Abstract
Approach-avoidance conflict is an important psychological concept that has been used extensively to better understand cognition and emotion. This review focuses on neural systems involved in approach, avoidance, and conflict decision making, and how these systems overlap with implicated neural substrates of anxiety disorders. In particular, the role of amygdala, insula, ventral striatal, and prefrontal regions are discussed with respect to approach and avoidance behaviors. Three specific hypotheses underlying the dysfunction in anxiety disorders are proposed, including: (i) over-representation of avoidance valuation related to limbic overactivation; (ii) under- or over-representation of approach valuation related to attenuated or exaggerated striatal activation respectively; and (iii) insufficient integration and arbitration of approach and avoidance valuations related to attenuated orbitofrontal cortex activation. These dysfunctions can be examined experimentally using versions of existing decision-making paradigms, but may also require new translational and innovative approaches to probe approach-avoidance conflict and related neural systems in anxiety disorders.
Keywords: approach, avoidance, conflict, decision making, anxiety disorder, neuroimaging, insula, prefrontal cortex, amygdala, striatum
Abstract
El conflicto aproximación-evitación es un importante concepto psicológico que se ha utilizado ampliamente para una mejor comprensión de la cognición y de la emoción. Esta revisión se focaliza en los sistemas neurales involucrados en la aproximación, la evitación y la toma de decisiones f rente al conflicto y cómo estos sistemas se traslapan con los sustratos neurales implicados en los trastornos ansiosos. Se discute en especial el papel de la amígdala, la ínsula, el estriado ventral y las regiones prefrontales en relación con las conductas de aproximación y de evitación. Se proponen tres hipótesis específicas que subyacen a la disfunción en los trastornos ansiosos, las que incluyen: 1) la sobre-representación que se le da a la valoración de la evitación, la cual se relaciona con la sobre-activación límbica, 2) la sub o sobrerepresentación para la valoración de la aproximación, la cual se relaciona respectivamente con una activación estriatal reducida o exagerada y 3) la integración insuficiente y arbitraria de las valoraciones para la aproximación y la evitación, las cuales se relacionan con una activación reducida de la corteza órbito-frontal. Estas disfunciones se pueden estudiar experimentalmente utilizando versiones de paradigmas de toma de decisiones ya existentes, pero se pueden emplear también nuevas e innovadoras propuestas translacionales para investigar el conflicto aproximación-evitación y los sistemas neurales relacionados con éste en los trastornos ansiosos.
Abstract
Le conflit de type approche-évitement est un concept psychologique important qui a été largement utilisé pour une meilleure compréhension de la cognition et des émotions. Cet article s'intéresse aux systèmes neuraux impliqués dans l'approche, l'évitement et le conflit décisionnel qui en résulte et au chevauchement de ces systèmes avec les substrats neuraux des troubles anxieux. Nous analysons en particulier le rôle de l'amygdale, de l'insula, du striatum ventral et des régions préfrontales par rapport aux comportements d'approche et d'évitement. Nous proposons trois hypothèses spécifiques soustendant le dysfonctionnement dans les troubles anxieux: 1) la sur-représentation de l'estimation de l'évitement liée à une suractivation limbique ; 2) la sous- ou la sur-représentation de l'estimation de l'approche liée à une activation striatale respectivement atténuée ou exagérée ; et 3) l'intégration et l'arbitrage insuffisants de l'estimation de l'approche et de l'évitement liés à une diminution de l'activation du cortex orbitofrontal. Ces dysfonctionnements peuvent être examinés de façon expérimentale en utilisant des versions de modèles existants de prise de décision, mais peuvent aussi nécessiter de nouvelles approches translationnelles et innovantes afin d'explorer le conflit approche-évitement et les systèmes neuraux qui y sont liés dans les troubles anxieux.
“Conflict” occurs when a person or animal is faced with opposing drives, ie, incentives to act, that are incompatible with one another.1-3 For example, conflict can be instigated when the same action is associated with both reward and punishment, as in the case of approachavoidance conflict, or when two distinct actions are associated with somewhat balanced rewards (approachapproach conflict) or punishments (avoidance-avoidance conflict). Conflict poses a unique challenge for comparing the value of available options in a decision-making situation. Individuals must integrate a variety of information concerning the value of potential rewards and punishments, and the likelihood and magnitude of those potential outcomes.4
Conflict between opposing internal or external drives was recognized as an important process for understanding psychopathology as early as the 1900s. Conflict was conceptualized in unique ways by two ancestral lines of psychology - psychoanalytical thought led by Sigmund Freud,5 and behavioral psychology led by Ivan Pavlov6 Although these two fields used disparate experimental approaches, the similarities between Freud's concept of psychic conflict and Pavlov's use of conflicting conditioned reflexes to produce “experimental neuroses” were soon recognized.7,8 Since that time, various experimental methods, paradigms, and self-report measures have been developed in attempts to further characterize animal and human conflict behavior and its relationship to psychopathology.3,9-12
Avoidance has been implicated as a cardinal symptom of anxiety disorders13 and is thought to be an underlying mechanism maintaining anxiety. The majority of psychotherapies used to treat anxiety (eg, cognitive-behavioral and exposure-based therapies) aim to decrease such avoidance behavior.14,15 Importantly, avoidance is an active choice process, ie, a decision that is made to sacrifice potential rewards in order to avoid potential negative outcomes. Individuals with strong avoidance drives in the absence of approach drives would most likely not experience distress and not present to the clinic - or would be given a diagnosis other than anxiety, such as Asperger's syndrome or schizoid personality disorder. Therefore, inherent in the notion of an anxiety disorder is conflict between approach-related drives (eg, to seek positive social interactions, to leave the house) and avoidance-related drives (eg, to prevent being humiliated or having a panic attack).
In this review, we propose that the approach-avoidance perspective provides an important framework for bridging the gap in knowledge about the relationship between brain and behavior, ie, to clarify the role of specific neural systems in anxiety. In particular, we review neural systems that, based on neuroimaging research related to approach, avoidance, and decision making, should be considered of utmost importance for approach-avoidance conflict processes. By combining knowledge regarding these neural systems with implications from current neuroimaging research in anxiety disorders, we will outline what important questions remain from an approach-avoidance perspective.
As this review focuses on a few brain regions likely to play a vital role in conflict decision making in anxiety disorders, we do not extensively cover every brain system potentially involved, nor do we discuss related neurotransmitter systems (eg, dopaminergic, serotonergic; for review see refs 2,16-19). Secondly, our discussion focuses on conflict decision-making paradigms and excludes paradigms in which prescribed behavior conflicts with automatic reactions (eg, inhibition or interference tasks20,10) and self-report measures of approachavoidance or behavioral inhibition-activation.21 Lastly, we will limit our discussion of anxiety disorders to generalized anxiety disorder (GAD), social anxiety disorder (SAD), panic disorder, specific phobia, and posttraumatic stress disorder (PTSD). We exclude obsessive-compulsive disorder because significant distinctions between obsessive-compulsive spectrum disorders and other anxiety disorders have been noted with respect to both symptom presentation and underlying neural substrates.22
Behavioral models of approach, avoidance, and decision making
Avoidance can be considered a drive motivated in response to stimuli and situations that threaten the integrity of the individual, ie, fear- or pain-inducing stimuli. Approach behavior can be considered a drive motivated by stimuli or situations that further ensure the integrity of the individual, ie, rewarding or pleasurable stimuli. Frequently, one has to make decisions among options that have both avoidance and approach features. We propose that understanding neural substrates of approach and avoidance processes and the arbitration of these values is necessary for understanding dysfunctions associated with anxiety disorders.
