Abstract
Traumatic brain injury (TBI) is a worldwide public health problem typically caused by contact and inertial forces acting on the brain. Recent attention has also focused on the mechanisms of injury associated with exposure to blast events or explosions. Advances in the understanding of the neuropathophysiology of TBI suggest that these forces initiate an elaborate and complex array of cellular and subcellular events related to alterations in Ca++ homeostasis and signaling. Furthermore, there is a fairly predictable profile of brain regions that are impacted by neurotrauma and the related events. This profile of brain damage accurately predicts the acute and chronic sequelae that TBI survivors suffer from, although there is enough variation to suggest that individual differences such as genetic polymorphisms and factors governing resiliency play a role in modulating outcome. This paper reviews our current understanding of the neuropathophysiology of TBI and how this relates to the common clinical presentation of neurobehavioral difficulties seen after an injury.
Keywords: neurotrauma, neurobehavior, traumatic brain injury, neuropsychiatry of TBI
Abstract
El daño cerebral traumático (DCT) es un problema de salud pública mundial causado característicamente por fuerzas de contacto o de inercia que actúan sobre el cerebro. La preocupación reciente se ha centrado en los mecanismos de daño asociado con la exposición al efecto de ráfagas o explosiones. Los avances en la comprensión de la neurofisiopatologáa del DCT sugieren que estas fuerzas inician y producen una serie compleja de acontecimientos celulares y subcelulares relacionados con alteraciones en la homeostasis y mecanismos de señales del Ca++. Además, hay un perfil bastante predecible de regiones cerebrales que son afectadas por el neurotrauma y los acontecimientos relacionados. Este perfil de daño cerebral predice con precisión las secuelas agudas y crónicas que sufren los supervivientes de un DCT, aunque existe bastante variación que sugiere que las diferencias individuales - como los polimorfismos genéticos y los factores que regulan la resiliencia - tienen un papel en la modulación de los resultados. Este artículo revisa la comprensión actual de la neurofisiopatología del DCT y cómo se relaciona ésta con la presentación clínica habitual de las dificultades neuroconductuales que se observan después de una lesión.
Abstract
La lésion cérébrale traumatique (LCT), problème de santé publique mondial, est provoquée par un contact et des forces d'inertie agissant sur le cerveau. Récemment, l'intérêt s'est porté aussi sur les mécanismes des lésions associées aux explosions ou aux phénomènes de souffle. Les avancées dans la compréhension de la neurophysiopathologie de la LCT laissent supposer que ces forces sont à l'origine d'une série élaborée et complexe d'événements cellulaires et sous-cellulaires liés aux altérations de l'homéostasie et du signal calciques. De plus, le profil des régions cérébrales touchées par les neurotraumatismes et les événements liés est assez prévisible. Le profil de la lésion cérébrale prédit précisément les séquelles aiguës et chroniques des survivants aux LCT, les variations étant néanmoins suffisantes pour suggérer que des différences individuelles (polymorphismes génétiques et facteurs de resilience) jouent un rôle dans la modulation de l'évolution. Cet article fait une mise au point sur notre compréhension actuelle de la neurophysiopathologie de la LCT et sur la façon dont on peut la rattacher aux problèmes neurocomportementaux observés après une lésion.
Traumatic brain injury (TBI) may be the brain disorder that best illustrates the perils of the mind/brain dualism and that breaks down the remaining conceptual barriers between the clinical disciplines of neurology and psychiatry. The forces that create neurotrauma typically result in a profile of regional brain dysfunction that maps nicely onto the neuropsychiatrie sequelae and functional distress encountered by survivors of such injury. In turn, the effects of living with these neurobehavioral sequelae, the meaning and the significance of being identified as “brain injured” greatly influence the quality of life of the individuals and their caregivers. Failure to appreciate these complex but predictable relationships impedes proper assessment and treatment of the individual with a TBI. This paper reviews the current knowledge of the neurobiological effects of TBI, with special emphasis on how these processes inform the understanding of the clinical presentation and treatment of a person with neurobehavioral complications of neurotrauma.
It is helpful to start with some clarification of the term “traumatic brain injury.” A variety of definitions have been put forth by various groups including the American Congress of Rehabilitation Medicine,1 the Centers for Disease Control, 2 and the World Health Organization.3 The most recent consensus definition is that proposed by the Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health.4 They posit that TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force”4 (p 1637). As with previous definitions, alteration in brain function can be manifest by loss or decreased level of consciousness, alteration in mental state, incomplete memory for the event, or neurological deficits. Examples of external forces include the head striking or being struck by an object, rapid acceleration or deceleration of the brain, penetration of the brain by a foreign object, and exposure to forces associated with blasts. The external force requirement separates TBI from other acquired brain injuries due to cerebrovascular, neoplastic, or neurodegenerative conditions. Two additional points are worth noting. Most definitions have distinguished brain injury from head injury, which might be limited to damage to the face or scalp. In addition, most groups have emphasized that sustaining a brain injury at some point in time is different from attributing current symptoms to that event. Many of the symptoms associated with TBI are nonspecific.5
Using any of the common definitions, TBI is a global health concern. For example 1 to 2 million Americans are injured each year, with 290 000 hospitalized and over 50 000 dying from their injuries.6 Other developed regions of the world have roughly similar rates,7 and although figures are harder to come by in developing nations, it is generally thought that TBI is a significant public health problem in these regions as well.
Many individuals with TBI, particularly those with moderate and severe TBI, are left with significant long-term neurobehavioral sequelae.8-10 The overarching theme of this article is that there is a clear relationship between these sequelae and the profile of brain injury seen in the typical TBI. Thus it is helpful to understand the forces involved in TBI, the brain regions at particular risk for damage from the forces, and the cascade of neurobiological changes precipitated by these forces in order to make sense of the clinical presentation of individuals with TBI and neurobehavioral difficulties. It is also helpful to distinguish between traumatic injuries involving penetration of the brain substance (“penetrating” injuries) and injuries that do not penetrate the brain (often referred to as “closed” head injuries). The main reasons for drawing this distinction is that the injury profiles can be quite different, and thus the associated neurobehavioral sequelae can be quite different. Broadly speaking, the profile of injury involving penetration of the brain substance will depend on the location and trajectory of the object that is involved, for example the entrance location, trajectory, and size of a bullet that enters the head will largely predict the neurobehavioral sequelae. In these injuries damage typically results from displacement or destruction of brain tissue by the projectile; fragmentation and deposition of bone or a projectile within brain tissue; or introduction of potential infectious material on the projectile.
Nonpenetrating or closed injuries are better understood based on how the typical biomechanical forces involved in causing injury interact with the material properties of the brain substance and its relationship to the bony structure (skull) in which it sits. The following discussion focuses primarily on the latter category of injury (closed or nonpenetrating). However, it is important to note that many injuries, particularly in the modern combat context, can be a combination of these different forces and injury types.
