Abstract
The human Bcl-2 family includes six antiapoptotic members (Bcl-2, Bcl-B, Bcl-W, Bcl-XL, Bfl-1, and Mcl-1) and many proapoptotic members, wherein a balance between the two determines cell life or death in many physiological and disease contexts. Elevated expression of various antiapoptotic Bcl-2 members is commonly observed in cancers, and chemical inhibitors of these proteins have been shown to promote apoptosis of malignant cells in culture, in animal models, and in human clinical trials. All six antiapoptotic members bind a helix from the proapoptotic family member Bim, thus quenching Bim's apoptotic signal. Here, we describe the use of a multiplex, high-throughput flow cytometry assay for the discovery of small molecule modulators that disrupt the interaction between the antiapoptotic members of the Bcl-2 family and Bim. The six antiapoptotic Bcl-2 family members were expressed as glutathione-S-transferase fusion proteins and bound individually to six glutathione bead sets, with each set having a different intensity of red fluorescence. A fluorescein-conjugated Bcl-2 homology region 3 (BH3) peptide from Bim was employed as a universal ligand. Flow cytometry measured the amount of green peptide bound to each bead set in a given well, with inhibitory compounds resulting in a decrease of green fluorescence on one or more bead set(s). Hits and cheminformatically selected analogs were retested in a dose–response series, resulting in three “active” compounds for Bcl-B. These three compounds were validated by fluorescence polarization and isothermal titration calorimetry. We discuss some of the lessons learned about screening a chemical library provided by the National Institutes of Health Small Molecule Repository (∼195,000 compounds) using high-throughput flow cytometry.
Introduction
The Molecular Libraries Initiative of the National Institutes of Health (NIH) in the United States supports high-throughput screening (HTS) for important target proteins and protein families, which are carried out by dedicated screening centers against a compound collection provided by the NIH Small Molecule Repository, presently consisting of ∼350,000 compounds. Multiplexing by flow cytometry gained prominence when commercial polystyrene bead sets with different fluorescence intensities, covalently attached to different antibodies to cytokines, were combined in suspension to simultaneously quantify 15 cytokines using a multiplexed flow cytometric assay.1 Glutathione-S-transferase fusion proteins (GST-fusion proteins) are widely used in cell biology because a small laboratory can easily construct a DNA sequence for the fusion protein, express the protein in bacteria, and partially purify the protein using commercial glutathione (GSH) affinity beads. Although GST-fusion proteins noncovalently bind to GSH with a fairly weak dissociation constant of about 10 nM, making it improbable that the bound proteins would stay on the bead during 1 h of incubation, in fact the fusion proteins dissociate from such beads at a slow rate, perhaps facilitated by dimerization of the proteins at the bead surface where high GSH densities reside (USP 7,785,900).2,3 When amino polystyrene bead sets of different red fluorescent intensities became commercially available, we envisioned opportunities for devising HTS assays wherein the bead surface would be covalently coated with GSH, allowing noncovalent binding of various GST-fusion proteins, which could then be used for quantitative measurement of the binding of various fluorochrome-conjugated analytes that associated with the immobilized GST-fusion proteins, such as peptides, nucleotides, and steroid hormones.
