Abstract
Major advances have been made in our understanding of the epidemiology of schizophrenia. We now know that the disorder is more common and severe in young men, and that the incidence varies geographically and temporally. Risk factors have been elucidated; biological risks include a family history of the disorder, advanced paternal age, obstetric complications, and abuse of drugs such as stimulants and cannabis. In addition, recent research has also identified social risk factors such as being born and brought up in a city, migration, and certain types of childhood adversity such as physical abuse and bullying, as well as social isolation and adverse events in adult life. Current research is focussing on the significance of minor psychotic symptoms in the general population, gene-environmental interaction, and how risk factors impact on pathogenesis; perhaps all risk factors ultimately impact on striatal dopamine as the final common pathway.
Keywords: schizophrenia, epidemiology, risk factor, gene-environment interaction, psychosis
Abstract
Se han realizado importantes avances en nuestra comprensión acerca de la epidemiología de la esquizofrenia. Actualmente se sabe que el trastorno es más común y grave entre los hombres jóvenes y que la incidencia varía geográfica y temporalmente. Se han aclarado los factores de riesgo; los riesgos biológicos incluyen una historia familiar de la enfermedad, avanzada edad paterna, complicaciones obstétricas y abuso de drogas como los estimulantes y el cannabis. Además, la investigación reciente también ha identificado factores de riesgo sociales como el haber nacido y haberse criado en una ciudad, la migración y ciertos tipos de adversidad infantil como el abuso físico y el bullying, al igual que el aislamiento social y situaciones adversas en la vida adulta. La investigación actual está orientada hacia los síntomas psicóticos menores en la población general, la interacción genes-ambiente y cómo los factores de riesgo impactan en la patogénesis; considerando que quizás todos los factores de riesgo finalmente impactan en la dopamina estriatal como la vía final común.
Abstract
Notre compréhension de l'épidémiologie de la schizophrénie a fait d'énormes progrès. Nous savons maintenant que la maladie est plus courante et plus sévère chez les hommes jeunes et que l'incidence de la maladie varie géographiquement et chronologiquement. Les facteurs de risque ont été identifiés ; les risques biologiques comprennent des antécédents familiaux de la maladie, un âge paternel élevé, des complications obstétricales et la consommation excessive de drogues comme les stimulants et le cannabis. De plus, la recherche récente a également identifié des facteurs de risque sociaux comme le fait d'être né et d'avoir été élevé dans une ville, l'immigration et certains types d'enfance malheureuse comme la maltraitance physique et les brimades, ainsi que l'isolation sociale et les événements négatifs au cours de la vie adulte. La recherche actuelle se concentre sur la signification de symptômes psychotiques mineurs dans la population générale, l'interaction gène-environnement et la façon dont les facteurs de risque influent sur la pathogenèse ; tous les facteurs de risque agissent peut-être en fin de compte sur la dopamine striatale, qui serait la voie finale commune.
The last decade has seen striking progress in our understanding of the epidemiology of schizophrenia. Some traditional beliefs have been confirmed, but others have been swept away, while recent data have implicated new risk factors for the disorder and have changed the way we conceptualize it.
Descriptive epidemiology
Lifetime prevalence
Schizophrenia affects just under 1% of the population at some point in their life. Perhaps the most comprehensive study to demonstrate this comes from Finland; Perala et al estimated lifetime prevalence, according to DSM-IV criteria, at 0.87% for schizophrenia, and 0.32% for schizoaffective disorder.1
Incidence
For many years the curious view held sway that the incidence of schizophrenia was constant both geographically and temporally2 However, we now know that this is not so.3,4 A systematic review5 showed that rates for the incidence of schizophrenia ranged from 7.7 to 43.0 per 100 000, a fivefold difference. There are fewer data concerning long-term trends, but it has been demonstrated that the operationally defined incidence of schizophrenia in South London doubled between 1965 and 1997.6
Age of onset
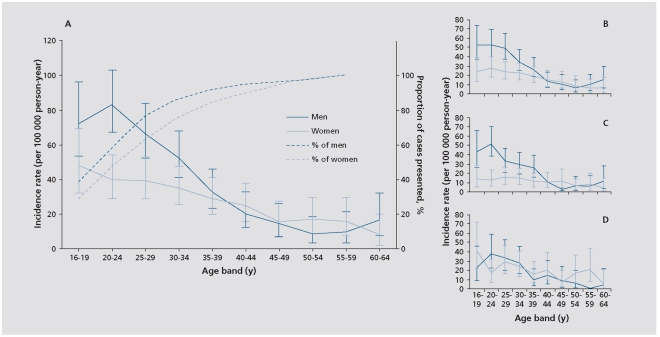
Kirkbride et al assessed the incidence of psychosis in three English cities as part of the large AESOP (Aetiology and Ethnicity of Schizophrenia and Other Psychoses) study. Figure 1 shows the age-specific incidence rates for psychosis as a whole and for the main diagnostic types. It can be seen (Figure 1c) that the peak incidence for schizophrenia in males was between 20 and 24 years, but 29 to 32 years in females; the latter showed a flatter curve with more cases presenting in later life.7 Thus, the AESOP study confirms previous evidence of an earlier age of schizophrenia onset in males. One of the most detailed studies of gender differences investigated 477 first-contact cases with schizophrenia including those presenting in later life. Though the mean age of onset was therefore later, males still had an earlier mean onset of illness than females (31.2 vs 41.1 years).8 Castle et al also showed that while the incidence was relatively equal in the two sexes for mild schizophrenia, as the diagnostic criteria were narrowed so there emerged an excess of males.8 Other studies confirm that narrowly defined schizophrenia tends to be more common (risk ratio 1.4:1), and the illness tends to be more severe, in men.3,7,9,10 The earlier age of onset in men has been attributed to the male brain's greater susceptibility to neurodevelopmental disorders,11 while the excess in women in the postmenopausal period could be secondary to loss of the antidopaminergic action of estrogens.12
Figure 1. Incidence of psychosis in the AESOP Study.