Mechanisms of injury
Contact forces
The biomechanical effects of nonpenetrating injuries may be divided broadly into two types, both of which are applicable across the spectrum of injury severity: contact and inertial. Contact injuries result when the brain, moving inside the skull, strikes the inner surface of skull. Movement of brain against the various ridges and bony protuberances of the anterior (frontal) and middle (temporal) fossae is particularly injurious to the temporal and frontal poles and the ventral anterior, medial, and lateral temporal cortices, and the frontal cortices.11-14
Inertial forces
Linear translation and rotational forces, which in combination produce angular acceleration or deceleration, can result in straining, shearing, and compression of brain tissue.15-22 When these forces exceed the tolerances of brain tissue, injury results. These forces tend to be maximal in brain areas that experience the highest angular acceleration or deceleration forces (superficial > deep and anterior > posterior), at the planes between tissues of different densities and elasticities (eg, the junction between gray and white matter), and at the rotational center of mass in the intracranial space (rostral brain stem). The effects of high-speed, long-duration acceleration or deceleration injuries are maximal on axonal projections and small blood vessels within and from the brain stem, the parasagittal white matter of the cerebrum, the corpus callosum, the gray-white junctions of the cerebral cortex,23 and especially at gray-white junctions in the ventral and anterior frontal and temporal lobes.12 Although this type of inertial injury usually is described as diffuse axonal injury, the term is somewhat misleading in that the actual pattern of injury is more accurately characterized as multifocal.23
Cellular response to injury
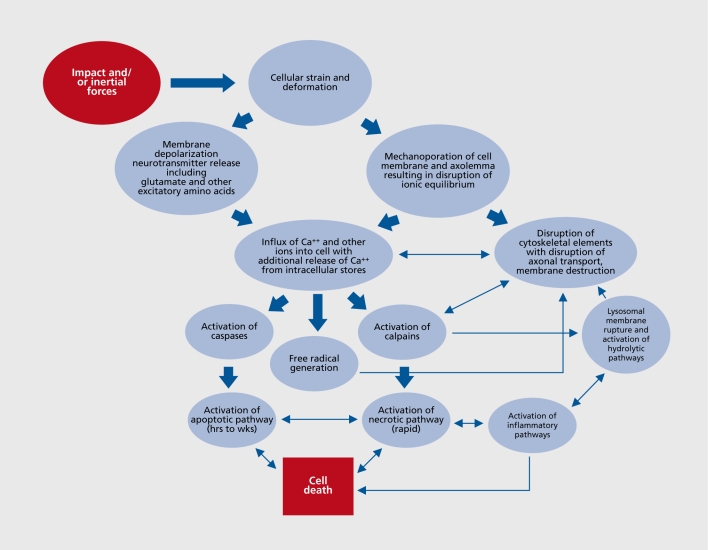
The above-described forces, whether in and around focal injuries such as contusions, or remote from the focal injury and attributable to inertial forces, a complex set of events is set in motion at the cellular and subcellular level that is only partially understood (Figure 1).24 Two initiating events related to Ca++ homeostasis appear to be of particular importance. First, at the time of injury mechanical perturbation of neurons is associated with a significant release of a host of neurotransmitters. Of particular importance is the release of glutamate and other excitatory amino acids with a resultant influx of extracellular Ca++ into the cell. This in turn releases additional Ca++ from intracellular stores, thus producing sufficient quantities of free intracellular Ca++ to initiate a host of intracellular reactions that can result in cytotoxic injury and eventually cell death. Second, mechanical perturbation of the neuron and its axon can result in mechanoporation of the cell membrane and axolcmma with subsequent influx of extracellular Ca++ and other ions into the cell and axon. The mechanical distortion of the membrane does not resolve immediately and the ultimate fate of the membrane and the neuron appears related to the degree of distortion and other factors, with some cells repairing and resealing, and others progressing on to further disruption and cell death.
Figure 1. Simplified summary of traumatic brain injury (TBI)-associated cellular injury cascades. Of note is that events are triggered at the time of injury but the full evolution of the process plays out over hours to weeks after injury. For the details see ref 24.
A variety of intracellular events attributable to this altered Ca++ homeostasis are set in motion (see refs 24-26). Most emphasis has been on the activation of two groups of cysteine proteases, the caspases and the calpains, and their role in the initiation of necrosis and apoptosis. Both pathways can result in cell death, and there are important linkages between the two mechanisms. However the necrosis pathway occurs rapidly, is a “passive” event related to energy failure and subsequent inability to maintain cellular homeostasis, is more closely associated with the calpain proteases, and triggers an inflammatory response, whereas the apoptotic pathway evolves over hours to weeks after injury, is an active process requiring energy, is more closely associated with the caspase proteases, and is less clearly linked to inflammatory responses. A variety of cytoskeletal elements including neurofilaments and spectrin are primary substrates for the calpains and thus activation of these proteases can lead to disruption of cell transport, destruction of cytoarchitecture and cell membrane elements, disruption of cell transport, and ultimately cell death. The apoptotic pathway evolves over hours to weeks after injury, is an active process requiring energy, is more closely associated with the caspase proteases, and is less clearly linked to inflammatory responses. Primary substrates for the caspases also include cytoskeletal elements as well as the capacity to activate other processes that can be toxic to the cell.25 Both families of proteases and hence both the necrotic and apoptotic pathways are under complex control of multiple modulators, the ultimate balance of which appear to determine cell survival.25
In addition to these processes, there is a growing appreciation for the role of other factors in the cytotoxic cascades such as the generation of free radicals, and the disruption of lysosomal membranes with the subsequent release of hydrolytic enzymes into the intracellular environment.24 The excessive release of neurotransmitters other than glutamate may also play a role in the elaboration of neurotrauma. For example cholinergic excess may amplify the destructive effects of excitatory amino acid excesses, and may be particularly injurious to brain areas where acetylcholine and excitatory amino acids are densely colocated (ie, hippocampus and frontal cortices).27 The effects of cerebral monoaminergic excesses in the cytotoxic cascade are not understood fully, although in experimental injury models traumatically induced elevations of cerebral serotonin seem to decrease cerebral glucose use,28,29 and serotonin agonists are not particularly helpful in improving post-traumatic neurobehavioral status or TBI outcome.30,31 Administration of catecholamine antagonists impedes recovery from brain injury32-34 and delay emergence from post-traumatic amnesia in humans,35 suggesting that blocking catecholamine excesses is not an effective means by which to mitigate the cytotoxic cascade after TBI.
