Abstract
Intelligence can be defined as a general mental ability for reasoning, problem solving, and learning. Because of its general nature, intelligence integrates cognitive functions such as perception, attention, memory, language, or planning. On the basis of this definition, intelligence can be reliably measured by standardized tests with obtained scores predicting several broad social outcomes such as educational achievement, job performance, health, and longevity. A detailed understanding of the brain mechanisms underlying this general mental ability could provide significant individual and societal benefits. Structural and functional neuroimaging studies have generally supported a frontoparietal network relevant for intelligence. This same network has also been found to underlie cognitive functions related to perception, short-term memory storage, and language. The distributed nature of this network and its involvement in a wide range of cognitive functions fits well with the integrative nature of intelligence. A new key phase of research is beginning to investigate how functional networks relate to structural networks, with emphasis on how distributed brain areas communicate with each other.
Keywords: intelligence, brain, gray matter, white matter, network, cognition
Abstract
La inteligencia se puede definir como una capacidad mental general para razonar, resolver problemas y aprender. Dada su naturaleza general, la inteligencia integra funciones cognitivas como perceptión, atención, memoria, lenguaje o planificatión. De acuerdo con esta definitión la inteligencia se puede medir confiablemente mediante pruebas estandarizadas en que los puntajes obtenidos predicen algunas repercusiones sociales generales como éxito educacional, rendimiento laboral, salud y longevidad. Una comprensión detallada de los mecanismos cerebrales a la base de esta capacidad mental general podría entregar significativos beneficios individuales y sociales. Los estudios de neuroimágenes estructurales y funcionales en general le han dado soporte a una red frontoparietal como relevante para la inteligencia. Esta misma red se ha encontrado a la base de las funciones cognitivas relacionadas con la perceptión, el almacenamiento de la memoria de corto plazo y el lenguaje. La forma en que se distribuye esta red y su participatión en una amplia gama de funciones cognitivas se ajusta bien con la característica integradora de la inteligencia. Se está iniciando una nueva fase clave de la investigatión para estudiar cómo se relacionan las redes funcionales con las redes estructurales, con un énfasis en cómo las áreas cerebrales dispersas se comunican unas con otras.
Abstract
L'intelligence peut se définir comme une capacité mentale générale de raisonnement, de résolution de problèmes et d'apprentissage. Sa nature généraliste lui permet d'intégrer des fonctions cognitives comme la perception, l'attention, la mémoire, le langage ou l'organisation. Selon cette définition, l'intelligence peut être mesurée de façon fiable par des tests standardisés dont les scores prédisent plusieurs données sociales importantes comme le niveau d'éducation, la performance professionnelle, la santé et la longévité. Une compréhension précise des mécanismes cérébraux sous-tendant cette aptitude mentale générale pourrait bénéficier de façon significative à l'individu et à la société. Des études de neuro-imagerie structurale et fonctionnelle sont dans l'ensemble en faveur d'un réseau frontopariétal pour l'intelligence. Ce même réseau est également à la base des fonctions cognitives liées à la perception, à la mémorisation à court terme et au langage. La nature multifocale de ce réseau et son implication dans de nombreuses fonctions cognitives cadre bien avec la démarche d'ensemble de l'intelligence. Une nouvelle phase clé de la recherche commence à s'intéresser aux rapports entre les réseaux fonctionnels et les réseaux structuraux, en insistant sur la façon dont les différentes aires cérébrales communiquent entre elles.
Human intelligence: definition, measurement, and structure
Reasoning, problem solving, and learning are crucial facets of human intelligence. People can reason about virtually any issue, and many problems may be solved. Simple and highly complex behavioral repertoires can be learned throughout the lifespan. Importantly, there are widespread individual differences in the ability to reason, solve problems, and learn which lead to human differences in the general ability to cope with challenging situations. These differences: (i) become more salient as the cognitive complexity of the situation becomes greater1-3; (ii) are stable over time4; and (iii) are partially mediated by genetic factors.5
Various definitions of intelligence tend to converge around similar notions designed to capture the essence of this psychological factor. Jensen6 notes Carl Bereiter's definition of intelligence: “what you use when you don't know what to do” (p 111). After their extensive survey, Snyderman and Rothman7 underscored reasoning, problem solving, and learning as crucial for intelligence. The “mainstream science on intelligence” report coordinated by Gottfredson8 highlights reasoning, planning, solving problems, thinking abstractly, comprehending complex ideas, learning quickly, and learning from experience. The American Psychological Association (APA) report on intelligence acknowledges that “individuals differ from one another in their ability to understand complex ideas, to adapt effectively to the environment, to learn from experience, to engage in various forms of reasoning, to overcome obstacles by taking thought” (p 77). 9
Humans perceive the environment, attend to relevant stimuli, memorize episodic and semantic information, communicate, and so forth. However, these activities must be integrated in some way for: (i) adapting our behavior to the environment; (ii) selecting the most appropriate contexts; or (iii) changing the world when adaptation and selection are not an option.10 In our view, the integration of cognitive functions and abilities is dependent on the very general mental ability we call “general intelligence” or g for short. This integration is consistent with g as ability11 or as an emergent property of the brain.12
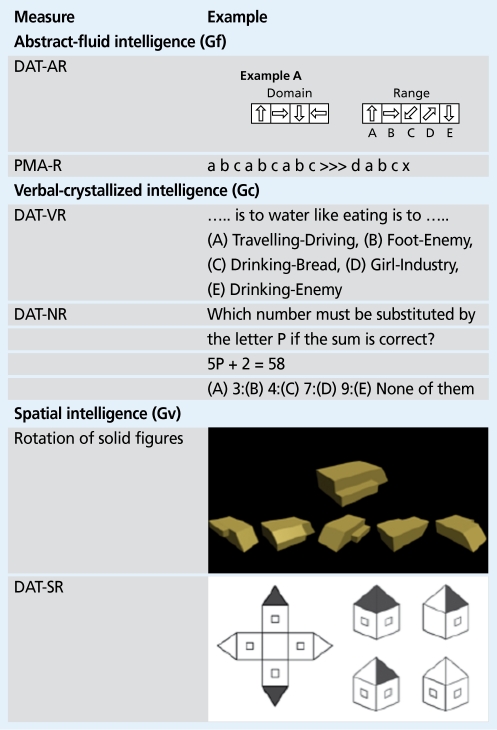
Any cognitive ability refers to variations in performance on some defined class of mental or cognitive tasks (Figure 1). Abilities reflect observable differences in individuals' performance on certain tests or tasks. However, this performance involves the synthesis of a variety of abilities: “spatial ability,” for instance, can be regarded as an inexact concept that has no formal scientific meaning unless it refers to the structure of abilities that compose it. The problem of defining (and measuring) intelligence is the problem of defining the constructs that underlie it and of specifying their structure.13-15
Figure 1. Examples of classes of mental tasks. DAT, differential aptitude test; AR, abstract reasoning; VR, verbal reasoning; NR, numerical reasoning; SR, spatial reasoning; PMA, primary mental abilities .