The antiapoptotic members of the Bcl-2 family have been found to be elevated in various cancers and/or become elevated during treatment. New chemical scaffolds that modulate Bcl-2 functions are attractive targets for chemical screening. In humans, the Bcl-2 family consists of many proapoptotic and six antiapoptotic members (Bcl-2, Bcl-B, Bcl-W, Bcl-XL, Mcl-1, and Bfl-1), where the balance between the two decides at what level of damage or stress a cell will undergo apoptosis.4 For this reason, antiapoptotic Bcl-2 family proteins are important players in cancer cell survival and are recognized as relevant targets in cancer treatment. Pro and antiapoptotic Bcl-2 family proteins exert their cellular functions at least in part by physically interacting with each other to either suppress or promote apoptosis. The best documented mode of interaction among Bcl-2 family proteins involves the association of an α-helical segment, called Bcl-2 homology region 3 (BH3), provided by proapoptotic proteins, with a complementary surface crevice found on the antiapoptotic proteins.5 Synthetic BH3 peptides mimic the cellular activities of many of the proapoptotic Bcl-2 family proteins, serving as antagonistic ligands for the antiapoptotic members Bcl-2, Bcl-XL, Mcl-1, Bcl-W, Bfl-1, and Bcl-B. Thus, it has been shown that simple HTS assays can be constructed, in which chemical libraries are screened for compounds that displace BH3 peptides from antiapoptotic Bcl-2 family proteins, providing a starting point for generation of chemical antagonists that might be useful as cancer therapeutics.6
BH3 domains of proapoptotic Bcl-2 family proteins can be either selective or broad spectrum in their interactions with antiapoptotic Bcl-2 family proteins. For example, though many proapoptotic Bcl-2 family proteins such as Bcl-G show preferential binding to particular antiapoptotic members of the family, some proapoptotic members of the family such as Bim possess BH3 domains that interact with all six antiapoptotic family members with high affinity.7 By analogy to BH3 peptides, synthetic nonpeptidyl chemical antagonists of antiapoptotic Bcl-2 family proteins can be either selective or broad spectrum in terms of their binding to and neutralization of these cytoprotective proteins. From a therapeutic standpoint, however, the ideal spectrum of binding activity of chemical antagonists to antiapoptotic Bcl-2 family proteins is debatable and probably varies depending on the clinical indication. On one hand, broad-spectrum inhibitors that neutralize all six family members might be preferred for many types of cancer to ensure cytotoxic activity, but could also confer unacceptable toxicities. On the other hand, highly selective inhibitors that only neutralize specific antiapoptotic members of the Bcl-2 family might be better tolerated, but also could be limited in their efficacy only to those tumors where the relevant members are expressed in isolation without other redundant members of the family. Indeed, examples of resistance to selective inhibitors have been documented for chemical antagonists of Bcl-2 and Bcl-XL, where high levels of either Mcl-1 or Bfl-1 have been demonstrated to negate cytotoxic activity against cancer and leukemia cells.8 Still other applications of Bcl-2 antagonists can be envisioned for diseases beyond cancer, such as autoimmunity, wherein antiapoptotic Bcl-2 family proteins are believed to play an important role in allowing the survival of destructive, autoreactive lymphocytes. For example, Bcl-B is highly expressed in plasma cells,9 which suggests that it might serve as a target for eradication of long-lived autoantibody-producing cells.
To identify chemical inhibitors with different spectrums of activity against the six antiapoptotic Bcl-2 family members, it would be ideal to have an HTS assay method for efficient and simultaneous analysis of all six targets in one well. Herein, we describe such a multiplexed assay using high-throughput flow cytometry. We present a combined approach of biological screening and cheminformatics data mining to identify small molecule modulators of the antiapoptotic members of Bcl-2 family within a large collection of compounds provided by the National Institute of Health's Molecular Libraries' Small Molecules Repository (MLSMR). The MLSMR is an important part of the NIH Roadmap initiative, which comprises a diverse collection of small molecules that generally display drug-like properties.10 By conducting a multiplexed assay for competitive displacement of fluorochrome-conjugated Bim BH3 peptide to each of the six antiapoptotic family members, we have been able to identify a number of different unrelated classes of compounds as candidate modulators of antiapoptotic Bcl-2 proteins. As an example, we focus on inhibitors of Bcl-B, which were validated by fluorescence polarization (FP) and isothermal calorimetry.
Materials and Methods
General
Unless otherwise noted, all reagents were of analytical grade and purchased from Sigma-Aldrich. The six GST-fusion proteins were constructed, in which the Bcl-2 moieties lacked only an ∼20-amino acid hydrophobic tail; these were bacterially expressed and affinity purified as previously described.11 Beads were delivered from a 384-well plate (Catalog No. 784201 from Greiner Bio-One) using an autosampler and a peristaltic pump (HyperCyt™, available from IntelliCyt). The details of this particle delivery system have been previously described.12,13 Briefly, the autosampler dips into a well for 900 ms, then travels through the air between wells for 0.5 s, and then samples the next well. These 384 clusters of beads are delivered to a CyAn flow cytometer (Dako Corporation, now Becton-Dickinson) and appear as 384 groups of beads or groups of “events” in a single time file. The flow cytometry file is analyzed using a commercial software, HyperView™ (Intellicyt), which can determine the mean green fluorescence on each bead set for each cluster (well) of beads.