Neurotransmitter excesses seem to wane over the first several weeks after TBI,36,37 although the time course of their resolution is not characterized fully. TBI in humans produces chronic cerebral cholinergic deficit via injury to ventral forebrain cholinergic nuclei38,39 and their cortical projections.39-41 It is possible that TBI also results in primary or secondary disturbances in monoaminergic systems,42 the effects of which may be amplified by individual genetically mediated variations in catecholamine metabolism.43
Role of secondary and systemic complications
In addition to the above primary effects of TBI, a variety of additional factors may complicate an injury including traumatic hematomas (eg, subdural, epidural, subarachnoid, and intraparenchymal hematomas), focal or diffuse cerebral edema, elevated intracranial pressure, obstructive hydrocephalus, hypoxic-ischemic injury, and infection. Because TBI frequently occurs in the context of other injuries (polytrauma) and medical complications such as volume depletion or blood loss, hypoperfusion, hypoxia, infection, and related problems can be seen and may increase post-traumatic mortality and morbidity.44
Blast injury
The emergence of explosive devices, particularly “improvised explosive devices” (IEDs), as a primary method of attack in recent conflicts, has called attention to “blast injury.” Explosions generate a rapidly moving wave of overheated expanding gases that compress surrounding air. The ongoing expansion of the heated gases eventually results in a drop in pressure, with resulting reversal of the pressure wave. These fluctuations in pressure are associated with strain and shear forces (barotrauma) that can be particularly damaging to air- and fluid-filled organs and cavities.45 For example the tympanic membrane can be ruptured with approximately a 30 % increase in atmospheric pressure and is a useful, though not always reliable, indicator of blast exposure.46 Blast can also be associated with significant brain injury.47-51 At this time it is not clear if injury associated with blast is due to the high pressure wave with distortion of vascular tissue, neural tissue or both, the inertia! effects of buffeting by the alternating high- and low-pressure events, or some other mechanism. Additional mechanisms often come into play, including impact mechanisms from the head coming into contact with an object or penetrating injuries from fragments and debris (referred to as secondary blast injury), and rapid acceleration or deceleration of the brain causing inertia! injury (tertiary injury), and exposure to toxic gas or chemicals as a result of the explosion (quaternary injury).46
Animal models suggest that primary blast injury can be associated with neural injury, although the underlying mechanism is not clear.52 For example Cernak et al47,50 exposed rats to either whole -body blast or localized pulmonary blast in which the brain was protected from the pressure wave with a steel plate. Both groups of animals showed hippocampal injury with neuronal swelling, cytoplasmic vacuolization, and loss of myelin integrity. These changes were associated with poorer performance on an active avoidance response task learned prior to the injury. This group has postulated that one potential mechanism is transmission of the pressure wave through cerebral vasculature with subsequent injury to perivascular neural tissue, axonal stretching, release of neurotransmitters and precipitation of the usual excitotoxic cascades,47,50,53 although this is not yet firmly established.
Summary of neuropathophysiology of TBI
Distilling the literature reviewed above, there are several points worth highlighting. The typical profile of injury involves a combination of focal and diffuse injury. Injury occurs at the time of the event (often referred to as “primary injury”) and additional damage (“secondary injury”) evolves over a variable period of time related to the elaborately choreographed injury cascades that play out at the cellular and subcellular level. Although each injury is necessarily unique, there are certain brain regions that are particularly vulnerable to damage including the frontal cortex and subfrontal white matter, the deeper midline structures including the basal ganglia and diencephalon, the rostral brain stem, and the temporal lobes including the hippocampi. Certain neurotransmitter systems, particularly the catecholaminergic42 and cholinergic systems,54 are altered in TBI. Both of these systems play critical roles in a variety of domains important in behavioral homeostasis including arousal, cognition, reward behavior, and mood regulation. This profile of structural injury and neurochemical dysregulation occurs along a spectrum of injury severity, including “mild” injury.55 The correspondence between the neuropathophysiology of TBI and the common and disabling neurobehavioral sequelae associated with it is now reviewed.
Relationship of neurobiology of TBI to neurobehavioral sequelae of TBI
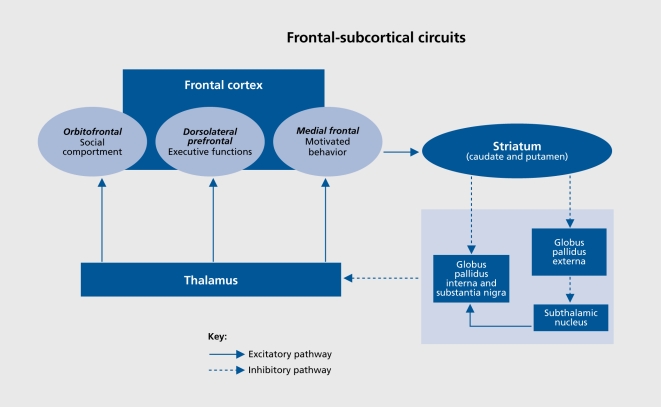
As noted, there are several high-risk regions vulnerable to the effects of neurotrauma, but it is important to note that these brain regions are important nodal points in frontal-subcortical circuits that subserve cognition and social behavior. In particular, three major frontal-subcortical circuits have significant roles in nonmotor forms of behavior56 (Figure 2). A circuit arising in the dorsolateral prefrontal cortex modulates executive functions, such as working memory, decision making, problem solving, and mental flexibility. Another, arising from cells in the orbitofrontal cortex, plays a critical role in intuitive reflexive social behaviors and the capacity to self-monitor and self-correct in real time within a social context. A third circuit starting in the anterior cingulate modulates motivated and reward-related behaviors. Although not a frontal subcortical circuit, per se, circuits traversing medial temporal regions play critical roles in episodic memory and new learning, as well as the smooth integration of emotional memory with current experience and real-time assessment of stimulus salience. Thus, the typical regions vulnerable to damage associated with TBI overlap significantly with key regions and nodal points in these frontal subcortical circuits, making it readily apparent that problems with cognition, social comportment, and executive function, as well as an increased relative risk of specific psychiatric disorders would be common after TBI (Table I, Figure 3).
Figure 2. Outline of frontal subcortical circuits relevant to common neurobehavioral sequelae of traumatic brain injury (TBI).
Adapted from ref 111: Arciniegas DB, Beresford TP. Neuropsychiatry: an introductory Approach. Cambridge, UK: Cambridge University Press; 2001:58. Copyright © Cambridge University Press, 2001
Table I. Neural substrates of common sequelae of TBI. TBI, traumatic brain injury; PTSD; post-traumatic stress disorder; GABA, γ-aminobutyric acid.
