Abstract
The classic fight-or-flight response to perceived threat is a reflexive nervous phenomenon thai has obvious survival advantages in evolutionary terms. However, the systems that organize the constellation of reflexive survival behaviors following exposure to perceived threat can under some circumstances become dysregulated in the process. Chronic dysregulation of these systems can lead to functional impairment in certain individuals who become “psychologically traumatized” and suffer from post-traumatic stress disorder (PTSD), A body of data accumulated over several decades has demonstrated neurobiological abnormalities in PTSD patients. Some of these findings offer insight into the pathophysiology of PTSD as well as the biological vulnerability of certain populations to develop PTSD, Several pathological features found in PTSD patients overlap with features found in patients with traumatic brain injury paralleling the shared signs and symptoms of these clinical syndromes.
Keywords: stress, psychological trauma, traumatic brain injury, PTSD, biological markers, psychopathology, pathophysiology
Abstract
La clâsica respuesta de ataque o huida ante la perceptión de una amenaza es un fenómeno nervioso reflejo que, obviamente en términos evolutivos, tiene ventajas para la supervivencia. Sin embargo, los sistemas que organizan la constelación de conductas reflejas de supervivencia que siguen a la exposición a la amenaza percibida en algunas circunstancias pueden constituirse en procesos mal regulados. La mala regulatión crónica de estos sistemas puede llevar a un deterioro funcional en ciertos individuos quienes pueden convertirse en “traumatizados psicológicamente” y presentar un trastorno por estrés postraumático (TEPT), Una gran cantidad de informatión acumulada en varias décadas ha demostrado alteraciones neurobiológicas en los patientes con TEPT, Algunos de estos hallazgos permiten adentrarse en la fisiopatologia asi como en la vulnerabilidad biológica de ciertas poblaciones que van a desarrollar un TEPT Algunas caracteristicas patológicas encontradas en patientes con TEPT se sobreponen con caracteristicas de patientes con daño cerebral traumático, estableciendo un paralelo de signos y sintomas compartidos entre estos sindromes clinicos.
Abstract
La réponse classique de lutte ou de fuite à une menace perçue est un phénomène nerveux réflexe dont les avantages pour la survie sont évidents en termes d'évolution. Cependant, les systèmes organisés en constellation de comportements réflexes de survie après exposition à une menace perçue peuvent se déréguler dans certaines circonstances. Une dysregulation chronique de ces systèmes peut entraîner un déficit fonctionnel chez certains sujets qui deviennent « psychologiquement traumatisés » ef souffrent de l'état de stress posi-traumatique (ESPT), Des données recueillies pendant des dizaines d'années montrent des anomalies neurobiologiques chez les patients souffrant d'ESPT, ce qui permet de mieux comprendre la physiopathologie de l'ESPT ainsi que la vulnérabilité biologique de certaines populations à développer un ESPT, Certaines caractéristiques pathologiques de l'ESPT se superposent à celles trouvées chez des patients atteints de lésion cérébrale traumatique, en parallèle avec les signes et les symptômes partagés par ces deux syndromes.
Overview of psychological trauma, post-traumatic stress disorder, and biological markers
Psychological trauma can result from witnessing an event that is perceived to be life-threatening or to pose the potential of serious bodily injury to self or others. Such experiences, which are often accompanied by intense fear, horror, and helplessness, can lead to the development of, and are required for the diagnosis of, post-traumatic stress disorder (PTSD).1 It was originallythought that PTSD represented a normative response, at the extreme end of a response continuum, the severity of which related primarily to trauma/stressor intensity. However, it has become clear over time that the response of an individual to trauma depends not only on stressor characteristics, but also on factors specific to the individual.2 For the vast majority of the population, the psychological trauma brought about by the experience of profound threat is limited to an acute, transient disturbance. Though transient, such reactions can be quite unpleasant and are typically characterized by phenomena that can be grouped for the most part into three primary domains: (i) reminders of the exposure (including flashbacks, intrusive thoughts, nightmares); (ii) activation (including hyperarousal, insomnia, agitation, irritability, impulsivity and anger); and (iii) deactivation (including numbing, avoidance, withdrawal, confusion, derealization, dissociation, and depression). As these reactions are self-limiting by definition, in general they provoke minimal functional impairment over time. On the other hand, for a significant minority of the population, the psychological trauma brought about by the experience of profound threat leads to a longer-term syndrome that has been defined, validated, and termed PTSD in the clinical literature. PTSD is often accompanied by devastating functional impairment.
PTSD is characterized by the presence of signs and symptoms in the three primary domains described above for a period extending beyond 1 month (such periods can in some cases occur long after the original, precipitating traumatic exposure). The signs and symptoms of PTSD, therefore, appear to reflect a persistent, abnormal adaptation of neurobiological systems to the stress of witnessed trauma. The neurobiological systems that regulate stress responses include certain endocrine and neurotransmitter pathways as well as a network of brain regions known to regulate fear behavior at both conscious and unconscious levels. Not surprisingly, much research has consequently focused on exploring these systems in more detail as well as attempting to elucidate the pathological changes that occur in patients who develop PTSD. More specifically, there have been and continue to be ongoing efforts to link neurobiological changes identified in patients who suffer from PTSD to the specific clinical features that constitute PTSD, including altered learning/extinction, heightened arousal, and intermittent dissociative behavior as examples relevant to each of the three primary domains. Efforts to identify neurobiological markers for PTSD originally presumed that abnormalities were acquired “downstream” from an exposure, as a consequence of traumatic experience. It could be, however, that certain abnormalities in the patient with PTSD simply represent pre-existing or “upstream” pathology that is functionally dormant until released by trauma exposure and detected thereafter upon investigation. Along these lines, recent interest has focused on factors that seem to modulate outcome variation in neurobiological systems following trauma exposure including genetic susceptibility factors, female gender, prior trauma, early developmental stage at the time of traumatic exposure, and physical injury (including traumatic brain injury - TBI) at the time of psychological trauma; these parameters likely contribute to vulnerability for, versus resilience against, developing PTSD.
Although the biological, psychological, and social ramifications of PTSD have been under scientific scrutiny for some time now, and treatment has improved dramatically, much remains unknown about this condition and controversy persists in both the neuroscientific as well as the clinical/treatment literature. In this text, we review the neurobiological impact of psychological trauma from the perspective that genetic, developmental, and experiential factors predispose certain individuals to the development of PTSD. More specifically, we review the current database as pertains to biological markers of PTSD and the possibility that some biological markers may not be acquired but, rather, may in fact predate trauma until functionally “unmasked” by stress. Where relevant, we also make note of similarities between PTSD and TBI, which extend beyond wellknown signs and symptoms (such as irritability and social withdrawal) to include abnormalities in the same neurobiological systems. Lastly, the article includes a short section on basic considerations for future direction. Ideas put forth in this communication are done so in the interest of developing a consistent model for conceptual purposes. It is recognized at the outset that numerous inconsistencies can be found in the literature that highlight the multifactorial and complex nature of this field.
The biology of PTSD
There are a number of factors that must be considered in contemplating the interplay between adverse environmental stimulation, stress responses/reactions, and pathology. In this section, basic findings are reviewed from endocrinology, neurochemistry, and brain circuitry research conducted on patients with a diagnosis of PTSD (Table I).
