Abstract
The integration of functional magnetic resonance imaging (fMRI) with cognitive and affective neuroscience paradigms enables examination of the brain systems underlying the behavioral deficits manifested in schizophrenia; there have been a remarkable increase in the number of studies that apply fMRI in neurobiological studies of this disease. This article summarizes features of fMRI methodology and highlights its application in neurobehavioral studies in schizophrenia. Such work has helped elucidate potential neural substrates of deficits in cognition and affect by providing measures of activation to neurobehavioral probes and connectivity among brain regions. Studies have demonstrated abnormalities at early stages of sensory processing that may influence downstream abnormalities in more complex evaluative processing. The methodology can help bridge integration with neuropharmacologic and genomic investigations.
Keywords: fMRI, BOLD, event-related, functional connectivity
Abstract
L'intégration de l'IRMf (imagerie par résonance magnétique fonctionnelle) aux paradigmes de neuroscience cognitifs et affectifs permet d'examiner des circuits cérébraux qui sous-tendent les déficits comportementaux observés dans la schizophrénie ; les études neurobiologiques utilisant l'IRMf dans cette maladie sont de plus en plus nombreuses. Cet article résume les caractéristiques de la méthodologie de l'IRMf et présente ses applications dans les études neurocomportementales sur la schizophrénie. Cette technique a permis de trouver des substrats neuraux potentiels des déficits cognitifs et affectifs en fournissant des mesures d'activation aux sondes neurocomportementales et en objectivant la connectivité dans les régions cérébrales. Des études ont montré des anomalies au stade précoce du traitement de l'information sensorielle pouvant induire en aval des anomalies dans certains processus de traitement plus complexes. Cette méthodologie peut permettre de relier les résultats des investigations neuropharmacologiques et génomiques.
Abstract
La integración de las imágenes de resonancia magnética funcional (IRMf) con paradigmas cognitivos y afectivos de las neurociencias permite el examen de los sistemas cerebrales que están a la base de los déficit conductuales que aparecen en la esquizofrenia. Se ha producido un notable aumento en el número de estudios que aplican las IRMf en los estudios neurobiológicos de esta enfermedad. Este artículo resume las características de la metodología de las IRMf y destaca su aplicación en los estudios neuroconductuales en la esquizofrenia. Dicho trabajo ha ayudado a dilucidar los potenciales sustratos neurales en déficit cognitivos y afectivos al proporcionar mediciones de activación para las exploraciones neuroconductuales y de conectividad entre regiones cerebrales. Los estudios han demostrado anormalidades en las etapas iniciales del procesamiento sensorial que pueden influir en una cascada de anormalidades en procesamientos de evaluatión más complejos. La metodología puede ayudar a generar puentes de integración con investigaciones neurofarmacológicas y genómicas.
The application of functional magnetic resonance imaging in neuroscience research
The introduction of magnetic resonance imaging (MRI) into neuroscience has instigated a revolution in the magnitude and type of research relating brain function to behavior. Functional MRI (fMRI) has been at the forefront of this effort for several reasons. Before MRI, functional neuroimaging was only feasible with radioisotopic tracers such as oxygen-15 labeled water or fluorine-18 labeled deoxy glucose, and the temporal resolution was in minutes. Such a time resolution precludes detailed mapping of cognitive operations that take place over much shorter epochs. In addition to improved temporal resolution down to about 2 to 16 seconds (duration of the “hemodynamic response”), fMRI has provided several other advantages relevant to its use in neuroscience: higher spatial resolution, noninvasiveness, lack of ionizing radiation, direct correlation with anatomical imaging, greater repeatability (without limitations of radiation exposure), feasibility in children, and affordability The relative disadvantages are: loud background noise generated by the gradients, need to adapt stimulus presentation and recording of performance to the magnet bore setting, low signal-to-noise ratio, lack of quantitation in physiologic units for the most abundant methods, and the need to exclude individuals with metal in their bodies or who have claustrophobia. With the increased utilization of the method, many of these disadvantages have been addressed through the use of specialized equipment compatible with the MRI environment. As a result, there has been an explosion of studies of fMRI across the neurosciences, both in healthy people and in patients with brain disorders.
Blood oxygenation level-dependent (BOLD) fMRI
This method is the most widely applied in fMRI studies. The technique relies on magnetic susceptibility effects of deoxyhemoglobin, which cause regional signal changes in imaging sequences that are sensitive to susceptibility (eg, echoplanar or routine gradient echo sequences). When the brain is activated by task demands, a net increase in signal intensity is observed in regions activated by the task. This is attributed to a greater increase in regional oxygenated blood flow that exceeds regional oxygen consumption. A variety of pulse sequences can be applied to obtain BOLD measures. Many activation paradigms have been tested, and activation has been observed with both fast and slow imaging. A typical response is a change in regional image intensity that develops over 2 to 16 seconds following stimulus presentation and response initiation. Susceptibility effects of deoxyhemoglobin are field-dependent. Thus, a scanner with 1.5 Tesla field strength would typically record signal changes with functional activation of about 0.25% to 5%, while at higher fields, (eg, 3 or 4 Tesla) changes up to 25% have been observed.
