Abstract
Maturation of hematopoietic stem cells (HSCs) from fetal to adult state and differentiation to progenitors are thought to follow a one-way street. In this issue of Genes & Development, He and colleagues (pp. 1613–1627) show that overexpression of Sox17 can convert adult multipotential progenitors to self-renewing HSCs that possess fetal properties. These findings challenge the irreversibility of hematopoietic development, and open up new perspectives to understand the different forms of HSC self-renewal at distinct stages of ontogeny and during transformation.
Keywords: Sox17, fetal, hematopoietic stem cell, self-renewal
Differentiation, dedifferentiation, and self-renewal
Development and differentiation are thought of as unidirectional processes in which cells commit to specific fates while their overall differentiation potential becomes more restricted. As proposed in Waddington's epigenetic landscape of development (Waddington 1957), these developmental fate decisions are thought to be highly stable. The rigidity of this model was challenged when Yamanaka and colleagues (Takahashi and Yamanaka 2006) questioned the irreversibility of developmental fate decisions in groundbreaking studies that showed reprogramming of differentiated skin cells to a pluripotent, embryonic state, by the ectopic expression of four pluripotency transcription factors. Since then, several studies have shown the potential of various adult cells to revert back to an embryonic, pluripotent state, or to transit from one “valley” to another upon induction of trans-differentiation by lineage-specific regulators. Examples of lineage reprogramming include conversion of fibroblasts to neurons (Vierbuchen et al. 2010), blood progenitors (Szabo et al. 2010), or cardiomyocytes (Ieda et al. 2010), as well as conversion of exocrine pancreas to insulin-secreting β cells (Zhou et al. 2008). These discoveries fueled new optimism for regenerative medicine with the hope of being able to tailor desired cell types and tissues from adult cells. However, a major challenge in using reprogrammed cells in regenerative medicine is to fine-tune the developmental stage of the reprogrammed cell to a tissue-specific stem cell that can self-renew. The ability to regulate development and differentiation states with such precision requires an in-depth understanding of the master regulators that govern the self-renewing state in tissue-specific stem cells.
There have been decades of effort to identify regulators that would sustain self-renewal of hematopoietic stem cells (HSCs) and thereby facilitate ex vivo expansion of HSCs, or even the generation of HSCs from pluripotent cells. However, “stemness” in the hematopoietic system is difficult to gain and easy to lose. It is thought that, once lost, self-renewal ability can only be reacquired through malignant transformation. In this issue of Genes & Development, He et al. (2011) show that adult hematopoietic progenitors can be induced to dedifferentiate into fetal-like HSCs and self-renew upon ectopic expression of Sox17. An earlier study from the Morrison laboratory (Kim et al. 2007) had identified Sox17 as a critical regulator of survival and self-renewal of fetal HSCs, but as dispensable for adult HSC maintenance. He et al. (2011) now demonstrate that Sox17 is truly a master regulator of fetal HSC identity and self-renewal programming, as overexpression of Sox17 was sufficient to confer fetal HSC properties on both adult HSCs and multipotential progenitors (Fig. 1). Ectopic expression of Sox17 was not initially leukemogenic, but in the long run resulted in transformation and megakaryocytic and/or erythroid leukemia. This study provides novel insights into the regulation of distinct HSC states during ontogeny, and opens new avenues to understand and manipulate self-renewal in normal and leukemic stem cells.
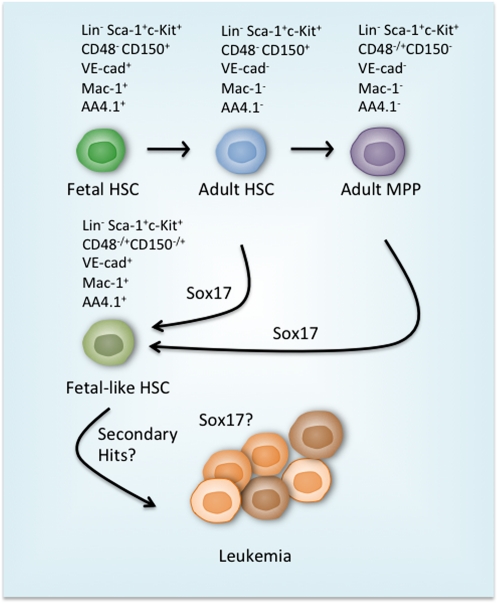
Figure 1.