Neuroimaging studies of avoidance-related processing have relied heavily on passively experienced fear- or anxiety- producing stimuli, including pictures, sounds, smells, etc. However, a few studies have also investigated neural correlates of emotion regulation, fear conditioning, and fear extinction.23,24 Approach-related processing can be investigated using passively experienced pleasurable or rewarding stimuli or appetitive conditioning.16,25-27 Human neuroimaging research related to anxiety has thus far relied heavily upon passive fear or anxiety processing paradigms.
Several decision-making paradigms have been used to delineate the processes associated with arbitrating approach or avoidance-related outcomes. Specifically, risk-taking paradigms have been used in which the same option could be associated with winning or losing reward,28,29 value-based decision-making tasks in which obtaining one reward requires sacrifice of another (eg, paying money for food items30), and delayed-discounting tasks in which decisions are made between immediate and delayed rewards of various values.31-33
Although neural mechanisms of reward-processing and decision making have been a focus of some areas of psychopathology research (eg, substance abuse), there has been a lack of related research in anxiety disorders. Behavioral research provides initial evidence that reward-based decision making may be dysfunctional in anxiety. PTSD has been associated with decreased expectancy and satisfaction of rewards,34 decreased willingness to exert effort to obtain rewards,35 and decreased ability to learn optimal responses during reward-based tasks.36 Research findings regarding decision-making processes in other anxiety disorders has not been as consistent. Individuals with high trait anxiety or specific phobia have reportedly exhibited impairment on the Iowa Gambling Task (IGT), a risk-based decision-making task(Aupperle RL et al, unpublished material).37,38 GAD has been associated with intact performance on the IGT,39 but increased errors during differential reward/punishment learning.40 SAD has been associated with intact performance on reward/punishment learning,40 but with exaggerated delayed discounting (greater preference for immediate over delayed rewards).41 Panic disorder has been associated with intact IGT performance,42 but with increased sensitivity to errors during a two-choice prediction task.43 Obviously these findings are mixed, making it difficult to draw any firm conclusions regarding the extent or specificity of decision-making dysfunction across anxiety disorders. Further behavioral and neuromaging research is warranted in order to elucidate potential decision-making dysfunction that may contribute to approach-avoidance conflict difficulties and the underlying mechanisms of anxiety disorders.
Neuroanatomy of approach, avoidance, and decision making
Neural substrates underlying approach, avoidance, and decision making are integrated here with a particular focus on anxiety disorders. Neuroanatomical research in animals and human neuroimaging research on fear processing have implicated a cortico-limbic circuitry including the amygdala, insula, and prefrontal cortex (PFC)44-46 - regions that have also been shown to exhibit dysfunction in anxiety disorders. Reward-processing and decision-making research has focused primarily on a corticostriatal circuitry involving ventral striatum/nucleus accumbens (NAcc) and frontal cortical regions - including the orbitofrontal cortex as well as more dorsal and lateral regions.4,26,47-49 It should be recognized that regions outside of these corticolimbic and cortocostriatal loops are also implicated in these processes, including hypothalamus, thalamus, hippocampus, midbrain, parietal, and brain stem regions (for review of reward-processing and decision-making networks see refs 16,31,50; for review of fear-processing networks see refs 45,46,51). For this review, we will focus on a few regions that: (i) have been shown to play vital roles in determining the value of stimuli or choices during decision making; and (ii) we believe are likely to underlie approach-avoidance dysfunction in anxiety disorders. These regions include the amygdala, ventral striatum, insula, and PFC.
Amygdala
Avoidance and approach processing
The amygdala has been a primary focus of animal and human research related to fear processing, conditioning, and extinction.52-54 Human neuroimaging studies implicate the amygdala in signaling fear- or anxiety-producing stimuli characteristics, including pictures, odors, and faces55-57 as well as in signaling changes in reinforcing properties of stimuli, such as occurs during fear conditioning58-60 or instructional and observational learning.61,62 However, human neuroimaging studies have also shown the amygdala to respond to positive, rewarding stimuli and during appetitive conditioning,16,26,27,63-68 suggesting this region may be involved in processing salience (eg, the emotional significance of stimuli), rather than simply negative valence per se.
Among the anxiety disorders literature, paradigms involving symptom provocation, anticipation of anxietyprovoking stimuli, fear conditioning/extinction, or processing of negative emotional faces, have been associated with exaggerated amygdala activation for GAD,69,71 SAD,72-78 panic,79-81 specific phobia,78,82-86 and PTSD46,78,87-92 patients. Many studies report amygdala activation to correlate with anxiety symptom severity (SAD75,94-96; PTSD87,88,96) and suggest that amygdala activation decreases in response to cognitive behavioral or pharmacologic treatment (SAD97; phobia98,99; PTSD100). These results suggest that amygdala dysfunction in anxiety disorders relates to aberrant signals concerning the presence of feared or negatively reinforcing stimuli - a dysfunction which can be at least partially rectified through treatment. However, it should be noted that some studies of GAD,101 SAD,102 phobia,103-105 and PTSD106-108 have failed to identify exaggerated amygala activation.
Although most neuroimaging studies of anxiety disorders do not explicitly aim to investigate responses to pleasurable or rewarding stimuli, many use such stimuli as “control” conditions and report neural activations during these conditions separately. These results are mixed, with some reporting no evidence of amygdala dysfunction (GAD70,101; SAD93,94; Phobia109; PTSD88) and others reporting exaggerated amygdala activation (SAD73,11073,110; phobia82; PTSD87) to positive emotional stimuli or faces. This suggests that while amygdala dysfunction may be most evident for anxiety disorders during processing of highly salient, negative stimuli, such dysfunction may relate to emotionally salient stimuli in general. This could result in not only increased urges to avoid negative outcomes but also increased urges to obtain rewards - leading to a “higher-stakes” experience of having a lot to gain and a lot to lose, increasing the level of approach-avoidance conflict.
Decision making
Animal research suggests that the amygdala, and PFCamygdala connections, play an important role in determining approach-avoidance behavior during conflict, delayed discounting (involving decisions between immediate smaller rewards and delayed larger rewards), and effort-based decision making (involving decisions between immediate easily attainable rewards vs larger rewards obtained after expending effort or energy)111-113 (see reviews in refs 2,114). Similarly, patients with amygdala damage have been shown to exhibit impaired riskrelated decision making,115,116 and amygdala activation has been reported during decision-making paradigms involving uncertainty or risk.117-119 A recent neuroimaging study implicated connectivity between amygdala/ hippocampus and PFC (anterior cingulate [ACC] in particular) in the use of episodic imagery of future events to increase delayed discounting.120 This suggests the amygdala may be involved in signaling risk and salience of future consequences.
The few studies that have examined neural substrates of decision making in anxiety disorders have, for the most part, not reported amygdala dysfunction. However, Krain et al121 examined a group of adolescents with either GAD or SAD during a decision-making task involving various levels of certainty and reported that self-reported intolerance of uncertainty was related to greater amygdala activation during uncertain, or riskier, conditions.
In summary, although the amygdala has been a focus of the fear-processing and fear-learning literature in particular, this region seems to play a more general role in signaling salience of stimuli rather than simply negative valence.122 The anxiety disorders literature provides evidence that the amygdala may be dysfunctional in signaling fear-related stimuli, but there is also initial evidence that this dysfunction may extend to salient stimuli in general.46,78 Although the amygdala has not been the focus of neuroimaging research related to decision making, there is evidence to suggest the amygdala is involved in signaling uncertainty or risk of decisions.117-117,121 We propose that the amygdala may have a primary role in signaling the presence, or potential future presence, of reinforcing stimuli as well as in gauging stimulus intensity. Such information is important in decision making and amygdala hyperactivation could relate to experiences of increased conflict or imbalances between approach and avoidance drives, such as observed in anxiety disorders.