Table I. Summary of neurobiological features with identified abnormalities and functional implications in patients with post-traumatic stress disorder. CRH, corticotropin-releasing hormone; 5HT, serotonin; GABA, y-aminobutyric acid; NPY, neuropeptide Y; ACTH, adrenocorticotropin; NE, norepinephrine; CSF, cerebrospinal fluid.
| Feature | Change | Effect |
| A. Neuroendocrine | ||
| Hypothalamic-pituitary-adrenal axis | Hypocortisolism | Disinhibits CRH/NE and upregulates response to stress |
| Drives abnormal stress encoding and fear processing | ||
| Sustained, increased level of CRH | Blunts ACTH response to CRH stimulation | |
| Promotes hippocampal atrophy | ||
| Hypothalamic-pituitary-thyroid axis | Abnormal T3: T4 ratio | Increases subjective anxiety |
| B. Neurochemical | ||
| Catecholamines | Increased dopamine levels | Interferes with fear conditioning by mesolimbic system |
| Increased norepinephrine levels/activity | Increases arousal, startle response, encoding of fear memories | |
| Increases pulse, blood pressure, and response to memories | ||
| Serotonin | Decreased concentrations of 5 HT in:
|
Disturbs dynamic between amygdala and hippocambus |
| Compromises anxiolytic effects | ||
| Increases vigilance, startle, impulsivity, and memory intrusions | ||
| Amino acids | Decreased GABA activity | Compromises anxiolytic effects |
| Increased glutamate | Fosters derealization and dissociation | |
| peptides | Decreased plasma NPY concentrations | Leaves CRH/NE unopposed and upregulates response to stress |
| Increased CSF b-endorphin levels | Fosters numbing, stress-induced analgesia, and dissociation | |
| C. Neuroanatomic | ||
| Hippocampus | Reduced volume and activity | Alters stress responses and extinction |
| Amygdala | Increased activity | Promotes hypervigilance and impairs discrimination of threat |
| Cortex | Reduced prefrontal volume | Dysregulates executive functions |
| Reduced anterior cingulate volume | Impairs the extinction of fear responses | |
| Decreased medial prefrontal activation | Unclear |
Endocrine factors
Core endocrine features of PTSD include abnormal regulation of Cortisol and thyroid hormones, though there is some disagreement about these findings in the literature. Of note, endocrine dysregulation is also found in patients diagnosed with TBI as a result of damage to the pituitary stalk.
The hypothalamic-pituitary-adrenal axis
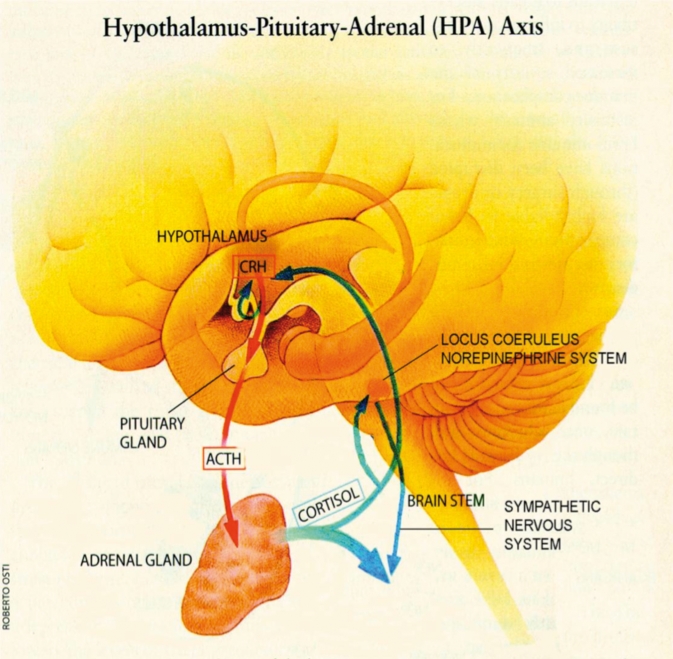
The hypothalamic-pituitary-adrenal (HPA) axis is the central coordinator of the mammalian neuroendocrine stress response systems, and as such, it has been a major focus of scrutiny in patients with PTSD (Figure 1.) In short, the HPA axis is made up of endocrine hypothalamic components, including the anterior pituitary, as well as an effector organ, the adrenal glands. Upon exposure to stress, neurons in the hypothalamic paraventricular nucleus (PVN) secrete corticotropin-releasing hormone (CRH) from nerve terminals in the median eminence into the hypothalamo-hypophyscal portal circulation, which stimulates the production and release of adrenocorticotropin (ACTH) from the anterior pituitary. ACTH in turn stimulates the release of glucocorticoids from the adrenal cortex. Glucocorticoids modulate metabolism as well as immune and brain function, thereby orchestrating physiological and organismal behavior to manage stressors. At the same time, several brain pathways modulate HPA axis activity. In particular, the hippocampus and prefrontal cortex (PFC) inhibit, whereas the amygdala and aminergic brain stem neurons stimulate, CRH neurons in the PVN. In addition, glucocorticoids exert negative feedback control of the HPA axis by regulating hippocampal and PVN neurons. Sustained glucocorticoid exposure has adverse effects on hippocampal neurons, including reduction in dendritic branching, loss of dendritic spines, and impairment of neurogenesis.3-5
Figure 1. The hypothalamic-pituitary-adrenal axis is the body's major response system for stress. The hypothalamus secretes CRH, which binds to receptors on pituitary cells, which produce/release ACTH, which is transported to the adrenal gland where adrenal hormones such as Cortisol are produced/released. The release of Cortisol activates sympathetic nervous pathways and generates negative feedback to both the hypothalamus and the anterior pituitary. This negative feedback system appears to be compromised in patients with post-traumatic stress disorder. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropin.
Although stressors as a general rule activate the HPA axis, studies in combat veterans with PTSD demonstrate decreases in Cortisol concentrations, as detected in urine or blood, compared with healthy controls and other com parator groups. This surprising finding, though replicated in PTSD patients from other populations including Holocaust survivors, refugees, and abused persons, is not consistent across all studies.6 It has been suggested that inconsistent findings may result from differences in the severity and timing of psychological trauma, the patterns of signs/symptoms, comorbid conditions, personality, and genetic makeup.7 Studies using low-dose dexamethasone suppression testing suggest that hypocortisolism in PTSD occurs due to increased negative feedback sensitivity of the HPA axis. Sensitized negative feedback inhibition is supported by findings of increased glucocorticoid receptor binding and function in patients with PTSD.6 Further, sustained increases of CRH concentrations have been measured in cerebrospinal fluid (CSF) of patients with PTSD. As such, blunted ACTH responses to CRH stimulation implicate a role for the downregulation of pituitary CRH receptors in patients with PTSD.6 In addition, reduced volume of the hippocampus, the major brain region inhibiting the HPA axis, is a cardinal feature of PTSD.8 Taken as a whole, these neuroendocrine findings in PTSD reflect dysregulation of the HPA axis to stressors.6
In the context of the above discussion, prospective studies suggest that low Cortisol levels at the time of exposure to psychological trauma may predict the development of PTSD.9,10 Therefore, hypocortisolism might be a risk factor for maladaptive stress responses and predispose to future PTSD. This hypothesis is supported in principle by the finding that exogenously administered hydrocortisone shortly after exposure to psychological trauma can prevent PTSD.11,12 In addition, it has been shown that simulation of a normal circadian Cortisol rhythm using exogenously introduced hydrocortisone is effective in the treatment of PTSD.13 In sum, it may be that decreased availability of Cortisol, as a result of or in combination with abnormal regulation of the HPA axis, may promote abnormal stress reactivity and perhaps fear processing in general. That said, it should be noted that glucocorticoids interfere with the retrieval of traumatic memories, an effect that may independently prevent or reduce symptoms of PTSD.14
The hypothalamic-pituitary-thyroid axis
The hypothalamic-pituitary-thyroid (HPT) axis is involved in regulating metabolic versus anabolic states and other homeostatic functions, which it does by controlling the blood level of thyroid hormones. A possible role for the HPT axis in stress-related syndromes has been suspected for some time because it is known that trauma can trigger thyroid abnormalities. To date, however, there has not been a significant research effort targeting the relationship between the HPT axis and PTSD. Studies have been conducted, however, on Vietnam Veterans with PTSD who were found to have elevated baseline levels of both tri-iodothyronine (T3) and thyroxine (T4). Of note, the level of '13 in these subjects was disproportionately elevated relative to T4, implicating an increase in the peripheral deiodinization process.15,16
These findings were replicated for the most part in a study of WWII Veterans with more longstanding PTSD diagnoses. In these individuals, isolated T3 levels were elevated whereas T4 levels were normal.17 Taken together, these studies suggest that over time the impact of trauma on T4 levels may abate. The authors suggest that elevated T3 may relate to subjective anxiety in these individuals with PTSD.
