Abstract
Subependymal nodules (SENs) and subependymal giant cell astrocytomas (SEGAs) are common brain lesions found in patients with tuberous sclerosis complex (TSC). These brain lesions present a mixed glioneuronal phenotype and have been hypothesized to originate from neural stem cells. However, this hypothesis has not been tested empirically. Here, we report that loss of Tsc1 in mouse subventricular zone (SVZ) neural stem/progenitor cells (NSPCs) results in formation of SEN- and SEGA-like structural abnormalities in the lateral ventricle, the consequence of abnormal migration of NSPCs following Tsc1 loss.
Keywords: neural stem/progenitor cells, subependymal giant cell astrocytomas (SEGAs), subependymal nodules (SENs), tuberous sclerosis complex (TSC), lateral ventricle (LV)
Tuberous sclerosis complex (TSC) is a multisystem genetic disease characterized by benign tumors in the brain, kidney, and other vital organs. The genetic cause of TSC is a loss-of-function mutation in either the TSC1 or TSC2 genes, which encode the interacting proteins hamartin (TSC1) and tuberin (TSC2), respectively (Kwiatkowski and Manning 2005).
Brain lesions, including cortical tubers, subependymal nodules (SENs), subependymal giant cell astrocytomas (SEGAs), and white matter abnormalities, develop in 90% of TSC patients and are the most common and debilitating aspect of the disease (Marcotte and Crino 2006; Rosser et al. 2006; Napolioni et al. 2009). These abnormalities are believed to be at the root of neurological manifestations including epilepsy, mental retardation, and autism. SENs present as multiple small nodules along the lateral ventricle walls, giving a characteristic “candle dripping” appearance. SEGAs are histologically indistinguishable from SENs, but are larger and tend to arise near the foramen of Monro. Neuroimaging studies have suggested that SEGAs arise from SENs (Morimoto and Mogami 1986; Fujiwara et al. 1989). Clinically, SEGAs present as benign, slow-growing tumors with a low mitotic index. However, SEGAs often obstruct cerebrospinal fluid flow, causing hydrocephalus and increasing intracranial pressure. SEGAs are initially characterized by strong immunoreactivity to astroglial cell markers, such as glial fibrillary acidic protein (GFAP) and S100β (Burger et al. 2002; Lopes et al. 2007). However, both dysmorphic glial cells and neural cell types are present within the tumor mass. Many cells in SEGAs are also found to be reactive to neuronal markers, such as neurofilaments and synaptophysin (Burger et al. 2002; Lopes et al. 2007). Given this mixed glioneuronal phenotype, the astrocytoma nature of SEGAs has been challenged, and they are now more often referred to as subependymal giant cell tumors (SGCTs) (Marcotte and Crino 2006; Buccoliero et al. 2009; Napolioni et al. 2009). Furthermore, this mixed glioneuronal phenotype and the recent finding that SEGAs contain cells that express glial and neural progenitor markers have led to the hypothesis that the developmental origin of SENs and SEGAs might be neural stem/progenitor cells (NSPCs) (Ess et al. 2005). However, to date, this hypothesis has not been tested experimentally.
Although TSC1/2 function in the brain has been studied in several murine models (Uhlmann et al. 2002; Meikle et al. 2007; Feliciano et al. 2011), the role of TSC1/2 in the NSPC population is not clear. In rodents and humans, postnatal neurogenesis occurs mainly in the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricle (Ming and Song 2005). Throughout adulthood, these stem cell niches continuously produce new neurons. In particular, SVZ stem cells differentiate into neuroblasts as they migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB), where they differentiate into olfactory interneurons. The SVZ–RMS–OB pathway therefore provides an excellent system for studying TSC1/2 function in NSPC migration and differentiation.
In this study, we used genetic tools to ablate Tsc1 in postnatal SVZ NSPCs. The resultant mice develop nodular protrusions on the brain lateral ventricle walls and small tumors near the interventricular foramen (IF) that recapitulate many features of human SENs and SEGAs. Further study revealed that development of these SEN- or SEGA-like structures resulted from abnormal aggregation and migration of NSPCs after Tsc1 loss. Our data provide experimental evidence that TSC1 is involved in NSPC migration, and that Tsc1 ablation in these cells leads to formation of SENs and SEGAs.
