Abstract
Suboptimal nutrition during prenatal and early postnatal development is associated with increased risk for type 2 diabetes during adult life. A hallmark of such diabetes risk is altered body composition, including reduced lean mass and increased adiposity. Since stem cell number and activity are important determinants of muscle mass, modulation of perinatal nutrition could alter stem cell number/function, potentially mediating developmentally programmed reductions in muscle mass. Skeletal muscle precursors (SMP) were purified from muscle of mice subjected to prenatal undernutrition and/or early postnatal high-fat diet (HFD)—experimental models that are both associated with obesity and diabetes risk. SMP number was determined by flow cytometry, proliferative capacity measured in vitro, and regenerative capacity of these cells determined in vivo after muscle freeze injury. Prenatally undernutrition (UN) mice showed significantly reduced SMP frequencies [Control (C) 4.8%±0.3% (% live cells) vs. UN 3.2%±0.4%, P=0.015] at 6 weeks; proliferative capacity was unaltered. Reduced SMP in UN was associated with 32% decrease in regeneration after injury (C 16%±3% of injured area vs. UN 11%±2%; P<0.0001). SMP frequency was also reduced in HFD-fed mice (chow 6.4%±0.6% vs. HFD 4.7%±0.4%, P=0.03), and associated with 44% decreased regeneration (chow 16%±2.7% vs. HFD 9%±2.2%; P<0.0001). Prenatal undernutrition was additive with postnatal HFD. Thus, both prenatal undernutrition and postnatal overnutrition reduce myogenic stem cell frequency and function, indicating that developmentally established differences in muscle-resident stem cell populations may provoke reductions in muscle mass and repair and contribute to diabetes risk.
Introduction
Nutritional or environmental stimuli acting during critical developmental windows can have a lasting impact on cellular structure/function and patterns of adult disease [1]. Indeed, a variety of prenatal nutritional stressors, including undernutrition, obesity, and placental insufficiency, may program metabolic adaptations that favor survival, but ultimately are detrimental to adult health. Moreover, prenatal nutritional stress is often followed by accelerated growth and fat accumulation during early childhood, which further increases risk for adult obesity, type 2 diabetes, and cardiovascular disease [2,3].
How suboptimal perinatal environments lead to adult disease has not been fully elucidated at a molecular level. However, a common physiological phenotype associated with developmentally mediated disease risk is altered body composition, with reduced lean body mass and increased fat mass observed in both humans and animal models [4–6]. Reductions in skeletal muscle mass may be particularly important as muscle is a key determinant of systemic metabolism and insulin sensitivity [7]. Moreover, humans with low birth weight, a marker of prenatal developmental history, demonstrate alterations in muscle fiber size and type and decreased oxidative capacity [8]. Similarly, experimental nutritional restriction during pregnancy alters offspring muscle mass and composition in sheep, in parallel with increased lipid accumulation and reduced oxidative capacity in muscle [9]. Such reductions in muscle mass and/or function may thus disrupt systemic metabolism and contribute to adult disease risk.
One determinant of skeletal muscle mass, as well as its appropriate maintenance with aging, is a quantitatively and qualitatively normal muscle stem cell population. Muscle stem cells, a subset of muscle satellite cells, are mononuclear cells residing between the plasma membrane and basal lamina of mature myofibers [10]; these cells are defined by their capacity to both self-renew and differentiate to generate mature, multinucleated muscle fibers, and are primary mediators of postnatal muscle regeneration [11]. Mouse models with impaired muscle stem cell function and number, such as Pax7-null mice, have significantly decreased muscle mass [12,13] and poor regenerative function after injury [14]. Moreover, satellite cells appear to be particularly sensitive to nutritional availability during periods of high growth. In poultry, early postnatal starvation yields reduced satellite cell proliferation, increased apoptosis, and sustained reductions in muscle mass despite normalization of food intake [15–17]. Reduced satellite cell number has also been observed in malnourished human children [18].
Given these interesting links between nutritional exposure during development, reduced muscle mass, and adult disease risk, an important question is whether muscle stem cell activity or regenerative capacity is altered as a function of perinatal nutrition and could contribute to adult disease in mammals.
