. These advances also make it possible to register brains at follow-up to baseline, in order to compare differences over time, and we are now able to use multiple imaging techniques in the same subject, which maximizes our opportunity to combine information from functional and structural measures of the brain.
New imaging tools today involve the application of imaging technology to map the structure and function of brain, the latter of which includes functional imaging, a topic which is not reviewed here as it is reviewed elsewhere in this issue. Most of the structural imaging studies, to date, have investigated gray matter, including longitudinal studies that show progression of gray matter abnormalities following first episode of illness (eg, refs 16,17), with far less attention to the role of white matter abnormalities in schizophrenia.
Diffusion imaging is introduced
The development of diffusion tensor imaging (DTI) has made it possible to investigate white matter in the brain, in vivo, in a manner not possible with conventional MRI.
The work that led to the first imaging of white matter in humans began with the work of Steiskal and Tanner18 in 1965, followed by the work of Le Bihan et al19 in 1986 who introduced diffusion MR, and Basser et al20 in 1994, who developed DTI. The first DTI of the human brain was conducted by Pierpaoli et al21 in 1996, and the first DTI study in patients with schizophrenia was conducted by Buchsbaum et al22 in 1998. These time periods are highlighted to emphasize just how recently this technology has been developed.
The basic principle underlying diffusion imaging is that the diffusion of water molecules is restricted equally in all directions in CSF (isotropic diffusion), and not restricted equally in white matter, where it exhibits strong anisotropic diffusion, or in gray matter, where it exhibits weak anisotropic diffusion. By calculating the distance that water diffuses from a given point in a given time period, in a number of directions, it is possible to construct a three-dimensional shape that describes the diffusion, ie, an ellipsoid, with the shape and size of the ellipsoid providing information about the underlying tissue. The two most common diffusion measures used are fractional anisotropy (FA, shape of ellipsoid) and mean diffusivity (MD, size of ellipsoid).
FA is a measure of the anisotropy or nonsphericity of the shape of the diffusion ellipsoid. FA varies between 0 and 1, with the most isotropic diffusion having a value of 0 and the most anisotropic diffusion having a value of 1. FA decrease is generally thought to reflect damage to myelin or axons, reduced axonal density, and/or reduced axonal coherence (see review in Kubicki et al23). In contrast, MD provides quantitative information about the size of the diffusion ellipsoid, or the average displacement of water molecules resulting from diffusion at a given point in time. MD is highest in tissues where there are fewer restrictions to diffusion (eg, CSF), and lowest in tissues where diffusion is restricted by densely packed tissue elements (ie, cells). In schizophrenia studies, reviewed later in this chapter, FA and MD are the most common measures used, with decreased FA and increased MD consistently evinced by patients with schizophrenia. As mentioned above, these diffusion abnormalities likely reflect subnormal levels of fiber coherence, demyelination/dysmyelination, and/or subnormal levels of axon packing density.
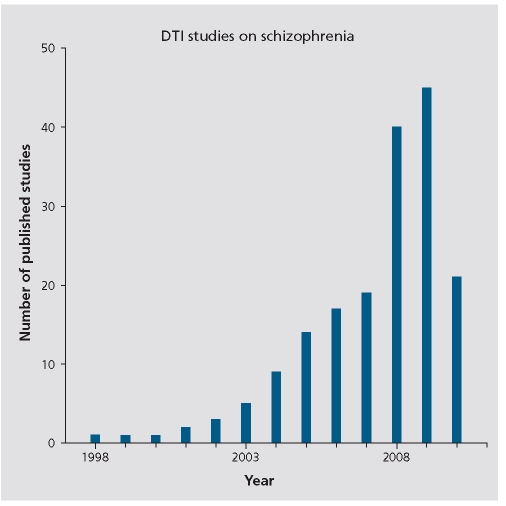
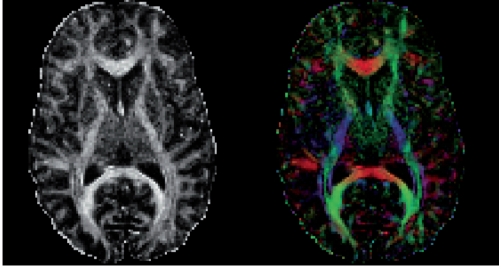
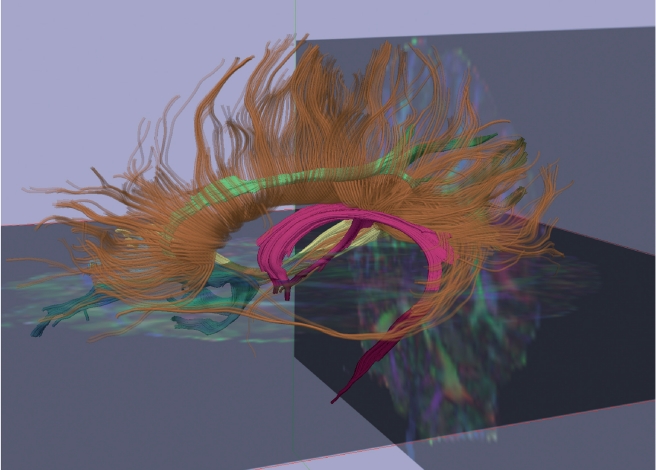
Figure 4 provides a graph depicting the number of diffusion imaging studies that have investigated white matter pathology in schizophrenia, each year, starting with Buchsbaum et al's22 first diffusion tensor imaging study in 1998 (Total =178 compared with 6305 MRI studies). The focus on white matter fiber bundles is important because for the first time it is possible to investigate connections between brain regions, which, along with functional measures, will likely lead to a new understanding of the neural circuits and their function and dysfunction in schizophrenia. Figure 5 shows a fractional anisotropy map (Panel A), as well as a color map (Panel B) that depicts the directionality of white matter along the x, y, and z axes. A 3D reconstruction of several white matter fiber tracts extracted from diffusion images of the brain is shown in Figure 6. This relatively new technology has become the major tool in neuroscience for investigating white matter, in vivo, and it shows great promise for elucidating further white matter fiber tracts that subserve neuroanatomical connections between both distant and proximal brain regions.