Initial BOLD studies have applied blocked conditions where signal change was integrated over periods ranging from 20 seconds to minutes. In such designs, stimuli are presented in blocks and activation maps are created by subtracting signals averaged across types of blocks. Thus, during data acquisition a sequence of stimuli is presented and the participant is requested to respond to different tasks. The task is different for each block, but a strong design will require that the physical characteristics of the stimuli and the difficulty of the task be as identical as possible.
Event-related fMRI is a variant of the BOLD technology where, rather than aggregating the tasks into blocks, an estimate of the hemodynamic response is obtained by interspersing the stimuli and contrasting the signal following stimulus presentation to that following a control stimulus or task. These contrasts provide information on brain regions whose activation is time-locked to the appearance of the specific stimulus class. Furthermore, an event-related analysis can identify brain regions whose activation is associated with correct responses, and separate them from brain regions associated with errors. This feature permits a closer link of brain activation with performance. A disadvantage of the event-related design is that to fully model the hemodynamic response, trials have to be separated by about 16 seconds. This limits the number of trials, thereby diminishing the power of the analysis, and also makes for a boring task that may not be well tolerated by participants. Compromises have been developed by carefully spacing stimulus classes, so that specific time-locked activations can still be modeled, yet many more stimuli are presented at varying intervals. Such “hybrid designs” can be applied where blocked analysis can identify brain regions engaged in specific tasks, and event-related analysis can be used to establish time-locked components of the hemodynamic response related to specific stimulus classes.
Analytic approaches
An fMRI study involves acquisition of vast amounts of data in a very short time. For illustration, a rather typical task that takes 5 minutes, where images are acquired every 10 seconds, will result in values for 10 000 (voxels) x 150 (acquisitions over 300 seconds) = 1 500 000 data points. Fortunately, public domain methods for analysis of fMRI have been increasingly standardized and typically include algorithms for motion correction, high-pass filtering, spatial smoothing, and scaling. Resulting translational motion parameters should be examined to ensure that there is not excessive motion. Non-brain areas can be removed and the functional images coregistered to the structural volume. The image is then usually transformed into a standard anatomical space and transformation parameters are later applied to statistical images, after analysis is carried out in the subject's space. An alternative spatial normalization procedure for fMRI data involves application of the high-dimensional deformations determined from the anatomic images to the coregistered fMRI data. This provides higher registration accuracy across subjects, and therefore potentially improved sensitivity in activation detection. This approach also allows correlating brain volumes with fMRI activity voxel-wise, as structural and functional images follow the same transformation to the standard space.
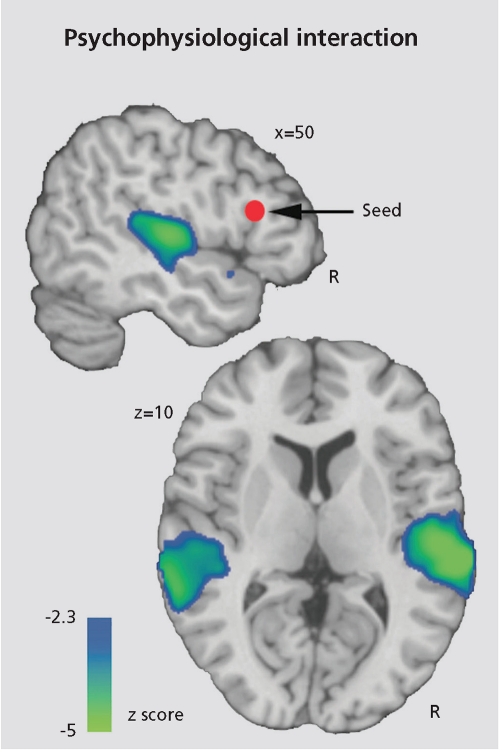
While most fMRI studies have focused on examining brain regions activated by specific tasks, increased attention is being drawn to examining intercorrelations among brain regions. Such “connectivity analyses” can help identify the strength of association among nodes in the network and to establish in clinical populations whether deficits relate to reduced connectivity.1 Defined as the temporal correlations in neural activity among brain regions, functional connectivity provides a conceptual and methodological framework for identifying spatially distributed patterns of brain activity and for quantifying inter-regional interactions. Several specific approaches have been implemented, but their relative merits have not been established. The time series correlation method examines interregional correlations within individual subjects over the time course of an experiment and has been effectively applied to measure functional connectivity across a wide range of cognitive and physiological states. For example, by examining correlations among timeseries, it was demonstrated that the amount of emotional information conveyed by a voice modulates opposing changes in activity in sensory areas of the temporal lobe where the voice is processed, and the frontal area where the results of this processing are evaluated.2 As the emotional information in the voice increases its intensity, sensory areas are increasingly activated, whereas the areas making the decision are relatively underactivated. Conversely, weaker signals require more decision-making effort, resulting in greater activation of frontal regions (Figure 1).