Insula
Avoidance and approach processing
The insula is thought to play an important role in monitoring internal bodily states, predicting future internal states in response to environmental changes, and in seeking to maintain homeostasis.123,124 The insula has been shown to activate in response to both pleasant and unpleasant somatosensory or emotional stimuli,55,125-133 as well as during anticipation of future events.140,141 The insula has been identified as important in the experience of drug craving and urges,134,135 but also for learning the aversiveness-predicting properties of stimuli.136-139
Insula hyperactivation has been identified during symptom provocation, processing or anticipation of negative emotional stimuli, or in response to negative emotional faces in SAD,78,142,143 phobia,78,82,84,109,144 and PTSD.46,78,90,145-147 Studies have reported insula activation to correlate with symptom severity (SAD,94 PTSD145) and phobia treatment has been shown to decrease insula activation.98,99,148 However, there have also been several studies failing to identify insula hyperactivation in anxiety disorders (eg, SAD74,93,95; PTSD106,108106,108).
The few studies reporting activations for positive emotional stimuli have, for the most part, either not reported on insula activation or have reported no insula dysfunction in anxiety disorders (GAD,149 SAD94). Additionally, Straube et al143 examined individuals with SAD and healthy controls and reported that while amygdala hyperactivation was observed for both happy and angry faces, insula activation was enhanced only for angry faces. This suggests that insula dysfunction may be circumscribed to negative valence in anxiety disorders.
Decision making
As mentioned, the insula is thought to signal potential changes in interoceptive state, and we propose that during conflict or decision making, the insula may be involved in predicting such changes to potential decisional outcomes. Animal and human studies of insula lesions have reported alterations in approach-avoidance behavior during effort-based and risk-related decisionmaking tasks.150-153 Similarly, human neuroimaging studies and a recent meta-analysis implicate the anterior insula for paradigms involving risk and uncertainty.29,154,155 An individual's predictions regarding interoceptive or emotional responses undoubtedly relate to his or her beliefs - developed through past experience or instructional/observational learning. There is some evidence that the insula plays a role in integrating information concerning current bodily state with cognitive information to make change predictions.156 A recent neuromaging study utilized a paradigm similar to animal models of approach-avoidance conflict, which involved various levels of monetary reward associated with differing probabilities of shock. This study found that connectivity between insula and orbitofrontal cortex (OFC) was related to individual variability in decision making during trials involving both reward and punishment.157 It is possible that insula-OFC connectivity is important for integrating individuals' preconceived beliefs about rewarding and punishing stimuli with information provided during the task to determine behavioral responses. In summary, the insula is thought to play an integral role in monitoring and predicting interoceptive state, particularly in response to affective stimuli.124,158,159 Insula dys function has been identified in anxiety disorders - primarily during processing of negative emotional stimuli. The insula, particularly in its connections with the OFC, is proposed to also play a role in integrating beliefs with the current bodily state in order to make change predictions related to various choices.156,157 This could be one way in which the brain estimates risk and influences decision making.29 We propose that insula dysfunction in anxiety disorders could relate to imbalances in difference calculations regarding current and future interoceptive state, which could influence risk estimations and approach-avoidance decision making.
Striatum
Avoidance and approach processing
The epicenter of dopaminergic neurons, the ventral striatum (including the nucleus accumbens), has been identified as important for signaling rewarding or reinforcing properties of stimuli. This conclusion has been supported through animal research16,160 as well as human neuroimaging research investigating responses to pleasant imagery,161 auditory stimuli,162 faces,163,164 sexual arousal,165,166 and food stimuli,64,167 and during appetitive learning.168-170 A metaanalysis reported that approximately 70% of studies involving provocation of pleasant emotion showed activation of the basal ganglia, including the striatum.171
Animal research suggests that, while some neurons within the ventral striatum respond to both rewarding and aversive stimuli,172 NAcc neurons increase or decrease in activation to reward- and punishment-predicting stimuli, respectively173 (for review see ref 16). Potentially related to this, human neuroimaging studies have reported striatal activation in response to aversive or unpleasant stimuli.164, 174-176
Decision making
Animal research suggests that anatomical or pharmacologic manipulation of ventral striatal neurons influences approach-avoidance behavior during conflict,177-179 delayed-discounting,180,181 effort -based,182,183 and riskrelated decision-making models184 (see review in ref 114). The directionality of lesion effects would suggest that this region is involved in orienting an organism towards reward. Potentially in concert with these findings, human neuroimaging studies report NAcc activation to correlate with the amount of risk involved in decisions185,186 and to signal prediction errors between expected and actual reward value187 (for review see refs 188,114). The few neuroimaging studies investigating decision making in anxiety disorders provide initial evidence of striatal dysfunction. In PTSD, attenuated nucleus accumbens activation and difficulty learning the optimal response pattern during risk-related decision making has been reported.36 Attenuated striatal activation in response to reward was also reported for PTSD during a wheel-of-fortune type task189 and this striatal attenuation was related to level of “numbing” symptoms (eg, symptoms involving difficulty experiencing positive emotions or feeling distant from others). Although somewhat unrelated to decision making, an implicit memory task known to elicit striatal activation was used to identify striatal dysfunction in SAD190; while the same task failed to identify such dysfunction in phobia.191 In summary, this research provides evidence that the ventral striatum is involved in signaling the rewarding value of outcomes.16,114,171,188 There is initial evidence of striatal dysfunction in PTSD and SAD,36,189,190 as well as suggestions that striatal dysfunction may be related to PTSDspecific symptoms such as numbing. However, we propose it may also be important for approach valuations in other anxiety disorders and that an imbalance between striatal and amygdala/insula signals could relate to increased conflict and dysfunctional approach-avoidance behavior.
Prefrontal cortex
Researchers of different specialty fields use varying terminologies when referring to regions of the PFC. We will delineate the medial PFC as suggested by Amodio and Frith,192 focusing on specializations of orbitomedial frontal cortex (OFC; including ventromedial PFC [vmPFC] and ventral anterior cingulate [ACC]) as distinguished from dorsomedial (dmPFC; dorsal ACC) and lateral (lateral OFC [lOFC], dorsalateral PFC [dlPFC]) regions.