Materials and Methods
Animal studies
Mice were housed in an Office of Laboratory Animal Welfare (OLAW)-approved facility, with controlled temperature, humidity, and light–dark cycle (07:00–19:00). Protocols were approved by the Joslin Diabetes Center Institutional Animal Use and Care Committee; “Principles of Laboratory Animal Care” (http://grants1.nih.gov/grants/olaw/references/phspol.htm) were followed.
Mice exposed to maternal undernutrition were generated as previously described [6,19–21] (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). In brief, 6–8-week-old ICR females were housed with ICR males, and pregnancies dated by vaginal plug (day 0.5). Pregnant dams were allowed ad lib access to regular chow (Purina 9F, 21% of calories from protein, 21% from fat, and 58% from carbohydrate) until day 12.5, when dams were randomly assigned to ad lib feeding (C) or 50% food restriction (undernutrition, UN) calculated based on food intake of control dams for the specific day of pregnancy. At birth, litters were equalized to 8 pups. During suckling, dams were provided regular chow ad libitum. Both C and UN pups were weaned on day 21 to either Purina 9F chow or high-fat diet (HFD) containing 60% of calories from fat (Research Diets, Inc., Open Source Diets, D12492). For additional experiments, C57/BL6 mice with normal prenatal nutritional exposure were obtained from Jackson Laboratories at 3 weeks of age, and fed ad libitum with either chow or HFD for 3 weeks.
Blood glucose was measured between 9 and 11 AM (fed) or after a 16-h fast in tail vein samples from 8 week old mice (Ascensia Elite; Bayer). Insulin was measured using rat insulin enzyme-linked immunosorbent assay with mouse standards (Crystal Chem) (Supplementary Table S1).
Body composition was analyzed by dual energy X-ray absorptiometry (Hologic).
Stem cell isolation and measurement of myogenic colony formation
Muscle stem cells were isolated as previously described [22]. In brief, after anesthesia with pentobarbital (150 mg/kg), hindlimb muscles were dissected and digested with 0.2% (w/v) collagenase type II in Dulbecco's modified Eagle's medium (Gibco BRL) for 90 min at 37°C, and individual muscle fibers were dissociated by repeat pipetting. Isolated myofibers were then digested in 10 volumes of F10 medium containing 0.05 U/mL dispase (Gibco) and 0.012% collagenase type II for 30 min at 37°C with agitation to isolate myofiber-associated cells. Skeletal muscle precursor (SMP) cells were isolated by fluorescence activated cell sorting (FACS)-based purification using the markers Sca1-, CD45-, Mac1-, CXCR4+, and β1integrin+ as described [23]; a representative set of FACS plots is provided in Supplementary Fig. S2. To set detection thresholds for FACS sort and analysis, aliquots of interstitial muscle cells were stained with all antibodies minus one; these cells were analyzed by flow cytometry to set a gating threshold specific for each antibody. To assess myogenic colony formation, FACS-purified SMP cells were plated clonally in 96-well plates precoated with laminin (10 mg/mL; Gibco) and collagen (1 mg/mL; Sigma) and cultured in F10 at 37°C; the presence or absence of myogenic colonies was evaluated by light microscopy 5 days after plating, and expressed as percentage of wells containing colonies relative to total number of wells.
Muscle injury and regeneration
After anesthesia with Avertin (tribromoethanol; Sigma, 800 μL of 1:80 dilution intraperitoneally), dry ice was placed directly on the belly of the tibialis anterior muscle for 5 s. Three or 10 days after injury, animals were euthanized (pentobarbital), and injured muscles dissected. Muscles were fixed in 4% paraformaldehyde for 1 h and embedded in paraffin. Multiple sections were obtained at 60 μm increments, and slides stained with hematoxylin and eosin. Areas of muscle regeneration were assessed using ImageJ software. Specifically, nonoverlapping photos of each muscle were taken at 100×magnification, and a grid composed of small open dots was overlaid. Two blinded observers scored each dot in the injured area and assigned one of the following categories: regenerating fiber (centrally located nucleus), inflammatory cell (nucleus without underlying fiber), injured (fiber without central nucleus), or other (blood vessel/empty space).