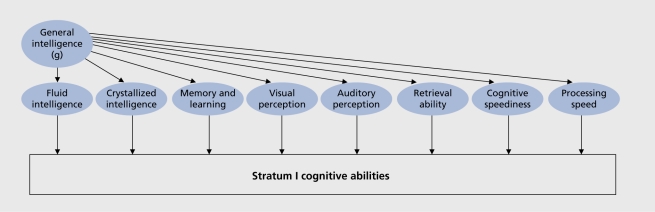
For more than a century, psychologists have developed hundreds of tests for the standardized measurement of intelligence with varying degrees of reliability and validity16 The resulting measures allowed for the organization of taxonomies identifying minor and major cognitive abilities. J. B. Carroll,17,18 for example, proposed a threestratum theory of intelligence after the extensive reanalysis of more than 400 datasets with thousands of subjects from almost 20 different countries around the world. Figure 2. shows a simplified depiction of the taxonomy of cognitive abilities.
Figure 2. Schematic representation of the three stratum taxonomy of intelligence .
This survey of factor analytic studies supports the view that intelligence has a hierarchical structure (ie, like a pyramid). There is strong evidence for a factor representing general intelligence (g) located at the apex of the hierarchy (stratum III). This g factor provides an index of the level of difficulty that an individual can handle in performing induction, reasoning, visualization, or language comprehension tests. At a lower order in the hierarchy (stratum II), several broad ability factors are distinguished: fluid intelligence, crystallized intelligence, general memory, visual perception, auditory perception, retrieval, or cognitive speed. Lastly, stratum I is based on specific abilities, such as induction, lexical knowledge, associative memory, spatial relations, general sound discrimination, or ideational fluency.
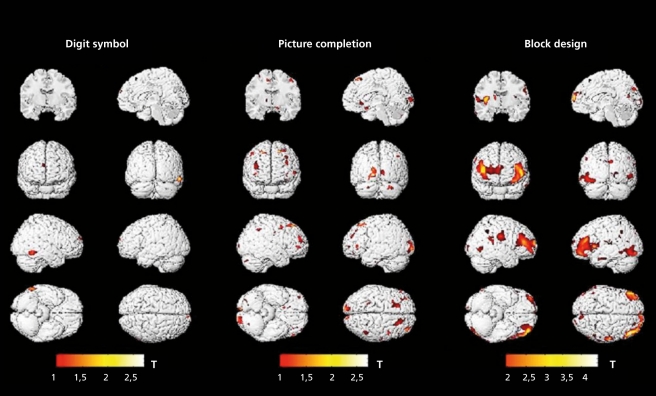
Factor analytic surveys reveal two main findings: (i) the g factor constitutes more than half of the total common factor variance in a cognitive test or task in samples representative of the population; and (ii) various specific cognitive abilities can be identified, including the cognitive domains of language, memory, and learning, visual perception, information processing, knowledge and so forth, indicating certain generalizations of abilities; actually, there are more than 60 specific or narrow abilities. Available test batteries (a good example would be the Wechsler Adult Intelligence Scale - WAIS) measure g in addition to several cognitive abilities and specific skills. We know how to separate these influences over cognitive performance by means of statistical analyses. There are some measures which are highly g-loaded (eg, the Vocabulary subtest of the WAIS), while others are less g-loaded (eg, the Digit Symbol Subtest of the WAIS). (Figure 3). shows how gray matter correlates become more prominent with increased g loadings of the intelligence measures. Moreover, the same measure can load differently on general and specific cognitive factors/abilities depending on the sample analyzed.19,20
Figure 3. Correlations between regional gray matter and digit symbol scores, picture completion, and block design (N =48). Color bar shows t values; maximum r=0.36:0.39:and 0.57 respectively .
Human intelligence and the brain
Exploring the relationships between human intelligence and the brain requires a careful consideration of the structure of human intelligence. As evident from above, when researchers state that they are measuring intelligence by means of the Standard Progressive Matrices Test (SPM - as another example) they are telling an imprecise story because the SPM measures g plus spatial and reasoning abilities plus SPM specificity. The exact combination of these “ingredients” for the analyzed sample must be computed before saying something clear about the measured performance. This requires that studies use a battery of tests rather than just one test. Although this was not usually done for the early functional imaging studies of intelligence,21-25 it is now more common.26-29 Results from the older and the newer studies, however, point to the importance of both whole brain and specific brain networks.
Brain size and human intelligence
Wickett et al30 state:
“There is no longer any doubt that a larger brain predicts greater intelligence. Several research teams, using differing scan protocols, populations, and cognitive measures, have all shown that IQ and brain volume correlate at about the 0.40 level ( ...) obviously replication of this effect is no longer required. What is required now is a more fine-grained analysis of why it is that a larger brain predicts greater intelligence, and what it is about intelligence that is most directly related to brain volume” (p 1096, emphasis added).