Sox17 as a regulator of fetal HSC fate and enhanced self-renewal. During early postnatal life, the highly proliferative fetal HSCs mature into quiescent adult HSCs to protect the longevity and integrity of the HSC pool. The fetal and adult HSCs can be distinguished by their unique surface marker profiles (listed above). The key step in CD150+CD48−LSK (CD150+CD48−Lin−Sca1+cKit+) HSC differentiation to CD48+LSK cells that contain transiently reconstituting multipotential progenitors (MPPs) is the loss of the ability to support long-term reconstitution. He et al. (2011) demonstrate that ectopic expression of Sox17 in adult CD150+CD48−LSK HSCs and CD48+LSK progenitors is sufficient to reprogram the cells into a fetal HSC-like state. The reprogrammed cells possess very similar properties to fetal HSCs, including the expression of fetal-specific surface markers VE-cadherin (VE-cad), Mac1, and AA4.1, and increased self-renewal and reconstitution potential. Ultimately, the prolonged expression of Sox17 results in leukemia due to unknown mechanisms.
Developmental maturation of HSCs—role of Sox17
Hematopoiesis during development has two major goals: to generate differentiated blood cells needed for the embryo's immediate survival and growth, and to establish a pool of self-renewing, multipotential HSCs for lifelong blood homeostasis. The establishment of the HSC pool is a complex process that involves several anatomical niches in extraembryonic and intraembryonic tissues. HSCs originate from the aorta–gonad–mesonephros region of the embryo proper, the placenta, and possibly the yolk sac, and then seed the fetal liver before establishing lifelong residence in the bone marrow (for reviews, see Mikkola and Orkin 2006; Orkin and Zon 2008). The change in the anatomical niches where HSCs reside is also accompanied by changes in their functional properties, surface phenotype, and cell-intrinsic regulatory machinery (Mikkola and Orkin 2006; Teitell and Mikkola 2006). How the nascent HSCs acquire and maintain their self-renewal properties has been a long-standing mystery.
HSC development starts in a subset of lateral plate mesoderm that undergoes hematopoietic specification and gives rise to unique “hemogenic” endothelium, from which HSCs emerge (Zovein et al. 2008; Chen et al. 2009). Once emerged, the nascent HSCs have to complete a poorly understood maturation process, during which they acquire the competence to engraft and self-renew in adult hematopoietic niches (Taoudi et al. 2008). The newly formed HSCs populate the fetal liver, where they undergo rapid expansion to establish a large pool of HSCs for postnatal life. At around birth in mice, the HSC pool relocates to the bone marrow, where HSCs ultimately adopt a quiescent, homeostatic state. The developmental maturation of HSCs continues also during postnatal life as the HSCs age and exhibit reduced reconstitution potential and changes in lineage output (Rossi et al. 2005). So far, there has been no indication that this developmental progression in HSC ontogeny could be reversed.
HSCs in the developing embryo can be distinguished from their adult counterparts by surface marker expression. Fetal HSCs in mice express many markers that are shared with their cellular precursor, endothelium, such as CD34 and VE-cadherin, as well as markers that are known as lineage-specific markers in the adult, such as CD11b/Mac1 and AA4.1 (Fig. 1). These markers become gradually down-regulated during fetal development and are no longer expressed in quiescent adult HSCs (for review, see Mikkola and Orkin 2006). Fetal HSCs are constantly cycling, and exhibit much higher repopulation ability during transplantation than adult HSCs (Morrison et al. 1995). It is thought that during homeostatic conditions in postnatal life, HSCs self-renew predominantly via asymmetric self-renewal divisions, which maintain but do not expand the HSC pool. This is in contrast to fetal HSCs that can undergo symmetric self-renewal divisions to expand the pool of HSCs. In adult life, HSCs can engraft only in G0, whereas during fetal life, HSCs that are in G1 are also capable of in vivo reconstitution (Passegue et al. 2005; Bowie et al. 2006). This suggests that not only the cell cycle regulation per se, but also the mechanisms that govern niche interactions in fetal and adult HSCs throughout cell cycle, may be distinct. The transition from highly proliferative fetal HSCs to a predominantly quiescent adult HSC state occurs between 3 and 4 wk after birth, and has been shown to be intrinsically regulated rather than simply induced by the microenvironment (Bowie et al. 2006, 2007). It has been unknown which factors regulate this important developmental switch.