Muscle fiber typing
After euthanasia, leg muscles were dissected, weighed, and frozen. Quadriceps sections were stained with succinyl dehydrogenase (Department of Pathology, Brigham and Women's Hospital), and 7 photos were taken at 400×. A blinded observer scored each fiber as type I (dark staining), type 2a (medium staining), or type 2b (little to no staining). Incomplete fibers at the border of photos were excluded.
Assessment of systemic and local inflammation
Systemic inflammation was assessed in plasma samples obtained via intracardiac puncture at 3 and 10 days after muscle injury. Tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1) were measured using multiplex enzyme-linked immunosorbent assay (Millipore). Local muscle inflammation (quadriceps) was assessed by both immunohistochemistry for the macrophage marker Mac2 (Cedarlane Labs) and quantitative real-time (RT)–polymerase chain reaction. In brief, RNA was extracted (Trizol; Invitrogen), cDNA generated from 1 μg RNA (Applied Biosystems), and RT–polymerase chain reaction analysis performed using SYBRGreen detection, with GAPDH as endogenous control (Applied Biosystems). Primer sequences are available in Supplementary Table S2.
Statistical analyses
Data are presented as mean±standard error of the mean. Multigroup comparisons of body or muscle weight, hormonal parameters, muscle area, and SMP frequencies were assessed by analysis of variance (Statview), with post hoc pairwise comparisons assessed by Fisher's protected least significant difference. Multigroup comparisons of regeneration and fiber type were assessed by χ2 analysis. P<0.05 was considered significant.
Results
Prenatal undernutrition alters body composition and reduces muscle mass
To determine the effect of antenatal or early postnatal nutrition on myogenic stem cell number and function, mice exposed to maternal undernutrition in utero were studied [6] (Supplementary Fig. S1). In this model, maternal food restriction during the final week of gestation results in a 14%–20% reduction in birth weight in UN pups as compared with controls (C).
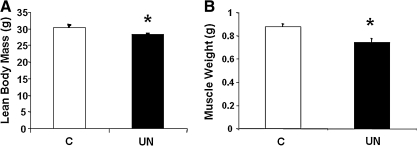
Despite reduced weight at birth, UN mice have similar weights to C mice by 6–7 weeks of age, reflecting catch-up growth in UN mice (C 38±0.7 g vs. UN 35.8±1 g; P=0.07). However, body composition analysis (dual energy X-ray absorptiometry) revealed that UN mice had persistent reductions in lean body mass as compared with C (C 30.4±0.7 g vs. UN 28.4±0.2 g; P=0.03) (Fig. 1A). Similarly, the combined weight of the quadriceps, gastrocnemius, soleus, tibialis anterior, and extensor digitalis longus muscles was significantly decreased in UN mice (C 0.88±0.02 g vs. UN 0.74±0.03 g; P=0.01) (Fig. 1B).
FIG. 1.
(A) Lean body mass, as assessed by dual energy X-ray absorptiometry, and (B) combined hindlimb muscle weight (quadriceps, gastrocnemius, soleus, TA, and EDL) at 6 weeks. (□C, ■UN). n=3–8 mice/group; *P=0.01 versus control. C, control; UN, undernutrition; TA, tibialis anterior; EDL, extensor digitorum longus.
UN mice have altered muscle fiber type
Alterations in fiber number or type have been previously described in low birth weight (LBW) humans and in humans at risk for diabetes based on obesity or diabetes family history [8,24,25]. Mice with UN exposure had no differences in fiber number per cross section of quadriceps muscle at either 6–7 weeks or 9 months of age. However, at 6–7 weeks, UN mice exhibited a 16% decrease in type I (slow oxidative) fibers (P<0.001), a 20% increase in type IIa (fast oxidative) fibers (P<0.001), and 6% increase in type IIb (fast glycolytic) fibers as compared with C (P<0.05) (Supplementary Table S3). At 9 months of age, type I fiber number did not differ, but UN mice had a 34% increase in type IIa and a 23% decrease in type IIb fibers (P<0.05 for both). Fiber number and type were also assessed in 9-month-old control and UN mice fed a HFD from weaning (C-HFD and UN-HFD, respectively). Neither fiber number nor fiber type distribution differed between C-HFD and UN-HFD muscle (Supplementary Table S3).