Reproduced from ref 7: Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center Aesop study. Arch Gen Psychiatry. 2006;63:250-258. Copyright © 2006 American Medical Association
Mortality
People with schizophrenia have, on average, a shorter life than the rest of the population. McGrath et al, who carried out a systematic review of mortality studies, reported that the standardized mortality ratio (SMR) was 2.6, with suicide and cardiovascular disease the major contributors. Sadly, they found that the SMR has been rising over recent decades.3
Risk factors
Risk factors for schizophrenia may be crudely divided into biological and social.
Biological risks
Genetics
The most widely replicated risk factor for schizophrenia is a family history of the disorder in a first-degree relative.13 Twin and adoption studies have shown that this is largely due to genetic factors rather than family environment.14,15,16 Assuming a model in which genes and environmental factors act additively, the heritability of schizophrenia can be calculated to be between 66% and 83 %.14 Current thinking implicates a large number of common genes of very small effect plus rarer variants such as copy number variations. However, as genetics is discussed in detail elsewhere in this issue, we will not consider this topic further here.
Parental age
In recent years, there has been a renewed interest inEdward Hare's observation that advanced paternal ageis a risk factor for schizophrenia in the offspring.17 Malaspina et al collected paternal birth data for 638 indi-viduals with schizophrenia in Israel and reported thatthe risk rose from 1/141 among those whose fathers wereless than 25 years at their birth to 1/47 for those whosefathers were 50 to 54 years (Figure 2). 18
Figure 2. Incidence of schizophrenia by paternal age.
Reproduced from ref 18: Malaspina D, Harlap S, Fennig S, et al Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361-367. Copyright © 2001 American Medical Association
Torrey et al conducted a meta-analysis of 10 studies ofthe pate rnal age effect and confirmed that risk of schiz-ophrenia rose with increased paternal age.19 There hasbeen much argument as to whether these findings aredue to biological or psychosocial factors. For instance,older fathers could produce a less favorable psychosocialenvironment for their children.20 However, the offspringof older fathers show subtle cognitive impairments. This,together with the fact that autism is also associated withincreased paternal age, and that both it and schizophrenia show an excess of copy number variations, has raisedthe possibility of mutations occurring during the repeatedmitosis in the progenitor sperm cells as men age.19,21,22
Pre- and perinatal events
Obstetric complications
Numerous studies have reported an excess of pregnancy and birth complications, collectively termed “obstetric complications” (OCs) in schizophrenic patients.23-29
Cannon et al conducted a meta-analysis of populationbased studies examining the relationship between OCs and later development of psychosis.30 They found significant associations with schizophrenia for ten individual complications, which they grouped into three categories: (i) complications of pregnancy (bleeding, pre-eclampsia, diabetes, rhesus compatibility); (ii) abnormal fetal growth and development (low birth weight, congenital malformations, small head circumference); (iii) complications of delivery (asphyxia, uterine atony, emergency cesarean section).
Season of birth
One of the most consistently replicated epidemiological features of schizophrenia is the small but significant excess of winter-spring births found in the Northern hemisphere (about 7% to 10%); patterns in the Southern hemisphere are less clear.31 Various theories have been put forward to explain this stubborn association; the most widely accepted postulates a teratogenic agent,32 or dietary deficiency, which impairs fetal brain development.
Considerable effort has been put into establishing whether the winter-spring birth excess could be due to exposure to influenza during fetal life33 but the results remain inconsistent. It has been found that the offspring exposed to prenatal maternal genital and reproductive infections were five times more likely to develop schizophrenia spectrum disorders than those who were not.34 Prenatal exposure to toxoplasmosis and to herpes simplex type 2 have also been blamed. In utero exposure to maternal malnutrition,35 maternal diabetes,30 smoking,36 and rhesus incompatibility,37 have also been considered. As yet none of these exposures to infectious or noninfectious agents can be taken as proven.
Hearing impairment increases the risk for psychosis.38 The underlying mechanism could be sensory deprivation39 or social isolation and defeat40 but hearing impairment and psychosis may be due to a common cause such as exposure to prenatal infections such as rubella.41
Drug abuse
Stimulants
The capacity of psychostimulants to produce psychotic symptoms is well known.42,43 Since the 1990s, methamphetamine abuse and the consequent psychosis has spread from Japan, Thailand, and Taiwan to California, and then eastwards across the USA. Both amphetamine and methamphetamine produce a picture almost identical to that of paranoid schizophrenia.44,45
Cannabis
Recently much more attention has been paid to the relationship between cannabis and psychosis. Initially it was widely believed that schizophrenic patients took cannabis as “self-treatment” either to alleviate negative or affective symptoms, or to counteract the dysphoric side effects of antipsychotics.46,47 However, acute ingestion of cannabis or its active ingredient tetrahydrocanabinol (THC) was found to precipitate acute psychotic episodes in experimental studies,48,49 and continuing use of cannabis is known to exacerbate existing psychotic illness.50
Andreasson et al followed up 45 570 conscripts into the Swedish army; those who abused cannabis at 18 years were more likely to be admitted to hospital with schizophrenia over the next decade and a half. There was a dose-response relationship such that the more cannabis consumed the greater was the likelihood of schizophrenia.51
For 15 years there was no attempt to replicate the Swedish Army study. However, since 2002 replications have come thick and fast,52-54 the most recent being thatby McGrath et al.55 Table I summarizes the main studies.