Figure 1.
This functional connectivity analysis map illustrates the negative interaction between the intensity of the vocal cue of an emotion and the mean time series of inferior frontal gyrus (IFG) seed region (red sphere). This map indicates that functiona connectivity between IFG and auditory processing regions in superior temporal gyrus (STG) is significantly modulated by cue saliency: decreasing cue saliency increases IFG-STG functional coupling, while increasing cue saliency decreases this coupling Reprinted with permission from ref 2: Leitman DI, Wolf DH, Ragland JD, et al. "It's not what you say, but how you say it": a reciprocal temporo-frontal network for affective prosody. Front Hum Neurosci 2010;4:19-31. Copyright © Frontiers Research Foundation 2010
The methodological developments in fMRI have offered investigators a wealth of interesting parameters of brain function. MR scanning facilities are widely available, and numerous studies have dissected complex behavior in schizophrenia.
fMRI studies in schizophrenia
There has been a rapid growth of fMRI studies in schizophrenia, and abnormal activity has been reported in motor tasks, working memory, attention, word fluency, emotion processing, and decision-making. An essential goal of such studies is to demonstrate how failure to activate a neural system leads to behavioral deficits in patients. To establish whether the neural system under investigation engages the same regions in patients with schizophrenia as in controls, it would be initially desirable to make the task easy so that patients and controls perform near perfection. Failure of patients to activate a specific region under these conditions indicates failure to recruit the requisite circuitry for that domain. However, in subsequent phases of the research the task needs to be made harder, to enable investigating whether individual differences in activation are correlated with individual differences in performance. Studies in schizophrenia have progressed from initial emphasis on cognition to the study of emotion and social cognition and motivation, focusing on the reward system.
Cognition
Schizophrenia has been characterized early on by its seemingly dementing features, yet until quite recently the focus of clinical evaluation and intervention has been on the positive symptoms associated with the disorder, such as hallucinations and delusions. Demonstration of significant deficits in several neurocognitive domains has prompted efforts to examine brain processes underlying information-processing cascades in schizophrenia. Diffuse deficits have been documented, with relatively greater impairment in executive functions and in learning and memory3-6 These deficits have been related to frontotemporal systems. Such impairments are core features of schizophrenia, important for elucidating underlying mechanisms. Their centrality to schizophrenia is buttressed by their prominence at initial presentation, limited amelioration with symptom relief, link to functional outcome, and utility as endophenotypes in genetic studies. Neurobehavioral probes with fMRI provide a powerful method for exploring the neural circuitry underlying such observed deficits. Abnormal activations in ventromedial and superior temporal lobe, prefrontal cortices, and limbic structures have been documented with memory and executive tasks. However, it is also important to note that these complex processes could be compromised as downstream effects of sensory integration deficits, and that fMRI offers tools for such investigations.
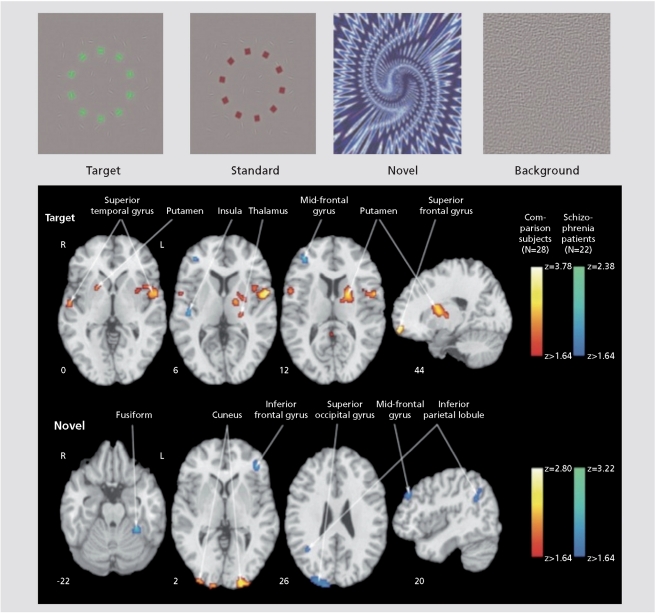
Data on early information processing in schizophrenia is relatively limited.7-9 Visual stimulation studies demonstrated normal activation of visual, motor, somatosensory, and supplementary motor regions to stimuli such as a flashing checkerboard with a simple motor response. However, abnormal activation was reported for more complex sensory conditions especially when requiring integration.3 A block design study presented simultaneous visual and auditory stimuli to first-episode neuroleptic-naïve patients. Reduced activation in parietal lobes and right thalamus and prefrontal cortex, implicated in the dorsal visual processing pathway, was observed in patients.10 Diminished activation in patients in prefrontal regions implicated in regulating inhibition was reported in a study of the inhibitory P300 for a NoGo condition.11 A three-stimulus auditory oddball task showed diffuse cortical and subcortical hypofunction during target detection and novelty processing in schizophrenia. Individuals with prodromal symptoms demonstrated smaller differential activation in frontal regions between relevant and irrelevant stimuli.12 In a visual oddball study, patients and healthy participants showed activation patterns specific to targets and novels, and activation of both neural systems was associated with faster performance.13 Specifically, reduced activation in regions involved in target and novelty processing in patients was accompanied by increased activation in circuits related to elaborated stimulus processing. For targets, abnormal activation was noted in regions related to ideational and visual association, and for novels patients overactivated sensory and frontal areas related to visualspatial processing and working memory ( Figure 2.) Thus, the attenuated electrophysiological response to targets seen in event-related potential studies may relate to insufficient top-down activation of target circuitry, while the attenuated evoked response to novel distractors reflects over-processing of bottom-up events. Notably, abnormal activation in patients was associated with more severe symptoms.