The meta-analysis by McDaniel31 studied the relationship between in vivo brain volume and intelligence. Thirty-seven samples comprising a total of 1530 participants were considered simultaneously. These were the main findings: (i) the average correlation is 0.33; (ii) subsets of the 37 studies that allow partitioning by gender revealed that the correlation is higher for females (0.40) than for males (0.34); and (iii) the correlation does not change across age (0.33). The report concludes that these results resolve a 169-year-old debate: it is clear that intelligence and brain volumes are positively related.
Going one step further, several studies measured the volume of regions of interest (ROIs) showing the most significant correlations (controlling for total brain volumes) in frontal, parietal, and temporal brain regions, along with the hippocampus and the cerebellum.32,33 Nevertheless, regional correlations are moderate (ranging from 0.25 to 0.50) which implies that measures of total or local brain size are far from telling the whole story.
From this perspective, gray and white matter must be distinguished. In keeping with this, voxel-by-voxel (a voxel is a volume element analogous to a pixel) analyses also showed specific areas where the amount of gray and white matter was correlated with intelligence scores.24,25 The amount of gray matter is considered to reflect number and density of neuronal bodies and dendritic arborization, whereas the amount of white matter is considered to capture number and thickness of axons and their degree of myelination. Gray matter could support information processing capacity, while white matter might support the efficient flow of information in the brain. Available reports are consistent with the statement that both gray and white matter volumes are positively related to intelligence, but that the latter relationship is somewhat greater (unweighted mean correlation values =.27 and .31 respectively).34 It is noteworthy that new studies using diffusion tensor imaging (DTI), which is the best method to date for assessing white matter, have reported DTI correlations with intelligence scores (see white matter section below).
A distributed brain network for human intelligence
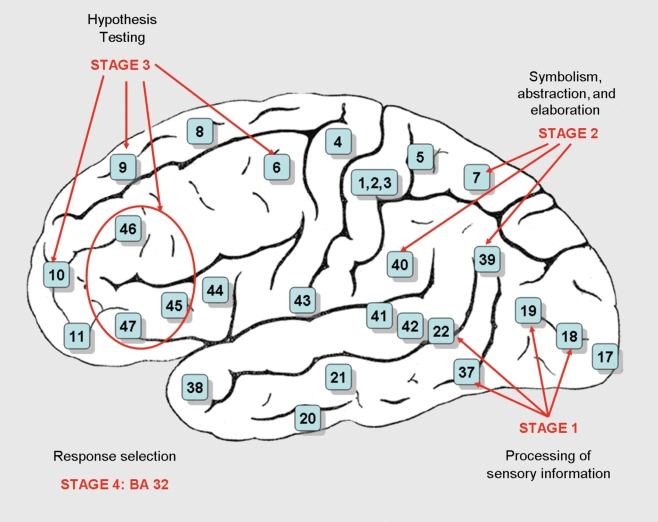
Jung and Haier35 reviewed 37 structural and functional neuroimaging studies published between 1988 and 2007. Based on the commonalities found in their analysis, they proposed the Parieto-Frontal Integration Theory (PFIT), identifying several brain areas distributed across the brain. These P-FIT regions support distinguishable information processing stages (Figure 4).
Figure 4. Processing stages proposed by the P-FIT model.35 .
This is a summary of the proposed stages.
Occipital and temporal areas process sensory information in the first processing stage: the extrastriate cortex (Brodmann areas - BAs - 18 and 19) and the fusiform gyrus (BA 37), involved with recognition, imagery and elaboration of visual inputs, as well as Wernicke's area (BA 22) for analysis and elaboration of syntax of auditory information.
Integration and abstraction of the sensory information by parietal BAs 39 (angular gyrus), 40 (supramarginal gyrus), and 7 (superior parietal lobule) correspond to the second processing stage.
The parietal areas interact with the frontal lobes in the third processing stage and this interaction underlies problem solving, evaluation, and hypothesis testing. Frontal BAs 6, 9, 10, 45, 46, and 47 are underscored by the model.
The anterior cingulate (BA 32) is implicated for response selection and inhibition of alternative responses, once the best solution is determined in the previous stage.
White matter, especially the arcuate fasciculus, is thought to play a critical role in reliable communication of information across the brain processing units. Nevertheless, note that the “Geschwind area” (underlying the angular gyrus) within the arcuate fasciculus may be even more important than the entire track.36
Frontal, parietal, temporal, and occipital areas are depicted in Figure 4. However, Jung and Haier35 suggest that not all these areas are equally necessary in all individuals for intelligence. Discrete brain regions of the dorsolateral prefrontal cortex (BAs 9, 45, 46, and 47) and the parietal cortex (BAs 7 and 40) could be considered most important for human intelligence.
A frontoparietal network may be relevant for intelligence, but also for working memory.37 A study by Gray et al38 tested whether fluid or reasoning ability (Gf) was mediated by neural mechanisms supporting working memory. Sixty participants performed verbal and nonverbal working memory tasks. They had to indicate if a current item matched the item they saw 3 items previously (3-back). Brain activity was measured by event-related functional magnetic resonance imaging (fMRI). The demand for working memory varied across trials. Results showed that: (i) participants scoring higher on the Progressive Matrices Test (a measure related to fluid g - Gf) were more accurate in the 3-back task; and (ii) only lateral prefrontal and parietal regions mediated the correlation between Gf and 3-back performance.