Neurochemical factors
Core neurochemical features of PTSD include abnormal regulation of catecholamine, serotonin, amino acid, peptide, and opioid neurotransmitters, each of which is found in brain circuits that regulate/integrate stress and fear responses. Of note, catecholamine and serotonin (as well as acetylcholine) dysregulation is also found in patients diagnosed with TBI, presumably as a result of diffuse axonal injury.
The catecholamines
the catecholamine family of neurotransmitters, including dopamine (DA) and norepinephrine (NE), derive from the amino acid tyrosine. Increased urinary excretion of DA and its metabolite has been reported in patients with PTSD. Further, mesolimbic DA has been implicated in fear conditioning. There is evidence in humans that exposure to stressors induces mesolimbic DA release, which in turn could modulate HPA axis responses. Whether or not DA metabolism is altered in PTSD remains unclear, though genetic variations in the DA system have been implicated in moderating risk for PTSD (see below). NE, on the other hand, is one of the principal mediators of autonomic stress responses through both central and peripheral mechanisms. The majority of CNS NE is derived from neurons of the locus ceruleus (LC) that project to various brain regions involved in the stress response, including the prefrontal cortex, amygdala, hippocampus, hypothalamus, periaqueductal grey, and thalamus. In addition, there is evidence for a feed-forward circuit connecting the amygdala and hypothalamus with the LC, in which CRH and NE interact to increase fear conditioning and encoding of emotional memories, enhance arousal and vigilance, and integrate endocrine and autonomic responses to stress. Like other stress pathways, this cascade is inhibited by glucocorticoids,18 which serve as a “brake” for the system. In the periphery, stress-induced sympathetic nervous system activation results in the release of NE and epinephrine from the adrenal medulla, increased release of NE from sympathetic nerve endings, and changes in blood flow to a variety of organs as needed for fight-or-flight behavior. The NE effects arc mediated via postsynaptic α1 β1 , and β2, receptors, whereas another NE-activated receptor, the α2 receptor serves as a presynaptic autoreceptor inhibiting NE release. Because of its multiple roles in regulating arousal and autonomic stress responses, as well as promoting the encoding of emotional memories, NE has been a central focus of many studies investigating the pathophysiology of PTSD.
A cardinal feature of patients with PTSD is sustained hyperactivity of the autonomic sympathetic branch of the autonomic nervous system, as evidenced by elevations in heart rate, blood pressure, skin conductance, and other psychophysiological measures. Accordingly, increased urinary excretion of catecholamines, and their metabolites, has been documented in combat veterans, abused women, and children with PTSD. In addition, patients with PTSD exhibit increased heart rate, blood pressure, and NE responses to traumatic reminders. Decreased platelet α2 receptor binding further suggests NE hyperactivity in PTSD.19,20 Administration of the α2 receptor antagonist yohimbine, which increases NE release, induces flashbacks and increased autonomic responses in patients with PTSD.21 Serial sampling revealed sustained increases in CSF NE concentrations and increased CSF NE responses to psychological stressors in PTSD:22,23 Taken together, there is an abundance of evidence that NF, accounts for certain classic aspects of PTSD symptomatology, including hyperarousal, heightened startle, and increased encoding of fear memories.20
Interestingly, prospective studies have shown that increased heart rate and peripheral epinephrine excretion at the time of exposure to trauma predict subsequent development of PTSD.10 Further, administration of the centrally acting β-adrenergic receptor antagonist propranolol shortly after exposure to psychological trauma has been reported to reduce PTSD symptom severity and reactivity to trauma cues.24 Although propranolol administration in this study did not prevent the development of PTSD, it may have blocked traumatic memory consolidation,25 and therefore may reduce the severity and/or chronicity of PTSD. It is important to note, however, that this finding contradicts those from an earlier study.26 Various antiadrenergic agents have been tested for their therapeutic efficacy in the treatment of PTSD in open-label trials; there is a paucity of controlled trials.20
Serotonin
Serotonin (5HT), is a monoamine neurotransmitter synthesized from the amino acid tryptophan. Neurons containing 5HT originate in the dorsal and median raphe nuclei in the brain stem and project to multiple forebrain regions, including the amygdala, bed nucleus of the stria terminalis, hippocampus, hypothalamus, and prefrontal cortex. 5HT has roles in regulating sleep, appetite, sexual behavior, aggression/impulsivity, motor function, analgesia, and neuroendocrine funtion. Not surprisingly, given its connectivity and broad homeostatic role, 5HT has been implicated in the modulation of affective and stress responses, as well as a role in PTSD. Although the mechanisms are not entirely clear, the effects of 5HT on affective and stress responses vary according to stressor intensity, brain region, and receptor type. It is believed that 5HT neurons of the dorsal raphe mediate anxiogenic effects via 5HT2 receptors through projections to the amygdala and hippocampus. In contrast, 5HT neurons from the median raphe are thought to mediate anxiolytic effects, facilitate extinction and suppress encoding of learned associations via 5HT1A receptors. Chronic exposure to stressors induces upregulation of 5HT2 and downregulation of 5HT1A receptors in animal models. Further, 5HT1A knockouts exhibit increased stress responses.
The 5HT system interacts with the CRH and NE systems in coordinating affective and stress responses.19,27 Indirect evidence suggests a role for 5HT in PTSDrelated behaviors including impulsivity, hostility, aggression, depression, and suicidally. In addition, 5HT presumably mediates the therapeutic effects of the selective serotonin reuptake inhibitors (SSRIs). A recent small and controversial study suggests that the street drug 3,4-Methylenedioxymetharnphetamine (also known as .MDMA or “ecstasy”), which alters central serotonin transmission, has therapeutic potential in the treatment of PTSD.28 Other evidence for altered 5 HT neurotransmission in PTSD includes decreased serum concentrations of 5HT, decreased density of platelet 5HT uptake sites, and altered responsiveness to CNS serotonergic challenge in patients diagnosed with PTSD.19,27 However, no differences in CNS 5HT1A receptor binding were detected in patients with PTSD compared with controls using PET imaging.28 Taken together, altered 5HT transmission may contribute to symptoms of PTSD including hypervigilance, increased startle, impulsivity, and intrusive memories, though the exact roles and mechanisms remain uncertain.