Results and Discussion
Tsc1Nestin conditional knockout (cKO) mice develop structural abnormalities in the lateral ventricle
We previously generated a tamoxifen (TMX)-inducible Nestin-CreERT2 transgenic mouse line that enables targeting of NSPCs at various developmental stages (Supplemental Fig. S1; Chen et al. 2009). To study the function of the TSC1/2 complex in NSPCs, we crossed Tsc1loxP mice (Meikle et al. 2007) with Nestin-CreERT2 mice. Progeny were induced with TMX at postnatal day 7 (P7) or 1 mo and examined at 3 mo and 6–7 mo, respectively (Supplemental Fig. S2A). For each cohort, TMX-treated Nestin-CreERT2;Tsc1loxp/loxp mice (Tsc1Nestin cKO mice) were compared with TMX-treated littermate wild-type mice and heterozygotes (Nestin-CreERT2;Tsc1loxp/+). The Tsc1Nestin cKO mice exhibited enlarged and heavier brains at both time points (Supplemental Fig. S2B,C). No body weight differences were observed between control and cKO groups.
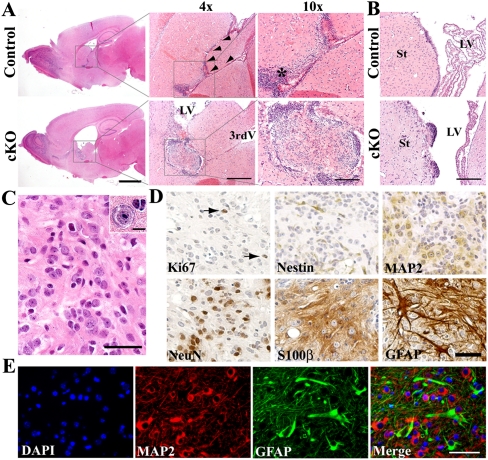
Further analysis revealed that the enlarged Tsc1Nestin cKO brains were accompanied by hydrocephalus, and to a lesser extent, an enlarged hippocampus (Fig. 1A; Supplemental Fig. S3A, left panels). Close examination of the dilated ventricles revealed the presence of abnormal structures in the lateral ventricles, specifically near the IF between the lateral and third ventricles (Fig. 1A,B; Supplemental Fig. S3). In the Tsc1Nestin cKO brain, the IF was noticeably dilated, in contrast to the narrow channel connecting the lateral and third ventricles on comparable sections from normal brain (Fig. 1A, arrowheads indicate the narrow connection between ventricles). Upon H&E staining, we also noticed that, next to the normal IF, a neural stem cell (NSC)-rich subventricular region was visible as a thick layer of cells with dense, hyperchromatic nuclei just beneath the ependymal cell layer (Fig. 1A, asterisk). This region displayed intense Cre activity upon TMX induction (Supplemental Fig. S1, right panels). However, in Tsc1Nestin cKO brain, this stem cell-rich region was not obvious, and instead we observed the presence of abnormal structures inside the ventricle. More often, these abnormal structures that developed near the IF contained a small but well-circumscribed tumor with distinct histological features (Fig. 1A; Supplemental Fig. S3A), which was sometimes accompanied by multiple cell masses nearby (Supplemental Fig. S3A, arrows). Among the mice examined, 93.3% (28 of 30) of cKO mice induced at P7 and 100% (19 of 19) of cKO mice induced at 1 mo were found to have abnormal structures in the ventricles close to the IF. Additionally, in cKO mice, we also frequently observed small nodular protrusions on the wall of the lateral ventricle on the side of the striatum. These structures were made up of discrete clusters of cells with distinctive “candle dripping” morphology (Fig. 1B; Supplemental Fig. S3B).
Figure 1.