Figure 4. Graph of DTI studies in schizophrenia between 1998 and Apri 201 0. DTI, diffusion tensor imaging .
Figure 5. The anisotropy map in the left panel shows increased fractiona anisotropy (FA) in areas where the water diffusion is restricted, such as in the corpus callosum. Areas with increased FA are visible as white. The color map in the right panel shows the directions of the diffusion in x, y z space, with the colors red, blue, and green, respectively.
Figure 6. Three-dimensional image reconstructed based on diffusion data acquired on a 3T GE scanner at Brigham and Women's Hospital, Harvard Medical School, Boston, MA. This image shows several major white matter fiber bundles identified through diffusion tensor imaging: fornix (magenta), right cingulum (green), right inferior longitudinal fasciculus (yellow), right uncinate fasciculus (blue), corpus callosum (orange). Adapted from ref 24: Shenton ME, Kubicki M. Structural brain imaging in schizophrenia. In: Sadock BJ, Sadock VA, Ruiz P, eds. Sadock's Comprehensive Textbook of Psychiatry. 9th ed. New York: NY: Lippincott Williams, and Wilkins; 2009:1494-1506. Copyright © Lippincott, Williams and Wilkins, 2009.
Summary
With respect to schizophrenia, the question is therefore not: “are brain abnormalities present in schizophrenia?” but, “what is the nature of these abnormalities and how can we use this information to understand better the neurobiology of schizophrenia so that we can develop more targeted treatments and perhaps neuroprotective agents to prevent the cascade of progressive changes that are often reported in chronic cases of schizophrenia?"3,23-26 Below, we review structural neuroimaging findings in schizophrenia, both MRI and DTI, although we refer the reader to several recent and more comprehensive reviews covering these same topics.3,23-26
MRI findings in schizophrenia
Prior to the advent of MRI, brain abnormalities in schizophrenia were based on crude measurements such as measuring the volume of plaster casts from postmortem brains, and pneumoencephalographic studies,27-30 both of which were used to measure ventricular size. The latter studies were quite invasive as they involved pushing air into the brain cavity. Many of these studies, nonetheless, along with CT studies (eg, ref 10), described above, showed enlarged ventricles in the brains of patients with schizophrenia.
MRI studies of schizophrenia have also observed ventricular enlargement in schizophrenia (see reviews, eg, refs 3,23-26), with approximately 80% of MRI studies reporting this enlargement.3 Enlarged lateral ventricles, however, are not pathognomonic of schizophrenia, as they are also observed in hydrocephalus, Alzheimer's disease, and other neurodegenerative diseases where brain is replaced by CSF Of further note, since the comprehensive review by the first author in 2001 ,3 MRI findings in schizophrenia are relatively similar in terms of the percentage of studies showing abnormalities. More specifically, in the 2001 review it was observed that 80% of the studies revealed enlarged lateral ventricles, 73% revealed enlarged third ventricles, and there was a preferential involvement of medial temporal lobe structures (74%) that included amygdala, hippocampus, parahippocampal gyrus, and neocortical temporal lobe structures, ie, superior temporal gyrus (100% if gray and white matter were differentiated), and moderate evidence for frontal lobe involvement (59% of studies), most notably prefrontal cortex and orbitofrontal cortex. Other brain regions involved, and reported in this earlier review, included parietal lobe abnormalities (60% of studies), particularly inferior parietal lobule, which includes supramarginal gyrus and angular gyrus. Other findings included subcortical abnormalities, including cavum septum pellucidum (92% of studies), basal ganglia (68% of studies), corpus callosum (63% of studies), thalamus (42% of studies), and cerebellum (31% of studies). These numbers have not changed appreciably with the increase in MRI studies since 2001, but they do highlight the fact that there are multiple focal brain regions that are abnormal in schizophrenia, which are not necessarily proximal but which may nonetheless be involved in brain circuits that are abnormal in schizophrenia.
Moreover, and as was noted in the 2001 review, the timing of these abnormalities is still not known, although more recent studies, reviewed below, suggest that changes occur over time, particularly soon after onset of illness, and these changes may also be evident before the onset of symptoms (ie, in the prodrome period).
Abnormalities are also observed, albeit in a more attenuated form, in family members of patients with schizophrenia.
The question, then, as noted above, and as noted by Harrison,31 is thus not whether there are brain abnormalities in schizophrenia, as this is clearly confirmed. The question is rather “what sort of brain disorder is schizophrenia? Is it static? Is it progressive?” Further, if brain abnormalities change over time, does this necessarily mean that schizophrenia is a neurodegenerative disorder, or is it more likely that schizophrenia is associated with the unfolding of neurodevelopmental processes, which may show progression over time?
Perhaps, for example, changes over time suggest abnormalities in brain maturation or a developmental lesion, which in some cases is limited but in other cases takes on a more neurodegenerative course, as postulated in the early part of the twentieth century by Kraepelin.1 Furthermore, are the two theories, neurodevelopmental versus neurodegenerative, necessarily mutually exclusive? Other questions to be addressed include: “Are some brain abnormalities more evident prior to the onset of schizophrenia, and in the prodrome period, or at first onset, while other brain abnormalities become evident later, as the disorder progresses?” - These are among the questions that need to be answered in order to understand further the neurobiology of schizophrenia.
To hold stringently, however, to the view that schizophrenia is either a neurodevelopmental disorder or a neurodegenerative disease is short-sighted. Development does not end at birth, but instead continues throughout the lifespan and involves critical periods of development, particularly in the periadolescent period, with the timing of disease sometimes linked to genetics, such as with Huntington's disease, which may not manifest itself until later in life.
Feinberg32 appreciated the notion that development is not static and that it occurs across the lifespan. He observed that development may proceed normally up to adolescence, which he viewed as a critical time period when faulty programming leads to abnormalities in synaptic pruning in those adolescents who go on to develop schizophrenia. Mednick and McNeil33 also had a broad view of neurodevelopment, and of the progression of illness. They described a “two-hit” model of schizophrenia, where genetics, and/or possible assaults during neurodevelopment, comprised the “first hit.” They believed, however, that schizophrenia did not develop without a “second hit,” which occurs later in development, around the time of adolescence or early adulthood. Thus a broad view of neurodevelopment, which includes the possibility of disease progression, has been proposed in the past and needs to be more appreciated today when we evaluate the role of brain abnormalities in schizophrenia.