Mice exposed to maternal UN have decreased muscle stem cell numbers with normal ex vivo myogenic capacity
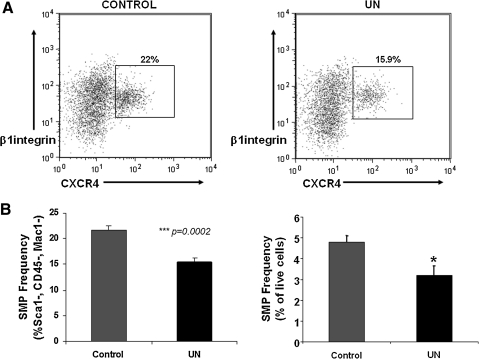
Muscle stem cells are crucial for muscle development, growth, and repair after injury [26]. To determine whether muscle stem cell number and/or function are altered in this experimental model, SMP cells were isolated from the myofiber-associated cell compartment of the hindlimbs of 6-week-old mice using FACS-based purification [22,23] based on the following markers: CD45-, Sca1-, Mac1-, CXCR4+, and β1 integrin+. Cells isolated using this protocol can self-renew, produce mature myotubes [23], and functionally engraft to enhance muscle function in mdx mice, a mouse model of Duchenne muscular dystrophy [22]. In UN mice (which show decreased muscle mass), SMP cell frequency was reduced by ∼33% (C 4.8%±0.3% vs. UN 3.2%±0.4%, P=0.02, percentage of live cells isolated) (Fig. 2A, B).
FIG. 2.
SMP frequency and function are reduced by prenatal undernutrition. (A) Representative FACS plots demonstrating final step in isolation of CXCR4+/β1 integrin+1, Sca1− cells from control mice (left panel) and mice exposed to maternal undernutrition (UN, right panel). (B) SMP frequency is decreased in UN mice compared with controls (n=6/group); data are expressed as % of the Sca1−, CD45−, Mac1−population on the left panel, and as % of live cells in the right panel. ***P<0.001, *P=0.02. SMP, skeletal muscle precursor; FACS, fluorescence activated cell sorting.
To assess whether reduced number of myogenic precursor cells in UN mice was accompanied by alterations in their ability to enter a myogenic program, SMPs were plated at 1 cell per well, and myogenic colonies were evaluated 5 days later by microscopic inspection. The number of myogenic colonies arising from the clonally plated SMPs did not differ between groups, suggesting that, on a per cell basis, muscle stem cells from UN mice retain their normal capacity to survive and initiate myogenesis, as assessed in a surrogate ex vivo culture environment.
In vivo regeneration after muscle injury is decreased in low-birth-weight mice
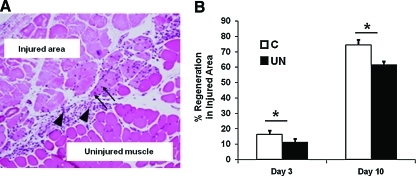
Because muscle stem cells play an important role in muscle repair, assessment of regeneration after injury is used as a measure of stem cell function [27]. To determine the functional consequence of reduced SMP numbers in UN mice, muscle histology and regeneration were assessed in 9-month-old UN or C mice at 3 or 10 days after in vivo freeze injury (representative sections, Fig. 3A). Consistent with differences in muscle weight observed as early as 6 weeks of life, overall muscle size was decreased in injured UN mice at 9 months of age, as determined by cross-sectional area of the tibialis anterior (day 3: C 10.1±0.7×106 vs. UN 6.1±0.7×106 pixels, P=0.004). The area of muscle injury was similar between experimental groups on both days 3 and 10 (Supplementary Fig. S3). However, at 3 days after injury, there was a 32% reduction in the number of regenerating fibers (Fig. 3B, left) in UN mice (C 16%±2.6% vs. UN 11%±2.2% of injured area; P<0.0001), and an 18% reduction at 10 days after injury (C: 74%±3%; UN 61%±2.2%; P<0.001) (Fig. 3B, right).