Amino acids
γ-Aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the brain. GABA has profound anxiolytic effects and dampens behavioral and physiological responses to stressors, in part by inhibiting the CRH/NE circuits involved in mediating fear and stress responses. GABA's effects are mediated by GABAA receptors, which are colocalized with benzodiazepine receptors that potentiate the inhibitory effects of GABA on postsynaptic elements. Uncontrollable stress leads to alterations of the GABA/benzodiazepine receptor complex such that patients with PTSD exhibit decreased peripheral benzodiazepine binding sites.29 Further, SPECT and PET imaging studies have revealed decreased binding of radiolabeled benzodiazepine receptor ligands in the cortex, hippocampus, and thalamus of patients with PTSD, suggesting that decreased density or receptor affinity may play a role in PTSD.30-31 However, treatment with benzodiazepines after exposure to psychological trauma does not prevent PTSD.32-33 Further, a recent study suggests that traumatic exposure at times of intoxication actually facilitates the development of PTSD.34 Although perhaps counterintuitive, the authors suggest that the contextual misperceptions which commonly accompany alcohol intoxication may serve to make stressful experiences more difficult to incorporate intellectually, thereby exacerbating fear. Taken together, while there are multiple studies strongly implicating the GABA/bcnzodiazepine receptor system in anxiety disorders, studies in PTSD are relatively sparse and conclusive statements would be premature.19
Glutamate is the primary excitatory neurotransmitter in the brain. Exposure to stressors and the release of, or administration of, glucocorticoids activates glutamate release in the brain. Among a number of receptor subtypes, glutamate binds to N -methyl D -aspartate (NMDA) receptors that are localized throughout the brain. The NMDA receptor system has been implicated in synaptic plasticity, as well as learning and memory, thereby contributing in all likelihood to consolidation of trauma memories in PTSD. The NMDA receptor system is also believed to play a central role in the derealization phenomena and dissocation associated with illicit and medical uses of the anesthetic ketamine. In addition to its role in learning and memory, overexposure of neurons to glutamate is known to be excitotoxic, and may contribute to the loss of neurons and/ or neuronal integrity in the hippocampus and prefrontal cortex of patients with PTSD. Of additional note, elevated glucocorticoids increase the expression and/or sensitivity of NMDA receptors, which may render the brain generally more vulnerable to excitoxic insults at times of stress.
Peptides
CRH neurons in the hypothalamic PVN integrate information relevant to stress and thereby serve as a major component of the HPA axis. CRH neurons are also found in widespread circuitry throughout the brain, including the prefrontal and cingulate cortices, central nucleus of the amygdala, the bed nucleus of the stria terminais, hippocampus, nucleus accumbens, periaqueductal gray, and locus coeruleus (LC) as well as both dorsal and median raphe. Direct injection of CRH into the brain of laboratory animals produces physiological stress responses and anxiety-like behavior, including neophobia (fear of new things or experiences), enhanced startle, and facilitated fear conditioning. Anxiety -like behaviors have been specifically linked with increased activity of amygdalar CRH-containing neurons that project to the LC. Of note, glucocorticoids inhibit CRH-induced activation of LC noradrenergic neurons, providing a potential mechanism by which low Cortisol may facilitate sustained central stress and fear responses. The effects of CRH are mediated primarily through two CRH receptor subtypes, CRH2., and CRH2. In animal experiments, both exogenous administration of a CRH1, receptor antagonist, and experimental knockout of the CRH1 receptor, produce attenuated stress responses and reduced anxiety. A recent experiment demonstrated that CRHj receptor blockade impacted not only gastrointestinal measures of chronic stress, but also prevented stress-induced hair loss in rodents.35 Thus, CRH] receptor stimulation may be involved in facilitating stress responses and anxiety. By contrast, CRH7 knockout mice demonstrate stress sensitization and increased anxiety, suggesting a role for CRH2 receptor activation in reducing stress reactivity.3 Given the central effects of CRH, as described in animal models, increased CNS CRH activity may promote certain of the cardinal features of PTSD, such as conditioned fear responses, increased startle reactivity, sensitization to stressor exposure, and hyperarousal. These results suggest that CRH] receptor antagonists and/or CRH, agonists might have important therapeutic potential in the treatment of PTSD.
Neuropeptide Y (NPY) may well be protective against the development of PTSD in that it has anxiolytic and stress-buffering properties. NPY has been shown to inhibit CRH/NE circuits involved in stress and fear responses and to reduce the release of NE from sympathetic neurons. As such, a lack of NPY may promote maladaptive stress responses and contribute to the development of PTSD. Indeed, patients with PTSD have been reported to exhibit decreased plasma NPY concentrations and blunted NPY responses to yohimbine challenge, compared with controls. Together, these findings suggest that decreased NPY activity may contribute to noradrenergic hyperactivity in PTSD.36 Moreover, it has been suggested that NPY may be involved in promoting recovery from, or perhaps resilience to PTSD, given that combat veterans without PTSD have been shown to exhibit elevated NPY levels compared with veterans with PTSD.6
Endogenous opioid peptides including the endorphins and enkephalins act upon the same CNS receptors activated by exogenous opioid molecules such as morphine or heroin. Endogenous opioids exert inhibitory influences on the HPA axis. Naloxone, an opioid receptor antagonist, increases HPA axis activation as evidenced by exaggerated HPA axis response to naloxone. PTSD patients exhibit increased CSF p-endorphin levels, suggesting increased activation of the endogenous opioid system. Alterations in endogenous opioids may be involved in certain PTSD symptoms such as numbing, stress-induced analgesia, and dissociation. Of additional interest, the nonselective opioid receptor antagonist, naltrexone, appears to be effective in treating symptoms of dissociation and flashbacks in traumatized persons.19,37 Further, the administration of morphine has been reported to prevent PTSD.38 Of note, an experiment investigating the hypothesis that PTSD may play an ctiologic role in fostering opioid addiction in an opioiddependent group of subjects rendered negative results.39
Brain circuitry
Characteristic changes in brain structure and function have been identified in patients with PTSD using brainimaging methods.40-42 Brain regions that arc altered in patients with PTSD include the hippocampus and amygdala as well as cortical regions including the anterior cingulate, insula, and orbitofrontal region. These areas interconnect to form a neural circuit that mediates, among other functions, adaptation to stress and fear conditioning. Changes in these circuits have been proposed to have a direct link to the development of PTSD.40 Recent work raises the question as to which CNS elements are involved in circuit changes resulting from stress, and suggests a critical role for myelin.43 Similar to PTSD, brain areas most impacted by TBI include inferior frontal and temporal lobes, and it is likely that myelinated circuits are subject to damage broadly as a result of shear forces.