Table I . Epidemiological studies examining cannabis use and risk of psychosis.
| Country in which the study was conducted | Study design | Number of participants | Follow-up | Odds ratio (95% CI) (adjusted risk) |
| United States (Tien & Anthony, 1990) | Population based | 4494 | NA | 2.4 (1.2-7.1) |
| Sweden (Andreasson et al, 1987; Zammit et al, 2002) | Conscript cohort | 50 053 | 15 years | 2.3 (1.0-5.3) |
| 27 years | 3.1 (1.7-5.5) | |||
| The Netherlands (NEMESIS) (van Os et al, 2002) | Population based | 4045 | 3 years | 2.8 (1.2-6.5 |
| Israel (Weiser, Knobler, Noy, & Kaplan, 2002) | Population based | 9724 | 4 -15 years | 2.0 (1.3-3.1) |
| New Zealand (Dunedin) (Arseneault et al, 2002) | Birth cohort | 1034 | 15 years | 3.1 (0.7-13.3) |
| New Zealand (Christchurch) (Fergusson et al, 2003) | Birth cohort | 1265 | 3 years | 1.8 ( 1.2-2.6) |
| The Netherlands (Ferdinand et al, 2005) | Population based | 1580 | 14 years | 2.8 (1.8-4.4) |
| Germany (EDSP) (Henquet et al, 2005) | Population based | 2437 | 4 years | 1.7 (1.1-1.5) |
| United Kingdom (Wiles et al, 2006) | Population based | 8580 | 18 months | 1.5 (0.5-3.9) |
| Greece (Stefanis et al 2004) | Birth Cohort | 3500 | NA | 4.3 (1.0-17.9) |
| Australia (McGrath et al, 2010) | Birth Cohort | 3800 | 21 years | 2.2 (1.1-4.5) |
The risk appears to be greater in those with a family history of psychosis or a psychosis-prone personality,56 those who start use early,57,58 and the longer and more frequently cannabis is used. Di Forti et al showed that the risk is especially increased in those who use high potency varieties of cannabis such as sinsemilla or skunk (which contain up to 18% THC).59 The exact mechanism whereby cannabis increases risk remains unclear,60 but it is known to have an effect on dopamine.61
Social risk
For the last quarter of the 20th century, etiological research interest in social factors in psychosis was virtually absent. However, from the late 1990s evidence has grown that social factors play an important role in the aetiology of schizophrenia.
Urban residence
Schizophrenia is over-represented in the most deprived sections of the population.62-65 In 1939 it was reported that there were higher admission rates for schizophrenia in the poorer central areas of Chicago compared with the suburbs.62 This pattern was consistently confirmed in other large cities in the USA and Europe, most recently in Ireland.66
For many years, this was widely believed to result from preschizophrenic individuals drifting into the deprived inner cities. However, studies from Sweden and the Netherlands have shown that the incidence of schizophrenia is greater among those born or brought up in urban areas.67,68 Pedersen and Mortensen demonstrated that in Denmark, the larger the town and the longer the individual has lived in a town, the greater the risk.69
The AESOP study, discussed above, demonstrated that the incidence of schizophrenia in South London was double that in Nottingham and Bristol,7 and that even within South London there were wide variations in the rates70; the highest rates were found in the areas with least social cohesion; this last finding echoes the original findings of Faris and Dunham who suggested that social isolation in socially disorganized parts of the city could increase the risk of schizophrenia.62
Migration
An increased risk of schizophrenia has been demonstrated among Surinamese migrants in the Netherlands,71 African refugees in Sweden,72 Greek migrants to Belgium,73 and Scandinavian migrants to Denmark.74 A systematic review confirmed a high incidence of schizophrenia among many migrants and ethnic minority groups, and especially black migrants to European countries.4
The AESOP study confirmed that all ethnic minority groups in England are at increased risk for schizophrenia, but that African-Caribbeans and black Africans show an especially high risk with a ninefold and sixfold increase in the incidence respectively compared with white Britons.75 Many previous studies in the UK have reported similar findings.76-82 This excess is not a consequence of misdiagnosis.83-85 Furthermore, African-Caribbeans do not show an increased risk of psychosis in the West Indies,86-88 indicating that genes alone cannot explain the findings. Hutchinson et al showed that among the siblings of Caribbean patients in the UK, the risk was much lower in those sibs mostly living in the West Indies compared with those mostly living in the UK.89 This implies some environmental factor operating in the UK but not in the West Indies.
Boydell et al demonstrated that as the proportion of non-white ethnic minorities in a given neighbourhood in London decreases, the incidence of schizophrenia in this minority increases.6,90 The finding was subsequently replicated in the Netherlands, and suggests an ameliorating effect of social support or of decreased exposure to adversities such as racial discrimination, in areas with relatively high proportions of ethnic minorities.91
Childhood adversity
Parental loss or separation
It has been noted that permanent separation from, or death of, one or both parents was associated with a more than threefold increased risk of schizophrenia (but not bipolar disorder).92 Similarly, it was observed in the AESOP study that psychotic cases were three times more likely than controls to have experienced a longterm separation from one or both parents and to have had a parent die before the age of 16. 93
Child abuse
Of course, parental separation and loss are associated with a range of adverse early experiences, including family conflict, socioeconomic disadvantage, and neglect and abuse.94 Evidence is emerging that childhood physical abuse may increase risk of later psychosis, but whether childhood sexual abuse is particularly culpable is contentious.95
Bullying
The association between bullying and severe mental health problems, including self-harm, violent behavior, and psychotic symptoms has attracted recent attention.96 In one study, the risk of psychotic symptoms was increased twofold among victims of bullying at ages 8 and/or 10 years, independent of other prior psychopathology, family adversity, or child's IQ, and was stronger for chronic or severe victimization.97
Adult adversity
Life events
Many studies have reported an excess of stressful life events before relapse of schizophrenic illness.98-101 The smaller number of studies of first-episode psychosis have also shown an increased rate of life events prior to the onset of illness.102 There is some evidence that intrusive life events such as assaults and victimization are especially likely to preceed psychosis.