Synthesis of GSH Beads
Amino polystyrene beads, 4 μm in diameter, were dyed with a proprietary red fluorophore to different intensities by Duke Scientific (now Thermo Fisher Scientific) and supplied at 1.4×108 particles/mL. The bifunctional chemical crosslinker succinimidyl 4-[N-maleimidomethyl]-cyclohexane-1-carboxylate (SMCC) was used to covalently react with the amino groups on the beads and then to GSH added later. About 3.6 mL of beads (5×108 beads) were spun at 1,500 g for 2 min and resuspended in 400 μL of 50 mM sodium phosphate buffer (pH 7.5) containing 0.01% Tween-20. Eight microliters of 100 mM SMCC in dimethyl sulfoxide (DMSO) was added and the beads were mixed gently for 30 min. The beads were spun and resuspended in 360 μL of fresh buffer and then 40 μL of 200 mM GSH (pH 7) and 4 μL of 100 mM EDTA (pH 7–8) were added. Nitrogen was bubbled slowly through the suspension for 5 min, the tube was capped to exclude oxygen, and the beads were gently mixed for 30 min. The beads were washed four times and stored at 1.4×108 beads/mL in 30 mM HEPES (pH 7.5), 100 mM KCl, 20 mM NaCl, 1 mM EDTA, and 0.02% NaN3 at 4°C.
Assay for Inhibitors of Fluoresceinated Bim BH3 Peptide Binding to Bcl-2 Family Proteins
This assay is described in detail in another report14 and an overview is given here. A schematic diagram of our strategy is shown as Figure 1. The assay buffer was 30 mM HEPES (pH 7.5), 100 mM KCl, 20 mM NaCl, 1 mM EDTA, 0.1% BSA, and 0.01% Tween-20. The six antiapoptotic Bcl-2 family members (Bcl-B, Bcl-XL, Bcl-2, Bcl-W, Bfl-1, and Mcl-1) were separately expressed as GST-fusion proteins, affinity purified, cleared of GSH, and mixed separately with the six GSH bead sets having different red intensities, and a seventh set of beads was left without any protein as a control for nonspecific binding.
Fig. 1.
Diagram of bead coating, mixing, and resolution by cytometry. (A) Six sets of beads are mixed separately with glutathione-S-transferase (GST)-Bcl-2 family proteins, and a seventh set serves as a control for nonspecific binding. (B) The seven bead sets are mixed together and distributed to appropriate wells. (C) The flow cytometer records red fluorescence for each bead, allowing the sets to be gated, after which the green fluorescence of beads in each gate can be measured.
Currently, HTS flow cytometry screening of 100 plates per day is performed with 4 people and three to four cytometers in our center. However, in this report, for screening 24 plates per day with a single operator and cytometer, 140 μL of each GSH bead set was added to 340 μL of assay buffer and mixed twice over 20 min at room temperature, giving seven suspensions of surface-passivated beads. An average of 20 μg of each GST-Bcl-2 family member was added to the passivated bead set and mixed twice on ice over 15 min, and then the seven suspensions were left on ice overnight for use the next day. The bead sets were resuspended, and for each batch of four 384-well plates, 75 μL of each coated bead set was spun down in a separate tube. The sets were then combined in 200 μL of fresh buffer and spun once more. The beads were diluted to 8.5 mL and transferred to the four 384-well assay plates in 5 μL of buffer per well.
The compounds to be tested were supplied as 1 mM DMSO stock solutions in 384-well plates and were added to the assay plates using a BioMek FXP robot (Beckman Coulter) equipped with a 384 pin tool optimized to transfer 0.1 μL of the sample. The fluoresceinated Bim (F-Bim) BH3 peptide was then added to all wells in 5 μL of buffer per well, the plates were mixed for 15 s at 2,000 rpm, and the beads were gently rotated end over end for 1 h at 4°C (surface tension keeps the suspensions in their wells). The plates were mixed again and delivered by the HyperCyt system to a flow cytometer for interrogation.
The flow cytometer was set to acquire forward scatter, side scatter, FL1 (fluorescein filter set, 488 nm excitation, 530±20 nm emission), FL8 (635 nm excitation, 665±10 nm emission), and time. Figure 1C shows the seven bead sets from one assay plate resolved from each other in red fluorescence and gated, following which the green fluorescence on each bead set is easily obtained. An arbitrary cutoff of 40% inhibition was taken to indicate a screening “hit” or possibly active compound. This was a deliberate use of low stringency and assumed that further analysis would be done on many false-positive hits. The assay can also be performed at a series of compound concentrations to form a dose–response curve (Fig. 3). Table 1 diagrams a step-by-step protocol for this assay.