More recent MRI findings, reviewed below, suggest that changes in the brain are observable at or even before the first onset of psychosis, with post-onset changes observed in a relatively short time period following illness onset. Progressive changes, however, in and of themselves, do not provide evidence that schizophrenia is a neurodegenerative disorder. More recent MRI findings have, nonetheless, rekindled an interest in neurodegenerative theories of schizophrenia, although it is clear that early motor abnormalities, neurological soft signs prior to illness onset, sulco-gyral pattern abnormalities and cavum septum pellucidum abnormalities commonly exhibited by patients with schizophrenia point to the important role of neurodevelopment in schizophrenia (see review in Shenton et al3). There is also no evidence for a loss of neurons and no gliosis in postmortem studies of schizophrenia.34 These findings, taken together, suggest that schizophrenia is not a neurodegenerative disorder, at least not as defined by conventional criteria.
A better question then, is: “Do some patients show progressive changes following onset of first episode of illness, while others do not? And if so, what factors contribute to these differences, and what are the implications with respect to treatment and outcome?” Other issues that need to be addressed include evaluating brain abnormalities in high-risk individuals and in the unaffected family members of patients with schizophrenia. Still another question that needs to be addressed is whether or not early treatment will make a difference in the course of the illness. Further, will treating high-risk individuals provide neuroprotective effects that prevent the development of schizophrenia? Finally, research needs to focus on determining the specificity of findings to schizophrenia. All of these issues need to be addressed as research moves toward unraveling what “sort of disease schizophrenia is”; a theme we will return to multiple times in this review.
MRI findings in chronic patients
There have been a large number of MRI studies investigating brain abnormalities in chronic schizophrenia. What is surprising is that from 2001 to the present,3 even though most studies have moved from 1.5T to 3T magnets, and the image resolution has increased, the findings, as noted previously, have not changed appreciably. Moreover, and importantly, most of these studies have focused on gray matter. This focus is understandable as it is more difficult to appreciate white matter fiber bundles with structural MRI. New measures that complement volume measures have also been introduced and are more commonplace now, including shape measures, cortical thickness measures, and sulcal-gyral and cortical folding measures (see reviews covering these issues, eg, refs 23-26).
The consistency of findings, nonetheless, is quite striking and reveals multiple brain regions that show gray matter abnormalities in chronic schizophrenia including brain regions within the prefrontal, temporal, parietal, and occipital lobe. The list of brain regions reported as abnormal is, in fact, quite long and includes: whole gray matter, whole white matter, CSF, lateral ventricles, third ventricle, ventricular CSF, subarachnoid CSF, prefrontal cortex, dorsolateral prefrontal cortex, orbitofrontal cortex, cavum septum pellucidum, cingulate gyrus, anterior cingulate gyrus, thalamus, amygdala, hippocampus, planum temporale, Heschl's gyrus, cerebellum, insular cortex, nucleus accumbens, striatum, superior temporal gyrus, olfactory bulb, basal ganglia, putamen, caudate, globus pallidus, parahippocampal gyrus, fusiform gyrus, cerebellar hemispheres, cerebellar vermis, parieto-occipital lobe, perigenual regions, pituitary, precentral sulcus, entorhinal cortex, occipital lobe, temporal lobe, pons, Sylvian fissure, adhesio interthalamica, and pineal gland (see reviews in refs 3,23-26). These abnormalities also include associations between brain abnormalities and cognitive/clinical symptoms in schizophrenia. The latter include, but are not limited to, deficit symptoms and prefrontal cortex abnormalities, impairments in insight and frontal-lobe abnormalities, impairments in social cognition and anterior cingulate abnormalities, impairments in error detection and cingulate gyrus abnormalities, impairments in face recognition and fusiform gyrus and amygdala abnormalities, hallucinations and superior temporal gyrus abnormalities, language disturbances and medial temporal lobe abnormalities, and formal thought disorder and both superior temporal gyrus and posterior temporal lobe abnormalities, to name just a few (see reviews, eg, refs 3,23-26).
It will be important, as we move forward, to begin associating such findings with other imaging modalities such as fMRI and white matter fiber tract measures derived from DTI, in order to understand further brain abnormalities and what they tell us about brain systems and circuits. We also need to tease apart more definitively what is intrinsically a signature, or marker, of the disorder, and what is associated with epiphenomena. Here, evaluating first-episode patients is important because the effects of long-term medication and chronicity can be ruled out as possible confounds, and putative markers can be discovered and better delineated from the epiphenomena of the illness. Additionally, investigating first-episode patients, and prodromal subjects (ie, those who are at high risk for schizophrenia but have as yet not developed it), may lead to important new inroads that will make early intervention possible in order to help prevent this disorder from taking hold and/or becoming chronic.
MRI findings in first-episode patients and longitudinal studies
DeLisi and colleagues35 were among the first to conduct cross-sectional and longitudinal studies of first-episode patients with schizophrenia. These investigators followed patients for 10 years and reported lateral ventricular enlargement and reduced volumes in the cerebrum, cerebellum, and corpus callosum. However, DeLisi and colleagues35 failed to identify volume reductions in the frontal or temporal lobes, or in more circumscribed regions of interest within these lobes, compared with healthy controls. In contrast, DeGreef et al36 reported no enlarged ventricles in first-episode patients, though their follow-up was only over a short period of 1 to 2 years. Also in contrast to DeLisi et al's findings, more recent studies have reported volume reduction in the frontal lobes, including Gur and colleagues37 who reported reduced volume in the frontal lobe at 2 to 3 years' followup in first-episode patients, and Ho and colleagues38 who reported similar findings. Additionally, Kasai and colleagues16 reported reduced volume in the left superior temporal gyrus as early as 1.5 years following first episode of illness, as well as reduced volume in left Heschl's gyrus and left planum temporale at 1.5 years follow-up.39 These latter findings suggest that progressive changes occur very early in the course of schizophrenia.