Tsc1Nestin cKO mice develop SEN- and SEGA-like abnormalities. (A) Tsc1Nestin cKO mice develop hydrocephalus and small tumors in the lateral ventricle, as shown by H&E staining on mice induced at P7 and examined at 3 mo of age. (Arrowheads) Interventricular foramen (IF); (*) stem cell-rich subventricular region; (LV) lateral ventricle; (3rdV) third ventricle. Bars: for whole forebrain pictures, 2 mm; for 4× pictures, 500 μm; for 10× pictures, 200 μm. (B) Tsc1Nestin cKO mice develop small nodular excrescences in the lateral ventricle (mice induced at P7, examined at 3 mo of age). (St) Striatum. Bar, 200 μm. (C) Histological features of the inner tumor cells near the IF. Bar, 40 μm. Inset shows a giant cell within the tumor mass. Bar, 10 μm. (D) Inner tumor cells are mostly cells immunoreactive for either neuronal (MAP2 and NeuN) or astrocytic (S100β and GFAP) markers. (Arrows) Ki67+ cells. Bar, 50 μm. (E) MAP2 (red) and GFAP (green) double-staining reveals mixed glioneuronal phenotype of cells inside the tumor and their fibrillated processes. Bar, 50 μm.
The location and morphology of these small nodular structures and tumors are reminiscent of idiopathic SENs and SEGAs that develop in TSC patients. The small tumors near the IF were well circumscribed with an outer layer of densely packed cells that contained hyperchromatic nuclei surrounded by scanty cytoplasm. These features resemble those of NSPCs that are normally located in the SVZ just beneath the ependymal cell layer. The inner cells within these tumors did not resemble any type of normal brain cell but had similarity to the histopathology of TSC-associated SENs and SEGAs. When examined under higher magnification, the inner tumor mass was found to be composed of a heterogeneous cell population containing small spindle-like cells, as well as cells that resembled differentiated neurons, with prominent nuclei and abundant eosinophilic cytoplasm (Fig. 1C). These various cell types were loosely gathered in a highly fibrillary background. More interestingly, among the cell mass, some cells were unusually large (Fig. 1C, inset). Further study revealed that the proliferative index among the inner tumoer mass was low, as measured by Ki67 staining (Fig. 1D, arrows). Although there were some Nestin-positive cells, consistent with the presence of stem/progenitor cells, most of the cells stained with either mature neuronal markers (MAP2 or NeuN) or astrocytic markers (S100β or GFAP) (Fig. 1D,E). Processes from both MAP2-positive and GFAP-positive cells contributed to the fibrillary matrix (Fig. 1E), indicating a mixed glioneuronal composition. Thus, our tumors present several key histological features of human SEGA, including the anatomic location, a mixed glioneuronal composition, and the presence of “giant” cells. However, we did not observe multinucleated cells within these tumor masses, as is typically seen in human SEGAs.
Tsc1 mutant SVZ stem/progenitor cells exhibit aberrant migration
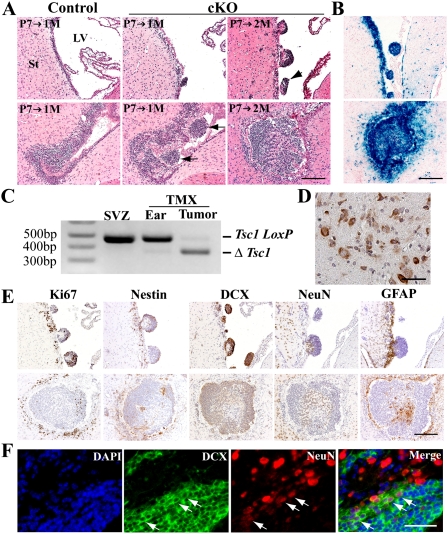
To investigate how these ventricular nodules and small tumors developed, we induced Tsc1 recombination in a group of mice at P7 and examined them at 1 and 2 mo of age. We observed that in Tsc1Nestin cKO mice, SVZ cells began to aggregate as they migrated to the lateral ventricle (Fig. 2A). The nodular protrusions on the lateral ventricle wall could be detected as early as 1 mo of age. By 2 mo of age, it was common to see multiple nodular structures present (Fig. 2A, top panels), with some nodular structures appearing to have detached from the ventricle wall and floating inside the ventricle (Fig. 2A, arrowhead). In the IF of mutant mice, we observed SVZ cell clusters forming as they migrated to the lateral ventricles at 1 mo of age (Fig. 2A, arrows), and by 2 mo of age, we often detected bigger cell masses at the same location (Fig. 2A, bottom panels). We believe that these initial tumor masses give rise to the SEGA-like structures seen later at 3 mo of age.