FIG. 3.
Muscle regeneration is reduced in UN mice. (A) Representative hematoxylin and eosin staining of skeletal muscle from C mice 3 days after injury. The area of injury, regeneration, and inflammation within the injured area was assessed for each muscle. Regenerating fibers are distinguished by their centrally located nuclei (arrows). Inflammation is more prevalent on day 3 (arrowhead). (B) Regeneration after freeze injury is decreased in muscle from UN mice at both 3 and 10 days after cold injury. (□, C; ■, UN) n=4–8 muscles/group. *P=0.0001 by χ2 analysis. Color images available online at www.liebertonline.com/scd
Since both inadequate and excessive inflammatory responses can hinder muscle regeneration [28,29], inflammation was assessed at both the local and systemic level in 9-month-old mice 3 days after cold injury. There were no significant differences in (1) serum levels of inflammatory markers (MCP1, TNF-α, IL-6, or PAI-1), (2) quadriceps muscle inflammatory gene expression (IL-1β, F4/80, MCP1, TNF-α, IL-6, and PAI-1), or (3) inflammatory cell infiltration in injured muscle, as indicated by immunohistochemical analysis of Mac2 (Supplementary Fig. S4A–C).
High-fat feeding decreases muscle stem cell number and function
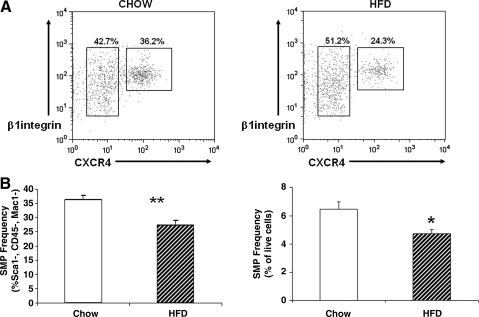
Since increased adiposity is a dominant phenotype in UN mice, observed as early as 3 weeks of age [21], it is possible that early life obesity per se could contribute to reduced muscle stem cell frequency. SMP frequency was measured in hindlimb muscle from mice with normal antenatal nutrition fed a HFD from ages 3 to 6 weeks postnatally. HFD-fed mice were heavier (chow 23.3±0.5 g vs. HFD 25.4±0.8 g, P=0.001), as expected, but had reduced muscle mass (hindlimb muscle weight: chow 1.29±0.04 g, HFD 1.21±0.03 g, P=0.008). Interestingly, SMP frequencies were reduced by 27% in HFD-fed mice (chow 6.4%±0.6% vs. HFD 4.7%±0.4%, expressed as percentage of live cells, P=0.03, Fig. 4).
FIG. 4.
Early life high-fat feeding reduces SMP frequency and function. (A) Representative FACS plots demonstrating final step in isolation of CXCR4+/β1 integrin 1, Sca1−cells from control mice (left panel) and HFD-fed mice (right panel). (B) SMP frequency is decreased in HFD mice compared with controls (n=6/group); data are expressed as % of the Sca1−, CD45−, Mac1−population on the left panel, and as % of live cells in the right panel. **P=0.006, *P=0.02. HFD, high-fat diet.
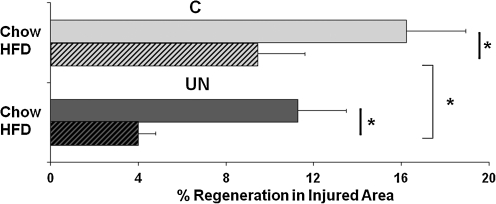
The impact of high-fat feeding in both C and UN mice on stem cell-initiated myogenesis was also assessed using in vivo regeneration assays. Muscle regeneration 3 days after cold injury was reduced by 42% in control mice fed a HFD (chow 16%±3% vs. HFD 9%±2%, P<0.0001). Strikingly, regeneration was reduced to an even greater extent in HFD-fed UN mice (64% reduction, UN 11%±2% vs. UN-HFD 4%±1%, P<0.0001) (Fig. 5).
FIG. 5.