Importantly, follow-up studies of chronic patients have also shown progressive changes in gray matter, including superior temporal gyrus.40 Thompson and colleagues41 have also reported an accelerated decrease in gray matter volume in early-onset schizophrenia. Of particular note, in a recent review of the literature, DeLisi et al42 reviewed evidence for progressive changes in both chronic and first-episode patients. They concluded that progressive changes over time in chronic patients are far less than what is observed in first-episode patients, again underscoring the fact that progressive changes in the early stages of illness may be more dramatic than changes observed later in the course of the illness.
Another recent review43 sheds further light on the issue of progressive changes following first episode. This review found that progressive changes following first episode were more pronounced in the first 20 years and less pronounced after this time period compared with healthy controls. Of further note, the changes observed included gray matter volume reductions in the frontal and temporal lobes, as well as increased lateral ventricles. In addition, these changes were associated with more progressive changes associated with poor outcome, more negative symptoms, and poor performance on neurocognitive measures.
A review by Pantelis and coworkers17 of longitudinal MRI studies of first-episode patients, prodromal patients, and high-risk individuals also suggests an acceleration of gray matter reduction early in the course of illness. Specifically, there is gray matter reduction in prefrontal regions, which these investigators believe leads to further progressive changes in medial temporal and orbitofrontal brain regions. These investigators also interpret findings, to date, as indicative of an early neurodevelopmental insult or lesion that likely “renders the brain vulnerable to later brain maturation processes” and which takes place during adolescence or early adulthood. These interpretations are reminiscent of Mednick and McNeil's 1968 two-hit theory of schizophrenia,33 and Feinberg's 198232 theory that schizophrenia results from abnormal synaptic pruning.
The number of first-episode studies is, however, relatively small compared with the number of chronic studies. Additionally, the selection of patients differs in that some studies include patients that have been ill for several years, and, while they may not be chronic per se, they might be better classified as reflecting “early schizophrenia” rather than as first-episode schizophrenia. Having said this, however, there are a number of highresolution longitudinal studies that have investigated brain abnormalities at first episode of illness. Some, including Gur and coworkers,37 and Kasai and coworkers,39 are noted above. Another study by Lieberman et al44 reported larger lateral ventricles and reduced volume of the hippocampus at baseline, but only increased lateral ventricles at follow-up 1 year later. Whitford and coworkers45 evaluated whole brain in a follow-up study of first-episode patients who were rescanned 2 years later. These investigators reported widespread gray matter reduction in frontal, parietal, temporal lobe (including superior temporal gyrus), cerebellum, and in portions of the occipital lobe.
Another interesting study by Nakamura and coworkers46 showed reduced neocortical gray matter, larger sulcal CSF, and increased lateral ventricles at 1.5-year followup in first-episode schizophrenics, compared with controls. Poorer outcome in this study was also associated with brain changes over time in the first-episode patients, whereas in first-episode patients with an affective disorder and psychotic features, there was an increase in neocortical gray matter volume (3.6%) at follow-up. These investigators concluded that the changes observed in neocortical gray matter in the affective group were likely not intrinsic to the disorder, but were instead associated with medication effects. In contrast, the changes observed at follow-up in the first-episode schizophrenia sample were interpreted as being intrinsic to the disorder. These latter findings are consistent with neuropil loss reported in postmortem studies.34
To summarize, findings from longitudinal studies of first episode schizophrenics suggest that brain abnormalities are present at first episode, and that some brain regions continue to show progression over a relatively short period of time. The follow-up in such studies, however, is variable and we therefore need more studies that follow patients over longer periods of time with intervals in between, ie, 10- to 15-year follow-up, with scans repeated 1 to 2 years post-onset. While this is a daunting task, it is necessary if we are to successfully delineate the brain regions affected at illness onset, and to determine which abnormalities are specific to schizophrenia, which progress over time, and what the implications of progression versus nonprogression might be. Additionally, because brain changes appear to be present in the early post-onset period, this is an important period in which to conduct research so as to understand better the processes taking place and the possibilities for early intervention.
Another area that needs further attention, and is discussed at further length in a separate review,24 is the study of cognitive impairments and clinical symptoms, and their association with brain abnormalities, as well as with progressive brain changes. While some studies report associations between brain regions and clinical and cognitive impairments (see reviews in refs 3,23-26), there is evidence that early in the course of illness cognitive and clinical symptoms may improve, while structural brain abnormalities are observed to progress. As we have noted previously: it is “important to understand why these two seemingly incongruous events take place, and to understand further their implications with respect to timing and progression, possible underlying mechanisms, and intervention and treatment strategies."24
Finally, as we learn more about brain abnormalities at first episode of illness, it becomes clear that it is important to learn also about brain abnormalities in individuals who are at risk for developing schizophrenia, and prior to the onset of illness. Focusing on what is known as “the prodromal period” will also make it possible to characterize a subset of individuals who are at risk and go on to develop schizophrenia, versus another subset of individuals who are at risk but who do not go on to develop schizophrenia. A focus on this group of subjects will also make it possible to learn more about the timing of brain abnormalities in schizophrenia, and to begin to develop putative brain markers or brain signatures that predispose an individual to develop schizophrenia. Another approach is to study family members of schizophrenic patients in order to discern brain abnormalities that are associated with genetically regulated variations in brain structure, but which are neither necessary nor sufficient for the development of psychosis. Some of these strategies, along with recent findings, are reviewed, below.
High-risk studies
To address the question of “what is the timing of brain abnormalities in schizophrenia?” it is useful to study individuals who are at high risk for developing schizophrenia, but who have not yet developed the disorder, ie, before psychosis begins. As noted above, this can be addressed to some extent with longitudinal studies, but can also be addressed by studying individuals who are at high risk for developing schizophrenia, as one can observe whether or not there are brain abnormalities present prior to onset of schizophrenia. This approach is quite appealing given that there is evidence to suggest that as many as 35% of individuals defined as being at ultra high-risk for schizophrenia convert to schizophrenia within the first year of being indentified17 (see also discussion below).
With respect to high-risk studies, two of the largest and best known research programs come to mind. The first is the Edinburgh High-Risk Study (EHRS),47 which evaluates individuals at risk for developing schizophrenia. The EHRS defines “at-risk” based on cognitive impairment measures from the Structural Interview for Schizotypy (SIS). Findings thus far indicate that indi viduals who are at risk and who also have schizotypal features tend to have increased right prefrontal cortical folding, which further predicts those individuals who develop schizophrenia. These investigators speculate that abnormalities in cortical folding reflect disordered connectivity in the right prefrontal lobe.