Fig. 3.
Representative dose–response curves showing active and inactive interactions. (A) Flow cytometry: CID 650929 is active against Bcl-B (•), not against Bfl-1 (■), Bcl-XL (▾), Bcl-W (♦), Mcl-1 (▴), or Bcl-2 (+); glutathione control beads are (o). (B) Fluorescence polarization: three compounds are active. The CIDs correspond to symbols as follows: 650929 (□), 1243212 (+), and 666339 (×). The y-axis is P×1,000, where P=(F∥−F⊥)/(F∥+F⊥), F∥=fluorescence intensity parallel to the excitation plane, and F⊥=fluorescence perpendicular to the excitation plane. (C) Isothermal titration calorimetry: compound accession identifier (CID) 650929 is active against Bcl-B, not Bcl-XL. The fluorescence polarization data (B) were obtained in duplicate, with the error bars showing the two readings. The flow cytometry (A) and isothermal titration calorimetry (C) data show single-point determinations. CID, PubChem compound accession identifier number.
Table 1.
Summary of High-Throughput Screening Flow Cytometry Multiplex Assay Protocol
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Bead mixture | 5 μL | Robot with 384 tips |
| 2 | Controls | 0.1 μL | Robot with pin tool; 10 μM |
| 3 | Library compounds | 0.1 μL | Robot with pin tool; 10 μM |
| 4 | F-Bim | 5 μL | Robot with 384 tips; 50 nM |
| 5 | Mix, incubate | 1–3 h | 4°C–10°C |
| 6 | Assay readout | FS, SS, FL1, FL8 | Flow cytometer |
1. Beads coated individually overnight, wash and mix in batches; not Cols 23, 24
2. Cols 1, 23; wash sequence: water, DMSO, EtOH
3. Wash sequence: water, DMSO, EtOH
4. The F-Bim retains 90% fluorescence for 8 h under laboratory fluorescent lights
5. The incubation can be 15 min at room temperature
6. After each plate, back flush and debubble
With 1 person and one cytometer, 24 plates per day containing a total of 7,680 compounds were screened. With 4 people and four cytometers, 100 plates per day can be screened.
DMSO, dimethyl sulfoxide; F-Bim, fluoresceinated Bim.
Time Binning, Data Reduction, and Analysis
The flow cytometry files for each plate were opened with HyperView software, singlet beads were gated in a standard forward scatter vs. side scatter dot plot, and then the individual bead sets were gated in a side scatter versus FL8 plot as shown in Figure 1C. The library of compounds (NIH Small Molecule Repository) was supplied in 320 wells of standard 384-well plates, leaving the first two columns and last two columns for controls. We chose to leave two columns free of beads at the end of the plate, so that the time file presents 16 groups (rows on the plate) of 22 clusters of events (wells with beads) in each row, totaling 352 rather than 384 clusters of events, to allow confidence in assigning a cluster to a plate position. The software identified the 352 clusters in about 3 s, and the series of 16 groups of 22 clusters was checked by eye in about 1 min: as beads are more homogeneous than cells, this check was rapid. The software then calculated the number of events and average green fluorescence associated with each bead for each bead set in each well and exported these reduced data as comma-separated value files, including the compound identification number. The resulting files were typically <1% the size of the original flow cytometry file. Hits were flagged for further testing. Dose–response curves were fitted using Prism (GraphPad Software).
Fluorescence Polarization
FP assays were performed similarly to previous reports using various Bcl-2-family proteins and fluorescein isothiocyanate (FITC)-conjugated Bim BH3 peptide.11 Briefly, 50 nM of various recombinant Bcl-2 family proteins were incubated with 10 nM of FITC-Bim BH3 peptide (FITC-Ahx-DMRPEIWIAQELRRIGDEFNAYYAR in 25 mM Bis-Tris buffer containing 1 mM tris(2-carboxyethyl) phosphine and 0.005% Tween 20 [pH 7.0]) in the dark. FP was measured using an Analyst TM AD Assay Detection System (LJL Biosystem) after 10 min. IC50 determinations were performed using GraphPad Prism software (GraphPad). A compound was described as active if it induced a ≥40% decrease in FP.
Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) was performed on an ITC200 calorimeter from Microcal. Two-microliter aliquots of solution containing 500 μM compound were injected into 200 μL cells containing 50 μM GST-Bcl-2 protein. Nineteen injections were made per titration. The experiments were performed at 23°C in buffer containing 20 mM HEPES (pH 7.0). Experimental data were analyzed using Origin software provided by Microcal. A compound was described as active when it displayed a sigmoidal dose–response curve, which corresponds to IC50<10 μM at the protein concentration used.
Compound Selection Scheme
Primary screening
The primary screening data were processed through Microsoft Excel using a template file designed specifically for this assay. This template segregated data for each protein and the fluorescence scavenger bead in the multiplex, assembled the desired statistics and graphs, and flagged possible hits for each molecular library plate in a batch mode. The analysis and assessment of the active compounds was made by considering the percentage of inhibition, a value calculated for each compound on each target using a simple algorithm and the Excel template. In the primary screening, a compound was considered “active” if the binding of F-Bim was inhibited by more than 40%.
Selection criteria for cherry picking the compounds to be tested in a dose–response assay
In selecting compounds for confirmation in a multiple concentration–response assay, we used a more relaxed selection criterion, that is, the percentage of inhibition should be greater than or equal to the average percentage of inhibition of all compounds plus three times the standard deviation of the mean.
Cluster generation and analysis
To establish structure–activity relationships among the actives, the Mesa Grouping Module was used to cluster the compounds that passed the defined criteria, to analyze the resulting families, and to prioritize the clusters (families) to be selected for a dose–response assay. Mesa Grouping Module is a structure-based clustering and classification software offered by MESA Analytics & Computing (Santa Fe; www.mesaac.com). Mesa Software is a suite of programs, and for our purposes, the Fingerprint Module was used to generate fingerprints for structures and the Measures program was employed to generate asymmetric matrices, using the Tversky measure with a dissimilarity threshold cutoff of 0.5. The output of Measures was directed to the Clustering Module, which uses asymmetric, symmetric, disjoint, or nondisjoint algorithms to group the compounds. In our case we used a disjoint algorithm and 50 different output files were generated using exclusion region thresholds between 0.01 and 0.5. Although the compounds active for Bcl-B were singletons, a number of compounds reported for other family members were obtained using cluster generation.
Dose–response results analysis
We analyzed the results of the dose–response assay in three steps using Microsoft Excel templates to select compounds with desirable properties. In the first stage, the compounds that produced a statistically significant competitive displacement of the F-Bim BH3 ligand were selected as follows. The first filter required that the standard error of log EC50 had to be lower than the absolute value of log EC50. The second filter required that the standard error of Hill slope had to be smaller than the absolute value of Hill slope. The third filter required that the biological response amplitude (span) had to be statistically significant: the bottom plus three times its standard deviation had to be less than the top minus three times its standard deviation.
In a second stage of analysis, we identified the compounds that were active on at least one target. The compounds annotated as “active” had to pass the following filters: first, the computed EC50 had to be between 10−8 and 10−4 M; second, the absolute value of the Hill coefficient had to be between 0.5 and 2.
The third stage of this analysis was dedicated to the identification of potentially “false hits.” Some compounds gave an increase in fluorescence on the GSH (or scavenger) beads, which would appear as an activator of binding. A compound was denoted a “false activator hit” if the span of the compound on GSH beads was greater than 50% of the span of the appropriate protein beads. Some compounds gave a decrease in fluorescence in the counter screen assay (GST to GSH interaction, PubChem AID 1324), in which GSH beads were coated overnight with 50 nM GST-green fluorescent protein, as in the coating of the beads with GST-Bcl-2 proteins, and a dose–response curve of compounds was performed to determine if GST-green fluorescent protein was competed off the beads. A compound was denoted a “false hit” if the span of the counter screen assay was greater than 50% of the span of the appropriate protein beads. To recover the active compounds and identify the “false hits,” we applied all the filters and selection criteria described above.