| Neurobehavioral sequelae | Predominant brain regions involved | Predominant neurotransmitter systems involved | Comment |
| Cognitive deficits | |||
| Working memory | Dorsolateral prefrontal, parietal, and cerebellar cortices; subcortial white matter | Dopamine, norepinephrine, ?acetylcholine | Overlaps with attentional deficit |
| Short-term memory | Frontal and hippocampal cortices | acetylcholine | Remote memory typically intact |
| Attention | Frontal, cingulate and parietal cortices, subcortical white matter, reticular activating system | Dopamine, norepinephrine acetylcholine | “Top-down” processing may be impaired in TBI of all severities, “bottom-up” (arousal) more often in severe TBI |
| Processing speed | Subcortical white matter tracts | Catecholamines, acetylcholine | Underlies complaints of “slowed thinking” |
| Dysexecutive syndromes | |||
| Disinhibition/social comportment | Orbitofrontal subcortical circuit | Complex interaction of GABA, catecholamines serotonin and others | Emotional responses including anger out of proportion to precipitant |
| Cognitive dysexecutive | Dorsolateral prefrontal cortex | Interaction of GABA, catecholamines and others | Overlaps with cognitive deficits described above |
| Disorders of motivated behavior | Medial frontal cortex, anterior cingulate, related reward circuitry | dopamine, norepinephrine | Often presents as apathy and can be confused with depression |
| Psychiatric disorders | |||
| Depression | ?left anterior frontal cortex, temporo-limbic circuitry | ? dopamine, norepinephrine serotonin | Associated with poor short and long-term outcome |
| Substance abuse | Components of reward circuitry (nucleus accumbens, frontal cortex) | Dopamine, norepinephrine, opiod system? | Often present before injury but can arise de novo |
| PTSD | Medial and orbitofrontal cortices, amygdala, hippocampus | ? serotonin, norepinephrine, dopamine | Cognitive deficits increase risk of PTSD |
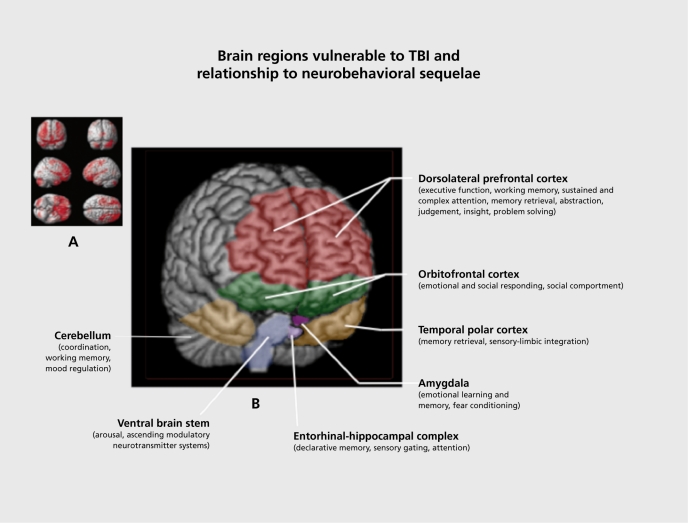
Figure 3. (A) Brain regions vulnerable to damage in a typical traumatic brain injury (TBI); (B) Relationship of vulnerable brain regions to common neurobehavioral sequelae associated with TBI.
(A) Adapted from ref 112: Bigler E. Structural imaging In: Silver J, McAllister T, Yudofsky S, eds. Textbook of Traumatic Brain Injury. Washington DC: American Psychiatric Press; 2005:87. Copyright © American Psychiatric Press, 2005. (B) Adapted from ref 111: Arciniegas DB, Beresford TP. Neuropsychiatry: an Introductory Approach. Cambridge, UK: Cambridge University Press; 2001:58. Copyright © Cambridge University Press, 2001
Changes in cognition
Initial and persistent cognitive deficits are the most common complaints after TBI57,58 and can present significant challenges to independent living, social readaptation, family life, and return to work.59,60 Frontal executive functions (problem solving, set shifting, impulse control, self-monitoring), attention, short-term memory and learning, speed of information processing, and speech and language functions are the cognitive domains typically impaired.61-67 Injury to medial temporal regions, the dorsolateral prefrontal cortex, and subcortical white matter connecting these regions readily account for these difficulties.
Changes in personality
The term “personality change” is often used by survivors and family/caregivcrs to describe alterations in emotional and behavioral regulation after brain injury. In some individuals, this presents as exaggeration of preinjury traits (eg, irritability). It is important in this context to ask about changes in the frequency and/or intensity of behaviors or traits that may have been present before the injury took place. Alternatively, these behaviors can present as fundamental changes in response patterns. Several common clusters of symptoms that characterize the “personality changes” are recognizable.
Impulsivity
This may be manifest in verbal utterances, physical actions, snap decisions, and poor judgment flowing from the failure to fully consider the implications of a given action. This is closely related to the concept of stimulus boundcdness, in which the individual responds to the most salient cue in the environment or attaches exaggerated salience to a particular cue, without regard to previously determined foci of attention or priorities, a syndrome commonly seen in individuals with frontal cortical damage or degeneration from a variety of disorders.
Irritability
Survivors are often described as more irritable or more easily angered. Responses can range from verbal outbursts to aggressive and assaultive behavior. Although a particular cue might be perceived as a legitimate aggravation, the response is characteristically out of proportion to the precipitating stimulus. This modulatory deficit differs in intensity, onset, and duration from the pre-injury pattern for many individuals. This behavioral disinhibition is most likely attributable to damage to orbital frontal regions and white matter connections along the orbitofrontal subcortical circuitry of social comportment.
Affective instability
Survivors and family/caregivers frequently describe exaggerated displays of emotional expression, out of proportion to the precipitating stimulus and the preinjury range of responses. Additional characteristics include a paroxysmal onset, brief duration, and subsequent remorse. This phenomenon occurs in other central nervous system disorders and has been called pathological affect, affective lability, pseudobulbar affect, and affective incontinence,61 and is most likely related to disruption of “top-down” modulation of limbic responses to emotional stimuli by frontal cortex.68
Apathy
Disorders of motivated behavior can be of concern to family members and can be a barrier to progress in rehabilitation programs. It is often misinterpreted as laziness or depression and may be linked to aggression when attempts to engage the individual in activities in which they have little interest can precipitate assaultive behavior.69 Kant et al70 found that apathy (mixed with depression) occurred in 60 % of their sample. Andersson et al71 found that almost half of their individuals with TBI had significant degrees of apathy. Deficits in motivated behavior can occur in association with injury to the circuitry of “reward.” 69,72 Key nodal points in this circuitry include the amygdala, hippocampus, caudate, entorhinal and cingulate cortices, the ventral tegmental area, and the medial forebrain bundle. Catecholaminergic systems, particularly the mcsolimbic dopaminergic system, appear to play critical roles in the modulation of the reward system.66,73
Lack of awareness of deficits
The personality changes described above are often more difficult to address because the injured individual may be unable to appreciate that his or her behavior is different after the injury.62,74 Of interest is that individuals with TBI are less likely to be aware of changes in behavior and executive function than changes in more concrete domains, such as motor function.67 Furthermore, the degree of awareness has been found to correlate with functional and vocational outcome in many,75-78 although not all,79 studies.