Figure 2.
Development of small nodular structures and tumors in the lateral ventricle involves aberrant aggregation and migration of SVZ NSPCs. (A) Tsc1Nestin cKO mice progressively develop abnormalities in the lateral ventricle, as shown by mice induced at P7 and examined at 1 and 2 mo of age. (Arrows) Clusters of cells show aberrant aggregation and migration from the SVZ to the lateral ventricle. Bar, 200 μm. (B) Both nodular structures and early tumor masses are composed of cells in which Cre activity has been induced, as visualized by X-gal staining on brain sections of Tsc1Nestin cKO mice bearing the R26-lacZ reporter allele. Bar, 200 μm. (C) PCR assay detected the recombined Tsc1 allele (ΔTsc1 allele) in tumor samples but not in controls (SVZ samples of untreated Nestin-CreERT2;Tsc1loxp/loxp mice and ear samples of TMX-treated Nestin-CreERT2;Tsc1loxp/loxp). Tsc1 loxP is the flox allele. (D) Phospho-S6 staining indicates strong mTOR signaling in the tumor mass. Bar, 50 μm. (E) Nodular structures and tumors that develop in the ventricle originate from NSPCs. Bar, 200 μm. (F) Neuroblasts retained in the lateral ventricle give rise to neurons, as shown by DCX (green) and NeuN (red) double-staining. (Arrows) Cells that are double-positive for NeuNdim and DCX. Bar, 50 μm.
Given the restricted activity of the Cre transgene to stem/progenitor cells and the absence of phenotype in TMX-treated controls, we concluded that the abnormal nodules and tumors originated from SVZ NSPCs that had lost the Tsc1 gene through Cre-mediated recombination. To confirm this, we examined Cre activity in the cells that comprise these abnormal structures using the R26-lacZ reporter line (Soriano 1999). We found that the nodular structures and the initial tumors were positive for X-gal staining, indicating that Cre activity had been induced in those cells (Fig. 2B). Indeed, as a consequence, we detected mainly recombined Tsc1 LoxP allele (ΔTsc1 allele) in the tumor sample, demonstrating that in tumor cells the Tsc1 gene had been disrupted by Cre-mediated recombination (Fig. 2C). In addition, consistent with human SEGA studies, we observed high levels of phospho-S6 expression within the tumor masses, indicating activated mTOR signaling, as a consequence of TSC1/2 complex loss of function (Fig. 2D; Kwiatkowski and Manning 2005). All of these data suggest that both the nodules and the tumors are likely derived from Nestin-positive NSPCs that have lost Tsc1.
Tsc1-null stem/progenitor cells undergo differentiation in the lateral ventricle
We next sought to evaluate how Tsc1-null NSPCs give rise to cell types that exhibit the histological features of human SENs and SEGAs. By immunostaining, we found that both the nodular structures and the initial tumors shared similar cell components (Fig. 2E). Both contained a subset of stem/progenitor marker-positive cells (Fig. 2E, Ki67 and Nestin staining), consistent with a stem/progenitor cell of origin. However, the majority of cells were Doublecortin (DCX)-positive, a marker for neuroblasts that derive from Nestin-positive NSPCs. Some of the inner cells of the initial tumor clearly displayed differentiated cell morphology. This was frequently observed in 2-mo-old cKO mice that were induced at P7. The more differentiated cell morphology was consistent with the reduced proliferation among the inner cell population of the tumor, as evidenced by Ki67 staining (Fig. 2E, Ki67 immunostaining in the bottom panels). Indeed, some of those cells expressed NeuN even when the cell mass was very small, suggesting that differentiated neurons were present in the cell mass. Interestingly, as shown by staining on adjacent sections of a small early tumor, there was some overlap of NeuN-positive and DCX-positive cells (Fig. 2E, NeuN and DCX immunostaining in the bottom panels). This was further confirmed by NeuN and DCX double-staining on many other tumor samples collected at 3 mo of age (Fig. 2F). Within the tumor mass, we clearly observed some dim NeuN-positive cells that retained DCX staining (Fig. 2F, arrows), suggesting that the DCX-positive neuroblasts retained in the lateral ventricle undergo further differentiation and give rise to neurons. These data provide evidence that the neuronal cell component of the tumors is derived from NSPCs, and not from entrapped nearby neurons.