Figure 2.
Examples of stimuli used in “oddball” studies of attentional processing (top) and contrast images of patients with schizophrenia and comparison subjects for target and novel stimuli. Greater activation in patients is depicted by the blue scale, whereas greater activation in comparison subjects is shown by the red scale. Images are in radiological convention (left hemisphere to viewer's right).
Reprinted with permission from ref 13: Gur RE, Turetsky BI, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442-449. Copyright © American Psychiatric Association 2007
Abnormal activation of frontotemporal regions has been further investigated in relation to more complex downstream processes.5,6 Verbal learning deficits are well established in schizophrenia, and fMRI studies have consistently demonstrated abnormalities during the learning phase in frontotemporal circuits. Most studies have reported decreased activation of the frontal cortex, especially the inferior prefrontal region, in schizophrenia. The data are less consistent with regard to the temporal lobe. Most studies have observed decreased activation in patients in the hippocampus and parahippocampal gyrus, but other studies have noted increased activation. As emphasized earlier, performance may be a contributing factor as the studies differ in their approach to performance. Event-related fMRI studies have shown deficits in working memory and cognitive control in schizophrenia. Hippocampal dysfunction was often concomitant with aberrations in prefrontal function, suggesting that frontotemporal connectivity is disrupted in schizophrenia. As summarized above, advances in image analysis methods enable measurement of the connectivity of distributed brain circuitry and test hypotheses implicating specific network models.
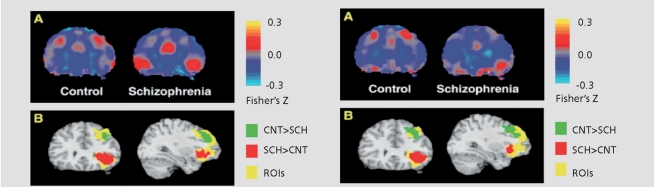
In a study examining within-subject correlations of frontal and temporal time series during verbal encoding, aberrant frontotemporal connectivity was noted in schizophrenia, confirming previous reports.14 The study also identified distinct alterations within dorsal and ventral prefrontal cortex. Relative to healthy controls, patients with schizophrenia had reduced connectivity between the dorsolateral prefrontal cortex and temporal lobe areas, including parahippocampus and superior temporal gyrus. Patients, however, showed increased connectivity between a region of ventrolateral prefrontal cortex and these same temporal lobe regions. Higher temporal dorsolateral prefrontal cortex connectivity during encoding was associated with better subsequent recognition accuracy in healthy participants, but not patients. Temporalventrolateral prefrontal cortex connectivity was uncorrelated with recognition accuracy in either group. The results suggest that reduced temporal-dorsolateral prefrontal cortex connectivity in schizophrenia could underlie encoding deficits, and increased temporal-ventrolateral prefrontal cortex connectivity may represent an ineffective compensatory effort (Figure 3).
Figure 3.
Group differences in left STG-DLPFC and STG-VLPFC connectivity (Left Column) and in left PHIP-DLPFC and PHIP-VLPFC connectivity (right column). For the left column, panel A: group-averaged STG timeseries correlation maps for control group (left) and schizophrenia group (right), from a representative coronal plane (y = 30). A region of DLPFC shows greater connectivity (measured as average Fisher's Z-transformed correlation coefficients) with STG in controls, while a region in VLPFC shows greater connectivity with STG in patients. Panel B results of voxel-by-voxel t-tests are overlaid on the two prefrontal regions of interest and a standard reference brain (Colin, MNI), in the same coronal plane. The group differences seen within DLPFC and VLPFC are statistically significant. For the right column, Panel A: groupaveraged PHIP timeseries correlation maps reveal a region within DLPFC showing greater connectivity with PHIP in controls, while a region within VLPFC shows greater connectivity with PHIP in patients. Panel B: group differences seen within DLPFC and VLPFC are statistically significant. STG, superior temporal gyrus; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; PHIP, parahippocampal gyrus; CNT, control; SCH, schizophrenia; ROI, region of interest.