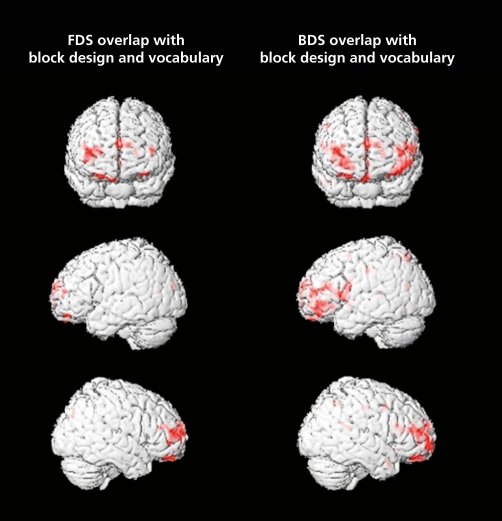
These fMRI results are consistent with the voxel-based morphometry (VBM) study reported by Colom et al (N = 48).39 In agreement with the well established fact that the g factor and working memory capacity are very highly correlated,40-45 these researchers predicted that g and working memory would share significant common neural networks. Therefore, using a VBM approach they quantified the overlap in brain areas where regional gray matter was correlated with measures of general intelligence and working memory, finding a common neuroanatomic framework supported by frontal gray matter regions belonging to BA 10 and by the right inferior parietal lobule (BA 40). Of note, this study also showed: (i) more gray matter recruitment for the more cognitively complex tasks (= more highly g loaded); and (ii) the complex span task (backward digit span) showed more gray matter overlap with the general factor of intelligence than the simple span task (forward digit span, (Figure 5). These results were interpreted after the theory proposed by Cowan,46 namely that parietal regions support “capacity limitations,” whereas frontal areas underlie the “control of attention.”
Figure 5. Overlap of correlations between gray matter and g (conjunction of block design and vocabulary) and gray matter and forward (FDS) and backward (BDS) digit span scores (P<01).39 .
A similar commonality between intelligence and working memory was found in animal studies. Matzel and Kolata47 reviewed several reports in which performance of laboratory mice was measured in a variety of attention and learning tasks. These are their most prominent conclusions:
The “positive manifold” (eg, scores on cognitive tasks of various kinds are positively correlated) found in humans also applied to mice
Storage and processing components of working memory accounted for the strong relationship between this cognitive function and g
Networks involved in working memory overlap with those relevant for intelligence. These findings support an evolutionary conservation process of the structure and determinants of intelligence beyond humans.48
Giftedness has been also investigated with related findings. Lee et al49 used an fMRI approach to investigate the neural bases of superior intelligence. Eighteen gifted and 18 nongifted adolescents were analyzed. They solved reasoning problems, having high (complex) and low (simple) loadings on g. Increased bilateral frontoparietal activations (lateral prefrontal, anterior cingulate, and posterior parietal cortices) were found for both groups, but the gifted subjects showed greater activations in the posterior parietal cortex. Furthermore, activations in BAs 7 and 40 (superior and intraparietal cortices) correlated with intelligence differences. Therefore, high intelligence was associated with increased involvement of the frontoparietal network through preferential activation of the posterior parietal regions.
Gläscher et al28 investigated the neural substrates of g in 241 patients with focal brain damage, using voxel-based lesion-symptom mapping. Statistically significant associations between g and damage within a distributed network in frontal and parietal brain regions were found. Further, damage of white matter association tracts in frontopolar areas was also shown to be associated with differences in g. They concluded that g draws on connections between regions integrating verbal, visuospatial, working memory, and executive processes.
Going one step further, Gläscher et al28 asked whether or not there was a neural region whose damage uniquely impacts g beyond subtests contributing to the general score. They examined this question by analyzing the nonoverlap between a disjunction of subtests and the reported lesion pattern for g. A single region was found in the left frontal pole (BA 10) showing a significant effect unique to g. This result complements the distributed nature of g and suggests a hierarchical control mechanism. This unique area for g may be involved in the allocation of the working memory resources necessary for successful performance on specific cognitive tasks. However, this finding should be placed within context since there are studies showing no decline in intelligence associated with prefrontal lobotomy, presumably including the frontopolar cortex.35 Therefore, future studies are necessary to determine the specific necessity of the frontal poles to g. The comparison between lesion cohorts and normal cohorts must be done carefully.
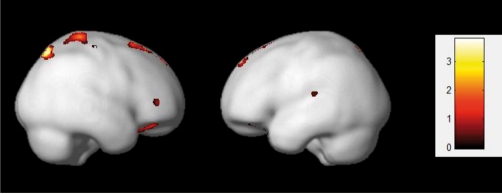
The structural studies reported by Colom et al27 and Karama et al50 are also consistent with the P-FIT model. In the first study (N =100) the general factor of intelligence was estimated after nine tests measuring reasoning, verbal, and nonverbal intelligence. Their VBM approach revealed several clusters of voxels correlating with individual differences in g scores. The main regions included the dorsolateral prefrontal cortex, Broca's and Wernicke's areas, the somatosensory association cortex, and the visual association cortex. The design matrix in this study controlled for sex, but when total gray matter was controlled for instead of sex, significant correlations were concentrated in frontal and parietal areas only (Figure 6):superior, middle, and frontal gyrus, along with the postcentral gyrus and the superior parietal lobule.
Figure 6. Regional correlations between gray matter density and individual differences in g (N =1 04). The design matrix controls for total gray matter .
Karama et al50 used an automated cortical thickness protocol (CIVET51) to analyze a large sample of children and adolescents representative of the population (N=216). The most consistent areas of association between g scores and cortical thickness were found in lateral prefrontal, occipital extrastriate, and parahippocampal areas. Similar to the study reported by Colom et al,27 Karama et al50 identified more brain regions related to g than those in the P-FIT model, likely resulting from the synthesizing nature of the P-FIT approach (ie, if all regions implicated in intelligence across all 37 studies were included, they would have numbered in the hundreds) as opposed to the experimental/exploratory approach employed by these studies.
There are three other studies applying a cortical thickness approach (the third will be discussed later). Shaw et al52 analyzed the trajectory of change in the thickness of the cerebral cortex on a sample of 307 children and adolescents. Intelligence was measured by four subtests from the Wechsler scales (vocabulary, similarities, block design, and matrix reasoning). They found that changes in thickness are more related to intelligence than thickness itself: negative correlations were found in early childhood, whereas the correlation was positive in late adolescence (these positive correlations were identified in frontal BAs 4, 6, 8, 10, 11, and 44-46, in parietal BAs 1-3, 5, 39, 40, in temporal BAs 21, 37, and in occipital BAs 17, 18, and 19). Further, intelligence differences were associated with the trajectory of cortical development in frontal brain regions. Finally, children with higher scores on intelligence showed more change in estimated cortical thickness along the developmental process.