Postnatal high-fat feeding reduces muscle regeneration in both control and UN mice. n=4–8 muscles/group. *P=0.0001 by χ2 analysis.
Discussion
Both prenatal undernutrition and early postnatal overnutrition are associated with reduced muscle stem cell number and reduced muscle regenerative capacity. These defects, while sustained during early life, persist into adulthood and may contribute to developmentally mediated reductions in muscle mass and altered body composition. Given that muscle mass is an important mediator of insulin-stimulated glucose uptake and systemic metabolism, reductions in stem cell number may contribute to associations between early life nutrition and developmental risk for adult disease.
Muscle growth and maintenance depend on adequate stem cell availability and repair. Muscle stem cells are normally quiescent, but upon muscle injury, can be activated to proliferate, self-renew, and differentiate into myoblasts. These myoblasts subsequently fuse with other myoblasts, as well as damaged muscle fibers, to form new functional muscle. This process may be particularly vulnerable to postnatal nutrition, as starvation reduces satellite cell proliferation and increases apoptosis in poultry [15–17] and satellite cell number is reduced in malnourished human children [18]. Reduced function of genes critical for postnatal satellite cell survival, such as Pax7, can cause accelerated muscle wasting soon after birth [14]. Thus, the reductions in stem cell number observed in UN mice may contribute directly to the decreased muscle mass observed during postnatal growth and in adulthood—a key phenotype in both UN mice and LBW humans [4,5]. Indeed, these experimental data confirm the hypothesis suggested by Cianfarani, who proposed that stem cells are critical for normal tissue maintenance and function and that prenatal malnutrition would decrease these populations leading to early tissue malfunction and contribute to LBW-associated disease phenotypes [30].
Alterations in stem cell number or function can also affect the ability of an organism to repair [14]. For example, mice with mutations in the dystrophin gene (mdx mice) have poor integrity of muscle fibers, increased vulnerability to mechanical stress, and thus need for perpetual repair, but also have reduced frequency of SMPs [22]. Together with the early observations of Schultz and colleagues that repeated stresses reduce the proliferative capacity of satellite cells [31], it is also possible that reductions in regeneration in UN mice could reflect not only early life reductions in SMP number, but also subclinical increases in muscle damage accumulated during life, which further magnify age-related reductions in repair capacity.
Tissue stem cell number and function are highly dependent on features of the systemic and local microenvironment (niche), as demonstrated for aging-related dysfunction. For example, in muscle, repair responses may convert to favor fibrogenic, rather than myogenic, processes with age [32]. Regeneration capacity can be restored in aging mice by exposure to the circulation of young mice, in part via reactivation of Notch signaling [27,33]. In accord with this concept, the present data suggest that alterations in the intrauterine or early postnatal nutritional/metabolic environment also affect muscle regenerative function. Since functional impairment was detected in vivo, reflected by reduced regeneration after muscle injury, but not during ex vivo myogenic colony formation assays, it is likely that reduced availability of myogenic stem cells in UN mice contributes to this process.
Since undernutrition in mice is associated with early onset adiposity and progressive glucose intolerance with aging, it is possible that obesity per se or other features of a diabetogenic microenvironment might contribute to reductions in stem cell frequency or function. Interestingly, early life obesity (produced by high-fat feeding) was associated with a 27% reduction in SMP frequency and reduced regeneration after muscle injury. Moreover, the effects of HFD to reduce regeneration after muscle injury were additive with prenatal undernutrition. Thus, an adverse prenatal metabolic environment, early life onset of nutritional obesity (or both), and chronic obesity may all be detrimental for stem cell activity and repair.