Relationship of TBI to psychiatric disorders
In addition to the changes in cognition, behavior, and personality described above, a significant body of evidence suggests that TBI results in an increased risk of developing psychiatric disorders, including mood and anxiety disorders,80 sleep disorders,81 substance abuse, and psychotic syndromes.82-85 For example, Kopenen et al85 studied 60 individuals 30 years after their TBI and found that almost half (48 %) developed a new Axis I psychiatric disorder86 after their injury. The most common diagnoses were depression, substance abuse, and anxiety disorders. In individuals with a TBI, rates of lifetime and current depression (26 %; 10 %), panic disorder (8 %; 6 %), and psychotic disorders (8 %; 8 %), were significantly higher than base rates found in the Epidemiologic Catchment Area (RCA) study.87 Hibbard et al83 studied 100 adults on average 8 years after TBI. A significant number of individuals had Axis I disorders before injury. After TBI, the most frequent diagnoses were major depression and anxiety disorders (ie, posttraumatic stress disorder [PTSD], obsessive-compulsive disorder, and panic disorder). Almost half (44 %) of individuals had two or more disorders. More recently, this group reported a longitudinal study of 188 individuals enrolled within 4 years of injury and assessed at yearly intervals on at least two occasions.88 Once again, they found elevated rates of psychiatric disorders (depression and substance abuse) before injury and increased rates of depression, PTSD, and other anxiety disorders subsequent to injury. This was particularly true of those with preinjury psychiatric disorders. Furthermore, the rates were greatest at the initial assessment point after injury and stabilized or decreased over time. Others have also reported increased indicators of psychiatric illness after TBI and increased medical costs associated with those indicators.89,90 More recently, Bryant et al91 have shown that there arc high rates of psychiatric illness in individuals hospitalized with traumatic injury of any sort (including mild TBI) 12 months after the event (31 %). Twenty-two percent suffered psychiatric disorders that they had never had before. Having a mild TBI was associated with higher rates of PTSD and other anxiety disorders. The combination of mild TBI and psychiatric illness was associated with greater degrees of functional impairment. Whelan-Goodinson et al92 also found a strong relationship between post-TBI depression, anxiety, and outcome. Furthermore, as with any potentially disabling condition, individuals with TBI report a variety of symptoms in different domains (discouragement, frustration, fatigue, anxiety, etc). Not all of these symptoms will rise to the level of a disorder. However, constellations of symptoms that are consistent and sustained over time (usually weeks), and that are of sufficient severity to interfere with social or occupational function or quality of life, are legitimately considered disorders. The consistent observation that individuals who sustain a TBI have higher base rates of psychopathology before injury also suggests that there is a reciprocal interaction: psychopathology predisposes to TBI, and TBI in turn predisposes the individual to develop psychiatric disorders. Although the link between TBI and psychiatric disorders holds for many conditions, the relationship of TBI to PTSD and dementia are worth additional comment.
Relationship to PTSD
Recent conflicts in Iraq and Afghanistan have focused attention on the relationship between psychological and biomcchanical trauma particularly in military populations (eg, see refs 93-95). Several recent studies highlight their complex interaction. Hoge et al96 found that higher rates of Iraq war returnees reporting a TBI with loss of consciousness met criteria for PTSD, relative to those reporting only altered mental status, other injuries, and or no injury. Much of the variance across these groups with respect to physical health outcomes and symptoms could be accounted for by the presence of PTSD and/or depression. It is important to point out that participants were assessed 3 to 4 months after deployment and thus reflect individuals with persistent symptoms. Schneidcrman ct al97 found that combat-incurred mild TBI approximately doubled the risk for PTSD and that a PTSD diagnosis was the strongest factor associated with persistent post-concussive symptoms. Belanger et al98 studied patients with mild and moderatc-to-scverc TBI and found, as expected, that mild TBI was associated with higher levels of postconcussion complaints approximately 2 years after injury. However, after adjusting for PTSD symptoms, these betwecn-group differences were no longer significant. These studies are consistent with the literature cited above that suggests that mild TBI may increase the relative risk for psychiatric disorders, and that these disorders can interfere with recover}' from the TBI.
There is reason to believe that part of the explanation for the complex interaction between biomechanical and psychological trauma relates to overlap in the neural substrates of both conditions (see refs 93-95,99 for discussion). For example mesial temporal structures are vulnerable in TBI from both contact/impact forces, as well as increased sensitivity to excitotoxic injury. Hippocampal and amygdala injury are common. Both of these regions play key roles in PTSD as well, both in terms of contextual memory consolidation and fear conditioning. The hippocampus is also felt to be vulnerable to the effects of chronic stress presumably through the mediating effects of the HPA axis. Thus biomechanical and neurochemically mediated damage could conceivably interact with neurohumoral dysrcgulation to create a milieu that lends itself to the development of PTSD. Orbitofrontal cortex is also vulnerable to TBI through impact forces as well as frontal subcortical axonal injury.
Relationship to dementia
Several studies have raised a concern about the relationship of TBI to progressive dementia.100 For example, TBI-associated disruption of axonal transport results in the rapid accumulation of amyloid precursor protein (APP) in animals100,101 and humans.102,103 APP, A-beta, and other proteins associated with Alzheimer's disease and other neurodegenerative disorders accumulate rapidly after a TBI.104-106 Some (but not all) autopsy studies have shown increased amyloid plaques and neurofibrillary tangles in individuals with TBI.106,107 This variation has prompted exploration of the role of genetic factors in modulating risk for Alzheimer's disease after TBI. For example, Mayeux et al108 retrospectively studied 113 older adults with AD, comparing them with a control group of 123 healthy older individuals. They found that the combination of APOR-e4 and history of TBI increased the risk of AD by a factor of 10. However, not all studies have found such a relationship. A large, prospective population-based study of 6645 individuals 55 years and older and free of dementia at baseline found that mild brain trauma was not a major risk factor for the development of AD. Moreover, brain trauma did not appear to increase the risk of developing AD in people carrying the APOR-e4 allele.109 One possibility is that diminished cognitive reserve associated with TBI facilitates earlier manifestation of dementia symptoms in individuals already at risk for AD.110 Therefore, although there are some compelling scientific reasons to consider the relationship of TBI to Alzheimer's disease and other neurodegenerative disorders, and some strong evidence suggesting clinical associations, the relationship between TBI and dementia needs further study.
Although the relationships between profile of injury and neurobehavioral sequelae are generally seen, there is a surprising amount of variance in long-term outcome after TBI. Some individuals with apparently severe injuries have remarkably good functional outcomes, whereas some individuals with injuries that judged “mild” at the time of the event suffer longstanding significant disability. A full discussion of the factors involved in outcome variance is beyond the scope of this paper; however, such observations have raised the question of whether individual differences, for example, polymorphisms in genes that modulate response to n eurotrauma (for instance at key points in the excitotoxic injury cascades), efficiency and extent of neural repair and plasticity, or baseline cognitive and behavioral functions might play a role in modulating outcome after TBI. Although this field is relatively new, several promising candidate polymorphic alleles in genes such as APOE, BDNF, DRD2/ANKK1, and others, suggest that this is in fact the case (see ref 86 for recent review) and may prove a fruitful line of inquiry.
Conclusions
TBI is a significant public health problem both because of the high incidence of injury events and because of the high prevalence of chronic neuropsychiatrie sequelae that can devastate the lives of survivors and their family caregivers. Related to the common mechanisms of injury such as motor vehicle crashes, falls, and assaults, there are two broad types of force that results in neurotrauma - contact and inertial. Both of these forces are associated with damage to predictable brain regions and both are also associated with damage that occurs at the time of the event and that precipitates a complex set of potentially excitotoxic cascades that evolves in the minutes to days after the event. In addition to these factors, other event-related processes such as hemorrhage, cerebral edema, and cerebral anoxia may further complicate the injury profile. Blast injury is an incompletely understood event that may have additional neuropathological processes, further complicated by the fact that inertial and contact mechanisms are also typically involved in explosion-related injuries.