The second large research program to evaluate individuals at risk for psychosis is the Melbourne Ultra HighRisk Studies, in collaboration with the Personal Assessment and Crisis Evaluation (PACE) clinic. This study investigates individuals at risk for developing psychosis. Pantelis and colleagues, (eg, ref 17) define individuals at ultra-high risk (UHR) for developing psychosis on the basis of several trait and state factors. As noted above, from this sample of UHR individuals, 35% went on to develop schizophrenia within 1 year. Those UHR individuals who go on to develop schizophrenia show medial temporal and prefrontal (particularly orbitofrontal) brain abnormalities, compared with UHR subjects who do not develop schizophrenia. These investigators, also as noted previously, suggest that the brain abnormalities observed in those who transition to schizophrenia reflect abnormal brain maturation, which occurs with other events such as substance abuse, stress, etc, and likely involves early neurodevelopmental insults to the brain. This abnormal maturation might then render the brain vulnerable to later abnormal processes, including accelerated gray matter loss in frontotemporal regions, and abnormal connectivity in prefrontal brain regions.
A focus on genetics in high-risk studies is also important. For example, the effects of the catechol-Omethyltransferase (COMT) gene on brain structure and function in high-risk individuals, reported by the Edinburgh group,48 suggests that the risk of developing schizophrenia in the high-risk group is increased in individuals with the COMT Val158Met polymorphism. Thus subtyping of high-risk individuals based on putative brain markers, genes, and outcome, while just beginning, will be an important direction for future studies.
Family studies: genetic high risk studies
An area of further inquiry is whether or not there are some brain abnormalities that are present in schizophrenia which are also present in nonaffected family members. Such findings would point to potential markers of genetic vulnerability to schizophrenia. In addition, studying nonaffected family members avoids the confounds of chronicity and medication, which characterize studies of chronic patients. Further, studying this population is independent of psychosis, thus avoiding the possible neurotoxic effects of psychosis, which may be brewing even in high-risk populations. Finally, a focus on nonaffected family members makes it possible to study genetic factors as well as environmental factors with respect to their roles in the etiology of schizophrenia.
Most of the MRI studies that have investigated nonaffected family members report the severity of brain abnormalities to be midway between healthy controls and patients with schizophrenia, and similar to what is observed in high-risk individuals.23-26 The brain region most commonly reported as abnormal is the hippocampus, although it should noted that the hippocampus is also one of the most commonly investigated brain region in the relatives of schizophrenic patients.
In a recent meta-analysis study by Boos and colleagues,49 25 MRI studies of nonaffected first-degree relatives of patients with schizophrenia were reviewed. The main finding was reduced left hippocampal volume, and increased third ventricle volume. Perhaps not surprisingly, hippocampal findings were associated with verbal and declarative memory deficits, similar to findings in schizophrenia. Szesko and coworkers,50 in 2005, also reported an association between hippocampal volume and brain-derived neurotropic factor (BDNF) val66met polymorphism in schizophrenia. Furthermore, in the same year, Callicott and colleagues51 reported that the DISC1 gene was associated with both structural and functional alterations in the hippocampus.
Other brain regions that have been associated with unaffected first-degree relatives of patients with schizophrenia include parahippocampal gyrus, which is also closely interconnected with hippocampus, and the thalamus. These findings provide additional evidence for vulnerability to schizophrenia that is independent of psychosis. Moreover, despite the fact that there have also been negative findings, and not all brain regions have been evaluated in the family members of patients with schizophrenia, the findings are nevertheless instructive, with the caveat that hippocampal abnormalities are not specific to schizophrenia.
Summary
To summarize, MRI findings in schizophrenia provide evidence that brain abnormalities involve multiple focal brain regions. There is also strong evidence to suggest that there are progressive changes that occur early in the course of illness and far greater attention needs to be given to research in this critical time period in order to develop targeted treatments that may halt the progression of disease. It is also evident that those individuals in the prodromal phase show brain abnormalities that are similar to those observed in nonaffected relatives, but are more attenuated than the abnormalities observed in patients with schizophrenia. Understanding why some at risk individuals who evince brain abnormalities convert to schizophrenia while others do not will be an important future area of scientific investigation. The hope here is that what we learn from “nonconverters” can lead to the development and implementation of new treatments that are neuroprotective in nature and thus may prevent the conversion to schizophrenia and perhaps prevent further progression in those with a first onset of illness.
Diffusion imaging findings in schizophrenia
While a great deal of attention has focused on gray matter abnormalities in schizophrenia, more recently there has been a growing interest in “the other half of the brain”52; white matter. This interest follows the advent of a new tool, DTI, which makes it possible to quantify and to visualize white matter structure. This ability was not possible previously with conventional MRI, where white matter appears quite homogeneous. White matter is comprised primarily of myelinated axon sheaths that form the infrastructure for the transmission of signals between populations of neurons, which can be proximal or distant spatially in the brain. These densely packed fibers interconnect gray matter regions of the brain and form neural circuits that, among other things, subserve cognitive functions that we associate with being human, ie, attention, motivation, emotion, awareness of self and others, etc. Lesion studies provide a multitude of examples of the consequences that result from damage to white matter, including akinetic mutism and aphasia (see review in Kubicki23).
Another reason for interest in white matter is that the multifocal nature of gray matter abnormalities in schizophrenia is consistent with earlier views of schizophrenia as a disturbance in the connections between brain regions.1-3,23 Weinberger and colleagues,53 in fact, speculate that temporal lobe abnormalities likely reflect a neurodevelopmental “disconnection” between temporo-limbic and prefrontal regions.
Additional reasons for investigating white matter pathology in schizophrenia come from neuropathological as well as genetic studies that suggest myelin involvement in schizophrenia (see recent reviews in refs 23-26). More specifically, oligodendrocytes, which are cells that produce myelin and provide both protection and facilitation of communication between brain regions, may be involved in the pathophysiology of schizophrenia. The latter has been described by Whitford et al25 as possibly related to conduction velocity abnormalities that may affect the action potential as it travels along myelinated axons,54 which could lead to modulations in the speed of conduction between spatially disparate populations of neurons. As discussed by Whitford et al, this could ultimately leading to confusions as to the origins of neural signals, and possibly to confusion when distinguishing between internally generated and externally generated events.