While the specific mechanisms mediating the effects of both the prenatal and postnatal nutrient environment on stem cell number and/or function remain unclear at this time, stem cell-independent mechanisms may also contribute to our findings of decreased regeneration in UN and/or HFD-fed mice, including the size of the injury, extent of inflammation, and other aspects of the systemic or local tissue milieu. While larger injuries, whether in absolute size or as a percent of the muscle, could slow the regenerative process (fewer satellite cells to repair the injury), there were no differences in the area of injury (percentage of cross-sectional area) in this model. Similarly, either reduced or excessive inflammation could also impair muscle growth, regeneration, and injury responses [28,34–36]. However, systemic or local inflammation was not altered in UN mice. While elevated glucose levels may alter differentiation of muscle stem cells [37], circulating glucose levels are consistently normal in our models at the age when stem cell number was assessed [6]. Whether nutritional or obesity-related alterations in amino acids, other metabolites, or nutritionally responsive growth factors critical for satellite cell development (e.g., insulin like growth factor 1 [IGF1]) could also contribute is an important question for future study [16,38]. Additional developmental signals or components of the systemic or tissue microenvironment, for example, stem cell niche [39,40], or growth factors produced locally by muscle fibers [16,41,42] could also contribute to reductions in stem cell frequency, function, and/or differences in myogenic versus adipogenic lineage development during regeneration [43], in the setting of UN exposure and obesity.
In summary, our studies demonstrate that low birth weight induced by prenatal undernutrition is associated with decreased frequency and in vivo functionality of muscle stem cells, as assessed by regeneration after injury, reduced muscle mass, and altered fiber type. Further, a HFD during early life is also associated with reduced stem cell number and decreased regenerative capacity, in both normal- and low-birth-weight mice. Thus, decreased number of stem cells and associated changes in regenerative capacity can result from adverse metabolic environments during both prenatal and early postnatal life. Such reductions in muscle stem cell number and function may contribute to alterations in muscle mass and body composition associated with developmentally mediated risk for adult disease.
Supplementary Material
Acknowledgments
We gratefully acknowledge research grant support from the American Diabetes Association (research grant and mentored postdoctoral fellowship awards to M.E.P.), the Graetz Foundation (to M.E.P.), individual NRSA (to M.W.), the Lawson-Wilkins Pediatric Endocrine Society (to E.I.), and a Burroughs Wellcome Fund Career Award, Harvard Stem Cell Institute Program Grant, and NIH grant 1RO1 AG033053 (to A.J.W.). This work was supported in part by the Joslin Diabetes Center Flow Cytometry Core, supported by the Harvard Stem Cell Institute and Joslin DERC (5P30 DK036836-23), and the Boston Area Claude D. Pepper Muscle Progenitor Core (P30 AG031679-02).
Author Disclosure Statement
The authors disclose no conflict of interest relevant to this article.
References
- 1.Gluckman PD. Hanson MA. Cooper C. Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava SK. Sachdev HS. Fall CH. Osmond C. Lakshmy R. Barker DJ. Biswas SK. Ramji S. Prabhakaran D. Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ. Osmond C. Forsen TJ. Kajantie E. Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 4.Hediger ML. Overpeck MD. Kuczmarski RJ. McGlynn A. Maurer KR. Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics. 1998;102:E60. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- 5.Wells JC. Chomtho S. Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Chillaron JC. Hernandez-Valencia M. Reamer C. Fisher S. Joszi A. Hirshman M. Oge A. Walrond S. Przybyla R. Boozer C. Goodyear LJ. Patti ME. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes. 2005;54:702–711. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA. Gunnarsson R. Bjorkman O. Olsson M. Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen CB. Storgaard H. Madsbad S. Richter EA. Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007;92:1530–1534. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]