Social isolation
Those with long-standing psychotic disorders experience very high rates of unemployment,103 more often live alone,104 and fail to establish long-term relationships,105 the consequence being social isolation and exclusion.106 Marwaha and Johnson, reviewing studies of first-episode psychosis,107 noted rates of employment at >40%; other studies report similar findings.108,109 Furthermore, in a study using Danish national data, it was found that, compared with controls, those who subsequently developed schizophrenia were more frequently unemployed and living alone for as long as 19 years before first hospital admission.110 Morgan et al compared the prevalence of a number of indicators of adult social disadvantage and isolation in first-episode psychotic cases and controls in the AESOP study. All current and long-term indicators (eg, unemployment, living alone, social housing) were associated with an increased odds of psychosis.111
It is uncertain whether the association between social disadvantage and psychosis is a consequence of the developing disorder itself, or a contributory cause of the illness. Possibly urban living may impact on risk by isolating individuals, a process compounded for those whose social development is disrupted by frequent moves, leading to a loss of potentially protective factors, such as social supports. In line with such reasoning, the number of changes of school during adolescence has been associated with an increased risk of psychosis in Denmark.69
Recent conceptual developments
Psychotic symptoms in the general population
Schizophrenia was originally conceived as a disease (or diseases) qualitatively different from the normal state. However, minor psychotic symptoms are reported by a surprising number of people in the general population.112 Furthermore, the factors associated with these minor psychotic symptoms are the same as those associated with risk for schizophrenia; youth, male sex, poor education, unemployment, membership of an ethnic minority, and cannabis use.113 Thus, migrant groups with high rates of schizophrenia, such as African-Caribbean people living in the UK,80,114 also show higher rates of minor psychosis-like phenomena.115,116
Many medical disorders such as hypertension or anemia are considered as occupying the extreme end of a continuum; a disease threshold is imposed (eg, a diastolic BP of 90 mm Hg) at a point beyond which intervention is beneficial. Such a continuum view means that it is useful not only to study individuals with the established disease, but also to examine what factors propel individuals along the continuum towards the threshold. Thus, the NEMESIS follow-up study in the Netherlands demonstrated that individuals from the general population who report childhood abuse are at increased risk of developing both minor psychotic symptoms and psychotic disorder.117
Gene-environment interaction
Research has begun to focus on the possibility of gene-environmental interaction whereby genes influence risk of disorder only in the presence of a particular environmental factor or vice versa.118 One report suggested an interaction between obstetric complications and several genes involved in hypoxia,119 while it has been suggested that cannabis may increase the risk of psychosis, particularly in those with the val/val genotype at the COMT locus.120 Neither of these reports have yet been replicated.
We noted earlier that heritability estimates for schizophrenia range up to 83%. However, it may be that such calculations from twin studies inflate the apparent role of genes since gene x common environment interactions are subsumed in the heritability figure. The fact that many of the environmental risk factors that operate upon schizophrenia are common to both twins in a pair (eg, urban living, migration) could be one reason for the relative failure of molecular genetics to identify susceptibility genes of large effect for the condition.
Integrating epidemiology with pathogenesis - do all roads lead to dopamine?
In summary, the epidemiological evidence suggests that schizophrenia is a multifactorial disorder in which genes interact with each other and with environmental factors to push individuals over a threshold into expression of the disorder.121 The environmental risk factors operate at various stages of life122 but until till now there has been little attempt to relate them to what we know of pathogenesis. This is unfortunate since in many medical disorders, epidemiology is integrated with etiology and pathogenesis; for example, the risk factors for myocardial infarction are known to facilitate the development of atheroma in the coronary arteries. Such integration has not yet happened in schizophrenia research. However, there is much evidence that dysregulation of striatal dopamine is the final common pathway underlying positive psychotic symptoms. One unifying view is therefore that ultimately all risk factors for schizophrenia impact on the dopamine system.123 Such a view is schematically portrayed in Figure 3.124
Figure 3. Developmental cascade towards schizophrenia. CNV, copy number variant; HPA, hypothalamic-pituitary-adrenal.
Here, dopamine dysregulation appears as the final step in a complex developmental cascade that starts early in life and ends with the onset of full-blown psychosis. Thus stimulant drugs are known to increase synaptic dopamine while animal studies show that isolation rearing is associated with an increase in basal dopamine levels.125 There is much evidence that stress is associated with hyperactivity of the HPA axis, and in turn high cortisol is known to impact on the dopamine system.
Conclusion
The two major theories of schizophrenia, the neurodevelopmental and the dopamine hypotheses, have hitherto been largely distinct and indeed independent of much of the epidemiological evidence concerning risk factors for the condition. However, these theories are now beginning to be integrated through the growing evidence that the major developmental risk factors for schizophrenia appear to act by facilitating dopamine dysregulation; this latter appears to be the final common pathway underlying psychosis. The challenge is now to delineate the exact chain of pathogenic mechanisms which connect such risk factors to dopamine dysregulation.
Contributor Information
Simona A. Stilo, Psychosis Clinical Academic Group, Institute of Psychiatry, King's Health Partners, King's College London, UK.
Robin M. Murray, Psychosis Clinical Academic Group, Institute of Psychiatry, King's Health Partners, King's College London, UK.