Narr et al53 studied a sample of 65 participants. They found positive associations between cortical thickness and intelligence bilaterally in prefrontal BAs 10/11 and 47, as well as in posterior temporal BAs 36/37. These researchers also analyzed males and females separately, finding that males showed correlations in temporaloccipital association cortices, whereas females exhibited correlations in prefrontal and temporal association cortices. These results are not entirely consistent with the parietofrontal framework and emphasize the importance of separate analyses for males and females.25,54,55
Functional networks and neurotransmitters
Using an fMRI approach, Bishop et al56 reported a study based on previous evidence showing that a polymorphism (val158met) in the catechol-O-methyltransferase (COMT) gene regulates catecholaminergic signaling in prefrontal cortex. The val158 allele is associated with higher COMT activity than the met158 allele-therefore, a lesser conten of dopamine. Twenty-two participants, genotyped for the COMT val158met polymorphism, performed verbal and spatial fluid intelligence (Gf) items, classified according to their cognitive complexity, as estimated from the loadings on g (see ref 57). These researchers were particularly interested in the analysis of the frontoparietal network related to fluid intelligence (the lateral prefrontal cortex, the presupplementary motor area/anterior cingulate cortex, and the intraparietal sulcus).
Findings revealed a positive effect of COMT val allele load upon the BOLD signal in regions belonging to this brain network when items showing distinguishable cognitive complexity were compared. This result suggests that the COMT val158met polymorphism impacts on the neural network supporting fluid intelligence. The finding is a demonstration that the effect of single genes can impact blood oxygen level dependent signal as assessed by fMRI. Further evidence linking catecholamine modulation within the identified network may help explain individual differences in the neural response to high levels of cognitive complexity, irrespective of the content domain (verbal or nonverbal).
White matter
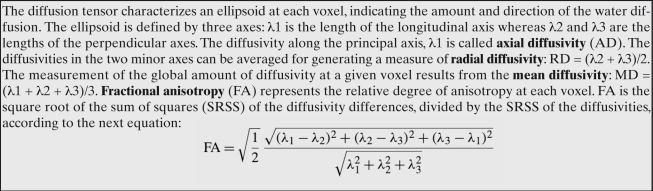
The relationship between human intelligence and the integrity of white matter has been much less investigated, although this trend is changing rapidly. Diffusion tensor imaging (DTI) is based on the diffusion of water molecules in the brain and provides information about the size, orientation, and geometry of myelinated axons. DTI can produce measures that include fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RA), and axial diffusivity (AD), which allow for the assessment of myelin and axonal integrity (see Figure 7).
Figure 7.
DTI is useful for fine-grained deterministic and probabilistic tractography to capture underlying cortical connectivity patterns. This can be used for the quantitative analysis of local and global network properties using graph-theoretical approaches (eg, analysis of small-world properties).58,59
Using DTI, Schmithorst et al60 analyzed the relationship between intelligence and white matter structure. The sample comprised 47 children and adolescents (age range 5 to 18). White matter structure was studied using fractional anisotropy (FA) and mean diffusivity (MD) indices. These indices were correlated with intelligence scores obtained from the Wechsler scales. These researchers found positive correlations bilaterally for FA in white matter association areas (frontal and parietooccipital areas). These correlations were thought to reflect a positive relationship between fiber organization-density and intelligence.
Also using a DTI approach, Yu et al61 computed correlations between the integrity of several tracts (corpus callosum, cingulum, uncinate fasciculus, optic radiation, and corticospinal tract) and intelligence. On the basis of their scores on the Wechsler scales, 79 participants were divided in two groups: average and high intelligence. White matter integrity was assessed by fractional anisotropy (FA). The results showed that high intelligence participants display more white matter integrity than average intelligence participants only in the right uncinate fasciculus. Therefore, the right uncinate fasciculus might be an important neural basis for intelligence differences. A sample of 15 participants with mental retardation was also analyzed. These participants were compared with the 79 healthy controls and they showed extensive damage in the integrity of several white matter tracts: corpus callosum, uncinate fasciculus, optic radiation, and corticospinal tract.
Tang et al62 used both DTI and fMRI during an n-back memory task in 40 young adults who had also completed a battery of intelligence tests. Correlations between the BOLD signal obtained from the n-back task and intelligence were mainly concentrated in the right prefrontal and bilateral parietal cortices. These correlations were negative (the higher the intelligence, the lower the activation during the n-back task) which supports the efficiency model of brain function. Further, white matter tracts connecting these areas also showed correlations to g. Specifically, integrity of interhemispheric connections was positively correlated to some intelligence factors in females but negatively correlated in males.
Chiang et al63 have reported the first study combining a genetic informative design and a DTI approach for analyzing the relationships between white matter integrity and human intelligence. Intelligence was assessed by the Multidimensional Aptitude Battery, which provides measures of general intelligence, verbal (information, vocabulary, and arithmetic), and nonverbal intelligence (spatial and object assembly). The sample comprised 23 pairs of identical twins and 23 pairs of fraternal twins. White matter integrity, quantified using FA, was used to fit structural equation models (SEM) at each point in the brain. Afterwards three-dimensional maps of heritability were generated. White matter integrity was found to be under significant genetic control in bilateral frontal, bilateral parietal, and left occipital lobes (values ranging from .55 to .85). FA measures were correlated with the estimate of general intelligence and with nonverbal intelligence in the cingulum, optic radiations, superior fronto-occipital fasciculus, internal capsule, the isthmus of the corpus callosum, and the corona radiata. Further, common genetic factors mediated the correlation between intelligence and white matter integrity which suggests a common physiological mechanism and common genetic determination.