- 9.Zhu MJ. Ford SP. Means WJ. Hess BW. Nathanielsz PW. Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575(Pt 1):241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins CA. Olsen I. Zammit PS. Heslop L. Petrie A. Partridge TA. Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Seale P. Sabourin LA. Girgis-Gabardo A. Mansouri A. Gruss P. Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 13.Oustanina S. Hause G. Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang S. Charge SB. Seale P. Huh M. Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halevy O. Geyra A. Barak M. Uni Z. Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- 16.Halevy O. Nadel Y. Barak M. Rozenboim I. Sklan D. Early posthatch feeding stimulates satellite cell proliferation and skeletal muscle growth in turkey poults. J Nutr. 2003;133:1376–1382. doi: 10.1093/jn/133.5.1376. [DOI] [PubMed] [Google Scholar]
- 17.Mozdziak PE. Evans JJ. McCoy DW. Early posthatch starvation induces myonuclear apoptosis in chickens. J Nutr. 2002;132:901–903. doi: 10.1093/jn/132.5.901. [DOI] [PubMed] [Google Scholar]
- 18.Hansen-Smith FM. Picou D. Golden MH. Muscle satellite cells in malnourished and nutritionally rehabilitated children. J Neurol Sci. 1979;41:207–221. doi: 10.1016/0022-510x(79)90040-6. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Chillaron JC. Hernandez-Valencia M. Lightner A. Faucette RR. Reamer C. Przybyla R. Ruest S. Barry K. Otis JP. Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Chillaron JC. Isganaitis E. Charalambous M. Gesta S. Pentinat-Pelegrin T. Faucette RR. Otis JP. Chow A. Diaz R. Ferguson-Smith A. Patti ME. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes. 2009;58:460–468. doi: 10.2337/db08-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isganaitis E. Jimenez-Chillaron J. Woo M. Chow A. Decoste J. Vokes M. Liu M. Kasif S. Zavacki AM. Leshan RL. Myers MG. Patti ME. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes. 2009;58:1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerletti M. Jurga S. Witczak CA. Hirshman MF. Shadrach JL. Goodyear LJ. Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherwood RI. Christensen JL. Conboy IM. Conboy MJ. Rando TA. Weissman IL. Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Marin P. Andersson B. Krotkiewski M. Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care. 1994;17:382–386. doi: 10.2337/diacare.17.5.382. [DOI] [PubMed] [Google Scholar]
- 25.Nyholm B. Qu Z. Kaal A. Pedersen SB. Gravholt CH. Andersen JL. Saltin B. Schmitz O. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–1828. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- 26.Wagers AJ. Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Conboy IM. Conboy MJ. Smythe GM. Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira CF. Zamuner SR. Zuliani JP. Fernandes CM. Cruz-Hofling MA. Fernandes I. Chaves F. Gutierrez JM. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve. 2003;28:449–459. doi: 10.1002/mus.10453. [DOI] [PubMed] [Google Scholar]
- 29.Bondesen BA. Mills ST. Kegley KM. Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 30.Cianfarani S. Foetal origins of adult diseases: just a matter of stem cell number? Med Hypotheses. 2003;61:401–404. doi: 10.1016/s0306-9877(03)00182-8. [DOI] [PubMed] [Google Scholar]
- 31.Schultz E. Jaryszak DL. Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech Ageing Dev. 1985;30:63–72. doi: 10.1016/0047-6374(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 32.Brack AS. Conboy MJ. Roy S. Lee M. Kuo CJ. Keller C. Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 33.Conboy IM. Conboy MJ. Wagers AJ. Girma ER. Weissman IL. Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 34.Thaloor D. Miller KJ. Gephart J. Mitchell PO. Pavlath GK. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol. 1999;277(2 Pt 1):C320–C329. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 35.Shoelson SE. Lee J. Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vignaud A. Ramond F. Hourde C. Keller A. Butler-Browne G. Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology. 2007;74:291–300. doi: 10.1159/000105812. [DOI] [PubMed] [Google Scholar]
- 37.Aguiari P. Leo S. Zavan B. Vindigni V. Rimessi A. Bianchi K. Franzin C. Cortivo R. Rossato M. Vettor R. Abatangelo G. Pozzan T. Pinton P. Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nierobisz LS. Felts V. Mozdziak PE. The effect of early dietary amino acid levels on muscle satellite cell dynamics in turkeys. Comp Biochem Physiol B Biochem Mol Biol. 2007;148:286–294. doi: 10.1016/j.cbpb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Kuang S. Gillespie MA. Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Brack AS. Conboy IM. Conboy MJ. Shen J. Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Waddell JN. Zhang P. Wen Y. Gupta SK. Yevtodiyenko A. Schmidt JV. Bidwell CA. Kumar A. Kuang S. Dlk1 is necessary for proper skeletal muscle development and regeneration. PLoS ONE. 2010;5:e15055. doi: 10.1371/journal.pone.0015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischoff R. Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn. 1994;201:41–54. doi: 10.1002/aja.1002010105. [DOI] [PubMed] [Google Scholar]
- 43.Uezumi A. Fukada S. Yamamoto N. Takeda S. Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.