Additionally, we observed GFAP-positive cells within the inner mass of the early tumors (Fig. 2E, GFAP immunostaining in the bottom panels). We hypothesized that those cells may have directly differentiated from Nestin-positive stem cells, as we observed Nestin-positive cells within the cell mass (Fig. 2E, Nestin immunostaining in the bottom panels). To evaluate whether loss of Tsc1 affects the ability of NSCs to differentiate into different neuronal lineages, we isolated Tsc1loxP/loxP SVZ NSCs from P7 pups and generated Tsc1-null SVZ NSCs by infecting the cells with Cre-GFP-expressing adenovirus. Loss of Tsc1 was confirmed by Western blot analysis (Supplemental Fig. S4A). In cultured NSCs, loss of Tsc1 did not result in obvious changes in morphology or proliferation (Supplemental Fig. S4B). Upon removal of growth factors, Tsc1-null NSCs were able to differentiate into various neural lineages, including neurons, astrocytes, and oligodendrocytes (Supplemental Fig. S4C). Therefore, in vitro, loss of Tsc1 does not affect the ability of NSCs to give rise to different neural lineages.
Impaired migration of OB granule neuron precursors
In wild-type mice, SVZ stem cells continuously generate DCX-expressing neuroblasts that migrate through the RMS to the OB, where they differentiate into granule or periglomerular interneurons (Ming and Song 2005). In the Tsc1Nestin cKO mice, we observed that the NSPCs aggregated in the lateral ventricle and differentiated into neuroblasts, resulting in the formation of nodular structures and small tumors in the ventricles. We therefore predicted that this should have repercussions on the state of interneurons in the OB. Indeed, although the overall structure of the OB remained intact in the Tsc1Nestin cKO mice, the cell density of the granule cell layer was significantly reduced compared with wild-type and heterozygote controls (Fig. 3A,B). This was not due to increased cell death; as shown by cleaved caspase-3 staining, although we observed a few apoptotic cells within the nodular structures, there was no obvious increase in apoptotic cells in either the SVZ or the OB of the Tsc1Nestin cKO mice (Supplemental Fig. S5A,B). We reasoned that the reduced number of granule neurons in the OB was due to the decrease in new neurons coming from the SVZ. To visualize all of the new OB neurons generated, we included the R26-LacZ allele in heterozygous and Tsc1Nestin cKO mice. Although β-gal-positive cells were present in the OB of Tsc1Nestin cKO mice, they were significantly reduced compared with the heterozygous controls (Fig. 3C,D). Thus, the reduction in granule neuron number coincides with the reduction in new neurons and is consistent with the observation that neuroblasts are retained in the lateral ventricle, where they form abnormal structures, rather than migrating to the OB.
Figure 3.
Tsc1Nestin cKO mice have fewer granule cells in the OB due to reduced migration. (A) Tsc1Nestin cKO mice have less granule cells in the OB. Bars: left panels, 500 μm; right panels, 100 μm. (B) Quantification of cell density in medial granule cell layer (GCL) of OB. n = 3 mice were examined for each genotype. Data are plotted by mean ± SEM and analyzed by Student's t-test; (**) P < 0.01. (C) Fewer newly generated granule cells in the OB were observed in Tsc1Nestin cKO mice. Bar, 50 bar. (D) Quantification of β-gal-positive cells in the medial granule cell layer. n = 3 pairs of mice were examined. Data are plotted by mean ± SEM and analyzed by Student's t-test; (**) P < 0.01.
The presence of β-gal-positive granule neurons in the OB of Tsc1Nestin cKO mice indicated that not all Tsc1-null neuroblasts migrate to the lateral ventricle. Some mutant neuroblasts were able to migrate to the OB and differentiate into mature neurons (Supplemental Fig. S6A). This is not likely to be due to incomplete recombination of the Tsc1 flox allele for two reasons. First, most of these β-gal-positive cells demonstrated higher phospho-S6 staining, a downstream marker of Tsc1 inactivation; and second, the cells were noticeably larger, a known consequence of TSC1/2 complex downstream hyperactivation (Supplemental Fig. S6B). These data indicate that some fraction of Tsc1-null neuroblasts do not exhibit migration deficits. Thus, either the precise timing of Tsc1 loss is critical to the cell, or other pathways are also involved in regulating neuroblast migration.