Establishment of the conditional knockout technology as well as stage- and cell type-specific Cre strains has facilitated the identification of stage-specific transcription factors that dictate HSC development or maintenance (for review, see Teitell and Mikkola 2006). Scl/Tal1 and Runx1 are critical for hematopoietic specification and hematopoietic stem and progenitor cell (HSPC) emergence from hemogenic endothelium, respectively, but become dispensable for the subsequent maintenance of the HSC pool (Mikkola et al. 2003; Chen et al. 2009). In contrast, Bmi1, Tel, and Gfi1 have important roles in HSC self-renewal and survival in the bone marrow, but are not essential for the establishment of the HSC pool during development (Teitell and Mikkola 2006). Little was known about the transcriptional regulation of HSCs during their highly expansive phase in the fetal liver, until the study from the Morrison laboratory (Kim et al. 2007), which identified Sox17 as a unique, fetal-specific HSC regulator that is needed for HSC survival/self-renewal, specifically during the fetal and neonatal period. These findings document that the fundamental properties of HSCs, such as self-renewal ability, are regulated by distinct transcriptional mechanisms during development and in postnatal life.
He et al. (2011) now show that Sox17 has an active role in determining fetal HSC identity and self-renewal properties. Overexpression of Sox17 by retroviral gene transfer or in tetracycline-inducible transgenic mice was sufficient to convert adult bone marrow cells to a fetal-like state, with significant up-regulation of the overall fetal HSC transcriptional program, including the fetal HSC surface markers VE-cadherin, Mac1, and AA4.1 (Fig. 1). Furthermore, Sox17-transduced HSCs were able to engraft at a higher frequency and showed increased self-renewal ability, similar to fetal HSCs. Moreover, the progeny of Sox17-expressing HSCs preferentially differentiated into erythroid, myeloid, and megakaryocytic lineages, similar to hematopoiesis in the fetal liver. These findings show that the intrinsic program driven by the overexpression of Sox17 is sufficient to impart fetal properties even in the adult—in this respect, overriding the regulation from the bone marrow niche, and documenting for the first time that developmental progression in HSCs can be reverted.
Given the strong correlation of fetal HSC phenotype and functional properties with Sox17 overexpression, it is not surprising that the expression pattern of Sox17 in the hematopoietic cells is highly restricted to CD150+CD48−LSK (CD150+CD48+Lin−Sca1+ckit+) and CD150−CD48−LSK fetal multipotential HSPCs that comprise ∼1% of fetal liver cells. He et al. (2011) show that the expression of Sox17 in fetal HSCs decreases during differentiation into lineage-restricted progenitors, and is also temporally regulated. By 2 wk after birth, Sox17 expression is no longer detected in HSPCs. As the transition of highly proliferative fetal HSCs to a quiescent adult-like state occurs between 3 and 4 wk of age (Bowie et al. 2006, 2007), it is tempting to speculate that down-regulation of Sox17 expression is the key event that induces the switch from the fetal state to a quiescent adult HSC.
Acquisition of self-renewal by Sox17
Perhaps the most surprising result from the study by He et al. (2011) was their finding that overexpression of Sox17 can also confer self-renewal properties to bone marrow CD48+LSK multipotential progenitors, which otherwise lack long-term self-renewal ability and would at best provide transient hematopoietic reconstitution. Ectopic expression of Sox17 in adult hematopoietic progenitors was also accompanied by increased expression of fetal HSC genes. However, it was not possible to revert the more differentiated, lineage-restricted progenitors to an HSC state, suggesting that Sox17 acts merely by inducing a fetal self-renewal program rather than by promoting dedifferentiation of lineage-committed cells.
The finding that Sox17 expression can induce self-renewal in otherwise non-self-renewing or poorly engrafting multipotential progenitors raises the question as to whether or not transient Sox17 overexpression could be used in a therapeutic setting. One major goal would be to improve the engraftment of cultured HSCs, which otherwise transplant poorly. Indeed, He et al. (2011) show diminished engraftment potential with ex vivo cultured HSCs that were transduced with control virus, whereas transduction with the Sox17 virus increased HSC activity. Previously, expression of HoxB4 during HSC culture has been shown to result in a net expansion of transplantable HSCs (Antonchuk et al. 2002). Nevertheless, Sox17 is the first regulator that has been documented to have the capacity to confer self-renewal ability to multipotential progenitors in the bone marrow that have clearly exited the HSC compartment.