Related to the profile of brain damage associated with these forces and related events, there is an equally predictable profile of neurobehavioral sequelae that survivors of brain injury often suffer from including cognitive deficits (memory, attention, executive function, speed of information processing), personality changes (best characterized as dysexecutive syndromes involving social comportment, cognition, and motivated behavior), and increased relative rates of psychiatric disorders, particularly depression, anxiety, and PTSD both in civilian and military populations. Our understanding of the ncuropathophysiology of TBI has outpaced advances in our ability to mitigate and treat the effects of neurotrauma both acutely (eg, neuroprotection trials) and chronically. Furthermore, although the patterns described arc the norm, there are surprising variations in outcome that suggest that individual factors such as genetic differences and factors modulating resiliency are worthy of much more study.
Acknowledgments
Supported in part by grants: NICHD R01 HD048176, 1R01HD047242, and 1R01HD48638; NINDS 1 R01 NS055020; CDC R01/CE001254.
REFERENCES
- 1.Kay T., Harrington DE., Adams R., et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- 2.Thurman D., Alverson C., Browne D., et al. Traumatic Brain Injury in the United States: A Report to Congress. Centers for Disease Control and Prevention. 1999 [Google Scholar]
- 3.Carroll LJ., Cassidy JD., Peloso PM., et al. Prognosis for mild traumatic brain injury: Results of the who collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;(43 suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 4.Menon D., Schwab K., Wright D., Maas A. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Iverson GL., Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10:137–144. doi: 10.1207/S15324826AN1003_02. [DOI] [PubMed] [Google Scholar]
- 6.Rutland-Brown W., Langlois JA., Thomas KE., Xi YL. Incidence of traumatic brain injury in the united states, 2003. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Tagliaferri F., Compagnone C., Korsic M., Servadei F., Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochirurgica. 2006;148:255-68; discussion 268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- 8.DHHS (US Department of Health and Human Services). Department Of Health And Human Services: Interagency Head Injury Task Force Report. 1989 [Google Scholar]
- 9.Levin HS., Gary HE., Eisenberg HM., et al. Neurobehavioral outcome 1 year after severe head trauma: Experience of the traumatic coma data bank. J Neurosurg. 1990;73:699–709. doi: 10.3171/jns.1990.73.5.0699. [DOI] [PubMed] [Google Scholar]
- 10.Sorenson SB., Kraus JF. Occurrence, severity, and outcome of brain injury. J Head Trauma Rehabil. 1991;5:1–10. [Google Scholar]
- 11.Halliday A. Pathophysiology. In: Marion D, ed. Traumatic Brain Injury. New York, NY: Thieme Medical Publishers, Inc. 1999:29–38. [Google Scholar]
- 12.Bigler ED. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome, [review]. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy J. Neuropathology. In: Silver JM, Yudofsky SC, Hales RE, eds. Neuropsychiatry of Traumatic Brain Injury. Washington, DC: American Psychiatric Press, Inc; 1994:43–79. [Google Scholar]
- 14.Levin HS., Mendelsohn D., Lilly MA., et al. Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: a test of the ommaya-gennarelli model. Neurosurgery. 1997;40:432-40; discussion 440-441. doi: 10.1097/00006123-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sabet A., Christoforou E., Zatlin B., et al. Deformation of the human brain induced by mild angular head acceleration. J Biomech. 2008;41:307–315. doi: 10.1016/j.jbiomech.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol. 2002;12:1237–1252. doi: 10.1007/s00330-002-1355-9. [DOI] [PubMed] [Google Scholar]
- 17.Ryan GA., McLean AJ., Vilenius AT., et al. Brain injury patterns in fatally injured pedestrials. J Trauma. 1994;36:469–476. [PubMed] [Google Scholar]
- 18.Misra J., Chakravart S. A study of rotational brain injury. J Biomech. 1984;17:459-66. doi: 10.1016/0021-9290(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 19.Fijalkowski RJ., Stemper BD., Pintar FA., Yoganandan N., Gennarelli TA. Biomechanical correlates of mild diffuse brain injury in the rat. Biomed Sci instrurn. 2007;43:18–23. [PubMed] [Google Scholar]
- 20.Fijalkowski R., Stemper B., Pintar F., Yoganandan N., Gennarelli T. Biomechanical correlates of mild diffuse brain injury in the rat. Biomed Sci Instrurn. 2007;43:18–23. [PubMed] [Google Scholar]
- 21.Fijalkowski R., Ellingson B., Stemper B., et al. Interface parameters of impact-induced mild traumatic brain injury. Biomed Sci Instrum. 2006;42:108–113. [PubMed] [Google Scholar]
- 22.Margulies SS., Thibault LE., Gennarelli TA. Physical model simulations of brain injury in the primate. J Biomechanics. 1990;23:823–836. doi: 10.1016/0021-9290(90)90029-3. [DOI] [PubMed] [Google Scholar]
- 23.Meythaler J., Peduzzi J., Eleftheriou E., et al. Current concepts: diffuse axonal injury associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 24.Farkas O., Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- 25.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 26.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips LL., Reeves TM. Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor Neurol Neurosci. 2001;19:213–235. [PubMed] [Google Scholar]
- 28.Pappius H. Involvement of indoleamines in functional disturbances after brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:353–361. doi: 10.1016/0278-5846(89)90124-3. [DOI] [PubMed] [Google Scholar]
- 29.Tsuiki K., Takada A., Nagahiro S., et al. Synthesis of serotonin in traumatized rat brain. J Neurochem. 1995;64:1319–1325. doi: 10.1046/j.1471-4159.1995.64031319.x. [DOI] [PubMed] [Google Scholar]
- 30.Meythaler JM., Depalma L., Devivo MJ., Guin-Renfroe S., Novack TA. Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain inj. 2001;15:321–331. doi: 10.1080/026990501750111274. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MS., Hamm RJ. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am J Phys Med Rehabil. 2002;81:364–372. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein L. Neurotransmitters and motor activity: effects on functional recovery after brain injury. NeuroRX. 2006;3:451–457. doi: 10.1016/j.nurx.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein LB. Neuropharmacology of TBI-induced plasticity. Brain Inj. 2003;17:685–694. doi: 10.1080/0269905031000107179. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein LB. Effects of amphetamines and small related molecules on recovery after stroke in animals and man. Neuropharmacology. 2000;39:852–859. doi: 10.1016/s0028-3908(99)00249-x. [DOI] [PubMed] [Google Scholar]
- 35.Rao N., Jellinek H., Woolston D. Agitiation in closed head injury: haloperidol effects on rehabilitation outcome. Arch Phys Med Rehabil. 1985;66:30–34. [PubMed] [Google Scholar]