Loss of Tsc1 in transit-amplifying neural progenitors results in abnormal growths in the ventricle
During our analysis of the Nestin-CreERT2-R26 mice, we noted that Cre activity was also present in the ependymal cells upon TMX treatment, as demonstrated by S100β and YFP double-staining (Fig. 4A, left panel). Ependymal cells are highlighted with S100β staining, and Cre activity upon TMX induction is reflected by YFP expression from the R26-YFP reporter allele (Srinivas et al. 2001). We believe that the Cre activity in ependymal cells reflects the nature of the Nestin promoter used in our Nestin-CreERT2 mouse line, as other reported transgenic lines using the same Nestin promoter also display transgene expression in ependymal cells (Yamaguchi et al. 2000; Imayoshi et al. 2008).
Figure 4.
Tsc1 loss in transit-amplifying neural progenitors results in a neuroblast migration deficit. (A) Cre activity was induced in both SVZ NSPCs and ependymal cells in Nestin-CreERT2 mice, while in the Ascl1-CreERTM mice, only a limited number of SVZ progenitor cells are targeted upon TMX induction. Ependymal cells are marked by S100β (red) staining. Cre activity is marked by YFP (green) staining. Nestin-CreERT2;Tsc1loxp/+;R26-YFP mice and Ascl1-CreERTM; Tsc1loxp/+;R26-YFP mice were treated with TMX at P7 (for Nestin-CreERT2 mice) or at P7, P9, and P11 (for Ascl1-CreERTM mice) and examined at 1 mo of age. Bar, 50 μm. (B) Diagram illustrating Ascl1 expression in transit-amplifying neural progenitor cells. (C) Gross examination revealed that Tsc1Ascl1 cKO mice develop small abnormal growths in the lateral ventricle near the IF. Bars: for whole forebrain pictures, 2 mm; for 4× pictures, 500 μm; for 10× pictures, 200 μm. (D) Abnormal growths in the lateral ventricle of Tsc1Ascl1 cKO mice are composed of Cre-positive cells. Cre activity is visualized by X-gal staining on brain sections of Tsc1AsclI heterozygous and cKO mice bearing the R26-lacZ reporter allele. Bar, 200 μm.
Ependymal cells and SVZ stem cells share the same lineage, both being derived from radial glial cells that exist during early developmental stages (Merkle et al. 2004; Spassky et al. 2005). Therefore, it is likely that in human patients, these two cell types could share the same somatic mutations. Additionally, it is known that ependymal cells provide an important niche for stem/progenitor cells to grow. We therefore wanted to determine whether, in our mouse model, loss of Tsc1 in the ependymal cells contributed to the migration deficit. To address this question, we crossed Tsc1 flox mice with the Ascl1-CreERTM transgenic line, which drives Cre expression in SVZ transit-amplifying neural progenitors but not in ependymal cells (Fig. 4A,B; Kim et al. 2007). Additionally, with the Ascl1-CreERTM line, only transit-amplifying neural progenitors are targeted, but not NSCs. In this way, using acute TMX treatment, we could temporally target a wave of neurogenesis. To target as many neural progenitors as possible, we performed the initial induction at P7 and boosted it at P9 and P11. Examination of mice at 1 mo of age with X-gal staining indicated successful targeting of a small percentage of SVZ forebrain progenitors, compared with the Nestin-CreERTM line (Fig. 4A; Supplemental Fig. S1). We then examined the Ascl1-CreERTM; Tsc1loxp/loxp cKO mice (herein referred to as Tsc1Ascl1 cKO mice) at 6 mo of age along with littermate controls. Gross examination of Tsc1Ascl1 cKO mice revealed no overt brain abnormalities and no obvious deficits in the SVZ or most of the ventricle region (Fig. 4C, left panels). However, more than half of the Tsc1Ascl1 cKO mice (eight of 14, 57.1%), had small abnormal growths in the lateral ventricle near the IF (Fig. 4C, boxed area); four of these eight mice had developed obvious hydrocephalus. We examined Cre activity using the R26-lacZ reporter line and saw many blue cells retained in the SVZ of Tsc1Ascl1 cKO mice. More importantly, the abnormal growths that developed in the ventricle were found to be