In the final stage of the process, a prioritization scheme was developed in which the information from the previous steps was combined with data obtained from querying chemical scaffolds in different databases to eliminate “unattractive” scaffolds from the list of active compounds. Scaffolds found to be active in many other assays from PubChem and the chemical literature were flagged as undesirable from a chemical perspective. After compounds with known reactive groups and undesirable scaffolds were eliminated from the clusters, the remaining compounds were considered suitable to consider as candidate Bcl-2 family protein modulators.
Results
Optimizing and Validating a Bead-Based Set of Assays
A diagram of the multiplex assay is shown in Figure 1, in which the GSH bead sets, consisting of different red fluorescent intensities, were mixed individually with a GST-fusion protein and then washed and mixed together with the fluorescent probe (F-Bim). After incubation, the bead samples were transported to a flow cytometer and resolved by red fluorescence.
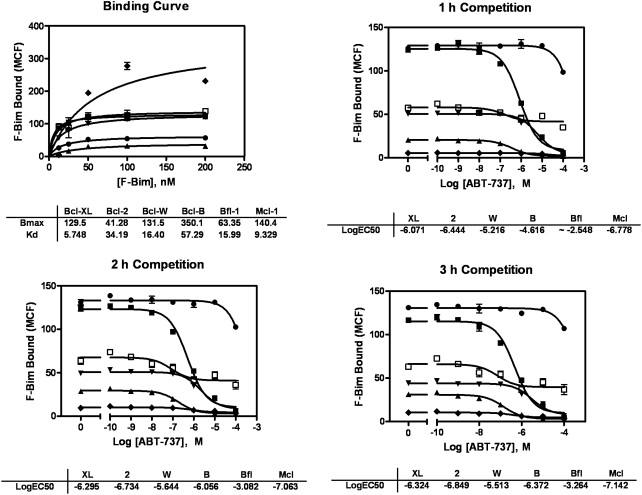
To validate the screening assay, we set out to reproduce a Kd that was determined by others and to reproduce an EC50 value determined by others using a known competitor, taking experimental differences into account. Multiplex binding data and inhibition of binding data are shown in Figure 2, and they additionally demonstrate the stability of the signal from 1 to 3 h. Binding curves for F-Bim to all six GST-Bcl-2 family proteins were determined, with results generally agreeing with the literature that Bim strongly engages all six antiapoptotic Bcl-2 proteins.15 It was fortunate that our Kd values covered only a 10-fold range from 6 to 60 nM, which allowed the F-Bim ligand to be added at a single intermediate concentration of 50 nM, wherein it displayed only 10-fold more sensitivity to an inhibitor of the most sensitive protein than to the least sensitive protein. It should be noted that for any assay describing the binding of one entity to another, results will differ among laboratories because of differences in time, temperature, ionic concentrations, source of materials, and other factors, so that absolute agreement of Kd values is rarely obtained. Nevertheless, the values obtained here are in general agreement with measures of the Kd of Bim and other BH3 peptide interactions with antiapoptotic Bcl-2 family proteins obtained by other methods.11,15,16
Fig. 2.
Binding and inhibition curves. Binding curves were obtained as described, including 1 h of incubation at 4°C with end-over-end mixing before interrogation by the cytometer. As with other data from our laboratory, above 100 nM fluorescein there appears to be clipping of the signal. The competition curves were obtained as described using 50 nM fluoresceinated Bim (F-Bim) after 1 year of storage and have been selected to show both the utility of the multiplex assay to demonstrate decreased activity of one protein in the multiplex (Bcl-B) as well as the stability of the response over 3 h. The proteins correspond to symbols as follows: Bcl-XL (■), Bcl-2 (▴), Bcl-W (▾), Bcl-B (♦), Bfl-1 (•), and Mcl-1 (□). All data were obtained in duplicate, with error bars showing the two readings.
This binding of F-Bim to each protein was also tested for competitive inhibition by ABT-737, a known Bcl-2 isosteric inhibitor.5 The concentration of ABT-737 was varied from 10−10 to 10−4 M, and the inhibitions obtained agreed with the literature, in particular that ABT-737 acts only weakly with Mcl-1 or Bfl-1.11 However, during the testing of ABT-737, it was observed that the preparation of Bcl-B had lost almost all activity during a year of storage at −80°C. This is seen in Figure 2, in which more F-Bim bound to Bcl-B than the other proteins in the binding curve, whereas less F-Bim bound to Bcl-B in the competition curves obtained a year later. This figure shows an advantage of multiplexing: with six individual dose–response series, one might be tempted to think that not enough F-Bim had been added to the Bcl-B series, but as all the beads at, for example, 10−9 M were incubated together in the same well, the lack of binding to the Bcl-B set is not a product of failure to deliver F-Bim to one series. As specific activities of the individual proteins do not have to be identical to obtain Z′ values over 0.5, a fresh sample of Bcl-B was obtained, in which the binding was similar to that of the other proteins (Fig. 3A), and used for the dose–response data submitted to PubChem.