REFERENCES
- 1.Peräla J, Suvisaari J, Samuli I, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. . Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. . The ICD-10 Classification of Mental and Behavioral Disorders. Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization 1992 [Google Scholar]
- 3.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. . Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 4.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. . Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 5.McGrath JJ. Variations in the incidence of schizophrenia: data versus dogma. . Schizophr Bull. 2006;32:195–197. doi: 10.1093/schbul/sbi052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boydell J, Van Os J, Lambri M, et al. Incidence of schizophrenia in south-east London between 1965 and 1997. . Br J Psychiatry. 2003;182:45–49. doi: 10.1192/bjp.182.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center Aesop study. . Arch Gen Psychiatry. 2006;63:250–258. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- 8.Castle D, Sham P, Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. . Schizophr Res. 1998;33:179–183. doi: 10.1016/s0920-9964(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 9.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. . PloS Med. 2005;2:413–433. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. . Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- 11.Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in Schizophrenia. . Psychol Med. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- 12.Häfner H, Riecher-Rössler A, An Der Heiden W, Maurer K, Fätkenheuer B, Löffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. . Psychol Med. 1993;23:925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 13.Tsuang M. Schizophrenia: genes and environment. . Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 14.Cardno AG, Marshall J, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. . Arch Gen Psych. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, McGuire M, Gruenberg AM, et al. The Roscommon Family Study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. . Arch Gen Psychiatry. 1993;50:527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman II, Shields J, Hanson DR. Schizophrenia: the Epigenetic Puzzle. Cambridge, UK: Cambridge University Press; 1982 [Google Scholar]
- 17.Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. . Br J Psychiatry. 1979;134:169–177. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- 18.Malaspina D, Harlap S, Fennig S, et al. Advancing paternal age and the risk of schizophrenia. . Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 19.Torrey EF, Buka S, Cannon TD, et al. Paternal age as a risk factor for schizophrenia: how important is it? . Schizophr Res. 2009;114:1–5. doi: 10.1016/j.schres.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Miller B, Messias E, Miettunen J, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. . Schizophr Bull. 2010. In press doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki K, Kyyrönen P. Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. . Epidemiology. 1999;10:747–751. [PubMed] [Google Scholar]
- 22.Perrin MC, Brown AS, Malaspina D. Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. . Schizophr Bull. 2007;33:1270–1273. doi: 10.1093/schbul/sbm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geddes JR, Verdoux H, Takei N, Lawrie SM, Murray RM. Individual patient data meta-analysis of the association between schizophrenia and abnormalities of pregnancy and labour. . Schizophr Bull. 1999;25:113–123. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SW, Murray RM. Obstetric complications, neurodevelopmental deviance and risk of schizophrenia. . J Psychiatric Res. 1987;21:413–421 . doi: 10.1016/0022-3956(87)90088-4. [DOI] [PubMed] [Google Scholar]
- 25.McGrath J, Murray RM. Risk factors for schizophrenia: from conception to birth. In: Hirsch S, Weinberger D, eds. . Schizophrenia. Oxford, UK: Blackwell; 1995:187–205. [Google Scholar]
- 26.McNeil TF, Kaij L. Obstetric factors in the development of schizophrenia: complications in the births of preschizophrenics and in reproductions by schizophrenic parents. in: Wynne LC, Cromwell RL, Matthysse S, eds. . The Nature of Schizophrenia: New Approaches to Research and Treatment. New York, NY: Wiley; 1978:401–429. [Google Scholar]
- 27.Murray RM, Lewis SW, Reveley AM. Towards an aetiological classification of schizophrenia. . Lancet. 1985;1:1023–1026. doi: 10.1016/s0140-6736(85)91623-x. [DOI] [PubMed] [Google Scholar]
- 28.O'Callaghan E, Gibson T, Colohan HA, et al. Risk of schizophrenia in adults born after obstetric complications and their association with early onset of illness: a controlled study. . BMJ. 1992;305:1256–1259. doi: 10.1136/bmj.305.6864.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parnas J, Schulsinger F, Teasdale TW, Schulzinger H, Feldman PM, Mednick SA. Perinatal complications and clinical outcome within the schizophrenia spectrum. . Br J Psychiatry. 1982;140:416–420. doi: 10.1192/bjp.140.4.416. [DOI] [PubMed] [Google Scholar]
- 30.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. . Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 31.McGrath JJ, Welham L. Season of birth and schizophrenia: a systematic review and meta-analysis of data from the Southern Hemisphere. . Schizophr Res. 1999;35:237–242. doi: 10.1016/s0920-9964(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 32.Jones P, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long term out come of pregnancy and delivery complications: a 28 year follow-up of the 1966 North Finland general population birth cohort. . Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 33.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. . Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 34.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal Exposure to Maternal Genital and Reproductive Infections and Adult Schizophrenia. . Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 35.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. . Am J Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 36.Sacker A, Done DJ, Crow TJ, et al. Antecedents of schizophrenia and affective illness. Obstetric complications. . Br J Psychiatry. 1995;166:734–741 . doi: 10.1192/bjp.166.6.734. [DOI] [PubMed] [Google Scholar]