mainly composed of cells that were X-gal-positive (Fig. 4D), indicating that the abnormal growths originated from the subset of transit-amplifying neural progenitor cells that had lost Tsc1. Additional examination demonstrated that these abnormal growths were similar to the tumors in the Tsc1Nestin cKO mice that developed in the same location (Supplemental Fig. S7). We interpret the lower frequency and smaller size of the abnormal growths in the Tsc1Ascl1 cKO mice, as compared with Tsc1Nestin cKO mice, to reflect the limited number of neural progenitor cells that were targeted. By the TMX pulse, we only induced Tsc1 recombination in a small proportion of transit-amplifying cells present at induction. Nevertheless, our data indicate that loss of Tsc1 in transit-amplifying neural progenitors is sufficient to cause a migration deficit in neuroblasts. Therefore, loss of Tsc1 in ependymal cells is not required for the abnormal migration behavior of NSPCs seen in the Tsc1Nestin cKO mice.
Considering the many TSC-associated brain lesions, the observation that loss of Tsc1 in the NSPC population alone is sufficient to cause aberrant neuroblast migration may have larger implications. Migration deficits following Tsc1 loss may be a general underlying mechanism for other TSC-related brain lesions as well. For example, cortical tubers, the most common TSC-associated brain lesion, are characterized by disrupted cortical lamination and the presence of aberrant glia and neurons within the tuber, possibly due to a migration problem of the NSCs during formation of the cortical layers. This idea is supported by a recent study in which inducing Tsc1 loss in a small subset of cortical progenitor cells by in utero electroporation at embryonic day 15 (E15) resulted in tuber-like structure formation (Feliciano et al. 2011). However, while we have demonstrated that Tsc1 loss in ependymal cells is not required for the Tsc1-null NSPC migration deficits, we do not exclude the possibility that loss of Tsc1 in ependymal cells can facilitate tumor formation or contribute to the development of hydrocephalus in our model.
While our tumors recapitulate several key features of human SEGAs, we did not observe pleomorphic multinucleated tumor cells that are frequently seen in human SEGAs. We note that mouse models of cortical tubers also possess some, but not all, histological features of tubers (Meikle et al. 2007; Feliciano et al. 2011). This may reflect differences between species, or it may be that some histological features take a longer time to develop, and in our mouse model, we have only captured the initial stages of SEGA development.
One interesting question is why SEGAs preferentially develop near the foramen of Monro. In our mouse model, nodular structures that developed on the lateral ventricle wall did not become very large and tended to “fall” into the ventricle, whereas similar cell masses that formed near the IF grew bigger and developed into small tumors. We believe this ventricular region might be advantageous for tumor development due to its stem cell-rich environment and the narrow ventricular space, which could facilitate formation of initial cell masses and provide a supportive growth environment. It is possible that multiple cell masses develop simultaneously and then merge into one large mass. This is supported by the observation of individual cell masses in this area (Fig. 2A, arrows; Supplemental Fig. S3A, arrows). This idea has also been proposed in human SEGA development; it is believed that SEGAs originate from SENs near the foramen of Monro (Morimoto and Mogami 1986; Fujiwara et al. 1989).
In summary, we show that Tsc1 loss specifically in NSPCs can drive the development of SEN- and SEGA-like structures in the ventricles, due to aberrant aggregation and migration of SVZ NSPCs following Tsc1 loss. Our study offers direct genetic evidence that the cells of origin of human SENs and SEGAs are the NSPCs, thus providing new insight into TSC1/2 function in the nervous system.