- 36.Markianos M., Seretis A., Kotsou A., et al. CSF neurotransmitter metabolites in comatose head injury patients during changes in their clinical state. Acta Neurochir (Wien). 1996;138:57–59. doi: 10.1007/BF01411725. [DOI] [PubMed] [Google Scholar]
- 37.Markianos M., Seretis A., Kotsou S., et al. CSF neurotransmitter metabolites and short-term outcome of patients in coma after head injury. Acta Neurol Scand. 1992;86:190–193. doi: 10.1111/j.1600-0404.1992.tb05064.x. [DOI] [PubMed] [Google Scholar]
- 38.Salmond CH., Chatfield DA., Menon DK., Pickard JD., Sahakian BJ. Cognitive sequelae of head injury: Involvement of basal forebrain and associated structures. Brain. 2005;128(Pt 1):189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch I., Nicoll JA., Graham Dl., Dewar D. Nucleus basalis of meynert pathology in the human brain after fatal head injury. J Neurotrauma. 2002;19:279–284. doi: 10.1089/08977150252807018. [DOI] [PubMed] [Google Scholar]
- 40.Dewar D., Graham Dl. Depletion of choline acetyltransferase but preservation of ml and m2 muscarinic receptor binding sites temporal cortex following head injury: a preliminary human postmorten study. J Neurotrauma. 1996;13:181–187. doi: 10.1089/neu.1996.13.181. [DOI] [PubMed] [Google Scholar]
- 41.Murdoch I., Perry EK., Court JA., Graham Dl., Dewar D. Cortical cholinergic dysfunction after human head injury. J Neurotrauma. 1998;15:295–305. doi: 10.1089/neu.1998.15.295. [DOI] [PubMed] [Google Scholar]
- 42.McAllister TW., Flashman LA., Sparling MB., Saykin AJ. Working memory deficits after mild traumatic brain injury: catecholaminergic mechanisms and prospects for catecholaminergic treatment - a review. Brain inj. 2004;18:331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- 43.Lipsky RH., Sparling MB., Ryan LM., et al. Role of COMT VAL1 58MET genotype in executive functioning following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17:465–471. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- 44.Marion D. Evidenced-based guidelines for traumatic brain injuries. Prog Neurol Surg. 2006;19:171–196. doi: 10.1159/000095191. [DOI] [PubMed] [Google Scholar]
- 45.Kocsis J., Tessler A. Pathology of blast-related brain injury. J Rehabil Res Dev. 2009;46:667–672. doi: 10.1682/jrrd.2008.08.0100. [DOI] [PubMed] [Google Scholar]
- 46.DePalma RG., Burris DG., Champion HR., Hodgson MJ. Blast injuries. N Engl J Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- 47.Cernak I., Wang Z., Jiang J., Bian X., Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- 48.Cernak I., Savic J., Malicevic Z., et al. Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma. 1996;40(3 suppl):S100–S104. doi: 10.1097/00005373-199603001-00023. [DOI] [PubMed] [Google Scholar]
- 49.Mayorga MA. The pathology of primary blast overpressure injury. Toxicology. 1997;121:17–28. doi: 10.1016/s0300-483x(97)03652-4. [DOI] [PubMed] [Google Scholar]
- 50.Cernak I., Wang Z., Jiang J., Bian X., Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y., Smith DH., Meaney DF. In-vitro approaches for studying blastinduced traumatic brain injury. J Neurotrauma. 2009;26:1–16. doi: 10.1089/neu.2008.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharjee Y. Shell shock revisited: solving the puzzle of blast trauma. Science. 2008;319:406–408. doi: 10.1126/science.319.5862.406. [DOI] [PubMed] [Google Scholar]
- 54.Arciniegas DB. The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Curr Psychiatry Rep. 2003;5:391-9. doi: 10.1007/s11920-003-0074-5. [DOI] [PubMed] [Google Scholar]
- 55.McAllister T. Mild traumatic brain injury. In: Silver J, McAllister T, Yudofsky S, eds. Textbook of Traumatic Brain Injury. 2nd ed. Washington DC: American Psychiatric Press, Inc. 2011 [Google Scholar]
- 56.Mega MS., Cummings JL. Frontal subcortical circuits. In: Salloway SP, Malloy PF, Duffy JD, eds. The Frontal Lobes and Neuropsychiatrie illness. Washington DC, American Psychiatric Publishing. 2001 [Google Scholar]
- 57.Lovell M., Franzen M. Neuropsychological assessment. In: Silver JM, Yudofsky SC, Hales RE, eds. Neuropsychiatry of Traumatic Brain injury. Washington, DC: American Psychiatric Press, Inc; 1994:133–160. [Google Scholar]
- 58.Whyte J., Polansky M., Cavallucci C., et al. Inattentive behavior after traumatic brain injury. J Int Neuropsychol Soc. 1996;2:274–281. doi: 10.1017/s1355617700001284. [DOI] [PubMed] [Google Scholar]
- 59.Ben-Yishay Y., Diller L. Cognitive remediation in traumatic brain injury: update and issues. Arch Phys Med Rehabil. 1993;74:204–213. [PubMed] [Google Scholar]
- 60.Cicerone K., Dahlberg C., Kalmar K., et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 61.Lehtonen S., Stringer AY., Millis S., et al. Neuropsychological outcome and community re-integration following traumatic brain injury: the impact of frontal and non-frontal lesions. Brain Inj. 2005;19:239–256. doi: 10.1080/0269905040004310. [DOI] [PubMed] [Google Scholar]
- 62.Mattson AJ., Levin HS., Mattson AJ., Levin HS. Frontal lobe dysfunction following closed head injury. A review of the literature. J Nerv Ment Dis. 1990;178:282–291. doi: 10.1097/00005053-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Rassovsky Y., Satz P., Alfano MS., et al. Functional outcome in TBI II: verbal memory and information processing speed mediators. J Clin Exp Neuropsychol. 2006;28:581-91. doi: 10.1080/13803390500434474. [DOI] [PubMed] [Google Scholar]
- 64.O'Jile JR., Ryan LM., Betz B., et al. Information processing following mild head injury. Arch Clin Neuropsychol. 2006;21:293–296. doi: 10.1016/j.acn.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 65.McMillan TM., Glucksman EE. The neuropsychology of moderate head injury. J Neurol Neurosurg Psychiatry. 1987;50:393–397. doi: 10.1136/jnnp.50.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 67.Mathias JL., Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: a meta-analytic review. Neuropsychology. 2007;21:212–223. doi: 10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- 68.Arciniegas D., Lauterbach E., Anderson K., et al. The differential diagnosis of pseudobulbar affect (PBA). CNS Spectr. 2005;10:1–14. doi: 10.1017/s1092852900026602. [DOI] [PubMed] [Google Scholar]
- 69.McAllister TW. Apathy. Sern Clin Neuropsychiatry. 2000;5:275-82. doi: 10.1053/scnp.2000.9557. [DOI] [PubMed] [Google Scholar]
- 70.Kant R., Duffy JD., Pivovarnik A. Prevalence of apathy following head injury. Brain inj. 1998;12:87–92. doi: 10.1080/026990598122908. [DOI] [PubMed] [Google Scholar]
- 71.Andersson S., Krogstad JM., Finset A. Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol Med. 1999;29:447–456. doi: 10.1017/s0033291798008046. [DOI] [PubMed] [Google Scholar]
- 72.Chau DT., Roth RM., Green Al. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- 73.Ruff RM., Levin HS., Mather S., et al. Recovery of memory after mild head injury: a three center study. In: Levin HS, Eisenberg HM, Benton AL, eds. Mild Head Injury. New York, NY: Oxford University Press. 1989:176–188. [Google Scholar]