Reprinted with permission from ref 1 3: Gur RE, Turetsky BI, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442-449. Copyright © American Psychiatric Association 2007
Social cognition
Social cognition refers to the processes involved in perception, interpretation, and processing of social information, most prominently emotions. Neurobiological studies of social cognition have confirmed that the processing of social information requires complex and synergistic interactions among several neural regions, which progress from basic perception of social stimuli to their later evaluation and ultimate response. The growth of affective neuroscience has generated several paradigms to probe emotion processing and social cognition in schizophrenia. Impaired emotional functioning is a prominent feature of schizophrenia. fMRI studies with block design report lack of amygdala activation for sad mood induction, also evident in unaffected siblings.15 Decreased activation and accuracy in identifying expressions of fear, anger, and disgust, contrasted with responses to mild happiness, especially in non-paranoids.16 Presentation of fearful and neutral faces showed a disconnection between brain activity and arousal in paranoid patients, where increased arousal was associated with decreased amygdala/medial prefrontal activity. In a study using a block design alternating between emotion and age identification, patients showed decreased activation in left amygdala and bilateral hippocampus for the emotion condition.17 In a study examining intensity judgment, patients had an exaggerated amygdala response: positive faces induced right amygdala activation in both groups, while negative faces activated only the right amygdala in controls and bilaterally in patients.18 Picture categorization (pleasant, unpleasant, neutral) produced less activity in amygdaloid-hippocampal and cortical-basal ganglia- thalamic circuitry in patients.19
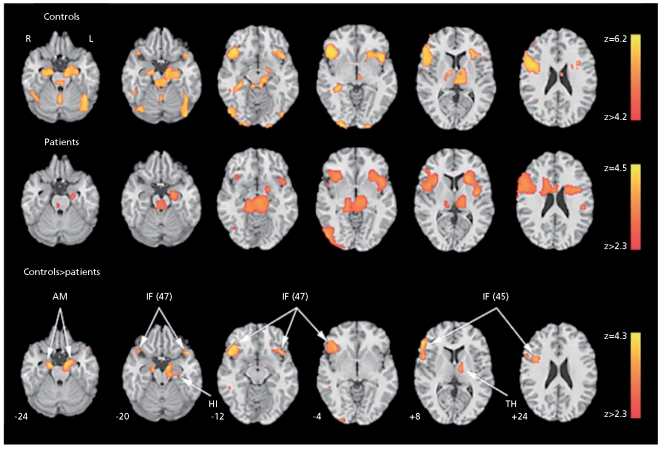
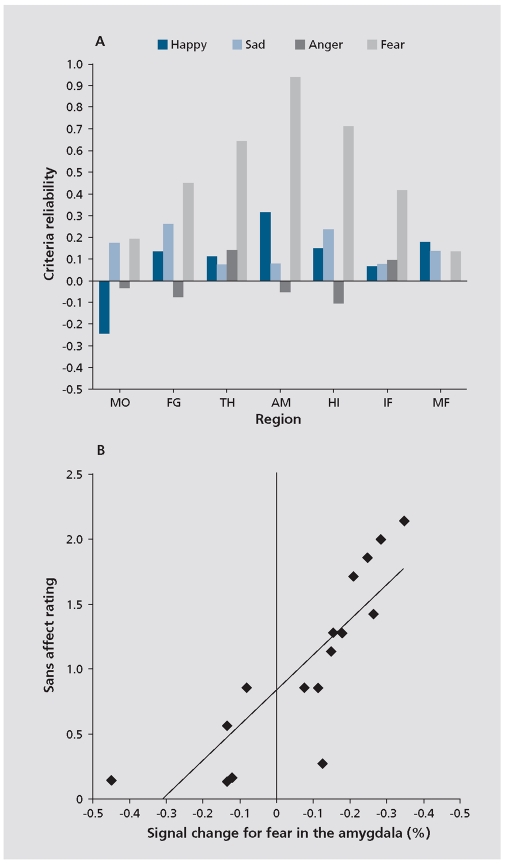
The role of limbic response in identification of facial emotions and its relation to symptoms is illustrated in Figure 4. 20 As can be seen in the middle row, top-down (task-related) limbic activation is diminished in schizophrenia. However, patients showed abnormally increased limbic activation time-locked to the appearance of threat-related facial emotions of anger and fear. Furthermore, increased amygdala activation for fear was associated in patients both with failure to identify the emotion and with more severe flat affect (Figure).
Figure 4.