Materials and methods
Animals and TMX treatment
Nestin-CreERT2 and Ascl1-CreERTM mice have been described previously (Kim et al. 2007; Chen et al. 2009). Tsc1loxP mice were a gift from Dr. David Kwiatkowski (Harvard Medical School, Boston) (Meikle et al. 2007). TMX (Sigma) was dissolved in sunflower seed oil (Sigma). To activate CreERT2 in Nestin-CreERT2 mice, 0.5 mg of TMX was intraperitoneally delivered to P7 mice, and 10 mg/20 g body weight of TMX was orally administered to 1-mo-old mice once a day for two consecutive days. To induce Cre activity in Ascl1-CreERTM mice, 0.5 mg of TMX was intraperitoneally delivered to P7 mice, and the induction was boosted at P9 and P11.
Microdissection and PCR assay for detecting recombined Tsc1 allele
Genomic DNA from tumor samples was isolated from paraffin sections by microdissection under the microscope. Tumor location was determined by methyl green staining on the section prior to microdissection. Genomic DNA from both the microdissected SVZ sample of untreated Nestin-CreERT2;Tsc1loxp/loxp mice and the ear sample of TMX-treated Nestin-CreERT2;Tsc1loxp/loxp served as control.
Histology and immunohistochemistry
Histology and immunohistochemistry are described in the Supplemental Material.
Acknowledgments
We thank Dr. David Kwiatkowski for the Tsc1loxP mice, and Drs. Dawen Zhao and Jian Chen for helpful discussion. This work was supported by the Simons Foundation (to L.F.P.). L.F.P. is an American Cancer Society Research Professor.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.16750211.
References
- Buccoliero AM, Franchi A, Castiglione F, Gheri CF, Mussa F, Giordano F, Genitori L, Taddei GL 2009. Subependymal giant cell astrocytoma (SEGA): is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology 29: 25–30 [DOI] [PubMed] [Google Scholar]
- Burger PC, Scheithauer BW, Vogel FS 2002. Surgical pathology of the nervous system and its coverings. Churchill Livingstone, New York. [Google Scholar]
- Chen J, Kwon CH, Lin L, Li Y, Parada LF 2009. Inducible site-specific recombination in neural stem/progenitor cells. Genesis 47: 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ess KC, Kamp CA, Tu BP, Gutmann DH 2005. Developmental origin of subependymal giant cell astrocytoma in tuberous sclerosis complex. Neurology 64: 1446–1449 [DOI] [PubMed] [Google Scholar]
- Feliciano DM, Su T, Lopez J, Platel JC, Bordey A 2011. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J Clin Invest 121: 1596–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Takaki T, Hikita T, Nishio S 1989. Subependymal giant-cell astrocytoma associated with tuberous sclerosis. Do subependymal nodules grow? Childs Nerv Syst 5: 43–44 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R 2008. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE 2007. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci 27: 12764–12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD 2005. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 14: R251–R258 doi: 10.1093/hmg/ddi260 [DOI] [PubMed] [Google Scholar]
- Lopes MBS, Wiestler OD, Stemmer-Rachamimov AO, Sharma MC 2007. Tuberous sclerosis complex and subependymal giant cell astrocytoma. In WHO classsification of tumours of the central nervous system, 4th ed (ed. Louis DN et al. ), pp. 218–221 International Agency for Research on Cancer (IARC), Lyon, France. [Google Scholar]
- Marcotte L, Crino PB 2006. The neurobiology of the tuberous sclerosis complex. Neuromolecular Med 8: 531–546 [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ 2007. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci 27: 5546–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A 2004. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci 101: 17528–17532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H 2005. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223–250 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Mogami H 1986. Sequential CT study of subependymal giant-cell astrocytoma associated with tuberous sclerosis. Case report. J Neurosurg 65: 874–877 [DOI] [PubMed] [Google Scholar]
- Napolioni V, Moavero R, Curatolo P 2009. Recent advances in neurobiology of Tuberous Sclerosis Complex. Brain Dev 31: 104–113 [DOI] [PubMed] [Google Scholar]
- Rosser T, Panigrahy A, McClintock W 2006. The diverse clinical manifestations of tuberous sclerosis complex: a review. Semin Pediatr Neurol 13: 27–36 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71 [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A 2005. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci 25: 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4 doi: 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH 2002. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 52: 285–296 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K 2000. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11: 1991–1996 [DOI] [PubMed] [Google Scholar]