In the primary screening campaign, the MLSMR library of 194,920 samples (194,829 unique compounds) was screened at a single concentration (10 μM) against GST-Bcl-2, GST-Bcl-B, GST-Bcl-W, GST-Bcl-XL, GST-Bfl-1, and GST-Mcl-1 using an F-Bim BH3 peptide, as shown in Table 2. The data analysis with user-defined criteria led to the identification of 518 compounds having a percentage of inhibition (response value) of >40%. These data are available from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/) under the assay identification numbers (AID numbers) presented in Table 2. The “response” value used in the PubChem database is the percentage of inhibition of F-Bim binding.
Table 2.
Primary Screening and Confirmatory Dose–Response Results
| |
|
Number of hits (PubChem AID) |
|
|||||
|---|---|---|---|---|---|---|---|---|
| Screening | Compounds tested | Bcl-2 | Bcl-B | Bcl-xL | Bcl-W | Bfl-1 | Mcl-1 | Total hitsa |
| Primary | 194,829 | 116 (950) | 142 (951) | 82 (1007) | 48 (952) | 237 (1008) | 196 (1009) | 385a |
| Confirmatory dose response | 834 | 9 (1328) | 6b (1327) | 6 (1322) | 18 (1330) | 97 (1320) | 3 (1329) | 27a |
| Powder dose response | 42 | 6 (2075) | 9/2c (2077) | 0 (2084) | 5 (2081) | 23 (2080) | 0 (2086) | 9a |
| FP (1) | 47 | 3 | Not tested | Not tested | Not tested | 3 | Not tested | 3 |
| FP (2) | 10 | 1 | 3 | 0 | Not tested | 0 | Not tested | 4d |
| ITC | 4 | Not tested | 3 | 0 | Not tested | Not tested | Not tested | 3 |
Some compounds that did not pass the threshold for being declared as active were nevertheless selected to be tested in follow-up assays.
Bfl-1 hits were not included in the number of total hits because the dose–response results from the original Bfl-1 preparation were not replicated with a new preparation in the FP assay dose–response analysis; total hits is thus the number of unique compounds affecting one or more of the other five antiapoptotic Bcl-2 family members. We hypothesized that redox-based modulation of cysteine 55 in the BH3 binding pocket could account for the discrepancy. However, the C55A mutant of Bfl-1 bound Bim normally and the compounds did not show the activity that they had against the original Bfl-1 preparation (data not shown). We then performed a multiplex analysis in which the original preparation of Bfl-1, the fresh preparation used in the FP assay, and the mutant protein were bound to individual bead set. These data recapitulated the activities of the individual preparations, and the fresh preparation set is being uploaded to PubChem to reconcile the earlier results.
Compound SID 85203040 was declared inactive in this assay, with an EC50=18.2 μM higher than the chosen threshold (10 μM).
Only two Bcl-B hits in this assay were inhibitors; seven other compounds “increased” the binding of the fluorescent peptide and were considered artifacts; compound SID 85203037 was declared inactive in this assay, with an EC50=16.3 μM higher than the chosen threshold (10 μM).
A benzoquinone reactive compound was also identified as hit on all protein targets, but was discarded.
AID, assay identification numbers; BH3, Bcl-2 homology region 3; FP, fluorescence polarization; ITC, isothermal titration calorimetry.
Dose–Response Data Confirm Three Compounds as Specific Bcl-B Inhibitors
This set of 518 compounds deemed active was subjected to structure-based clusterization, and 834 compounds covering the entire Bcl-2 family were obtained as stock solutions in DMSO and assayed in a dose–response series. The results of the confirmatory assay (Table 2) have been uploaded in PubChem under the assay identifier numbers presented in this table and also AID 1324 for the GST-GSH counter screen, which eliminates inhibition due to disruption of the GSH to GST binding.