Regions activated for emotion identification task relative to baseline (block analysis) in controls (upper row), patients (middle row), and the controls-patients contrast (bottom row). No patients-controls contrast survived correction. Significance thresholds are based on spatia extent using a height of z>3.1 and a cluster probability of P<.05. Images are displayed over a Talairach-normalized template in radiological convention (left hemisphere to viewer's right). The z-level coordinates are provided. AM, amygdala; IF (47), inferior frontal (Brodmann area 47); HI, hippocampus; IF (45), inferior frontal (Brodmann area 45); TH, thalamus.
Reprinted with permission from ref 1 3: Gur RE, Turetsky BI, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442-449. Copyright © American Psychiatric Association 2007
Figure 5.
Association between brain activity and clinical measures. A, Correlations between event-related activation for the 4 emotional expressions in activated regions and severity of clinical ratings for flat affect. B, Scatterplot of the association between percentage of signal change for the appearance of fear expressions and severity of flat affect. MO, Mid-occipital; FG,=fusiform gyrus AM, amygdala; IF (47), inferior fronta (Brodmann area 47); HI, hippocampus; IF (45), inferior fronta (Brodmann area 45); TH, thalamus.
Reprinted with permission from ref 13: Gur RE, Turetsky BI, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442-449. Copyright © American Psychiatric Association 2007
Studies of emotion processing in schizophrenia vary in methodology and design. Nonetheless, there seems to be considerable convergence of evidence that patients show abnormal activation in amygdala and associated regions. It also appears that the abnormalities are more pronounced for negatively valenced stimuli, although no further differentiation for specific emotions has been established. In most studies patients performance was carefully examined and tasks were often constructed so as to minimize performance difference, to avoid confounding of physiologic measures. In all cases where performance was evaluated, it did not explain the difference in activation patterns. However, reduced task-related (top-down) activation could reflect bottom-up interference from abnormally increased amygdala activation related to stimulus valence. Thus, if the task is to identify emotions, sensitivity of the amygdala for specific stimuli may disrupt cortical processes required for categorization and response.
Frontotemporal connectivity was examined by considering the pattern of correlations among activation parameters obtained from regions recruited for specific tasks. Controls have high specific connectivity for activation to the top-down task and for bottom-up activation associated with correct responding. Patients, by contrast, show abnormally high connectivity associated with activation to threat-related stimuli leading to incorrect responding.
Thus, disrupted operation of a top-down process in schizophrenia could be linked to excessive connectivity for bottom-up amygdala activation related to stimulus valence. Such disruptive activation could not only reduce connectivity among regions participating in the topdown process, but also trigger recruitment of other regions, including cortical ones, that may further disrupt task-related processing. Therefore, abnormal amygdala activation will lead to dysfunctional activation in other regions involved in information processing and disrupt downstream processes involving complex behavior. This may manifest in cognitive deficits. A likely specific consequence of abnormal bottom-up activation of amygdala is the disruption of memory processes. There is considerable evidence that the amygdala interacts closely with the hippocampus in the formation of episodic memory, not only when affective valence is prominent. This area merits further investigation.
Rigorous studies with fMRI in healthy people have identified modulators of affect regulation. Emerging findings from meta-analyses support the notion of a fear specific response of the amygdala. Potential contributions of modulating processes such as attention, habituation, arousal, and anxiety, learning, and extinction have been examined. Overall, while the amygdala response to fearful expressions can be influenced by such factors, the response is quite robust and specific for fear, and could be neither explained nor completely extinguished by such manipulations. There is evidence that threat-related stimuli are especially potent activators of the amygdala, although cognitive processing modulates stimulus effects. The amygdala's extensive connections with subcortical regions are likewise essential for its role in affect processing. It has been proposed that rapid visual input from a subcortical thalamic pathway provides an early alarm signal for threat detection, whereas slower input through a geniculostriate pathway enables more detailed evaluation of environmental input. This slower pathway could explain why amygdala activation seems to habituate rapidly in passive viewing but shows no signs of habituation when the task involves top-down processing where affective valence is relevant.
Studies attempting to elucidate mechanisms underlying the fear response have often used faces to link with animal evolutionary approaches. Affective information on the face was systematically manipulated by methods including spatial frequency, binocular suppression, chimeric faces, masked fearful eye whites, and gaze direction. These methods have suggested that amygdala response can be potentiated or suppressed by such factors. For example, amygdala response is stronger when information is presented in low rather than high spatial frequency, and the effect is especially pronounced for fear in the upper part of the face, presumably the eyes region. Notably, bilateral amygdala lesions diminish specifically the patient's ability to utilize fear-related information in the eyes region, and directing the patient to note the eyes could temporarily reverse this deficit. This finding is encouraging in that rehabilitation could possibly ameliorate deficits.