- 37.Hollister JM, Laing P, Mednick SA. Rhesus incompatibility as a risk factor for schizophrenia in male adults. . Arch Gen Psychiatry. 1996;53:19–24. doi: 10.1001/archpsyc.1996.01830010021004. [DOI] [PubMed] [Google Scholar]
- 38.Van der Werf M, Van Boxtel M, Verhey F, Jolles J, Thewissen V, Van Os J. Mild hearing impairment and psychotic experiences in a normal aging population. . Schizophr Res. 2007;94:180–186. doi: 10.1016/j.schres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Mason OJ, Brady F. The psychotomimetic effects of short-term sensory deprivation. . J Nerv Ment Dis. 2009;197:783–785. doi: 10.1097/NMD.0b013e3181b9760b. [DOI] [PubMed] [Google Scholar]
- 40.Selten JP, Cantor-Graae E. Hypothesis: social defeat is a risk factor for schizophrenia? . Br J Psychiatry (Suppl). 2007;51:9–12. doi: 10.1192/bjp.191.51.s9. [DOI] [PubMed] [Google Scholar]
- 41.Dalman C, Allebeck P, Gunnell D, et al. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. . Am J Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- 42.Murray RM, Doody GA, Philips P, et al. The relationship between substance abuse and schizophrenia. In: Murray RM, Jones PB, Susser E, et al, eds. The Epidemiology of Schizophrenia. Cambridge, UK Cambridge University Press 2003:317–342. [Google Scholar]
- 43.Curran C, Byrappa N, McBride A. Stimulant psychosis. . Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 44.Chen CK, Lin SK, Sham PC, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. . Psychol Med. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- 45.Chen CK, Lin SK, Sham PC, Ball D, Loh el-W, Murray RM. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. . Am J Med Genet B Neuropsychiatr Genet. 2005;136B:87–91 . doi: 10.1002/ajmg.b.30187. [DOI] [PubMed] [Google Scholar]
- 46.Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. . Acta Psychiatr Scand. 1992;85:127–130. doi: 10.1111/j.1600-0447.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 47.Hambrecht M, Häfner H. Substance abuse and the onset of schizophrenia. . Biol Psychiatry. 1996;40:1155–1163. doi: 10.1016/S0006-3223(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 48.D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. . Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 49.Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. . Psychol Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 50.Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. . Eur Psychiatry. 2005;20:349–353. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Andreasson S, Allebeck P, Engstromm A, Rydberg U. Cannabis and schizophrenia: a longitudinal study of Swedish conscripts. . Lancet. 1987:1483–1485. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 52.Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. . BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. . Schizophrenia Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 54.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. . Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 55.McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. . Arch Gen Psychiatry. 2010;67:440–447. doi: 10.1001/archgenpsychiatry.2010.6. [DOI] [PubMed] [Google Scholar]
- 56.Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM. Gene-environment interplay between cannabis and psychosis. . Schizophr Bull. 2008;34:1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. . Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 58.Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. . BMJ. 2005;330:7481–7511. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Forti M, Morgan C, Dazzan P, et al. High potency cannabis and the risk of psychosis. . Br J Psychiatry. 2009;195:488–491 . doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison PD, Murray RM. From real-world events to psychosis: the emerging neuropharmacology of delusions. . Schizophr Bull. 2009;35:668–674. doi: 10.1093/schbul/sbp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. . Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 62.Faris REL, Dunham Warren H. Mental Disorders in Urban Areas. An Ecological Study of Schizophrenia and Other Psychoses. Chicago; IL: The University of Chicago Press; 1939;270 [Google Scholar]
- 63.Hollingshead A, Redlich RC. Social Class and Mental Illness. London, UK: Wiley 1958 doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison J, Barrow S, Creed F. Social deprivation and psychiatric admission rates among different diagnostic groups. . Br J Psychiatry. 1995;167:450–462. doi: 10.1192/bjp.167.4.456. [DOI] [PubMed] [Google Scholar]
- 65.Allardyce J, Gilmour H, Atkinson J, Rapson T, Bishop J, McCreadie RG. Social fragmentation, deprivation and urbanicity: relation to first-admission rates for psychoses. . Br J Psychiatry. 2005;187:401–406. doi: 10.1192/bjp.187.5.401. [DOI] [PubMed] [Google Scholar]
- 66.Kelly BD, O'Callaghan E, Waddington JL, et al. Schizophrenia and the city: A review of literature and prospective study of psychosis and urbanicity in Ireland. . Schizophr Res. 2010;116:75–89. doi: 10.1016/j.schres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Lewis G, David A, Andreasson S, Allebeck P. Schizophrenia and city life. . Lancet. 1992;340:137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 68.Marcelis M, Navarro Mateu F, Murray R, Selten JP, Van Os J. Urbanization and psychosis: a study of 1942-1978 birth cohorths in the Netherlands. . Psychol Med. 1998;28:871–879. doi: 10.1017/s0033291798006898. [DOI] [PubMed] [Google Scholar]