Avoidance and approach processing
Medial prefrontal and particularly OFC regions are thought to play a role in regulating or inhibiting limbic regions and behavioral responses during fear processing. Neuroimaging studies in nonclinical populations report OFC and dorsomedial PFC (specifically dorsal and rostral ACC) activation in response to emotional pictures55,133,171,193 and emotional faces55,194,195 and provide evidence these regions are important for fear learning.24,60,61,139,196,197 Animal and human studies provide some evidence of an inhibitory relationship between prefrontal regions (including OFC, dmPFC, and lateral PFC) and amygdala during fear extinction or emotional regulation.198-202
Human neuroimaging research supports implications from animal studies by showing the OFC to play a primary role in reward processing.50,203 This region (as well as dmPFC) has been shown to activate in response to rewarding and reward-predicting stimuli, such as money, appetizing food, pleasant smells or music, attractive facial stimuli, and sexual arousal.26,64,171,204,205
PFC dysfunction has been repeatedly implicated across anxiety disorders, though the direction of dysfunction differs depending upon the paradigm and the anxiety disorder being examined. In response to symptom provocation or negative emotional stimuli, OFC and dmPFC (and occasionally lOFC and dlPFC) hyperactivation has been identified for GAD,69,70,101 SAD,72,74,206 phobia,84,85,105,144,207 and panic.79,208,209 Directional effects within the PFC have been mixed for PTSD,47,78,89,96,210-214 though the majority of studies and meta-anlyses support hypoactivation of OFC and ventromedial regions.78
Experimental approaches involving instructed downregulation of negative emotion have identified attenuated activity within OFC, dlPFC, and dmPFC regions in anxiety disorders (SAD,201,215 PTSD216). These results have been taken as evidence that anxiety disorders are associated with decreased propensity to recruit PFC regions to regulate limbic activity and/or emotional responses. Additionally, SAD has been associated with an negative relationship between ventrolateral PFC and amygdala activation,102 and PTSD has been associated with an negative relationship between mPFC and amygdala activation96 during symptom provocation. Treatment of PTSD and phobia has been associated with increased dmPFC and/or OFC activations,100,217-219 though other studies report phobia treatment to result in decreased prefrontal activation.99,104,148
Neuroimaging studies using positive emotional stimuli have, for the most part, either not reported or failed to find evidence of prefrontal dysfunction in GAD70,101,149 and SAD.72,220 However, Campbell et al221 reported SAD to be associated with delayed dlPFC and dmPFC activation in response to happy faces compared with nonanxious controls. Additionally, panic disorder patients exhibited enhanced mid-ACC activation in response to happy faces222 - the opposite of the attenuated ACC activation reported for fearful faces.223
Decision making
Decision-making research suggests the OFC is important for integrating information concerning the value of various stimuli or choice characteristics in order to bias the system towards one decision versus another. Animal research suggests the OFC plays a role in approachavoidance conflict,224-226 delayed discounting,112,227,228 and risk-related decision making.114,228,229 In human research, both OFC and dlPFC regions have been implicated in comparing values of various choices188,230,231 and for ensuring successful decision making during the Iowa Gambling Task (dlPFC,232,233 vmPFC232,233). Neuroimaging research has shown OFC to activate proportionally to the subjective value of stimuli during decision making,31 and indicates it may be important for integrating sensory stimuli with cognitive information/beliefs to signal subjective value of stimuli.234-237 Studies also suggest dlPFCOFC connectivity may be involved in weighting various stimuli characteristics during decision making (eg, taste vs health characteristics of food238). The importance of the OFC in approach-avoidance conflict was also confirmed by Talmi et al,157 who reported reward-prediction to be associated with OFC activation and individual variability during trials involving both reward and punishment to relate to insula-OFC connectivity.
Researchers have attempted to tease apart specific roles of various PFC subregions in processing decision-making characteristics, such as risk or delay calculations versus effort or action-based calculations. Animal research suggests that the OFC plays more of a role in the former, while dorsal PFC regions play more of a role in the latter.16,31,114,239 This distinction, however, does not seem quite as clear in human neuroimaging research, as some studies support the OFC's role in calculating both the value of potential reward as well as the effort needed to obtain those rewards (eg, energy expense, receipt of shock31,157,187). Other studies support the dorsal PFC's role in risk-taking and delayed-discounting.29,31,33,240,241 Human neuroimaging research has partially supported the distinctions between ventral and dorsal PFC by providing evidence that, while OFC regions are important for calculating value of choices, dmPFC regions are involved in selecting actions during decision making and detecting errors in those actions.241
The few studies investigating neural substrates of decision making in anxiety disorders have implicated mPFC dysfunction. PTSD has been associated with attenuated mPFC activation during risk-related decision making.36 Self-reported intolerance of uncertainty in adolescents with GAD or SAD was associated with greater OFC (rostral/subgenual ACC) activation during uncertain, or risky, conditions of a decision-making task.121 Additionally, SAD patients exhibited attenuated dmPFC activation during a “trust” decision-making game involving risk when contrasting conditions in which the other “player” was human versus a computer.242 However, with this study, it is difficult to ascertain whether findings relate to risk processing or to the salience associated with supposed human interaction.
In summary, mPFC and OFC are considered important for processing both approach- and avoidance-related stimuli. These regions are thought to play an important role in negotiating and reconciling signals from other brain regions (eg, limbic, striatal, dlPFC) in order to calculate net values of stimuli and choices during decision making.31 Medial PFC and OFC regions also play a role in regulating limbic and behavioral responses, particularly in the case of fear-provoking stimuli.202 Anxiety disorders have exhibited OFC, dmPFC, and lateral PFC dysfunction during processing of negative emotional stimuli,47,78 instructed emotion regulation (eg, refs 215,216), and decision-making processes.36,121,242 We propose that OFC and mPFC dysfunction in anxiety disorders could be associated with difficulties in integrating signals from other brain regions concerning the various characteristics of a decision-making situation. Dysfunction of mPFC, striatal, and/or limbic regions could each have unique influences on approachavoidance and conflict processes. Below, we present specific hypotheses to be tested by future anxiety research.
Summary: neural circuitry of avoidance, approach, and decision making
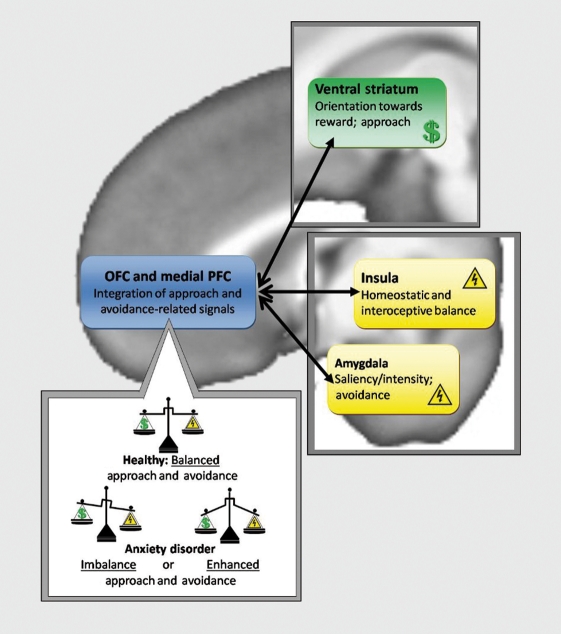
This review highlights the primary roles of amygdala, ventral striatum, insula, and prefrontal regions (OFC, dmPFC) in approach, avoidance, and decision-making processes (see (Figure 1) for pictoral representation of the proposed model). These neural substrates aid in computations of approach and avoidance valuations in decision-making situations. The valuation itself is a dynamic process, and is related to current and predicted internal state. For example, if a stimulus predicts an outcome that challenges the integrity of the individual, eg, a drop in body temperature or shock applied to the skin, that option is evaluated as to be avoided. However, if the same option also results in reception of reward, the option has both avoidance and approach value. Thus, the individual needs to arbitrate between potential aversive and rewarding outcomes when faced with such a decision. We propose that approach-avoidance valuation may be dysfunctional for individuals with anxiety disorders. The precise type of approach-avoidance dysfunction awaits further experimental testing. Among the proposed hypotheses are: (i) over-representation of avoidance valuation; (ii) under- or over-representation of approach valuation; and (iii) insufficiency in integrating and arbitrating approach- and avoidance-related valuations.
Figure 1. This figure summarizes the neural systems proposed to be integral for approach and avoidance processing as well as decision making, including the amygdala, insula, ventral striatum, and medial prefrontal (mPFC) and orbitofrontal cortex (OFC). Also represented are the proposed imbalance or enhancement of approach-avoidance signals that may underlie anxiety disorders.