Another important question to address is whether Sox17 promotes the functional maturation of HSCs and establishes the transcriptional program that confers self-renewal and in vivo engraftment ability in developing HSCs. If Sox17 has such a role, induction of Sox17 expression could confer self-renewal properties to nascent hematopoietic progenitors or hemogenic endothelial cells derived from embryonic stem (ES) cells. Generation of truly functional HSCs with in vivo engraftment and self-renewal ability from pluripotent cells has turned out to be a major challenge for the field, despite our ability to differentiate various blood cell types (for review, see Murry and Keller 2008). Prior studies had indicated that transient overexpression of HoxB4 was sufficient to induce engraftment ability to mouse ES cell-derived hematopoietic progenitors, albeit with a strong bias to form myeloid cells at the expense of lymphoid cells (Kyba et al. 2002). Furthermore, this approach has not been successful with human ES cells (Wang et al. 2005). Another possible avenue to use Sox17 is to coexpress it to promote self-renewal during lineage programming of fibroblasts or other adult cells to HSPCs (Szabo et al. 2010). However, although Sox17 is an interesting candidate for the master regulator of the establishment and maintenance of self-renewal in HSCs during development, a recent study reported that inducible overexpression of Sox17 during blood specification from mouse ES cells reduced the proliferation of CD41+ hematopoietic precursors and increased cell death (Serrano et al. 2010). The effects of Sox17 may therefore be highly developmental stage- and cell type-specific, and/or dependent on the dose of Sox17 protein. Regardless of whether induction of Sox17 expression during ES cell differentiation helps to establish HSC self-renewal and engraftment properties, using its expression as a marker to identify cells that have the potential to develop into functional HSCs, or to estimate the developmental state (fetal vs. adult) of engrafted HSCs, may be of use.
It remains to be shown to what degree the Sox17-transduced bone marrow progenitors have become reprogrammed to true HSCs, and whether sustained expression of Sox17 is required to maintain their self-renewal and engraftment. The study by He et al. (2011) did not detect induction of endogenous Sox17 expression in adult cells, arguing against a complete reprogramming of adult HSPCs to the fetal state.
Mechanisms of action of Sox17
The remarkably interesting finding of induction of the fetal HSC state and enhanced self-renewal ability upon Sox17 expression calls for future studies to identify the specific factors that govern Sox17 expression or are directly regulated by Sox17. Sox17 expression in the embryo is initiated in the early endoderm and subsequently in vascular and hematopoietic cells (Engert et al. 2009). Interestingly, targeting Cre into exon 1 of the Sox17 gene directs the expression of the reporter gene in arterial endothelium but not the endoderm, suggesting that distinct promoters drive its expression in endodermal and mesodermal cells (Liao et al. 2009). A 10-factor chromatin immunoprecipitation (ChIP) sequencing study in the mouse ES cell-derived HPC7 hematopoietic progenitor cell line suggests that many key hemato-vascular transcription factors, including Erg, Fli1, Scl, Lmo2, Runx1, Gata2, Gfi1b, Meis, and Pu.1, are capable of binding the Sox17 gene (Wilson et al. 2010). However, it was not assessed whether Sox17 expression in arterial endothelium or fetal HSCs is dependent on any of these individual factors. Identification of the key factors that initially activate Sox17 and maintain its expression highly restricted to fetal HSCs will help in understanding the mechanisms by which fetal-type HSC self-renewal is confined to the correct cells.
Further analysis of the programs downstream from Sox17 will provide a deeper understanding of the fetal HSC state and the molecular and cellular basis for the unique self-renewal and engraftment properties of fetal HSCs. Is increased self-renewal simply a result of more proliferation without further differentiation? It is not yet clear whether overexpression of Sox17 actually alters the cell cycle status of adult HSPCs. However, it is unlikely that increased proliferation of Sox17-expressing HSCs alone would result in an increased number of engraftable HSCs, as exit from G0 is thought to compromise the engraftment of bone marrow HSCs. In contrast, fetal HSCs can also engraft, even though they are in cell cycle (Passegue et al. 2005; Bowie et al. 2006). Further investigation of the unique developmental stage-specific engraftment mechanisms may provide answers to the increased self-renewal and engraftment of Sox17-expressing cells.