Gaze direction is another modulator of amygdala response. In healthy people, directed fear and averted anger expressions produced greater amygdala response than averted fear and directed anger. This effect was interpreted to suggest that the amygdala is especially sensitive to threat-related ambiguity. Since poor eye contact is a major feature of impaired social interactions in schizophrenia, gaze direction and evaluation of the patient's ability to use information in the eyes region seems the most appropriate target for the next phase of investigation. Although scan-path studies reported a restricted range of visual scanning in schizophrenia for happy, sad, and neutral faces, these studies have not indicated a difference between the eyes and the mouth region. However, fear and anger stimuli have not been used. Because amygdala damage leads to avoidance of the eyes region in fearful faces, and the eyes region is important for distinguishing anger from fear, the difficulty of patients in identifying fear could be related to impairment in recognizing changes in the eyes region, thereby diminishing amygdala response to fear in the eyes relative to the mouth region. As with other bottom-up activation abnormalities, we expect the severity of these abnormalities to be associated with poorer eye contact and affective flattening.
Pharmacology
The literature on the possible effects of antipsychotic agents on BOLD signal change is relatively limited.21,22 Few studies have examined neuroleptic-naïve patients, and fewer still applied a pre -post paradigm. Furthermore, small samples and methodologically limited designs have precluded systematic examination of the possible effects of therapeutics. Examples of studies include a working memory evaluation with an n-back paradigm after patients switched from first-generation antipsychotics to a second generation agent. Increased dosolateral prefrontal cortex and parietal cortex activity were reported in the patients with the new treatment.23 Similarly, normalization of brain activity was reported in patients treated with long-acting risperidone compared with conventional depot medication while performing an n-back task.24 Normalization of prepulse inhibition was noted in patients treated with olanzapine and risperidone compared with those on first-generation antipsychotics.25 Studies that examined motor control in relation to medications have also noted improved brain activity in patients treated with second-generation agents compared with first generation antipsychotics.
Given the limited number and scope of the available literature, there is no conclusive evidence, and double-blind studies are needed. Thus, it is difficult to conclude that reported "normalizing" effects of medications are attributable to that specific medication rather than reflecting clinical stabilization or practice effects. Furthermore, as no absolute measures of brain activity are provided with BOLD, the relative change needs to be interpreted with caution. Pharmacologic fMRI is a potentially powerful methodology when integrated with well-designed and informative pharmacologic paradigms.
Neuroimaging genomics
The application of fMRI in genetic paradigms is growing rapidly. Initial studies have examined genetic effects indirectly by comparing activation patterns in probands with schizophrenia, unaffected siblings, and healthy comparison subjects with no family history of schizophrenia. Such studies have demonstrated, for example, that there were abnormalities in siblings that were less severe than those seen in affected individuals. This supports the application of fMRI as a quantitative phenotypic marker of schizophrenia.4,15,26,27
The draft sequence of the human genome offers unprecedented opportunities for direct evaluation of the effects of genetic variability on brain activity. Early work exploiting this potential has demonstrated such effects in healthy people by comparing activation patterns between genotypically characterized groups. Studies applying genetic strategies used functional polymorphisms to group individuals for comparisons. For example, a common Val108/158Met substitution in the gene for catechol-O-methyltransferase (COMT) leads to decreased activity of this enzyme in dopamine catabolism and has been linked to decreased prefrontal cortical activity. Studies therefore examined COMT val/met polymorphism and prefrontal cortex activation.28,29 Individuals homozygous for the met allele had diminished prefrontal and hippocampal engagement while performing episodic memory encoding and retrieval compared with val/val subjects.
In schizophrenia, where disease risk is likely conferred by multiple interacting susceptibility genes, it is necessary to study convergent potential pathways from gene effects to clinical manifestations. Several at-risk genes implicated in schizophrenia are related to neuronal function including COMT, dysbindin, neuregulin 1 (NRG1), BDNF, RGS4, and DISC-L Initial work in schizophrenia demonstrated effects of the COMT polymorphism on cognition and prefrontal function and risk for schizophrenia. Using a similar approach a risk haplotype was examined in GRM3, a gene encoding a metabotropic glutamate receptor. The findings were of reduced neuronal function in prefrontal cortex and impaired activation in the hippocampus during performance of a verbal memory task. The risk allele in the NRG 1 promoter region was associated with decreased activation of prefrontal and temporal lobe cortex. This research has great potential for constructing mechanistic models for the pathophysiology of schizophrenia.30,31 The potential contribution of fMRI to neuroimaging genomics is especially powerful because it can be used in children and therefore applied in establishing neurodevelopmental trajectories.
The incorporation of functional neuroimaging into genetic studies is challenging. Since the expected effects of any single polymorphism are small, it is essential to pay close attention to experimental design in neuroimaging genomics. As highlighted above, it is important to use well-characterized neurobehavioral probes, and to evaluate and control confounding variables such as age, gender, basal abilities, performance, and clinical status. In addition, neuroimaging genomic studies require large samples that can optimally be obtained by multisite studies. Such efforts introduce the additional variability related to scanner characteristics and image acquisition. Nonetheless, the field is making rapid progress and is poised to make new discoveries that will illuminate neural pathways to specific features of schizophrenia in ways that will lead to novel interventions.