Over-representation of avoidance valuations would presumably relate to dysfunction within amygdala and/or insular regions. Enhanced signals regarding salience of stimuli and expected emotional and interoceptive state changes could serve to overpower the system and orbitofrontal regions attempting to integrate these with other signals. This could, in essence, create a “signal-tonoise” problem243 in which other important information is under-represented. We suspect that amygdala/insula dysfunction is likely to play a role in any observed conflict or decision-making dysfunction in anxiety disorders - as there is already plenty of evidence to implicate these regions in avoidance-related processing in anxiety79 However, one hypothesis to be tested is whether such limbic overactivation is the primary issue interrupting the dynamic approach-avoidance balance. If so, one may expect that aberrations in decision making would only be observed when the salience/magnitude of outcomes reaches a level in which there is significant recruitment of limbic regions or when the paradigm involves a component of risk for which the insula has been strongly implicated.29
Dysfunctional representation of approach valuation would most likely relate to ventral striatal dysfunction. Although only a few studies have reported striatal dysfunction in anxiety disorders,36,189,190 there have also been very few studies attempting to examine neural dysfunction of anxiety during reward or decision-making processes. The hypothesis that striatal activation is involved in conflict decision making in anxiety populations is therefore one worthy of testing in its own right. If there is dysfunction in ventral striatal regions there remains the possibility of either attenuation or exaggeration of activation, and we propose that these two findings could relate to different symptom presentations or disorder subtypes. Attenuated activation could result in under-representation of approach valuation and relate to symptoms of numbness or depression often experienced as comorbid with some anxiety disorders.244-246 At the experimental level, such striatal attenuation could result in decreased motivaton during effort-based decision-making tasks. Enhanced striatal activation could relate to over-representation of approach valuation. Concurrent enhanced representation of both approach and avoidance valuations could result in these signals “battling it out” against one another in order to influence the OFC and subsequent decision making. This in turn could relate to increased experiences of conflict and anxiety within decision-making contexts. Such dysfunction could theoretically relate to intolerance of uncertainty, difficulty making decisions, and tendencies towards perfectionism, which are symptoms often associated with anxiety disorders.247-249 At the experimental level, concurrently enhanced approach and avoidance valuation would theoretically result in increased reaction time and indecisiveness during decision-making paradigms - particularly those involving both reward and punishment, such as risk-based paradigms or approachavoidance conflict.
Dysfunction in the integration and arbitration of approach and avoidance valuations would likely relate to OFC and/or mPFC dysfunction. The OFC is the prefrontal region most implicated in integrating information concerning various stimuli and outcome characteristics.31 Rolls and Grabenhorst49 have suggested that reinforcers must have “approximately equal potency at their maximal value to ensure that different rewards are chosen sometimes, and that behavior is not always directed towards a few superpotent specific rewards.” We propose that the OFC may be responsible for scaling signals from various brain regions in order to enable comparisons to be made between them. By doing so, the OFC can then produce a signal that accurately reflects the net value of each potential outcome, biasing the system accordingly towards one behavior or another, and ensuring that responses represent a balance between approach- and avoidance-motivated signals. Attenuated OFC activation or a weakening in the correlation between OFC and limbic/striatal activation in anxiety disorders would suggest this is a primary site of approach-avoidance dysfunction, whereas enhanced OFC activation would most likely represent attempts to compensate for dysfunction in other regions within the proposed cortico-striatal-limbic system.
The hypotheses we set forth concerning OFC and mPFC, amygdala, insula, or striatal dysfunction in approach-avoidance processes in anxiety disorders can be examined on three different levels. First, specific behavioral experiments using decision-making paradigms can be used to disentangle effects of approach and avoidance from that of inefficient arbitration. For example, dysfunctions of approach-avoidance conflict may be examined during risk-related decision-making paradigms - particularly those modified to include affective-related outcomes (such as that used by Talmi et al157). Concurrent examination regarding the influence of effort and delay characteristics could be used to more fully delineate decision-making behavior. Second, functional neuroimaging can be used to determine whether the proposed segregation between approach and avoidance neural substrates and their relative dysfunction can be supported experimentally. In particular, neuroimaging research could utilize the framework of approach-avoidance conflict and decision making to more specifically delineate the role such dysfunction plays in determining the behavioral responses that are an integral part of anxiety disorders. Third, computational models developed within the decision-making literature31,250 can be used to examine more formally the internal representation of approach and avoidance and its impact on computing behavioral alternatives. In closing, we recognize that much knowledge has been gained over the past 15 to 20 years of neuroimaging research concerning frontolimbic dysfunction underlying fear and anxiety processing in anxiety disorders. However, more needs to be done to integrate these findings with that of animal studies, and to link more directly with anxiety disorder symptoms, behaviors, and treatment effects.
Selected abbreviations and acronyms
- ACC
anterior cingulate cortex
- GAD
generalized anxiety disorder
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- SAD
social anxiety disorder
Contributor Information
Aupperle Robin L., Department of Psychiatry, University of California, San Diego (UCSD), California, USA.
P. Paulus Martin, Psychiatry Service, Veterans Affairs San Diego Health Care System, San Diego, California, USA.
REFERENCES
- 1.Gray JA. Brain systems that mediate both emotion and cognition. Cogn Emot. 1990;4:269–288. [Google Scholar]
- 2.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 3.Carver CS., White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 4.Balleine BW., Delgado MR., Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freud SA. A General Introduction to Psychoanalysis. New York, NY: Boni and Liveright; 1920 [Google Scholar]
- 6.Pavlov PI. Conditioned reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex. London, UK: Oxford University Press; 1927 doi: 10.5214/ans.0972-7531.1017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JS. Principles of intrapersonal conflict. J Conflict Resolut. 1957;1:135–154. [Google Scholar]
- 8.French TM. Interrelations between psychoanalysis and the experimental work of Pavlov. Am J Psychiatry. 1933;89:1165–1203. [Google Scholar]
- 9.Millan MJ., Brocco M. The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- 10.Rinck M., Becker ES. Approach and avoidance in fear of spiders. J Behav Ther Exp Psychiatry. 2007;38:105–120. doi: 10.1016/j.jbtep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Vogel JR., Beer B., Clody DE. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 12.Geller I., Seifter J. The effects of meprobamate, barbiturates, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacologia. 1960;1:482–492. [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 14.Foa EB., Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 15.Barlow DH. Anxiety and its Disorders: the Nature and Treatment of Anxiety and Panic. New York, NY: Guilford Press; 2002 [Google Scholar]
- 16.Schultz W. Behavioral theories and the neurophysiology of reward. Ann Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 17.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 18.Mueller D., Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 20.Melcher T., Falkai P., Gruber O. Functional brain abnormalities in psychiatric disorders: Neural mechanisms to detect and resolve cognitive conflict and interference. Brain Res Rev. 2008;59:96–124. doi: 10.1016/j.brainresrev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Smith M., Duda J., Allen J., Hall H. Contemporary measures of approach and avoidance goal orientations: similarities and differences. Br J Educ Psychol. 2002;72(Pt 2):155–190. doi: 10.1348/000709902158838. [DOI] [PubMed] [Google Scholar]
- 22.Stein DJ., Fineberg NA., Bienvenu OJ., et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 23.Delgado MR., Nearing KI., LeDoux JE., Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelps EA., Delgado MR., Nearing KI., LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 25.O'Doherty J., Rolls ET., Francis S., Bowtell R., McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 26.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Gottfried JA., O'Doherty J., Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vorhold V. The neuronal substrate of risky choice: an insight into the contributions of neuroimaging to the understanding of theories on decision making under risk. Ann N Y Acad Sci. 2008;1128:41–52. doi: 10.1196/annals.1399.006. [DOI] [PubMed] [Google Scholar]