Interestingly, He et al. (2011) identify several transcription factors previously associated with the regulation of HSPC proliferation—such as Gata2, Evi1, Pbx1, and Egr1—as being up-regulated in Sox17-overexpressing HSPCs. While Gata2 and Evi1 seem to have a positive effect on HSC proliferation (Ling et al. 2004; Goyama et al. 2008), expression of Egr1 and Pbx1 in bone marrow HSCs have been associated with the regulation of niche interactions and HSC quiescence rather than enhanced proliferation (Ficara et al. 2008; Min et al. 2008). It remains to be determined whether the increased self-renewal and engraftment ability in Sox17-overexpressing HSCs and endogenous fetal HSCs is a result of a different type of self-renewal division (symmetric vs. asymmetric) and/or whether expression of Sox17 promotes different types of niche interactions that are facilitated by the fetal HSC surface proteins. He et al. (2011) do report increased extramedullary hematopoiesis with Sox17-expressing cells, suggesting that Sox17 expression may allow HSPCs to use secondary niches for their survival and proliferation.
In addition to the molecules that were expressed at higher levels in Sox17-expressing CD48+LSK cells, some lineage-specific genes, such as Rag1 and Rag2 that are essential for lymphoid differentiation, were down-regulated in these cells. Down-regulation of these critical lymphoid differentiation genes offers a possible explanation as to why the Sox17-expressing cells predominantly give rise to myelo-erythroid and platelet reconstitution at the expense of lymphoid cells. Once ChIP technologies have been optimized for a small number of cells, ChIP sequencing can be performed on purified fetal HSCs to define which regulatory molecules are bound and directly activated or repressed by Sox17.
Another important future goal is to identify cooperating mechanisms that allow Sox17 to access its target genes, as these may be the limiting factors dictating which cells can be induced to self-renew by overexpression of Sox17. Does Sox17 require specific cofactors to interact with? What is the chromatin state in Sox17 target genes that makes these genes accessible to Sox17? Understanding the regulation and mechanisms of action for Sox17 in fetal HSCs may ultimately enable the development of improved methods to enhance self-renewal of cultured HSCs for therapeutic purposes.
Side effects of excessive self-renewal
HSC self-renewal has to be tightly regulated, as perturbations in the regulation of proliferation can result in leukemia or HSC exhaustion. During postnatal life, HSCs are dormant and divide infrequently to replenish the pool of HSCs. Based on one study, HSC self-renewal was estimated to occur, on average, every 145 d (Wilson et al. 2008), and in another study, every 57 d (Cheshier et al. 1999). In the adult, loss of HSC quiescence is detrimental, and on one hand can lead to profound HSC engraftment defects and HSC exhaustion, and on the other hand to development of leukemia (for review, see Jude et al. 2008). HSC quiescence is thought to protect the genome and minimize the chance of acquiring mutations in long-lived HSCs, which in the long run could result in transformation and leukemia.
The study by He et al. (2011) did not find direct evidence that Sox17 overexpression would lead to engraftment defects; on the contrary, the frequency of engraftable HSPCs was higher. However, prolonged expression of Sox17 in adult HSPCs did result in leukemic transformation (Fig. 1). Mice transplanted with Sox17 developed megakaryocytic/erythroid leukemia several months after transplantation, and eventually died as a result of the disease. However, it is unknown whether the leukemia developed due to direct induction of an oncogenic program by Sox17, or due to secondary mutations that accumulated because Sox17 may have induced increased cycling of HSCs.
Until now, Sox17 has not been recognized as a leukemia oncogene, and there are no reports documenting the overexpression of Sox17 in leukemia patients. Interestingly, dysregulation of Sox17 has been implicated in many solid tumors, such as gastrointestinal, hepatocellular, and breast cancers (Du et al. 2009; Fu et al. 2010; Jia et al. 2010). The mechanism of Sox17 function in endodermal tissue-derived cancers differs from the leukemia caused by ectopic Sox17 expression, as Sox17 expression was silenced rather than overexpressed in many of these cancers. In endodermal tissues, Sox17 is thought to act as a tumor suppressor by antagonizing Wnt signaling (Du et al. 2009; Jia et al. 2010). Indeed, enforced expression of Sox17 in hepatocellular cancer cells suppressed colony formation and tumor growth (Jia et al. 2010). He et al. (2011) did not detect changes in Wnt signaling upon Sox17 overexpression in HSPCs, suggesting that Sox17 regulates different target genes and downstream pathways depending on the tissue type.