Hippocampus
A hallmark feature of PTSD is reduced hippocampal volume. The hippocampus is implicated in the control of stress responses, declarative memory, and contextual aspects of fear conditioning. Not surprisingly, the hippocampus is one of the most plastic regions in the brain. As mentioned above, prolonged exposure to stress and high levels of glucocorticoids in laboratory animals damages the hippocampus, leading to reduction in dendritic branching, loss of dendritic spines, and impairment of neurogenesis.4 Initial magnetic resonance imaging (M.RI) studies demonstrated smaller hippocampal volumes in Vietnam Veterans with PTSD and patients with abuse-related PTSD compared with controls.44-47 Small hippocampal volumes were associated with the severity of trauma and memory impairments in these studies. These findings were generally replicated in most but not all subsequent work. Studies using proton magnetic resonance spectroscopy further observed reduced levels of N-acctyl aspartate (NAA), a marker of neuronal integrity, in the hippocampus of adult patients with PTSD.40 Of note, NAA reductions were correlated with Cortisol levels.48 Interestingly, reduced hippocampal volume has been observed in depressed women with a history of early life trauma49 but not in children with PTSD.50
Hippocampal volume reduction in PTSD may reflect the accumulated toxic effects of repeated exposure to increased glucocorticoid levels or increased glucocorticoid sensitivity, though recent evidence also suggests that decreased hippocampal volumes might be a pre-existing vulnerability factor for developing PTSD.24 Indeed, hippocampal deficits may promote activation of and failure to terminate stress responses, and may also contribute to impaired extinction of conditioned fear as well as deficits in discriminating between safe and unsafe environmental contexts. Studies using functional neuroimaging have further shown that PTSD patients have deficits in hippocampal activation during a verbal declarative memory task.51 Both hippocampal atrophy and functional deficits reverse to a considerable extent after treatment with SSRIs,52 which have been demonstrated to increase neurotrophic factors and neurogenesis in some preclinical studies,5 but not others.53
Amygdala
The amygdala is a limbic structure involved in emotional processing and is critical for the acquisition of fear responses. The functional role of the amygdala in mediating both stress responses and emotional learning implicate its role in the pathophysiology of PTSD. Although there is no clear evidence for structural alterations of the amygdala in PTSD, functional imaging studies have revealed hyper-responsiveness in PTSD during the presentation of stressful scripts, cues, and/or trauma reminders.41 PTSD patients further show increased amygdala responses to general emotional stimuli that are not trauma-associated, such as emotional faces.41 The amygdala also seems to be sensitized to the presentation of subliminally threatening cues in patients with PTSD,54-56 and increased activation of the amygdala has been reported in PTSD patients during fear acquisition in a conditioning experiment.57 Given that increased amygdala reactivity has been linked to genetic traits which moderate risk for PTSD,58,59 increased amygdala reactivity may represent a biological risk factor for developing PTSD.
Cortex
The medial prefrontal cortex (PFC) comprises the anterior cingulate cortex (ACC), subcallosal cortex, and the medial frontal gyrus. The medial PFC exerts inhibitory control over stress responses and emotional reactivity in part by its connections with the amygdala. It further mediates extinction of conditioned fear through active inhibition of acquired fear responses.41 Patients with PTSD exhibit decreased volumes of the frontal cortex,60 including reduced ACC volumes.61,62 This reduction in ACC volume has been correlated with PTSD symptom severity in some studies. In addition, an abnormal shape of the ACC,63 as well as a decrease of NAA levels in the ACC,64 has been reported for PTSD patients. A recent twin study suggests that, unlike the hippocampus, volume loss in the ACC is secondary to the development of PTSD rather than a pre-existing risk factor.65 Functional imaging studies have found decreased activation of the medial PFC in PTSD patients in response to stimuli, such as trauma scripts,66,67 combat pictures and sounds,68 trauma-unrelated negative narratives,69 fearful faces,70 emotional stroop,71 and others, though there are also discordant findings.41 Reduced activation of the medial PFC was associated with PTSD symptom severity in several studies and successful SSRI treatment has been shown to restore medial prefrontal cortical activation patterns.41 Of note, in the abovementioned conditioning experiment,57 extinction of conditioned fear was associated with decreased activation of the ACC, providing a biological correlate for imprinted traumatic memories in PTSD. Not surprisingly, given the connectivity between the amygdala and medial PFC, interactions in activation patterns between these regions have been reported in PTSD, though the direction of the relationship is inconsistent across studies.41
The origin of neurobiological abnormalities in PTSD
A number of studies have investigated the fundamental question as to whether the neurobiological changes identified in patients with PTSD represent markers of neural risk to develop PTSD upon exposure to extreme stress as opposed to abnormalities acquired through traumatic exposure or, most likely, a combination of both. As an example, low Cortisol levels at the time of a trauma predict subsequent development of PTSD. Thus, low levels of Cortisol might be a pre-existing risk factor that engenders the development of PTSD; low levels of Cortisol could disinhibit CRH/NE circuits and thereby promote unopposed autonomic and neuroendocrine responses to stress, as well as augmented fear conditioning and traumatic memory consolidation. Similarly, the reduced size of the hippocampus in PTSD has remained an unresolved question for many years. There has been considerable debate as to whether this brain region shrinks as a result of trauma exposure, or whether the hippocampus of PTSD patients might be smaller prior to trauma exposure. Studies in twins discordant for trauma exposure have provided a means to address this question, though without complete resolution. Gilbertson and colleagues72 studied 40 pairs of identical twins, including Vietnam Veterans who were exposed to combat trauma and their twins who did not serve in Vietnam, and measured hippocampal volumes in all subjects. As expected, among Vietnam Veterans, the hippocampus was smaller in those diagnosed with PTSD as compared with those without a diagnosis. However, this brain region was abnormally smaller in non-PTSD twins as well, despite the absence of trauma exposure and diagnosis. These findings suggest that a smaller hippocampus could be a pre-existing, potentially genetic, neurodevelopmental, and almost surely multifactorial vulnerability factor that predisposes to the development of PTSD (and perhaps other stress-spectrum disorders). Recent results from the same study group indicate, as above, that gray matter loss in the ACC seems on the contrary to be an acquired feature.65 Studies are needed to identify the timing and/or etiology of other hallmark neurobiological features of PTSD.
Risk and resilience for developing PTSD
Individuals exposed to an event that either threatens serious injury/death, or is perceived as such, respond in different ways. Most will experience minimal (seconds) to brief (hours) to short-term (days/weeks) abnormalities while a smaller number will suffer from significant psychopathology over longer-term (months) and chronic (lifetime) time frames. In short, not all individuals who face potentially catastrophic trauma go on to develop PTSD. Why some individuals will develop PTSD following trauma, whereas others do not, is of paramount importance. Because the majority of trauma survivors do not go on to develop PTSD, it is crucial going forward to understand vulnerability and resiliency factors. In this section, the role of genetic factors, gender differences, and early developmental stress experiences in moderating risk for developing PTSD in response to psychological trauma are discussed as is the increased risk for developing PTSD in the context of co-occurring physical traumas (including TBI).