- 69.Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. . Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 70.Kirkbride JB, Fearon P, Morgan C, et al. Neighbourhood variation in the incidence of psychotic disorders in Southeast London. . Soc Psychiatry Psychiatr Epidemiol. 2007;42:438–445. doi: 10.1007/s00127-007-0193-0. [DOI] [PubMed] [Google Scholar]
- 71.Selten JP, Slaets JPJ, Kahn RS. Schizophrenia in Surinamese and Dutch Antillean immigrants to the Netherlands: evidence of an increased incidence. . Psychol Med. 1997;27:807–811. doi: 10.1017/s0033291797005199. [DOI] [PubMed] [Google Scholar]
- 72.Johansson LM, Sundquist J, Johansson SE, Bergman B. Immigration: moving house and psychiatric admissions. . Acta Psychiatr Scand. 1998;98:105–111. doi: 10.1111/j.1600-0447.1998.tb10050.x. [DOI] [PubMed] [Google Scholar]
- 73.Charalabaki E, Bauwens F, Stefos G, Madianos MG, Mendlewicz J. Immigration and psychopathology: a clinical study. . Eur Psychiatry. 1995;10:237–244. doi: 10.1016/0924-9338(96)80300-2. [DOI] [PubMed] [Google Scholar]
- 74.Mortensen PB, Cantor-Graae E, McNeil TF. Increased rates of schizophrenia among immigrants: some methodological concerns raised by Danish findings. . Psychol Med. 1997;27:813–820. doi: 10.1017/s0033291797004741. [DOI] [PubMed] [Google Scholar]
- 75.Fearon P, Kirkbride JB, Morgan C, et al. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP study. . Psychol Med. 2006;36:1541–1550. doi: 10.1017/S0033291706008774. [DOI] [PubMed] [Google Scholar]
- 76.Bebbington PE, Hurry J, Tennant C. Psychiatric disorder in selected groups in Camberwell. . Soc Psychiatry. 1981;16:43–51. [Google Scholar]
- 77.Castle D, Wessely S, Der G, Murray RM. The incidence of operationally defined schizophrenia in Camberwell, 1965-1984. . Br J Psychiatry. 1991;159:790–794. doi: 10.1192/bjp.159.6.790. [DOI] [PubMed] [Google Scholar]
- 78.Cochrane R, Bal R. Mental hospital admission rates of immigrants to England: a comparison of 1971 and 1981. . Soc Psychiatry. 1989;24:2–11. doi: 10.1007/BF01788193. [DOI] [PubMed] [Google Scholar]
- 79.Dean G, Walsh D, Downing H, Shelley E. First admissions of native born and immigrants to psychiatric hospitals in South East England 1976. . Br J Psychiatry. 1981;139:506–512. doi: 10.1192/bjp.139.6.506. [DOI] [PubMed] [Google Scholar]
- 80.Harrison G, Owens D, Holten A, Neilson D, Boot D. A prospective study of severe mental disorder in Afro-Caribbean patients. . Psychol Med. 1988;18:643–657. doi: 10.1017/s0033291700008321. [DOI] [PubMed] [Google Scholar]
- 81.McGovern D, Cope R. First psychiatric admission rates of first and second generation Afro-Caribbeans. . Soc Psychiatry. 1987;22:139–149. doi: 10.1007/BF00583848. [DOI] [PubMed] [Google Scholar]
- 82.Wessely S, Castle D, Der G, Murray R. Schizophrenia and AfroCaribbeans. A case-control study. . Br J Psychiatry. 1991;159:795–801. doi: 10.1192/bjp.159.6.795. [DOI] [PubMed] [Google Scholar]
- 83.Hickling FW, McKenzie K, Mullen R, Murray R. A. Jamaican psychiatrist evaluates diagnoses at a London psychiatric hospital. . Br J Psychiatry. 1999;175:283–285. doi: 10.1192/bjp.175.3.283. [DOI] [PubMed] [Google Scholar]
- 84.Lewis G, Croft-Jeffreys C, David A. Are British psychiatrists racist? . Br J Psychiatry. 1990;157:936–937. doi: 10.1192/bjp.157.3.410. [DOI] [PubMed] [Google Scholar]
- 85.McGovern D, Hemmings P, Cope R, Lowerson A. Long-term follow-up of young Afro-Caribbean Britons and white Britons with a first admission diagnosis of schizophrenia. . XX. 1994;29:8–19. doi: 10.1007/BF00796443. [DOI] [PubMed] [Google Scholar]
- 86.Bhugra D, Hilwig M, Hossein B, et al. First-contact incidence rates of schizophrenia in Trinidad and one-year follow-up. . Br J Psychiatry. 1996;169:587–592. doi: 10.1192/bjp.169.5.587. [DOI] [PubMed] [Google Scholar]
- 87.Hickling FW, Rodgers-Johnson P. The incidence of first contact schizophrenia in Jamaica. . Br J Psychiatry. 1995;167:193–196. doi: 10.1192/bjp.167.2.193. [DOI] [PubMed] [Google Scholar]
- 88.Mahy GE, Mallett R, Leff J, Bhugra D. First-contact incidence rate of schizophrenia on Barbados. . Br J Psychiatry. 1999;175:28–33. doi: 10.1192/bjp.175.1.28. [DOI] [PubMed] [Google Scholar]
- 89.Hutchinson G, Takei N, Fahy TA, et al. Morbid risk of schizophrenia in first-degree relatives of white and African-Caribbean patients with psychosis. . Br J Psychiatry. 1996;169:776–780. doi: 10.1192/bjp.169.6.776. [DOI] [PubMed] [Google Scholar]
- 90.Boydell J, Van Os J, McKenzie K, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. . BMJ. 2001;323:1336–1338. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veling W, Susser E, van Os J, Mackenbach JP, Selten JP, Hoek HW. Ethnic density of neighbourhoods and incidence of psychotic disorders among immigrants. . Am J Psychiatry. 2007;165:66–73. doi: 10.1176/appi.ajp.2007.07030423. [DOI] [PubMed] [Google Scholar]
- 92.Agid O, Shapira B, Zislin J, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. . Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 93.Morgan C, Kirkbride J, Leff J, et al. Parental separation, loss and psychosis in different ethnic groups: a case-control study. . Psychol Med. 2007;37:495–503. doi: 10.1017/S0033291706009330. [DOI] [PubMed] [Google Scholar]
- 94.Rutter M. Genes and Behaviour: Nature-Nurture Interplay Explained. Oxford, UK: Blackwell Publishing; 2006 [Google Scholar]