Dedifferentiation to a more fetal-like state and the acquisition of self-renewal properties in non-self-renewing cells are hallmarks of many cancers (Hu and Shivdasani 2005; Krivtsov et al. 2006). The finding that long-term overexpression of Sox17 reproducibly induces leukemia opens up many important questions about the leukemogenic process in HSCs. Does maintenance of these leukemias require continuous Sox17 expression, or have newly acquired mutations made the self-renewal of leukemic cells Sox17-independent? Does endogenous Sox17 get activated in the leukemic cells? Is Sox17 a leukemia oncogene also in human, and are Sox17 or its key downstream target genes dysregulated in human leukemias? Is the down-regulation of Sox17 and the transition from the fetal to adult HSC state a necessary switch to prevent leukemic transformation? Unraveling the mechanisms by which prolonged Sox17 expression induces leukemogenesis will be important for understanding what makes HSPCs susceptible to transformation, and in determining whether Sox17 overexpression, even transient, could be used in a clinical setting to improve self-renewal and engraftment of HSCs.
Acknowledgments
A.C. was supported by a Jonsson Cancer Center Foundation Fellowship, and H.K.A.M was supported by a NIH RO1 HL097766-01 and CIRM New Faculty Award.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.17328611.
References
- Antonchuk J, Sauvageau G, Humphries RK 2002. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109: 39–45 [DOI] [PubMed] [Google Scholar]
- Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ 2006. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest 116: 2808–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ 2007. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci 104: 5878–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA 2009. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457: 887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL 1999. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci 96: 3120–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YC, Oshima H, Oguma K, Kitamura T, Itadani H, Fujimura T, Piao YS, Yoshimoto T, Minamoto T, Kotani H, et al. 2009. Induction and down-regulation of Sox17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology 137: 1346–1357 [DOI] [PubMed] [Google Scholar]
- Engert S, Liao WP, Burtscher I, Lickert H 2009. Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis 47: 603–610 [DOI] [PubMed] [Google Scholar]
- Ficara F, Murphy MJ, Lin M, Cleary ML 2008. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell 2: 484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DY, Wang ZM, Li C, Wang BL, Shen ZZ, Huang W, Shao ZM 2010. Sox17, the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer Res Treat 119: 601–612 [DOI] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M 2008. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell 3: 207–220 [DOI] [PubMed] [Google Scholar]
- He S, Kim I, Lim MS, Morrison SJ 2011. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev (this issue). doi: 10.1101/gad.2052911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Shivdasani RA 2005. Overlapping gene expression in fetal mouse intestine development and human colorectal cancer. Cancer Res 65: 8715–8722 [DOI] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D 2010. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yang Y, Liu S, Herman JG, Lu F, Guo M 2010. SOX17 antagonizes WNT/β-catenin signaling pathway in hepatocellular carcinoma. Epigenetics 5: 743–749 [DOI] [PubMed] [Google Scholar]
- Jude CD, Gaudet JJ, Speck NA, Ernst P 2008. Leukemia and hematopoietic stem cells: balancing proliferation and quiescence. Cell Cycle 7: 586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ 2007. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130: 470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. 2006. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442: 818–822 [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ 2002. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109: 29–37 [DOI] [PubMed] [Google Scholar]
- Liao WP, Uetzmann L, Burtscher I, Lickert H 2009. Generation of a mouse line expressing Sox17-driven Cre recombinase with specific activity in arteries. Genesis 47: 476–483 [DOI] [PubMed] [Google Scholar]
- Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW, Dzierzak E 2004. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med 200: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola HK, Orkin SH 2006. The journey of developing hematopoietic stem cells. Development 133: 3733–3744 [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421: 547–551 [DOI] [PubMed] [Google Scholar]
- Min IM, Pietramaggiori G, Kim FS, Passegue E, Stevenson KE, Wagers AJ 2008. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2: 380–391 [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL 1995. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci 92: 10302–10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G 2008. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132: 661–680 [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL 2005. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med 202: 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci 102: 9194–9199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AG, Gandillet A, Pearson S, Lacaud G, Kouskoff V 2010. Contrasting effects of Sox17- and Sox18-sustained expression at the onset of blood specification. Blood 115: 3895–3898 [DOI] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M 2010. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468: 521–526 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A 2008. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 3: 99–108 [DOI] [PubMed] [Google Scholar]
- Teitell MA, Mikkola HK 2006. Transcriptional activators, repressors, and epigenetic modifiers controlling hematopoietic stem cell development. Pediatr Res 59: 33R–39R doi: 10.1203/01.pdr.0000205155.26315.c7 [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M 2010. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH 1957. The strategy of the genes. Geo Allen and Unwin, London, UK [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, Cerdan C, Levac K, Bhatia M 2005. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med 201: 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, Kaimakis P, Chilarska PM, Kinston S, Ouwehand WH, Dzierzak E, et al. 2010. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7: 532–544 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. 2008. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3: 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]