Genetic risk factors for PTSD
Studies on the genetics of PTSD have been hampered by a variety of factors, such as genetic heterogeneity (similar phenotypes develop from different genotypes) and incomplete phenotypic penetrance (a person with genetic risk for PTSD, who is not exposed to trauma, will not develop PTSD). Despite these confounds, there is accumulating evidence that risk for PTSD is heavily influenced by genetic factors. Evidence from family and twin studies has long suggested a heritable contribution to the development of PTSD. In addition, there is evidence for heritable contributions to some of the neurobiological endophenotypes of PTSD as discussed above, such as decreased hippocampal volume72 or exaggerated amygdala reactivity.58 Although it is beyond the scope of this review to comprehensively discuss the genetics of PTSD, it should be noted that there is an emerging literature on genetic variations in those neurobiological systems that drive responses to trauma and, consequently, risk versus resilience to develop PTSD.73
One study has linked a polymorphism in the DA transporter gene to PTSD risk. In this study, PTSD patients were found to have an excess of the SLC6A39 repeat allele. This finding suggests that genetically determined features of DA transmission may contribute to the development of PTSD among trauma survivors.74 Several studies have suggested polymorphisms in the D2 receptor as possible elements of PTSD risk, though results have not been consistent.73 In addition, there is evidence linking a low expression variant of the serotonin transporter to stress responsiveness and risk for developing depression in relation to life stress, particularly in the presence of low social support.59 This finding is intriguing as the same polymorphism is associated with increased amygdala reactivity58 as well as the trait of neuroticism,75 which is another risk factor for PTSD. It must be noted, however, that these findings of genetic risk with regard to the serotonin transporter have recently been questioned.76
Particularly exciting are findings that a genetic variation of the glucocorticoid receptor cochaperone protein, FKBP5, moderates risk of developing PTSD in relation to childhood abuse.77 This study tested interactions of childhood abuse, adulthood trauma, and genetic polymorphisms in the FKBP5 gene in 900 nonpsychiatric, general internal medicine clinic patients. Childhood abuse and adulthood trauma each predicted PTSD symptoms and FKBP5 polymorphisms significantly interacted with childhood abuse to predict adult PTSD symptoms. The FKBP5 genotype was further linked to enhanced glucocorticoid receptor sensitivity, as reflected by dexamethasone hypersuppression, a hallmark feature of PTSD.77 Most recently, Ressler and colleagues have demonstrated that a female-specific elevation of pituitary adenylate cyclase-activating peptide (PACAP) correlated not only with fear physiology and the diagnosis of PTSD78 but also a specific single nucleotide repeat on an estrogen response element in the same subjects. These findings and this type of work may shed new light not only on the well-known differences in PTSD risk between men and women that are discussed in the next section, but on our mechanistic understanding of PTSD in general.
Gender differences and risk for PTSD
Women more frequently suffer from PTSD than men for reasons that are not entirely clear. Women and men are, in general, subjected to different types of trauma, though the differences in PTSD frequency (reportedly 2:1) arc unlikely to be explained solely on the basis of exposure type and/or severity alone. In addition to those findings by Ressler described above, a number of gender-related differences in the neurobiological response to trauma have been documented.79 Rodent studies suggest that females generally exhibit greater magnitude and duration of HPA axis responses to stress than males,80 though findings in humans are not entirely consistent.81 Sex differences in neuroendocrine stress responses have been attributed to direct effects of circulating estrogen on CRH neurons.82 Sex steroids also interact with other neurotransmitter systems involved in the stress response, such as the serotonin system.83 Progesterone has been implicated in modulating these systems as well.84 However, gender differences in HPA responses to stress have also been observed independent of acute gonadal steroid effects.85
Factors that might determine gender differences in the stress response include genomic differences (as above) and/or devclopmentally programmed effects of gonadal steroids.81,85,86 Of note, a very recent study of female Veterans demonstrated that pregnancy raises the risk of PTSD above that for nonpregnant females.87 In addition, sex steroids play a role in structural plasticity across the lifespan of several brain regions, including areas involved in stress responsiveness such as the hippocampus and amygdala.86 Functional imaging studies have identified gender differences in the brain's response to fear stimuli.88 Over time our understanding of this constellation of processes may eventually converge to allow for a better description of the basis for gender differences and, specifically, how the consequences of trauma translate into differential risk for PTSD.
Early developmental factors and PTSD
Previous experience moderates risk for developing PTSD in response to trauma, particularly when exposure to stress occurs early in life. Thus, childhood adversity is associated with increased risk to develop PTSD in response to combat exposure in Vietnam Veterans.51 There is a burgeoning literature documenting that early adverse experience, including prenatal stress and stress throughout childhood, has profound and long-lasting effects on the development of neurobiological systems, thereby “programming” subsequent stress reactivity and vulnerability to develop PTSD.89-91 As an example, children with a history of date violence have recently been shown at risk of developing future PTSD.92 Further, a study of child survivors from the Hurricane Katrina disaster indicates significantly increased risk of PTSD.93 Along these lines, nonhuman primates exposed to a variable foraging demand condition, which causes unpredictable maternal care in the infant, leads to an adult phenotype with sensitization to fear cues, CRH hyperactivity and low Cortisol levels, a pattern of the classic features found in PTSD.94 Consistent with these findings, adult women with childhood trauma histories exhibit sensitization of both neuroendocrine, and autonomic stress responses.95 Studies are needed that identify particular sensitive periods for the effects of early stress, determine parameters for their reversal, and scrutinize the interactions of dispositional factors (genes, gender) with developmental features in determining neurobiological vulnerability to PTSD.
The influence of physical trauma (and TBI) on the development of PTSD
It has been known for some time that physical injury concomitant with psychological trauma increases risk for the development of PTSD. In studies of Vietnam Veterans,96,97 and more recently in a study of Iraq and Afghanistan Veterans,98 it was found that physical injury increased the risk of PTSD at least twofold. Similarly, a literature review of patients with documented TBI and program evaluation data from surveys of US Marines following blast exposures in Iraq99 demonstrate that TBI presents an increased risk for the development of PTSD. Though differentiating the risk of developing PTSD in patients with TBI is complicated by the subjective and objective abnormalities common to both clinical entities, it is striking that each shares common endocrine, neurochemical, and circuit abnormalities (see above, The biology of PTSD ). As such, it would follow that the existence of both diagnoses in an individual patient might be additive if not multiplicative from a clinical standpoint. For example, in the context of TBI (with frontal lobe damage and behavioral disinhibition) it would be reasonable to expect a very high violence risk profile for a patient suffering from the irritability and anger characteristic of comorbid PTSD. Of additional note, the helplessness that accompanies certain physical injuries (perhaps most notably TBI) is certain to compound issues of limited self-efficacy (and the overall lost sense of agency) that characterize PTSD. The psychological challenges of TBI may thereby introduce an additional chronic risk for the victimization that fosters PTSD in those patients with a tendency to become increasingly dependent over time.
A basic model of PTSD neurobiology
The biological perturbations observed in patients suffering from PTSD are numerous, and likely reflect an enduring dysregulation of multiple stress-mediating systems that occurs as a result of a psychological “shock.” These pathophysiological perturbations presumably occur in patients with genetic, epigenetic, and experiential predispositions when exposed to certain extreme conditions. Presumably these changes signify an indelible sensory imprint of a maladaptively processed experience that co-opts an imbalanccd degree of emotional importance and thereafter releases (or restrains) behavioral reactions that focus on defending against future trauma via activation (or deactivation) in a losing effort to secure homeostasis.