- 29.Mohr PN., Biele G., Heekeren HR. Neural processing of risk. J Neurosci. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rangel A., Camerer C., Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangel A., Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Wittmann M., Leland DS., Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka SC., Doya K., Okada G., Ueda K., Okamoto Y., Yamawaki S. Prediction of immediate and future rewards differentially recruits corticobasal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 34.Hopper JW., Pitman RK., Su Z., et al. Probing reward function in posttraumatic stress disorder: Expectancy and satisfaction with monetary gains and losses. J Psychiatr Res. 2008;42:802–807. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elman I., Ariely D., Mazar N., et al. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Res. 2005;135:179–183. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Sailer U., Robinson S., Fischmeister FP., et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Werner NS., Duschek S., Schandry R. Relationships between affective states and decision-making. Int J Psychophysiol. 2009;74:259–265. doi: 10.1016/j.ijpsycho.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Miu AC., Heilman RM., Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biol Psychol. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Mueller EM., Nguyen J., Ray WJ., Borkovec TD. Future-oriented decisionmaking in Generalized Anxiety Disorder is evident across different versions of the Iowa Gambling Task. J Behav Ther Exp Psychiatry. 2010;41:165–171. doi: 10.1016/j.jbtep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.DeVido J., Jones M., Geraci M., et al. Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP). Psychol Med. 2009;39:1153–1161. doi: 10.1017/S003329170800487X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rounds JS., Beck JG., Grant DM. Is the delay discounting paradigm useful in understanding social anxiety? Behav Res Ther. 2007;45:729–735. doi: 10.1016/j.brat.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Cavedini P., Riboldi G., D'Annucci A., Belotti P., Cisima M., Bellodi L. Decision-making heterogeneity in obsessive-compulsive disorder: ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia. 2002;40:205–211. doi: 10.1016/s0028-3932(01)00077-x. [DOI] [PubMed] [Google Scholar]
- 43.Ludewig S., Paulus MP., Ludewig K., Vollenweider FX. Decision-making strategies by panic disorder subjects are more sensitive to errors. J Affect Disord. 2003;76:183–189. doi: 10.1016/s0165-0327(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 44.Myers KM., Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 45.Quirk GJ., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin LM., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haber SN., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 49.Rolls ET., Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progr Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNally GP., Westbrook RF. Predicting danger: the nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis M., Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 53.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 54.Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- 55.Britton JC., Taylor SF., Sudheimer KD., Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Hariri AR., Tessitore A., Mattay VS., Fera F., Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 57.Paradiso S., Johnson DL., Andreasen NC., et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- 58.Buchel C., Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 59.Knight DC., Nguyen HT., Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez RP., Biggs A., Chen G., Pine DS., Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsson A., Nearing KI., Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phelps EA., O'Connor KJ., Gatenby JC., Gore JC., Grillon C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 63.Hamann S., Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- 64.O'Doherty JP., Deichmann R., Critchley HD., Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 65.Garavan H., Pendergrass JC., Ross TJ., Stein EA., Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- 66.Liberzon I., Phan KL., Decker LR., Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- 67.Fitzgerald DA., Angstadt M., Jelsone LM., Nathan PJ., Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Baxter MG., Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 69.McClure EB., Monk CS., Nelson EE., et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 70.Monk CS., Telzer EH., Mogg K., et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nitschke JB., Sarinopoulos I., Oathes DJ., et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blair K., Geraci M., DeVido J., et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry. 2008;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans KC., Wright CI., Wedig MM., Gold AL., Pollack MH., Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 74.Phan KL., Fitzgerald DA., Nathan PJ., Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Tillfors M., Furmark T., Marteinsdottir I., Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52:1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- 76.Guyer AE., Lau JY., Clure-Tone EB., et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schneider F., Weiss U., Kessler C., et al. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 1999;45:863–871. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- 78.Etkin A., Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Heuvel OA., Veltman DJ., Groenewegen HJ., et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 80.Maddock RJ., Garrett AS., Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfleiderer B., Zinkirciran S., Arolt V., Heindel W., Deckert J., Domschke K. fMRI amygdala activation during a spontaneous panic attack in a patient with panic disorder. World J Biol Psychiatry. 2007;8:269–272. doi: 10.1080/15622970701216673. [DOI] [PubMed] [Google Scholar]
- 82.Wendt J., Lotze M., Weike AI., Hosten N., Hamm AO. Brain activation and defensive response mobilization during sustained exposure to phobiarelated and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 83.Larson CL., Schaefer HS., Siegle GJ., Jackson CA., Anderle MJ., Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fearrelevant stimuli. Biol Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 84.Dilger S., Straube T., Mentzel HJ., et al. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neurosci Lett. 2003;348:29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- 85.Schienle A., Schafer A., Walter B., Stark R., Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 86.Straube T., Mentzel HJ., Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 87.Armony JL., Corbo V., Clement MH., Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 88.Protopopescu X., Pan H., Tuescher O., et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 89.Shin LM., Wright CI., Cannistraro PA., et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 90.Vermetten E., Schmahl C., Southwick SM., Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- 91.Bremner JD., Vermetten E., Schmahl C., et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milad MR., Pitman RK., Ellis CB., et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blair K., Shaywitz J., Smith BW., et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah SG., Klumpp H., Angstadt M., Nathan PJ., Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 95.Tillfors M., Furmark T., Marteinsdottir I., et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158: 1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 96.Shin LM., Orr SP., Carson MA., et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 97.Furmark T., Tillfors M., Marteinsdottir I., et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 98.Goossens L., Sunaert S., Peeters R., Griez EJ., Schruers KR. Amygdala hyperfunction in phobic fear normalizes after exposure. Biol Psychiatry. 2007;62:1119–1125. doi: 10.1016/j.biopsych.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 99.Schienle A., Schafer A., Hermann A., Rohrmann S., Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia: an fMRI study on exposure therapy. Eur Arch Psychiatry Clin Neurosci. 2007;257:486–493. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- 100.Felmingham K., Kemp A., Williams L., et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 101.Monk CS., Nelson EE., McClure EB., et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 102.Kilts CD., Kelsey JE., Knight B., et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 103.Hermann A., Schafer A., Walter B., Stark R., Vaitl D., Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biol Psychol. 2007;75:124–130. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Paquette V., Levesque J., Mensour B., et al. "Change the mind and you change the brain": effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage. 2003;18:401–409. doi: 10.1016/s1053-8119(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 105.Straube T., Mentzel HJ., Glauer M., Miltner WH. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004;372:204–208. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 106.Bremner JD., Narayan M., Staib LH., Southwick SM., McGlashan T., Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lanius RA., Frewen PA., Girotti M., Neufeld RW., Stevens TK., Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Phan KL., Britton JC., Taylor SF., Fig LM., Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 109.Wright CI., Martis B., McMullin K., Shin LM., Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- 110.Yoon KL., Fitzgerald DA., Angstadt M., McCarron RA., Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 111.Kopchia KL., Altman HJ., Commissaris RL. Effects of lesions of the central nucleus of the amygdala on anxiety-like behaviors in the rat. Pharmacol Biochem Behav. 1992;43:453–461. doi: 10.1016/0091-3057(92)90176-g. [DOI] [PubMed] [Google Scholar]