Networks for human intelligence
As noted above, gray matter supports information processing capacity and white matter promotes efficient flow of information across the brain. Connections are relevant for intelligence and these connections might be organized in networks. From this perspective, Li et al64 reported a study testing the hypothesis that high levels of intelligence involve more efficient information transfer in the brain.21,65,66 Studying a sample of 79 participants, brain anatomical networks were constructed by means of diffusion tensor tractography. These networks included intrahemispheric and interhemispheric connections. Six white-matter tracts were further constructed: the genu of the corpus callosum, the body of the corpus callosum, the splenium of the corpus callosum, the cingulum, the corticospinal tract, and the inferior fronto-occipital fasciculus. Thereafter, they calculated the topological properties of the networks for every participant. The sample was divided between average and high intelligence according to scores on the Wechsler scales. Higher global efficiencies were revealed for the latter group: higher intelligence was found to display shorter characteristic path length and a higher global efficiency of the networks. This was interpreted as a characteristic of a more efficient parallel information transfer in the brain anatomy. Therefore, the efficiency of brain structural organization could be an important biological basis for human intelligence, as originally proposed by Haier et al.21,66
Song et al67 analyzed 59 adults for studying the relationships between spontaneous brain activity at rest and individual differences in intelligence. Intelligence was assessed by the Wechsler scales. Using fMRI, the bilateral dorsolateral prefrontal cortices were the seed regions for investigating the correlations across subjects between individual intelligence scores and the strength of the functional connectivity between the seed regions and the remaining brain regions. These researchers found that brain regions in which the strength of the functional connectivity significantly correlated with intelligence scores were distributed in the frontal, parietal, occipital and limbic lobes. Furthermore, functional connectivity within the frontal lobe and between the frontal and posterior brain regions predicted differences in intelligence. These results are consistent with the relevance of a network view for human intelligence.
van den Heuvel et al68 used resting state fMRI and graph analysis for exploring the presumed organization of the brain network. Functional connections of this brain network were analyzed computing correlations among the spontaneous signals of different brain regions during rest. The sample comprised 19 subjects and intelligence was measured by the Wechsler scales. They found associations between global communication efficiency - more long-distance connections - and scores of intelligence. This was interpreted as suggesting that a difference in the efficiency with which the brain integrates information between brain regions is related to differences in human intelligence. The strongest effects were found in frontal and parietal regions. Furthermore, intelligence differences were not related to the level of local information processing (local neighborhood clustering) and to the total number of functional connections of the brain network.
Beyond these specific studies, the so-called “connectome project” deserves close attention.69 There is strong agreement regarding the fact that the human brain comprises a wide variety of functional systems. Obtaining brain images during rest shows large-amplitude spontaneous low frequency fluctuations in the fMRI signal. These fluctuations are related across areas sharing functions and the correlations show up as an individual's functional connectome. Biswall et al69 report findings obtained from 1414 participants from 35 laboratories. Their main results were: (i) there is a universal functional architecture; (ii) there are substantial sex differences and age-related gradients; and (iii) it is possible to establish normative maps for the functional boundaries among identified networks.
Integration of intelligence and cognitive findings
The frontoparietal network is relevant for intelligence, but also for other cognitive functions.70 Thus, for instance, Wager and Smith71 reported a meta-analysis of 60 positron-emission tomography (PET) and fMRI studies of working memory. The effect of three content domains (verbal, spatial, and object), three executive functions (updating, temporal order, and manipulation) along with their interactions were analyzed. Brain areas most involved in all these cognitive facets were located in the frontal and parietal lobes: (i) spatial and nonspatial contents were separated in posterior, but not anterior areas; (ii) executive manipulation evoked more frontal activations, but with some exceptions; and (iii) the parietal cortex was always implicated in executive processing. The meta-analysis by Wager, Jonides, and Reading72 after 31 PET and fMRI studies of shifting attention also highlights this fronto-parietal network (medial prefrontal, superior and inferior parietal, medial parietal, and premotor cortices).
Similarly, Marois and Ivanoff 3 analyzed the capacity limits of information processing in the brain. Three basic limitations for perception, working memory, and action were explicitly considered. Their revision was based mainly on fMRI evidence and these were the basic conclusions: (i) perception and action limitations are related to fronto-parietal brain networks; and (ii) working memory capacity limitations are associated to parieto-ccipital brain networks. The lateral prefrontal cortex may support general target consolidation and response selection, using a flexible coding system for processing relevant information in any given task. In contrast, the lateral parietal cortex might provide support to more specific processing goals. This brain region is more sensitive to perception than to action.
Thus, core cognitive functions (especially working memory) and intelligence share a frontoparietal brain network. If this network is involved for most individuals, it could be possible to predict individual differences in intelligence based on brain data.74 This was attempted by Choi et al75 using structural (cortical thickness) and functional magnetic resonance imaging. Their regression model explained 50% of the variance in IQ scores. Even when this figure may be questioned on several grounds, the main approach underscores that brain images might be employed for estimating intelligence levels in some instances using a neurometric approach.
Finally, experimental confirmatory approaches should be welcomed to increase refinement of ongoing research efforts. In this regard, transcranial magnetic stimulation (TMS) may help test hypotheses aimed at determining whether or not specific brain regions are really important for understanding individual differences in human intelligence. TMS induces transient changes in brain activity noninvasively. It does this by producing changes in a magnetic field that, in turn, evoke electric currents in the brain which promote depolarization of cellular membranes. Cognitive neuroscience often relies on a correlation approach, whereas TMS allows studying (almost) causal brain-behavior relationships in higher cognitive functions.76,77 The study reported by Aleman and van't Wout78 exemplifies this approach using a working memory task (forward and backward digit span). Working memory (and intelligence) performance is partially supported by the dorsolateral prefrontal cortex. Using repetitive TMS (rTMS) - adapted in the Hz band for suppressing cognitive processing - over the right dorsolateral prefrontal cortex, a significant decrease of performance in the forward and backward digit span test was found. Thus, regional suppression (or enhancement) might be produced to experimentally test specific predictions.
Conclusion
Regardless of the use of exploratory (correlation) or confirmatory (experimental) approaches, we do agree with Kennedy79: “as with more _eras', it is the underlying technology that makes the era possible [...] new advances in acquisition, analysis, databasing, modeling, and sharing will continue to be necessary.” This is especially true for analyzing human intelligence because this psychological factor is undoubtedly rooted in widely distributed regions in the brain. Frontal and parietal lobes likely comprise crucial processing areas for intelligence, but integrity of hard connections across the entire brain or spontaneous harmonic coactivation among distant regions appear also to be relevant. Creating a comprehensive picture for what can be called “neuro-intelligence”80 should prove as challenging as it is exciting.