Considering neurobiological findings in PTSD patients with this overview in mind, a relative lack of baseline Cortisol at the time of a psychological trauma may facilitate overactivation of the central CRH-NE cascade, resulting in enhanced and prolonged stress responses.6,95 This increased stress responsiveness may be further accentuated by inadequate regulatory effects of GABA, serotonin, and NPY. Additionally, altered norpinephrine and stress hormone activity may be critically involved in processes of learning and extinction, both of which are abnormal in PTSD; for example, norepinephrine enhances the encoding of fear memories and glucococorticoids block the retrieval of emotional memories. The constellation of elevated noradrenergic activity and relative hypocortisolism may lead to the enhanced encoding of traumatic memories and the lack of inhibition of memory retrieval both of which presumably trigger re-experiencing phenomena in PTSD.12
Further, an abnormally functioning hippocampus may account for some of the cognitive symptoms of PTSD, such as declarative memory deficits. In addition, because the hippocampus is critical for context conditioning, an impaired hippocampus may facilitate generalization of learned fear in contexts unrelated to a previous traumatic exposure and impair the ability to discriminate between safe and unsafe stimuli. In combination with exaggerated amygdalar responses seen in patients with PTSD, a limited capacity for discerning threat due to hippocampal and amygdalar dysfunction may promote paranoia, hypervigilance, behavioral activation, exaggerated stress responses, and further acquisition of fear associations. Disrupted prefrontal cortical function may then serve to facilitate PTSD pathology further as a result of deficient suppression of stress responses, fear associations, and extinction.
Future directions
In this article, we have selected findings from a broad range of the PTSD literature to consider the impact of psychological trauma on neurobiological systems. As described, some neurobiological findings in patients with PTSD are controversial and need to be further examined. In addition, there are a number of understudied yet important topics in the field such as factors that impact resiliency and vulnerability. For example, stress-protective neurobiological factors such as activity in oxytocin and NPY-containing circuits could, in principle, be manipulated to promote resilience. In addition, there is a general need to explore further the molecular biology of PTSD; identifying interactions between dispositional factors (genetic and epigenetic) and trauma exposure is critical to understand PTSD risk, gauge illness course, and predict treatment response. The effects of trauma on neurotrophic factors (in the hippocampus), neural plasticity (CNS-wide), circuit remodeling (myelination patterns) and gene expression need to be assessed in detail across illness duration. Though difficult, such studies will necessitate accessing, assaying and following populations at risk for exposure to trauma before any exposure occurs (ideally, predeployment soldiers). Where possible, the distinction between PTSD and TBI must also be better understood. Though the presumed mechanism of injury from psychological trauma as opposed to brain trauma is overtly different, the etiologic abnormalities seem to involve similar neurobiological systems and produce overlapping clinical syndromes.
Acknowledgments
The authors would like to thank Ms Cynthia CriderVega, Ms Magaly Gomez, and Ms Carmen Alsina for their outstanding administrative assistance.
Selected abbreviations and acronyms
- 5 HT
serotonin
- CRH
corticotropin-releasing hormone
- DA
dopamine
- GABA
y-aminobutyric acid
- HPA
hypothalamic-pituitary-adrenal
- NE
norepinephrine
- NPY
neuropeptide Y
- PTSD
post-traumatic stress disorder
Contributor Information
Jonathan E. Sherin, Department of Psychiatry and Behavioral Sciences, University of Miami, Leonard M. Miller School of Medicine, Miami, Florida, USA; Department of Mental Health and Behavioral Sciences, Miami VA Healthcare System, Miami, Florida, USA.
Charles B. Nemeroff, Department of Psychiatry and Behavioral Sciences, University of Miami, Leonard M. Miller School of Medicine, Miami, Florida, USA.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- 2.Yehuda R., LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:1932. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Arborelius L., Owens MJ., Plotsky PM., Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E., Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci . 2000;12:2211–2214. doi: 10.1046/j.1460-9568.2000.00130.x. [DOI] [PubMed] [Google Scholar]
- 5.Nestler EJ., Barrot M., DiLeone RJ., Eisch AJ., Gold SJ., Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N YAcadSci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 7.Meewisse ML., Reitsma JB., de Vries GJ., Gersons BP., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD., Elzinga B., Schmahl C., Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–86. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resnick HS., Yehuda R., Pitman RK., Foy DW. Effect of previous trauma on acute plasma Cortisol level following rape. Am J Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R., McFarlane AC., Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- 11.Schelling G., Kilger E., Roozendaal B., et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.De Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–47. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- 13.Aerni A., Traber R., Hock C., et al. Low-dose Cortisol for symptoms of posttraumatic stress disorder. Ami J Psychiatry. 2004;16:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 14.De Quervain DJ., Margraf J. Glucocorticoids for the treatment of posttraumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 15.Prang AJ. Thyroid axis sustaining hypothesis of posttraumatic stress disorder. Psychosom Med. 1999;61:139–140. doi: 10.1097/00006842-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Prang AJ. Thyroid axis sustaining hypothesis of posttraumatic stress disorder. Psychosom Med. 1999;61:139–140. doi: 10.1097/00006842-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Wang S., Mason J. Elevations of serum T3 levels and their association with symptoms in WWII veterans with combat-related posttraumatic stress disorder: replication of findings in Vietnam combat veterans. Psychosom Med. 1999;61:131–138. doi: 10.1097/00006842-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pavcovich LA., Valentino RJ. Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci. 1997;17:401–408. doi: 10.1523/JNEUROSCI.17-01-00401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermetten E., Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 20.Strawn JR., Geracioti TD. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 21.Southwick SM., Bremner JD., Rasmusson A., Morgan CA 3rd., Arnsten A., Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 22.Geracioti TD Jr., Baker DG., Ekhator NN., et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Ami Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 23.Geracioti TD Jr., Baker DG., Kasckow JW., et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Pitman RK., Sanders KM., Zusman RM., et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 25.Brunet A., Orr SP., Tremblay J., Robertson K., Nader K., Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Stein MB., Kerridge C., Dimsdale JE., Hoyt DB. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof -of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 27.Ressler K., Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Bonne O., Bain E., Neumeister A., et al. No change in serotonin type 1Â receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162:383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- 29.Gavish M., Laor N., Bidder M., et al. Altered platelet peripheral-type benzodiazepine receptor in posttraumatic stress disorder. Neuropsychopharmacology. 1996;14:181–186. doi: 10.1016/0893-133X(95)00078-R. [DOI] [PubMed] [Google Scholar]
- 30.Bremner JD., Innis RB., Southwick SM., Staib L., Zoghbi S., Charney DS. Decreased benzodiazepine receptor binding in prefrontal cortex in combatrelated posttraumatic stress disorder. Am J Psychiatry. 2000;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 31.Geuze E., van Berckel BN., Lammertsma AA., et al. Reduced GABAÂ benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry. 2008;13:74–83. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- 32.Gelpin E., Bonne O., Peri T., Brandes D., Shalev AY. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57:390–394. [PubMed] [Google Scholar]
- 33.Mellman TA., Bustamante V., David D., Fins A. Hypnotic medication in the aftermath of trauma (letter). J Clin Psychiatry. 2002;63:1183–1184. doi: 10.4088/jcp.v63n1214h. [DOI] [PubMed] [Google Scholar]