Evaluating white matter pathology may also shed light on the pattern and number of gray matter abnormalities observed in schizophrenia, including, but not limited to, frontotemporal tracts. Further, a focus on white matter fiber bundles is a move away from evaluating isolated gray matter regions and a move toward evaluating neural systems and networks that are biological substrates of cognition, social cognition, emotion, attention, and, in general, behavior.
DTI findings in schizophrenia
There are now more than 178 DTI studies of white matter pathology in schizophrenia (see also Figure 4). The first DTI study, as noted previously, was by Buchsbaum and coworkers.22 These investigators evaluated whole brain in a relatively small sample, ie, 5 chronic patients and 6 controls. Other early studies were also relatively small, with 20 or fewer patients. Even with these small sample sizes, however, reductions in anisotropy were reported within the majority of fasciculi (including frontotemporal, frontooccipital, temporo-occipital, thalamocortical, and interhemispheric connections; see also several recent reviews, eg, refs 23-26). A main focus of DTI studies in schizophrenia has been fronto-temporal connections in the brain (see recent reviews in refs 23-26). Kubicki et al,55 from our laboratory, was one of the first to use DTI to investigate frontotemporal connections in schizophrenia. In this study the uncinate fasciculus (UF) was investigated. This fiber bundle connects orbitofrontal and inferior frontal gyri with the anterior pole and the amygdala, and it is involved functionally in decision making, autobiographical and episodic memory, as well as in social behavior. These investigators reported a decrease in left>right FA asymmetry in chronic patients compared with healthy controls. This decreased FA asymmetry in the UF was correlated with declarative-episodic verbal memory in the patients, but not the controls. UF decrease has since been confirmed in two whole brain studies that used voxel based morphometry (VBM) measures.56-57
Another frontotemporal white matter connection that has been frequently investigated in schizophrenia is the cingulum bundle (CB). This fiber tract connects paralimbic-neocortical brain regions, and it also interconnects limbic structures including dorsolateral prefrontal cortex, cingulate gyrus, parahippocampal gyrus, and amygdala. The CB is involved in a number of functions, including pain perception, emotion, self-monitoring, and spatial orientation and memory. Kubicki and coworkers58 reported reduced FA in CB in patients compared with controls. Furthermore, FA was found to be correlated with errors in executive functions relevant to performance monitoring in schizophrenia. This finding has been reported also by other investigators, eg, refs 59-61. Another study by Mori et al62 evaluated both UF and CB in schizophrenia using VBM and also found FA decreases which they then confirmed using region of interest measures. These FA reductions were negatively correlated with duration of illness, suggesting possible medication-related white matter deterioration.
Several other white matter tracts connecting frontotemporal lobes have also been investigated. These tracts include the arcuate fasciculus (AF), a white matter fiber tract connecting superior temporal and inferior parietal regions with inferior frontal gyrus. This tract is important in language processing. Findings in chronic schizophrenia have shown left-lateralized reductions in anisotropy in this brain region (eg, refs 63-65). Another tract that has shown FA reduction in schizophrenia is the inferior longitudinal fasciculus (ILF), which connects the anterior temporal with parietal and occipital regions.66 Further, in a study that subdivided patients into those with and without auditory hallucinations, FA was reduced in the AF, UF, and ILF in patients without auditory hallucinations, while FA was increased in the AF and corpus callosum in patients with hallucinations (compared with patients without hallucinations).67 These findings, taken together, suggest that white matter fiber bundles that connect the frontal and temporal lobes are particularly abnormal in patients with schizophrenia. Other white matter fiber tracts that have been investigated in schizophrenia include the anterior limb of the internal capsule, the corpus callosum, occipital lobe white matter, hippocampus, frontal white matter, prefrontal white matter, and the fornix (see recent reviews in refs 23-26).
Finally, while most DTI studies of white matter fiber bundles have been conducted in patients with chronic schizophrenia, at least 15 studies have focused on firstepisode patients with schizophrenia (see recent reviews in refs 23-26). While the sample sizes have generally been small, the results suggest that the FA abnormalities are present in first-episode patients, but are milder than those exhibited by chronic patients. Given the subtlety of these DTI abnormalities at first episode, variability in the methods used may have impacted the consistency of the reported findings even more than in chronic patients. Another line of research has focused on populations that are in some way related to schizophrenia, in the hope of better characterizing schizophrenia phenotypes. These studies include subjects with increased risk for schizophrenia, including prodromes, schizotypal personality disorder, and velocardiofacial syndrome (see recent reviews in refs 23-26). As with MRI studies, investigating subjects who are at greater risk for developing schizophrenia is an important line of research as it will likely help to tease apart factors intrinsic to schizophrenia versus epiphenomena or factors necessary but not sufficient for onset of illness.
Diffusion tractography
The DTI studies reviewed above used voxel-based morphometry (VBM) and/or region of interest measures to define the fiber tracts. Fiber tractography is a promising new method to visualize white matter fiber bundles. This method follows the trajectory of fiber bundles along the principal diffusion direction, and provides an estimate of the local diffusion properties in the tensor field. It is important to point out, however, that at today's image resolution, this method is not able to trace along the trajectories of individual axons. The main advantage of DTI tractography, as compared with VBM and region-of-interest methods, is that diffusion can be quantified along entire fiber bundles. Furthermore, fascicle tributaries that might be misregistered or even excluded during the process of spatial normalization in VBM, can also be tracked and quantified, as can crossing fiber bundles. A great deal of work is currently underway in an effort to move from one-tensor models of fiber tractography to two-tensor models which are even more promising with respect to dealing with fiber crossings, eg, ref 68.
Jones et al published the first DTI tractography study in schizophrenia.69 This has been followed by a small handful of tractography studies which suggest that the most prominent white matter abnormalities in schizophrenia are in connections between frontotemporal lobe, including the UF and CB, and in the inferior and superior longitudinal fasciculi, and corpus callosum.