Acknowledgments
RC was partly supported by grant PSI2010-20364 from the Ministerio de Ciencia e Innovación (Spain).
Contributor Information
Roberto Colom, Universidad Autónoma de Madrid, Spain.
Sherif Karama, McGill University, Montreal, Quebec, Canada.
Rex E. Jung, The MIND Research Network, Albuquerque, New Mexico, USA.
Richard J. Haier, University of California, Irvine, California, USA.
REFERENCES
- 1.Gottfredson L. Intelligence: is it the epidemiologists' elusive “fundamental cause” of social class inequalities in health? J Person Soc Psychol. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- 2.Lubinski D. Introduction to the special section on cognitive abilities: 100 years after Spearman's (1904) “General Intelligence, Objectively Determined and Measured”. J Person Soc Psychol. 2004;86:96–111. doi: 10.1037/0022-3514.86.1.96. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt F, Hunter J. General mental ability in the world of work: occupational attainment and job performance. J Person Soc Psychol. 2004;86:162–173. doi: 10.1037/0022-3514.86.1.162. [DOI] [PubMed] [Google Scholar]
- 4.Deary IJ, Whalley LJ, Lemmon H, Crawford JR, Starr JM. The stability of individual differences in mental ability from childhood to old age: followup of the 1932 Scottish Mental Survey. Intelligence. 2000;28:49–55. [Google Scholar]
- 5.Bouchard T. Genetic influence on human intelligence (Spearman's g): how much? Ann Hum Biol. 2009;36:527–544. doi: 10.1080/03014460903103939. [DOI] [PubMed] [Google Scholar]
- 6.Jensen AR. The g Factor. The Science of Mental Ability. Westport, CONN: Praeger. 1998 [Google Scholar]
- 7.Snyderman M, Rothman S. Survey of expert opinion on intelligence and aptitude testing. Am Psychol. 1987;42:137–144. [Google Scholar]
- 8.Gottfredson L. Mainstream science on intelligence: an editorial with 52 signatories, history, and bibliography. Intelligence. 1997;24:13–23. [Google Scholar]
- 9.Neisser U, Boodoo G, Bouchard TJ, et al. Intelligence: knowns and unknowns. Am Psychol. 1996;51:77–101. [Google Scholar]
- 10.Sternberg R. The Triarchic Mind. London, UK: Penguin Books. 1988 [Google Scholar]
- 11.Spearman C. General intelligence objectively determined and measured. Am J Psychol. 1904;15:201–293. [Google Scholar]
- 12.van der Maas H, Dolan CV, Grasman RPPP, Wicherts JM, Huizengan HM, Raijmakers MEJ. A dynamical model of general intelligence: The positive manifold of intelligence by mutualism. Psychol Rev. 2007;113:842–861. doi: 10.1037/0033-295X.113.4.842. [DOI] [PubMed] [Google Scholar]
- 13.Hunt EB. Human Intelligence. Cambridge,UK: Cambridge University Press. In press. [Google Scholar]
- 14.Johnson W, Bouchard T. The structure of human intelligence: It is verbal, perceptual, and image rotation (VPR), not fluid and crystallized. Intelligence. 2005;33:393–416. [Google Scholar]
- 15.McGrew K. CHC theory and the human cognitive abilities project: standing on the shoulders of the giants of psychometric intelligence research. Intelligence. 2009;37:1–10. [Google Scholar]
- 16.Jensen AR. Bias in Mental Testing. New York, NY: Free Press; 1980 [Google Scholar]
- 17.Carroll JB. Human Cognitive Abilities A Survey of Factor Analytic Studies. Cambridge, UK: Cambridge University Press; 1993 [Google Scholar]
- 18.Carroll JB. The higher-stratum structure of cognitive abilities: Current evidence supports g and about 10 broad factors. In Nyborg H, ed. The Scientific Study of General Intelligence: Tribute to Arthur R. Jensen. Amsterdam, the Netherlands: Pergamon. 2003:5–21. [Google Scholar]
- 19.Colom R, Abad FJ, García LF, Juan-Espinosa M. Education, Wechsler's Full Scale IQ, and g. Intelligence. 2002;30:449–462. [Google Scholar]
- 20.Colom R, Thompson, PM. Understanding human intelligence imaging the brain. In Chamorro-Premuzic T, Furnham A, von Stumm S, eds. Handbook of Individual Differences. London, UK: Wiley-Blackwell. In press. [Google Scholar]
- 21.Haier RJ, Siegel, BV Jr, Nuechterlein KH, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–21. [Google Scholar]
- 22.Haier RJ. Cerebral glucose metabolism and intelligence. In Vernon PA, ed. Biological Approaches to the Study of Human Intelligence. Norwood, NJ: Ablex Publishing. 1993:317–331. [Google Scholar]
- 23.Haier RJ, White NS, Alkire MT. Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence. 2003;31:429–441. [Google Scholar]
- 24.Haier RJ, Jung RE, Yeo RA., et al. Structural brain variation and general intelligence. NeuroImage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Haier RJ, Jung RE, Yeo RA, et al. The neuroanatomy of general intelligence: sex matters. NeuroImage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Colom R. Intelligence? What intelligence? Behav Brain Sci. 2007;30:155–56. [Google Scholar]
- 27.Colom R, Haier RJ, Head K, et al. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- 28.Gläscher J, Rudrauf D, Colom R, et al. The distributed neural system for general intelligence revealed by lesion mapping. PNAS. 2010;10:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haier RJ, Colom R, Schroeder DH, et al. Gray matter and intelligence factors: Is there a neuro-g? Intelligence. 2009;37:136–144. [Google Scholar]
- 30.Wickett JC, Vernon PA, Lee DH. Relationships between factors of intelligence and brain volume. Person Indiv Diff. 2000;29:1095–1122. [Google Scholar]
- 31.McDaniel MA. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 32.Luders E, Narr KL, Thompson PM, Toga AW. Neuroanatomical correlates of intelligence. Intelligence. 2009;37:156–163. doi: 10.1016/j.intell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toga AW, Thompson PM. Genetics of brain structure and intelligence. Ann Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- 34.Gignac G, Vernon PA, Wicket JC. Factors influencing the relationship between brain size and intelligence. In: Nyborg H, ed. The Scientific Study of General Intelligence. Oxford, UK: Pergamon Press. 2003:93–106. [Google Scholar]