- 95.Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma - a critical review. . Schizophr Bull. 2007;33:3–10. doi: 10.1093/schbul/sbl053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: 'much ado about nothing'? . Psychol Med. 2010;40:717–729. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- 97.Schreier A, Wolke D, Thomas K, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. . Arch Gen Psychiatry. 2009;66:527–536. doi: 10.1001/archgenpsychiatry.2009.23. [DOI] [PubMed] [Google Scholar]
- 98.Brown GW, Birley JLT. Crises and life changes and the onset of schizophrenia. . J Health Soc Behav. 1968;9:203–214. [PubMed] [Google Scholar]
- 99.Day R, Neilsen JA, Korten A, et al. Stressful life events preceding the acute onset of schizophrenia: a cross national study from the World Health Organisation. . Cult Med Society. 1987;11:123–206. doi: 10.1007/BF00122563. [DOI] [PubMed] [Google Scholar]
- 100. Malla AK, Cortese L, Shaw TS, Ginsberg B. Life events and relapse in schizophrenia: a one year prospective study. . Soc Psychiatry Psychiatric Epidemiol. 1990;25:221–224. doi: 10.1007/BF00782965. [DOI] [PubMed] [Google Scholar]
- 101.Ventura J, Nuechterlein KH, Lukoff D, Hardisty JP. A prospective study stressful life events and schizophrenic relapse. . J Abnormal Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- 102.Bebbington P, Wilkins S, Jones PB, et al. Life events and psychosis, Initial results from the Camberwell Collaborative Psychosis Study. . Br J Psychiatry. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- 103.hornicroft G, Strathdee G, Phelan M, et al. Rationale and design. PRiSM Psychosis Study I. . Br J Psychiatry. 1998;173:363–370. doi: 10.1192/bjp.173.5.363. [DOI] [PubMed] [Google Scholar]
- 104.Harvey CA, Pantellis C, Taylor J, et al. The Camden schizophrenia surveys. II. High prevalence of schizophrenia in an inner London borough and its relationship to socio-demographic factors. . Br J Psychiatry. 1996;168:418–426. doi: 10.1192/bjp.168.4.418. [DOI] [PubMed] [Google Scholar]
- 105.Walsh E, Leese M, Taylor PJ, et al. Psychosis in high security and general psychiatric services: report from the UK700 and Special Hospitals' Treatment Resistant Schizophrenia groups. . Br J Psychiatry. 2002;180:351–357. doi: 10.1192/bjp.180.4.351. [DOI] [PubMed] [Google Scholar]
- 106.Social Exclusion Unit. Mental Health and Social Exclusion. . Social Exclusion Unit Report. London, UK: Office of the Deputy Prime Minister; 2004 [Google Scholar]
- 107.Marwaha S, Johnson S. Schizophrenia and employment: a review. . Soc Psychiatry Psychiatric Epidemiol. 2004;39:337–349. doi: 10.1007/s00127-004-0762-4. [DOI] [PubMed] [Google Scholar]
- 108.Barnes TR, Hutton SB, Chapman MJ, Mutsatsa S, Puri BK, Joyce EM. West London first-episode study of schizophrenia. Clinical correlates of duration of untreated psychosis. . Br J Psychiatry. 2000;177:207–211. doi: 10.1192/bjp.177.3.207. [DOI] [PubMed] [Google Scholar]
- 109.Craig TKJ, Garety P, Power P, et al. The Lambeth Early Onset Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. . BMJ. 2004;329:1067–1069. doi: 10.1136/bmj.38246.594873.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Agerbo E, Byrne M, Eaton WW, Mortensen PB. Marital and labour market status in the long run in schizophrenia. . Arch Gen Psychiatry. 2004;61:28–33. doi: 10.1001/archpsyc.61.1.28. [DOI] [PubMed] [Google Scholar]
- 111.Morgan C, Kirkbride J, Hutchinson G, et al. Cumulative social disadvantage, ethnicity and first-episode psychosis: a case-control study. . Psychol Med. 2008;38:1–15. doi: 10.1017/S0033291708004534. [DOI] [PubMed] [Google Scholar]
- 112.Van Os J, Linscott RJ, Delespaul MG, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. . Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 113.Johns LC, Cannon M, Singleton N, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. . Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- 114.Sharpley M, Hutchinson G, Murray RM, McKenzie K. Understanding the excess of psychosis among the African-Caribbean population in England. Review of current hypotheses. . Br J Psychiatry. 2001;40:60–68. doi: 10.1192/bjp.178.40.s60. [DOI] [PubMed] [Google Scholar]
- 115.Sharpley MS, Peters ER. Ethnicity, class and schizotypy. . Soc Psychiatry Psychiatr Epidemiol. 1999;34:507–512. doi: 10.1007/s001270050168. [DOI] [PubMed] [Google Scholar]
- 116.Johns LC, Nazroo JY, Bebbington P, Kuipers E. Occurrence of hallucinatory experiences in a community sample and ethnic variations. . Br J Psychiatry. 2002;180:174–178. doi: 10.1192/bjp.180.2.174. [DOI] [PubMed] [Google Scholar]
- 117.Janssen I, Krabbendam L, Bak M, et al. Childhood abuse as a risk factor for psychotic experiences. . Acta Psychiatr Scand. 2004;109:38–45. doi: 10.1046/j.0001-690x.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 118.Van Os J, Rutten BPF, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. . Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nicodemus KK, Marenco S, Batten AJ, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia . risk. Mol Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 120.Caspi A, Mffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. . Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 121.Van Os J, Marcelis M. The ecogenetics of schizophrenia. . Schizophr Res. 1998;32:127–135. doi: 10.1016/s0920-9964(98)00049-8. [DOI] [PubMed] [Google Scholar]
- 122.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. . Int J Neuropsychopharmacology. 2004;7:7–13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- 123.Murray RM, Lappin Julia, Di Forti Marta. Schizophrenia: from developmental deviance to dopamine dysregulation. . Eur Neuropsychopharmacol. 2008;18:129–134. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia - all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17:101–107. doi: 10.1016/j.euroneuro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 125.Hall FS, Wilkinson LS, Humby T, et al. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. . Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]