DTI tractography is thus a promising tool to understand how specific brain regions are connected, and to understand further how this connectivity may be relevant to functional abnormalities. It is a relatively new investigative tool that will likely be used more in the next few years, particularly in conjunction with functional measures of the brain.
Implications and future directions for research
Based on a review of structural neuroimaging studies in schizophrenia, it is clear that schizophrenia is a brain disorder that shows marked, albeit subtle, neuroanatomical abnormalities that are multifocal in nature and which likely involve brain circuits and networks which subserve cognition and behavior. Evidence also suggests that schizophrenia involves a disorder of neural and cognitive integration, an idea that has long been proposed in the schizophrenia literature. Some of the more recent theories that touch upon disordered integration include the dysmetria theory proposed by Andreasen et al,70 the failure of integration proposed by Gruzelier et al,71 Feinberg's30 theory of aberrant synaptic pruning, Friston's72 theory of abnormal synaptic modulation, and Bartzokis's73 theory which suggests that the underlying cause of neural integration may be abnormalities in myelination processes that occur in the periadolescent period.
Despite promising findings, however, we are still far from understanding the neuropathology of schizophrenia. Some of the outstanding questions that remain to be addressed if we are to understand the etiology of schizophrenia are: When do structural brain abnormalities first occur? What are the microstructural underpinnings of these structural brain abnormalities? Is there a causative relationship between gray matter abnormalities observed with MRI and white matter abnormalities observed with DTI? Do all of the structural brain abnormalities associated with schizophrenia progress with illness, or are some stable over time? Is it possible to arrest these progressive neuroanatomical changes once they have begun? As brain abnormalities in patients with schizophrenia have been shown to be associated with cognitive and clinical symptoms, how do we understand improvement in cognitive and clinical symptoms which are often concomitant with progressive volume reductions following the first episode of illness? What is the relationship between the structural brain abnormalities characteristic of schizophrenia and the equally well documented functional brain abnormalities (eg, such as observed with PET, fMRI, etc)? Can neuroanatomical, neuropsychological, or clinical phenotypes be used to predict who will go on to develop schizophrenia and who will not? These are among the questions that we need to address in future studies.
While these questions are daunting, there is nonetheless reason for optimism. The technological advances in neuroimaging that have led to new discoveries about the brain and its role in schizophrenia will continue. Additionally, future studies will likely involve combining multiple in vivo methodologies (ie, structural MRI, MTR, DTI on the one hand, and functional MRI, PET, and spectroscopy on the other) in order to investigate brain systems and networks in schizophrenia, and their function and dysfunction. Such studies are already beginning to emerge, and they will likely replace single-modality approaches within the next few years. As stated most recently: “This change from single to multimodal imaging will significantly increase our understanding of the relationship between functional and structural brain abnormalities in schizophrenia, and also lay the foundation for linking such findings to signature cognitive impairments and susceptibility genes."24 The next decade will thus be an exciting one. New developing technologies will be used in a multimodal fashion across patients' lifespans (ie, from prodrome to first episode to chronic), which will lead to a better understanding of what brain systems and networks are abnormal in schizophrenia, and when these abnormalities occur. Such knowledge will, in turn, lead to insight into why these abnormalities occur and how they might best be treated. Our common hope is that this will lead to a greater understanding of brain abnormalities in schizophrenia, with a particular focus on the critical period following first episode, where progressive changes in the brain are most profound. This may lead to a greater understanding of cognitive impairments, clinical symptoms, and genetic underpinnings of schizophrenia, which will ultimately lead to more rational and efficacious treatment strategies than are available today.
Acknowledgments
This study was supported, in part, by grants from the Department of Veterans Affairs Merit Award (MES), and from a VA Schizophrenia Center Grant (MES). Support also comes from the National Institute of Mental Health (K05 MH070047 and R01 MH 50740 to MES, P50MH 080272-CIDAR award to MES), the National Alliance for Medical Image Computing (NA-MIC), the latter a grant supported through the National Institutes of Health Roadmap for Medical Research (U54 EB005149 to MK), and from an Overseas-Based Biomedical Training Fellowship from the National Health and Medical Research Council of Australia (NHMRC 520627) through the University of Melbourne (TW).
Contributor Information
Martha E. Shenton, VA Boston Healthcare System, Brockton Campus, and Department of Psychiatry, Harvard Medical School, Brockton, MA, USA; Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Surgical Planning Laboratory, MRI Division, Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Thomas J. Whitford, Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Melbourne Neuropsychiatry Centre, Department of Psychiatry, University of Melbourne, and Melbourne Health, Melbourne, Australia.