- 74.Freedman PE., Bleiberg J., Freedland K., et al. Anticipatory behaviour deficits in closed head injury. J Neurol Neurosurg Psychiatry. 1987;50:398–401. doi: 10.1136/jnnp.50.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binder LM., Rohling ML., Larrabee J. A review of mild head trauma. Part I: Meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol. 1997;19:421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- 76.Binder LM. A review of mild head trauma. Part 2: Clinical implications [review]. J Clin Exp Neuropsychol. 1997;19:432–457. doi: 10.1080/01688639708403871. [DOI] [PubMed] [Google Scholar]
- 77.Binder LM. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol. 1986;4:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- 78.Hart T., Whyte J., Millis S., et al. Dimensions of disordered attention in traumatic brain injury: further validation of the moss attention rating scale. Arch Phys Med Rehabil. 2006;87:647–655. doi: 10.1016/j.apmr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Cripe LI. The neuropsychological assessment and management of closed head injury: general guidelines. Cogn Rehabil. 1987;5:18–22. [Google Scholar]
- 80.Rapoprt M. Depression complicating traumatic brain injury. Psychiatric Annals. 2010;30:581–587. [Google Scholar]
- 81.Vaishnavi S., McCann U., Rao V. Sleep disturbance after traumatic brain injury. Psychiatr Ann. 2010;40:553–559. [Google Scholar]
- 82.Deb S., Lyons I., Koutzoukis C. Neuropsychiatrie sequelae one year after a minor head injury. J Neurol Neurosurg Psychiatry. 1998;65:899–902. doi: 10.1136/jnnp.65.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hibbard MR., Uysal S., Kepler K., Bogdany J., Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 84.van Reekum R., Cohen T., Wong J. Can traumatic brain injury cause psych iatr ic disorders? J Neuropsychiatry Clin Neurosci. 2000;12:316–327. doi: 10.1176/jnp.12.3.316. [DOI] [PubMed] [Google Scholar]
- 85.Koponen S., Taiminen T., Portin R., et al. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry. 2002;159:1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 86.McAllister TW. Genetic factors modulating outcome after neurotrauma. Phys Med Rehabil. 2010;2:S241–S252. doi: 10.1016/j.pmrj.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Bourdon KH., Rae DS., Locke BZ., Narrow WE., Regier DA. Estimating the prevalence of mental disorders in U.S. adults from the epidemiologic catchment area survey. Pub Health Rep. 1992;107:663–668. [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald BC., Flashman LA., Saykin AJ. Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabiiitation. 2002;17:333–344. [PubMed] [Google Scholar]
- 89.Fann JR., Burington B., Leonetti A., et al. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. [see comment]. Arch Gen Psychiatry. 2004;61:53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- 90.Wei W., Sambamoorthl U., Crystal S., Findley PA. Mental illness, traumatic brain injury, and medicaid expenditures. Arch Phys Med Rehabil. 2005;86:905–911. doi: 10.1016/j.apmr.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bryant R., O'Donnell M., Creamer M. The psychiatric sequelae of traumatic injury. Am J Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 92.Whelan-Goodinson R., Ponsford J., Schonberger M. Association between psychiatric state and outcome following traumatic brain injury. J Rehabil Med. 2008;40:850–857. doi: 10.2340/16501977-0271. [DOI] [PubMed] [Google Scholar]
- 93.Stein MB., McAllister TW. Exploring the convergence of post traumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166:768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- 94.McAllister T. Psychopharmacological issues in the treatment of TBI and PTSD. Clin Neuropsychologist. 2009;23:1338–1367. doi: 10.1080/13854040903277289. [DOI] [PubMed] [Google Scholar]
- 95.Vasterling JJ., Verfaellie M., Sullivan KD. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuro-science. Clin Psychol Rev. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Hoge C., McGurk D., Thomas J., et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 97.Schnelderman A., Braver E., Kang H. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167:1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- 98.Belanger HG., Kretzmer T., Vanderploeg RD., French LM. Symptom complaints following combat-related traumatic brain injury: relationship to traumatic brain injury severity and posttraumatic stress disorder. J Int Neuropsychol Soc. 2010;16:194–199. doi: 10.1017/S1355617709990841. [DOI] [PubMed] [Google Scholar]
- 99.McAllister TW., Stein MB. Effects of psychological and biomechanical trauma on brain and behavior. Ann NY Acad Sci. 2010;1208:46–57. doi: 10.1111/j.1749-6632.2010.05720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Den Heuvel C., Thornton E., Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 101.Van Den Heuvel C., Blumbergs PC., Finnie JW., et al. Upregulation of amyloid precursor protein messenger rna in response to traumatic brain injury: an ovine head impact model. Exp Neurol. 1999;159:441–450. doi: 10.1006/exnr.1999.7150. [DOI] [PubMed] [Google Scholar]
- 102.Blumbergs PC., Scott G., Manavis J., et al. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12:565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- 103.Graham Dl., Gentleman SM., Lynch A., Roberts GW. Distribution of betaamyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol. 1995;21:27–34. doi: 10.1111/j.1365-2990.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 104.Uryu K., Chen X., Martinez D., et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uryu K., Chen XH., Graham DJ. Short-term accumulation of beta-amyloid in axonal pathology following traumatic brain injury in humans. Neurobiol Aging. 2004;25(suppl 1):P2–P250. [Google Scholar]
- 106.Chen X., Johnson V., Uryu K., Trojanowski J., Smith D. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braak H., Braak E. Frequency of stages of Alzheimer-related lesions in different age categoriesfrequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 108.Mayeux R., Ottman R., Maestre G., et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with alzheimer's disease. Neurology. 1995;45(3 Pt 1):555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 109.Mehta K., Ott A., Kalmijn S., et al. Head trauma and risk of dementia and alzheimer's disease: the Rotterdam study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 110.Starkstein SE., Jorge R. Dementia after traumatic brain injury. international Psychogeriatrics. 2005;17(suppl 1):S93–S107. doi: 10.1017/s1041610205001973. [DOI] [PubMed] [Google Scholar]
- 111.Arciniegas DB., Beresford TP. Neuropsychiatry: an Introductory Approach. Cambridge, UK: Cambridge University Press. 2001;58 [Google Scholar]
- 112.Bigler E. Structural imaging In: Silver J, McAllister T, Yudofsky S, eds. Textbook of Traumatic Brain injury. Washington DC: American Psychiatric Press. 2005;87 [Google Scholar]
- 113.Arciniegas DB., Beresford TP. Neuropsychiatry: an introductory Approach. Cambridge, UK: Cambridge University Press. 2001;370 [Google Scholar]