- 112.Winstanley CA., Theobald DEH., Cardinal RN., Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moller C., Wiklund L., Sommer W., Thorsell A., Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 114.Floresco SB., St Onge., Jr, Ghods-Sharifi S., Winstanley CA. Cortico-limbicstriatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- 115.Brand M., Grabenhorst F., Starcke K., Vandekerckhove MM., Markowitsch HJ. Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia. 2007;45:1305–1317. doi: 10.1016/j.neuropsychologia.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 116.Bechara A., Damasio H., Damasio AR., Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsu M., Bhatt M., Adolphs R., Tranel D., Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 118.Vorhold V., Giessing C., Wiedemann PM., Schntz H., Gauggel S., Fink GR. The neural basis of risk ratings: evidence from a functional magnetic resonance imaging (fMRI) study. Neuropsychologia. 2007;45:3242–3250. doi: 10.1016/j.neuropsychologia.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 119.Cohen MX., Ranganath C. Behavioral and neural predictors of upcoming decisions. Cogn Affect Behav Neurosci. 2005;5:117–126. doi: 10.3758/cabn.5.2.117. [DOI] [PubMed] [Google Scholar]
- 120.Peters J., Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010; 66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 121.Krain AL., Gotimer K., Hefton S., et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 122.McClure SM., York MK., Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 123.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 124.Critchley HD., Wiens S., Rotshtein P., Ohman A., Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 125.Singer T., Critchley HD., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 126.Damasio AR., Grabowski TJ., Bechara A., et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 127.Nielen MM., Heslenfeld DJ., Heinen K., et al. Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain Cogn. 2009;71:387–396. doi: 10.1016/j.bandc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 128.Anders S., Lotze M., Erb M., Grodd W., Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp. 2004;23:200. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hua QP., Zeng XZ., Liu JY., Wang JY., Guo JY., Luo F. Dynamic changes in brain activations and functional connectivity during affectively different tactile stimuli. Cell Mol Neurobiol. 2008;28:57–70. doi: 10.1007/s10571-007-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ochsner KN., Zaki J., Hanelin J., et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brooks JC., Zambreanu L., Godinez A., Craig AD., Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 132.Critchley HD., Rotshtein P., Nagai Y., O'Doherty J., Mathias CJ., Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 133.Lane RD., Reiman EM., Ahern GL., Schwartz GE., Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 134.Paulus MP. Neural basis of reward and craving--a homeostatic point of view. Dialogues Clin Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naqvi NH., Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dunsmoor JE., Bandettini PA., Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- 137.Marschner A., Kalisch R., Vervliet B., Vansteenwegen D., Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Critchley HD., Mathias CJ., Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 139.Buchel C., Morris J., Dolan RJ., Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 140.Lovero KL., Simmons AN., Aron JL., Paulus MP. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simmons A., Matthews SC., Stein MB., Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- 142.Gentili C., Gobbini MI., Ricciardi E., et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull. 2008;77:286–292. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 143.Straube T., Kolassa IT., Glauer M., Mentzel HJ., Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004D;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 144.Straube T., Mentzel HJ., Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 145.Hopper JW., Frewen PA., van der Kolk BA., Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 146.Simmons AN., Paulus MP., Thorp SR., Matthews SC., Norman SB., Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lindauer RJ., Booij J., Habraken JB., et al. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with posttraumatic stress disorder: a randomized clinical trial. Psychol Med. 2008;38:543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- 148.Straube T., Glauer M., Dilger S., Mentzel HJ., Miltner WH. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Schienle A., Schafer A., Pignanelli R., Vaitl D. Worry tendencies predict brain activation during aversive imagery. Neurosci Lett. 2009;461:289–292. doi: 10.1016/j.neulet.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 150.Forget B., Pushparaj A., Le FB. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010. In press doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 151.Clark L., Bechara A., Damasio H., Aitken MR., Sahakian BJ., Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.eller JA., Levin IP., Shiv B., Bechara A. The effects of insula damage on decision-making for risky gains and losses. Social Neuroscience. 2009;4:347–358. doi: 10.1080/17470910902934400. [DOI] [PubMed] [Google Scholar]
- 153.Jones CL., Ward J., Critchley HD. The neuropsychological impact of insular cortex lesions. J Neurol Neurosurg Psychiatry. 2010;81:611–618. doi: 10.1136/jnnp.2009.193672. [DOI] [PubMed] [Google Scholar]
- 154.Preuschoff K., Quartz SR., Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.arinopoulos I., Grupe DW., Mackiewicz KL., et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gray MA., Harrison NA., Wiens S., Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One. 2007;2:e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Talmi D., Dayan P., Kiebel SJ., Frith CD., Dolan RJ. How humans integrate the prospects of pain and reward during choice. J Neurosci. 2009;29:14617–14626. doi: 10.1523/JNEUROSCI.2026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paulus MP., Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 159.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 160.Wickens JR., Budd CS., Hyland BI., Arbuthnott GW. Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix. Ann N Y Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- 161.Costa VD., Lang PJ., Sabatinelli D., Versace F., Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain's reward circuitry. Hum Brain Mapp. 2010;31:1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Menon V., Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 163.Chakrabarti B., Kent L., Suckling J., Bullmore E., Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci. 2006;23:1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- 164.Liang X., Zebrowitz LA., Zhang Y. Neural activation in the GÇreward circuitGÇ shows a nonlinear response to facial attractiveness. Soc Neurosci. 2010;5:320–334. doi: 10.1080/17470911003619916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karama S., Lecours AR., Leroux JM., et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sabatinelli D., Bradley MM., Lang PJ., Costa VD., Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- 167.Berns GS., McClure SM., Pagnoni G., Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Valentin VV., O'Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. J Neurophysiol. 2009;102:3384–3391. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- 169.Bray S., Shimojo S., O'Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci. 2007;27:7498–7507. doi: 10.1523/JNEUROSCI.2118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McClure SM., Berns GS., Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 171.Phan KL., Wager TD., Taylor SF., Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 172.Ravel S., Legallet E., Apicella P. Tonically active neurons in the monkey striatum do not preferentially respond to appetitive stimuli. Exp Brain Res. 1999;128:531–534. doi: 10.1007/s002210050876. [DOI] [PubMed] [Google Scholar]
- 173.Roitman MF., Wheeler RA., Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 174.Delgado MR., Locke HM., Stenger VA., Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 175.Jensen J., Smith AJ., Willeit M., et al. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jensen J., McIntosh AR., Crawley AP., Mikulis DJ., Remington G., Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 177.Beaufour CC., Le BC., Hamon M., Thiebot MH. Extracellular serotonin is enhanced in the striatum, but not in the dorsal hippocampus or prefrontal cortex, in rats subjected to an operant conflict procedure. Behav Neurosci. 2001;115:125–137. doi: 10.1037/0735-7044.115.1.125. [DOI] [PubMed] [Google Scholar]
- 178.lvarez EO., Ruarte MB. Role of glutamate receptors in the nucleus accumbens on behavioural responses to novel conflictive and non-conflictive environments in the rat. Behav Brain Res. 2001;123:143–153. doi: 10.1016/s0166-4328(01)00190-5. [DOI] [PubMed] [Google Scholar]
- 179.Johansson AK., Hansen S. Increased alcohol intake and behavioral disinhibition in rats with ventral striatal neuron loss. Physiol Behav. 2000;70:453–463. doi: 10.1016/s0031-9384(00)00284-5. [DOI] [PubMed] [Google Scholar]