Marek Kubicki, Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
REFERENCES
- 1.Kraepelin E. Dementia Praecox. In: Barclay BBE, ed, tran . 1919-1971 New York, NY: Churchill Livingston Inc; [Google Scholar]
- 2.Bleuler E. Dementia Praecox or The Group of Schizophrenias. New York, NY: International Universities Press . 1911-1950 [Google Scholar]
- 3.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benes F. Is there a neuroanatomical basis for schizophrenia? An old question revisited. Neuroscientist. 1995;1:104–115. [Google Scholar]
- 5.Bogerts B. Neuropathology of schizophrenias. Fortschr Neurol Psychiatr. 1984;52:428–437. doi: 10.1055/s-2007-1002212. [DOI] [PubMed] [Google Scholar]
- 6.Bogerts B. The neuropathology of schizophrenic diseases: historical aspects and present knowledge. Eur Arch Psychiatry Clin Neurosci. 1999;249:2–13. doi: 10.1007/pl00014181. [DOI] [PubMed] [Google Scholar]
- 7.Chua SE, McKenna PJ. Schizophrenia brain disease? A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry. 1995;166:563–582. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 9.Plum F. Prospects for research on schizophrenia. 3. Neurophysiology. Neuropathological findings. Neurosci Res Program Bull. 1972;10:384–388. [PubMed] [Google Scholar]
- 10.Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 11.Smith RC, Calderon M, Ravichandran GK, et al. Nuclear magnetic resonance in schizophrenia: a preliminary study. Psychiatry Res. 1984;12:137–147. doi: 10.1016/0165-1781(84)90013-1. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen NC, Nasrallah HA, Dunn V, et al. Structural abnormalities in the frontal system in schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 1986;43:136–144. doi: 10.1001/archpsyc.1986.01800020042006. [DOI] [PubMed] [Google Scholar]
- 13.Shenton ME, Kikinis R, Jolesz FA, et al. Left temporal abnormalities in schizophrenia and thought disorder: a quantitative MRI study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield P, Grannell PK. NMR "diffraction" in solids? J Phys C. 1973;6:422–426. [Google Scholar]
- 15.Lauterbur PC. Image formation by induces local interactions. Examples of employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 16.Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left posterior superior temporal gyrus gray matter volume in patients with firstepisode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 18.Stejskal ED, Tanner JE. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 19.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: Applications to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 20.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. MR diffusion spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 22.Buchsbaum MS, Tang CY, Peled S, et al. MRI white matter diffusion and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatric Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenton ME, Kubicki M. Structural brain imaging in schizophrenia. In: Sadock BJ, Sadock VA, Ruiz P, eds Sadock's Comprehensive Textbook of Psychiatry. 9th ed. New York: NY: Lippincott Williams, and Wilkins;2009:1494–1506. [Google Scholar]
- 25.Whitford TW, Kubicki M, Shenton ME. Structural imaging of schizophrenia. In: Shenton ME, Turetsky B, eds Understanding Neuropsychiatric Disorders: Insights from Neuroimaging. New York, NY: Cambridge University Press. In press [Google Scholar]
- 26.Kubicki M, Shenton ME. DTI and its application to schizophrenia and related disorders. In: Johansen-Berg H, Behrens T, eds Imaging Brain Pathways - Diffusion MRI: from Quantitative Measurement to In-Vivo Neuroanatomy. New York, NY: Elsevier Publishers;2009:251–270. [Google Scholar]
- 27.Jacobi W, Winkler H. Encephalographische studien an chronische schizophrenen. Archiv Psychiatr Nervenkrankheiten. 1927;81:299–332. [Google Scholar]
- 28.Jacobi W, Winkler H. Encephalographische studien an schzophrenica. Arch Psychiatrie. 1928;84:208–226. [Google Scholar]
- 29.Haug JO. Pneumoencephalographic studies in mental disease. Acta Psychiatr Neurol. 1962;165:11–104. [PubMed] [Google Scholar]
- 30.Haug JO. Pneumoencephalographic evidence of brain atrophy in acute and chronic schizophrenic patients. Acta Psychiatr Scand. 1982;66:374–383. doi: 10.1111/j.1600-0447.1982.tb06719.x. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PJ. Brains at risk of schizophrenia. Lancet. 1999;353:3–4. doi: 10.1016/S0140-6736(05)74878-9. [DOI] [PubMed] [Google Scholar]
- 32.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 33.Mednick SA, McNeil TF. Current methodology in research on the etiology of schizophrenia: Serious difficulties which suggest the use of the high-risk-group method. Psychol Bull. 1968;70:681–693. doi: 10.1037/h0026836. [DOI] [PubMed] [Google Scholar]
- 34.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 35.DeLisi LE, Stritzke PH, Holan V, et al. Brain morphological changes in 1st episode cases of schizophrenia: are they progressive? Schizophr Res. 1991;5:206–208. doi: 10.1016/0920-9964(91)90076-4. [DOI] [PubMed] [Google Scholar]
- 36.Degreef G, Ashtari M, Wu H, Borenstein M, Geisler S, Lieberman J. Follow-up MRI study in first-episode schizophrenia. Schizophr Res. 1991;5:204–206. doi: 10.1016/0920-9964(91)90075-3. [DOI] [PubMed] [Google Scholar]
- 37.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 2003;160:156–164. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 38.Ho BC, Andreasen NC, Nopoulos P, et al. Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 39.Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in schizophrenia - a longitudinal study of first-episode patients. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 41.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLisi LE, Szulc KU, Bertisch HC, Majche M, Brown K. Understanding structural brain changes in schizophrenia: clinical research. Dialogues Clin Neurosci. 2006;8:71–78. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulshoff Pol HE, Kahn, RS What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 45.Whitford TJ, Grieve SM, Farrow TFD, et al. Progressive grey matter atrophy over the first 2-3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32:511. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Salisbury DF, Hirayasu H, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: A cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005:18618–18625. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- 48.McIntosh AM, Baig BJ, Hall J, et al. elationship of catechol-Omethyltransferase variants to brain tructure and function in a population at high risk of psychosis. Biol Psychiatry. 2007;61:1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Boos HBM, Aleman A, Cahn W, Hulshoff-Pol H, Kah RS. Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 50.Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotropic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 51.Callicott JH, Straub RE, Pezawas L, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fields R. The other half of the brain. Sci Am. 2004;290:54–61. doi: 10.1038/scientificamerican0404-54. [DOI] [PubMed] [Google Scholar]
- 53.Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dys-function of a prefrontal limbic network in schizophrenia: a magnetic res-onance imaging and regional cerebral blood flow study of discordantmonozygotic twins. Am J Psychiatry. 1992;169:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 54.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 55.Kubicki M, Westin C-F, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szesko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 57.Sussman JE, Lymer GK, McKirdy J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:8–11. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 58.Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujiwara H, Mural T. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 60.Wang F, Sun Z, Cui L, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 61.Manoach DS, Ketwaroo GA, Polli FE, et al. Reduced microstructural integrity of the white matter underlying anterior cingulated cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 62.Mori T, Kunugi H. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Burns J, Job D, Bastin ME, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 64.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 65.Kubicki M, Park HJ, Westin CF, et al. DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 67.Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 68.Malcolm J, Shenton ME, Rathi Y. Neural tractography using anunscented Kalman filter. Information Processing in Med Imaging (IPMI). 2009:126–138. doi: 10.1007/978-3-642-02498-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones DK, Catani M, Pierpaoli C, et al. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005;13:1092–1099. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- 70.Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcorticalcerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 71.Gruzelier J. Commentary on neuropsychological and information processing deficits in psychosis and neuropsychological syndrome relationships in schizophrenia. In: Takahashi R, Flor-Henry P, Gruzelier J, Niwa S, eds Cerebral Dynamics, Laterality and Psychopathology. Amsterdam, the Netherlands: Elsevier; 1987;23 [Google Scholar]
- 72.Friston K. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 73.Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]