- 35.Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- 36.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain, Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 37.Gray J, Thompson PM. Neurobiology of intelligence: Science and ethics. Nat Rev. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 38.Gray J, Chabris, C, Braver, T. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 39.Colom R, Jung RE, Haier RJ. General intelligence and memory span: Evidence for a common neuro-anatomic framework. Cogn Neuropsychol. 2007;24:867–878. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- 40.Colom R, Rebollo I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. . Intelligence. 2004;32:277–296. [Google Scholar]
- 41.Colom R, Abad FJ, Rebollo I, Shih PC. Memory span and general intelligence: A latent-variable approach. Intelligence. 2005;33:623–642. [Google Scholar]
- 42.Colom R, Abad FJ, Quiroga MA, Shih PC, Flores-Mendoza C. Working memory and intelligence are highly related constructs, but why? Intelligence. 2008;36:584–606. [Google Scholar]
- 43.Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci. 2002;11:19–23. [Google Scholar]
- 44.Kane MJ, Hambrick DZ, Conway ARA. Working memory capacity and fluid intelligence are strongly related constructs: comment on Ackerman, Beier, and Boyle (2005). Psychol Bull. 2005;131:66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- 45.Oberauer K, Schulze R, Wilhelm O, Süb H. Working memory and intelligence--Their correlation and their relation: comment on Ackerman, Beier, and Boyle (2005). Psychol Bull. 2005;131:61–65. doi: 10.1037/0033-2909.131.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Cowan N. Working Memory Capacity. New York, NY: Psychology Press; 2005 [Google Scholar]
- 47.Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neurosci Biobehav Rev. 2010;34:23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolata S, Wu J, Light K, Schachner M, Matzel LD. Impaired working memory duration but normal learning abilities found in mice that are conditionally deficient in the close homolog of L1. J. Neurosci. 2008;28:13505–13510. doi: 10.1523/JNEUROSCI.2127-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KH, Choi YY, Gray JR, et al. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. NeuroImage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 50.Karama S, Ad-Dab'bagh Y, Haier RJ, et al. the Brain Development Cooperative Group Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ad-Dab'bagh Y, Lyttelton O, Muehlboeck JS, et al. The CIVET imageprocessing environment: A fully automated comprehensive pipeline for anatomical neuroimaging research. In Corbetta M, ed. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Florence, Italy: Elsevier. 2006 [Google Scholar]
- 52.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 53.Nar KL, Woods RP, Thompson PM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163 – 2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 54.Haier RJ, Benbow CP. Sex differences and lateralization in temporal lobe glucose metabolism during mathematical reasoning. Dev Neuropsychol. 1995;11:405–414. [Google Scholar]
- 55.Jung RE, Haier RJ, Yeo RA, et al. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. NeuroIimage. 2005;26:965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 56.Bishop SJ, Fossella J, Croucher CJ, Duncan J. COMT val158met genotype affects recruitment of neural mechanisms supporting fluid intelligence. Cereb Cortex,. 2008;doi:10.1093/cercor/bhm240. doi: 10.1093/cercor/bhm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duncan J, Seitz JR, Kolodny J, et al. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 58.Gong G, Rosa-Neto P, Carbonell F, et al. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;2:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PCM. MRI Atlas of Human White Matter. Amsterdam, the Netherlands: Elsevier; 2005 [Google Scholar]
- 60.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Map. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu C, Li J, Liu Y, et al. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. NeuroImage. 2008;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 62.Tang, CY, Eaves EL, Ng, JC, et al. Brain networks for working memory and factors of intelligence assessed in males and females with fMRI and DTI. Intelligence. 2010;38:293–303. [Google Scholar]
- 63.Chiang M, Barysheva M, Shattuck DW, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, Liu Y, Li J, et al. Brain anatomical network and intelligence. Comp Biol. 2009. 2009;5:1–17. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neubauer AC, Fink A. Intelligence and neural efficiency: Measures of brain activation versus measures of functional connectivity in the brain. Intelligence. 2009;37:223–229. [Google Scholar]
- 66.Haier RJ, Siegel BV, MacLachlan A, Soderling E, Lottenberg S, Buschsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- 67.Song M, Zhou Y, Li J, et al. Brain spontaneous functional connectivity and intelligence. NeuroImage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 68.van den Heuvel MP, Stam CJ, Kahn RS, Pol H. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biswal BB, Mennes M, Zuo XM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Wager TD, Smith, EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 72.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 73.Marois, R. and Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Haier RJ. What does a smart brain look like? Inner views show how we think. Sci Am Mind. 2009 Nov [Google Scholar]
- 75.Choi YY, Shamosh NA, Cho SH , et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;41:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of 'virtual lesions'. Phil Trans R Soc London B. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sack AT. Transcranial magnetic stimulation, causal structure-function mapping and networks of functional relevance. Curr Opin Neurobiol. 2006;16:593–599. doi: 10.1016/j.conb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 78.Aleman A, van't Wout M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiol. 2008;57:44–48. doi: 10.1159/000129666. [DOI] [PubMed] [Google Scholar]
- 79.Kennedy DN. Making connections in the connectome era. Neuroinformatics, 2010;8:61–62. doi: 10.1007/s12021-010-9070-1. [DOI] [PubMed] [Google Scholar]
- 80.Haier RJ. Neuro-intelligence, neuro-metrics and the next phase of brain imaging Studies. Intelligence. 2009;37:121–123. [Google Scholar]
- 81.Colom R, Jung R, Haier RJ. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]