- 180.Cardinal RN., Pennicott DR., Sugathapala CL., Robbins TW., Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 181.Acheson A., Farrar AM., Patak M., et al. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behav Brain Res. 2006;173:217–228. doi: 10.1016/j.bbr.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cousins MS., Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 183.Salamone JD., Cousins MS., McCullough LD., Carriero DL., Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 184.Cardinal RN., Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tom SM., Fox CR., Trepel C., Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 186.Kuhnen CM., Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 187.Hare TA., O'Doherty J., Camerer CF., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rushworth MF., Mars RB., Summerfield C. General mechanisms for making decisions? Curr Opin Neurobiol. 2009;19:75–83. doi: 10.1016/j.conb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 189.Elman I., Lowen S., Frederick BB., Chi W., Becerra L., Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sareen J., Campbell DW., Leslie WD., et al. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biol Psychiatry. 2007;61:396–404. doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 191.Martis B., Wright CI., McMullin KG., Shin LM., Rauch SL. Functional magnetic resonance imaging evidence for a lack of striatal dysfunction during implicit sequence learning in individuals with animal phobia. Am J Psychiatry. 2004;161:67–71. doi: 10.1176/appi.ajp.161.1.67. [DOI] [PubMed] [Google Scholar]
- 192.Amodio DM., Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 193.Phan KL., Taylor SF., Welsh RC., et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry. 2003;53:211–215. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- 194.Gorno-Tempini ML., Pradelli S., Serafini M., et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 195.Chen AC., Welsh RC., Liberzon I., Taylor SF. 'Do I like this person?' A network analysis of midline cortex during a social preference task. NeuroImage. 2010;51:930–939. doi: 10.1016/j.neuroimage.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gottfried JA., Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 197.Milad MR., Wright CI., Orr SP., Pitman RK., Quirk GJ., Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 198.Quirk GJ., Likhtik E., Pelletier JG., Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Quirk GJ., Garcia R., Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 200.Ohira H., Nomura M., Ichikawa N., et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 201.Goldin PR., McRae K., Ramel W., Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Quirk GJ., Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 203.O'Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- 204.McLean J., Brennan D., Wyper D., Condon B., Hadley D., Cavanagh J. Localisation of regions of intense pleasure response evoked by soccer goals. Psychiatry Res: Neuroimaging. 2009;171:33–43. doi: 10.1016/j.pscychresns.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 205.Phan KL., Wager T., Taylor SF., Liberzon I. Functional Neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 206.Amir N., Klumpp H., Elias J., Bedwell JS., Yanasak N., Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 207.Goossens L., Schruers K., Peeters R., Griez E., Sunaert S. Visual presentation of phobic stimuli: amygdala activation via an extrageniculostriate pathway? Psychiatry Res. 2007;155:113–120. doi: 10.1016/j.pscychresns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 208.Maddock RJ., Buonocore MH., Kile SJ., Garrett AS. Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport. 2003;14:325–328. doi: 10.1097/00001756-200303030-00006. [DOI] [PubMed] [Google Scholar]
- 209.Bystritsky A., Pontillo D., Powers M., Sabb FW., Craske MG., Bookheimer SY. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12:3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- 210.Bremner JD., Staib LH., Kaloupek D., Southwick SM., Soufer R., Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bremner JD., Vythilingam M., Vermetten E., et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 212.Britton JC., Phan KL., Taylor SF., Fig LM., Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 213.Lanius RA., Williamson PC., Densmore M., et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 214.Lindauer RJ., Booij J., Habraken JB., et al. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 215.Goldin PR., Manber-Ball T., Werner K., Heimberg R., Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.New AS., Fan J., Murrough JW., et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 217.Peres JF., Newberg AB., Mercante JP., et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37:1481–1491. doi: 10.1017/S003329170700997X. [DOI] [PubMed] [Google Scholar]
- 218.Seedat S., Warwick J., van HB., et al. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J Affect Disord. 2004;80:45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 219.Schienle A., Schafer A., Stark R., Vaitl D. Long-term effects of cognitive behavior therapy on brain activation in spider phobia. Psychiatry Res. 2009;172:99–102. doi: 10.1016/j.pscychresns.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 220.Stein MB., Goldin PR., Sareen J., Zorrilla LT., Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 221.Campbell DW., Sareen J., Paulus MP., Goldin PR., Stein MB., Reiss JP. Timevarying amygdala response to emotional faces in generalized social phobia. Biol Psychiatry . 2007;62:455–463. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 222.Pillay SS., Rogowska J., Gruber SA., Simpson N., Yurgelun-Todd DA. Recognition of happy facial affect in panic disorder: an fMRI study. J Anxiety Disord. 2007;21:381–393. doi: 10.1016/j.janxdis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 223.Pillay SS., Gruber SA., Rogowska J., Simpson N., Yurgelun-Todd DA. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrusamygdala connection. J Affect Disord. 2006;94:173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 224.Resstel LBM., Souza RF., Guimarpes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav. 2008;93:200–205. doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 225.Lacroix L., Spinelli S., Heidbreder CA., Feldon J. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci. 2000;114:1119–1130. doi: 10.1037//0735-7044.114.6.1119. [DOI] [PubMed] [Google Scholar]
- 226.Shah AA., Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- 227.Rudebeck PH., Walton ME., Smyth AN., Bannerman DM., Rushworth MFS. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 228.Mobini S., Body S., Ho MY., et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl). 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 229.Pais-Vieira M., Lima D., Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience. 2007;145:225–231. doi: 10.1016/j.neuroscience.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 230.Wunderlich K., Rangel A., O'Doherty JP. Neural computations underlying action-based decision making in the human brain. Proc Natl Acad Sci U S A. 2009;106:17199–17204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kahnt T., Heinzle J., Park SQ., Haynes JD. Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. NeuroImage. 2010In press doi: 10.1016/j.neuroimage.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 232.Northoff G., Grimm S., Boeker H., et al. Affective judgment and beneficial decision making: ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp. 2006;27:572–587. doi: 10.1002/hbm.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fellows LK., Farah MJ. Different underlying impairments in decisionmaking following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 234.de A., I, Rolls ET., Velazco MI., Margot C., Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 235.Grabenhorst F., Rolls ET., Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 236.Plassmann H., O'Doherty J., Shiv B., Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci U S A. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McCabe C., Rolls ET., Bilderbeck A., McGlone F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci. 2008;3:97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hare TA., Camerer CF., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 239.Rudebeck PH., Behrens TE., Kennerley SW., et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Knoch D., Gianotti LR., Pascual-Leone A., et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fellows LK. The cognitive neuroscience of human decision making: a review and conceptual framework. Behav Cogn Neurosci Rev. 2004;3:159–172. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- 242.Sripada CS., Angstadt M., Banks S., Nathan PJ., Liberzon I., Phan KL. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport. 2009;20:984–989. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Savage CR. The role of emotion in strategic behavior: insights from psychopathology. In: Barrett LF, Salovey P, Mayer J, eds. The Wisdom in Feeling: Psychological Processes in Emotional Intelligence. New York, NY: Guilford Press; 2002:211–236. [Google Scholar]
- 244.Kashdan TB., Elhai JD., Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behav Res Ther. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 245.Feeny NC., Zoellner LA., Fitzgibbons LA., Foa EB. Exploring the roles of emotional numbing, depression, and dissociation in PTSD. J Trauma Stress. 2000;13:489–498. doi: 10.1023/A:1007789409330. [DOI] [PubMed] [Google Scholar]
- 246.Kaufman J., Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;(suppl 12):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 247.Saboonchi F., Lundh LG., Ost LG. Perfectionism and self-consciousness in social phobia and panic disorder with agoraphobia. Behav Res Ther. 1999;37:799–808. doi: 10.1016/s0005-7967(98)00183-1. [DOI] [PubMed] [Google Scholar]
- 248.Antony MM., Purdon CL., Huta V., Richard PS. Dimensions of perfectionism across the anxiety disorders. Behav Res Ther. 1998;36:1143–1154. doi: 10.1016/s0005-7967(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 249.Boelen PA., Reijntjes A. Intolerance of uncertainty and social anxiety. J Anxiety Disord. 2009;23:130–135. doi: 10.1016/j.janxdis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 250.O'Doherty JP., Hampton A., Kim H. Model-based fMRI and its application to reward learning and decision making. Ann N Y Acad Sci. 2007;1104:35–53. doi: 10.1196/annals.1390.022. [DOI] [PubMed] [Google Scholar]