Conclusions
Considerable advances with fMRI have been made in efforts to elucidate the neurobiology of schizophrenia, and fMRI has become a dominant method to examine brain systems. It provides noninvasive measures with high anatomic resolution, acceptable temporal resolution, and increasingly reliable quantitation. Abnormalities in schizophrenia have been documented at multiple levels of neurobehavioral processing and across several neural systems. These abnormalities are manifested in failure of some regions to activate to a task, while other regions overactivate and there are alterations in the connectivity among regions. A clearer picture of these abnormalities and their relation to genetic vulnerability, clinical manifestation, and the potential modulation with treatment will require the combined application of cross-modal measures. In this context fMRI will play a critical role by offering procedures for establishing whether a specific neural system is adequately recruited for its role in the information processing cascade and whether it communicates adequately with other systems on which it depends, or that may depend on its output.
Acknowledgments
Supported by NIH grants MH64045 and MH60722.
Contributor Information
Raquel E. Gur, Departments of Psychiatry, Neurology and Radiology, University of Pennsylvania Medical Center, Philadelphia, Pennsylvania, USA.
Ruben C. Gur, Departments of Psychiatry, Neurology and Radiology, University of Pennsylvania Medical Center, Philadelphia, Pennsylvania, USA.
REFERENCES
- 1.Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. . PLoS One. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leitman DI, Wolf DH, Ragland JD, et al. "It's not what you say, but how you say it": a reciprocal temporo-frontal network for affective prosody. . Front Hum Neurosci. 2010;4:19–31. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barch DM, Csernansky JG. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domain-general executive dysfunction. . Am J Psychiatry. 2007;164:1090–1098. doi: 10.1176/ajp.2007.164.7.1090. [DOI] [PubMed] [Google Scholar]
- 4.Becker TM, Kerns JG, Macdonald AW 3rd, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. . Neuropsychopharmacology. 2008;33:2619–2625. doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- 5.Gur RE, Keshavan MS, Lawrie SM. Deconstructing psychosis with human brain imaging. . Schizophr Bull. 2007;33:921–931. doi: 10.1093/schbul/sbm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. . Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. . Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haenschel C, Bittner R A, Haertling F, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. . Arch Gen Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- 9.Tost H, Wolf I, Brassen S, Ruf M, Schmitt A, Braus DF. Visual motion processing dysfunction in schizophrenia: a "bottom up" or "top down" deficit? . Neuroimage. 2004;22:374–380. [Google Scholar]
- 10.Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. . Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- 11.Mathalon DH, Whitfield S L, Ford J M. Anatomy of an error: ERP and fMRI. . Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 12.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. . Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gur R E, Turetsky B I, Loughead J, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. . Am J Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- 14.Wolf DH, Turetsky BI, Loughead J, et al. Auditory oddball fMRI in schizophrenia: association of negative symptoms with regional hypoactivation to novel distractors. . Brain Imaging Behav. 2008;2:132–145. doi: 10.1007/s11682-008-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habel U, Klein M, Shah NJ, et al. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. . Am J Psychiatry. 2004;161:1806–813. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- 16.Phillips ML, Williams L, Senior C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. . Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 17.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. . Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 18.Kosaka H, Omori M, Murata T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. . Schizophr Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Koeda M, Oda K, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. . Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. . Arch Gen Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 21.Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. . Am J Psychiatry. 2006;163:2184–2186. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]
- 22.Wise, RG, Tracey I. The role of fMRI in drug discovery. . J Magn Reson Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 23.Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. . Proc Natl Acad Sci U S A. 1999;96:13432–13437. doi: 10.1073/pnas.96.23.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surguladze SA, Chu EM, Evans A, et al. The effect of long-acting risperidone on working memory in schizophrenia: a functional magnetic resonance imaging study. . J Clin Psychopharmacol. 2007;27:560–570. doi: 10.1097/jcp.0b013e31815a256c. [DOI] [PubMed] [Google Scholar]
- 25.Kumari V, Soni W, Sharma T. Prepulse inhibition of the startle response in risperidone-treated patients: comparison with typical antipsychotics. . Schizophr Res. 2002;55:139–146. doi: 10.1016/s0920-9964(01)00276-6. [DOI] [PubMed] [Google Scholar]
- 26.Callicott J, Egan MF, Mattay V, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. . Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 27.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. . Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val 108/1 58 Met genotype on frontal lobe function and risk for schizophrenia. . Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan MF, Straub RE, Goldberg TE, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. . Proc Natl Acad Sci U S A. 2004;10:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hariri AR, Weinberger DR. Imaging genomics. . Br Med Bull. 2003;65:259. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. . Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]