- 34.Bisby JA., King JA., Brewin CR., Burgess N., Curran HV. Acute effects of alcohol on intrusive memory development and viewpoint dependence in spatial memory support a dual representation model. Biol Psychiatry. 2010;68:280–286. doi: 10.1016/j.biopsych.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang LX., Million M., Rivier J., et al. CRF receptor antagonist astressin-B reverses and prevents alopecia in CRF over-expressing mice. PLoS One. In press. doi: 10.1371/journal.pone.0016377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmusson AM., Hauger RL., Morgan CA., Bremner JD., Charney DS., Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 37.Newport DJ., Nemeroff CB. Neurobiology of posttraumatic stress disorder. Cum Opin Neurobiol. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 38.Holbrook TL., Galarneau MR., Dye JL., Quinn K., Dougherty AL. Morphine use after combat injury in iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 39.Boscarino JA., Rukstalis M., Hoffman SN., et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US healthcare system. Addiction. 2010;105:1776–1782. doi: 10.1111/j.1360-0443.2010.03052.x. [DOI] [PubMed] [Google Scholar]
- 40.Rauch SL., Shin LM., Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Shin LM., Rauch SL., Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD., Elzinga B., Schmahl C., Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartzokis G., Po HL., Turner J., Saunders CS. Adjunctive risperdone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 2004;57:474–479. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 44.Bremner JD., Randall P., Scott TM., et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bremner JD., Randall P., Vermetten E., et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse- a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurvits TV., Shenton ME., Hokama H., et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein MB., Koverola C., Hanna C., Torchia MG., McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 48.Neylan TC., Schuff N., Lenoci M., Yehuda R., Weiner MW., Marmar CR. Cortisol levels are positively correlated with hippocampal N-acetylaspartate. Biol Psychiatry. 2003;54:1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vythilingam M., Heim C., Newport J., et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2081. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Bellls MD., Keshavan MS., Clark DB., et al. AE Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 51.Bremner JD., Vythilingam M., Vermetten E., et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 52.Bremner JD., Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann N Y Acad Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- 53.Hanson ND., Nemeroff CB., Owens MJ. Lithium, but not fluoxetine or the corticotropin-releasing factor receptor 1 receptor antagonist R121919, Increases cell proliferation in the adult dentate gyrus. J Pharmacol Exp Ther. 2011;337:180–186. doi: 10.1124/jpet.110.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendler T., Rotshtein P., Yeshurun Y., et al. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 55.Rauch SL., Whalen PJ., Shin LM., et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 56.Bryant RA., Kemp AH., Felmingham KL., et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bremner JD., Vermetten E., Schmahl C., et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hariri AR., Mattay VS., Tessitore A., et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 59.Kilpatrick DG., Koenen KC., Ruggiero KJ., et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 60.Rauch SL., Shin LM., Segal E., et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 61.Yamasue H., Kasai K., Iwanami A., et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci USA. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodward SH., Kaloupek DG., Streeter CC., Martinez C., Schaer M., Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 63.Corbo V., Clément MH., Armony JL., Pruessner JC., Brunei A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry. 2005;58:119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 64.De Bellls MD., Keshavan MS., Spencer S., Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 65.Kasai K., Yamasue H., Gilbertson MW., Shenton ME., Rauch SL., Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin LM., Orr SP., Carson MA., et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 67.Britton JC., Phan KL., Taylor SF., Fig LM., Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Bremner JD., Narayan M., Staib LH., Southwick SM., McGlashan T., Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanius RA., Williamson PC., Hopper J., et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 70.Shin LM., Wright CI., Cannistraro PA., et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 71.Bremner JD., Vermetten E., Vythilingam M., et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Gilbertson MW., Shenton ME., Ciszewski A., et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broekman BF., Olff M., Boer F. The genetic background to PTSD. Neurosci BiobehavRev. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Segman RH., Cooper-Kazaz R., Macciardi F., et al. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- 75.Lesch KP., Bengel D., Heils A., et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 76.Risch N., Herrell R., Lehner T., et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Binder EB., Bradley RG., Liu W., et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ressler KJ., Mercer KB., Bradley B., et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becker JB., Monteggia LM., Perrot-Sinal TS., et al. Stress and disease: is being female a predisposing factor?. J Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhodes ME., Rubin RT. Functional sex differences ('sexual diergism') of central nervous system cholinergic systems, vasopressin, and hypothalamicpituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 81.Kudielka BM., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Vamvakopoulos NC., Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bethea CL., Mirkes SJ., Su A., Michelson D. Effects of oral estrogen, raloxifene and arzoxifene on gene expression in serotonin neurons of macaques. Psychoneuroendocrinology: 2002;27:431–445. doi: 10.1016/s0306-4530(01)00054-3. [DOI] [PubMed] [Google Scholar]
- 84.Centeno ML., Reddy AP., Smith LJ., et al. Serotonin in microdialysate from the mediobasal hypothalamus increases after progesterone administration to estrogen primed macaques. Eur J Pharmacol. 2007;555:67–75. doi: 10.1016/j.ejphar.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roca CA., Schmidt PJ., Deuster PA., et al. Sex-related differences in stimulated hypothalami ic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endo Metab. 2005;90:4224–4231. doi: 10.1210/jc.2004-2525. [DOI] [PubMed] [Google Scholar]
- 86.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 87.Mattocks KM., Skanderson M., Goulet JL. Pregnancy and mental health among women veterans returning from Iraq and Afghanistan. J Womens Health (Larchmt). 2010;19:2159–2166. doi: 10.1089/jwh.2009.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schienle A., Schafer A., Stark R., Walter B., Vaitl D. Gender differences in the processing of disgust- and fear-inducing pictures: an fMRI study. Neuroreport. 2005;16:277–280. doi: 10.1097/00001756-200502280-00015. [DOI] [PubMed] [Google Scholar]
- 89.Seckl JR., Meaney MJ. Glucocorticoid “programming” and PTSD risk. Ann N Y Acad Sci. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- 90.Meaney MJ., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- 92.Wolitzky-Taylor KB., Ruggiero KJ., Danielson CK., et al. Prevalence and correlates of dating violence in a national sample of adolescents. .Mm. Acad Child Adolesc Psychiatry. 2008;47:755–762. doi: 10.1097/CHI.0b013e318172ef5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLaughlin KA., Fairbank JA., Gruber MJ., et al. Trends in serious emotional disturbance among youths exposed to Hurricane Katrina. J Am Acad Child Adolesc Psychiatry. 2010;49:990–1000. doi: 10.1016/j.jaac.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coplan JD., Andrews MW., Rosenblum LA., et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heim C., Newport DJ., Heit S., et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 96.Kulka RA. . Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. New York, NY: Brunner/Mazel. 1990 [Google Scholar]
- 97.Pitman RK., Altman B., Macklin ML. Prevalence of post- traumatic stress disorder in wounded Vietnam veterans. Am J Psychiatry. 1989;146:667–669. doi: 10.1176/ajp.146.5.667. [DOI] [PubMed] [Google Scholar]
- 98.Koren D., Norman D., Cohen A., Berman J., Klein EM. Increased PTSD risk with combat-related injury: a matched comparison study of injured and uninjured soldiers experiencing the same combat events. Am J Psychiatry. 2005;162:276–282. doi: 10.1176/appi.ajp.162.2.276. [DOI] [PubMed] [Google Scholar]
- 99.Van Reekum R., Cohen T., Wong J. Can traumatic brain injury cause psychiatric disorders?. J Neuropsychiatry Clin Neurosci. 2000;12:316–327. doi: 10.1176/jnp.12.3.316. [DOI] [PubMed] [Google Scholar]