Following the analysis of the dose–response data with the defined criteria and prioritization algorithm, singletons and families of compounds having members with EC50 values <10 μM were identified. One hundred eight compounds were found as potential modulators of Bcl-2 family of proteins. This number included Bfl-1 hits, which later were found to be false-positives (see Discussion section).
For follow-up studies, 37 modulators representing the most active members for each representative scaffold and 10 singletons were chosen. Forty-two compounds were obtained as dry powders and were subjected to liquid chromatography–mass spectroscopy analysis (which passed the quality control criteria that they were >95% pure by high-performance liquid chromatography and that they displayed the correct mass by liquid chromatography/mass spectrometry and high-resolution mass spectrometry). This set was retested in the dose–response assay and six compounds were reconfirmed as active against Bcl-B in a dose–response assay, shown in PubChem bioassay AID 2075.
Figure 3A shows an example of a dose–response curve for one of these compounds, with PubChem compound accession identifier (CID) number 650929, in multiplex on all six Bcl-2 family members. The weak response of the earlier preparation of Bcl-B has been corrected. It is clear that this compound inhibits the binding of F-Bim to Bcl-B much more than to any of the other family members. We emphasize that, for example, all beads with 10−5 M compound were in the same well. When the powders were tested, three of them had log EC50 values below −5 (EC50<10 μM), which we considered reliably active. They showed <20% inhibition of the other five antiapoptotic Bcl-2 family members at the highest concentration tested, 100 μM. Active compounds were also identified for other antiapoptotic family members.
FP Confirms the Flow Cytometry Active Compounds
Two rounds of FP dose–response assays were performed to validate the flow cytometry data, using not just the F-Bim probe, but also one with a different fluorophore, Cy5-Bim. Figure 3B shows an example of an FP dose–response curve for 10 compounds against GST-Bcl-B with F-Bim, in which only 3 compounds were declared active. Quantitative results for the three active compounds against Bcl-B are shown in Table 3, which correspond to the second FP assay in Table 2. The different fluorophore made little difference in log EC50 values obtained by FP, with the values differing only by 0.3 units and not all in the same direction. The flow cytometric log EC50 values averaged 0.3 units higher than the FP data, but agree that all compounds significantly inhibit F-Bim binding. Multiple tests on different days showed that the coefficient of variation of flow cytometry log EC50 values was <10%. Control FP experiments corroborated the specificity of binding to Bcl-B, with no binding to Bcl-XL, Bcl-2, or Bfl-1 (data not shown).
Table 3.
Fluorescence Polarization and Isothermal Titration Calorimetry Confirm the Flow Cytometry Data for Bcl-B
| Compound | FP Cy5-Bim log EC50 | FP F-Bim log EC50 | Flow F-Bim log EC50 | ITC compound log Kd |
|---|---|---|---|---|
| CID 650929 | −5.7 | −6.0 | −5.3 | −6.7 |
| CID 1243212 | −5.3 | −5.1 | −5.6 | −5.5 |
| CID 666339 | −6.0 | −6.0 | −5.7 | −5.6 |
| SID 85203040 | −6.0 | −6.0 | −5.7 | −5.6 |
ITC Confirms the Flow Cytometry Active Compounds
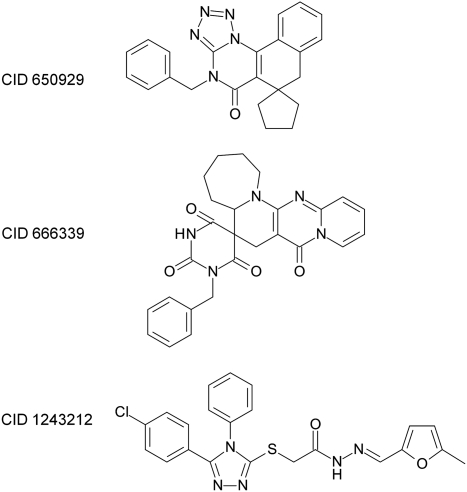
Isothermal calorimetry uses a different physical principle to measure binding and was used to confirm or deny binding of the three compounds to Bcl-B. Figure 3C shows an example of an ITC dose–response curve against Bcl-B, in which CID 650929 was declared active, and against Bcl-XL, in which the compound was declared inactive. Table 3 shows that the ITC log EC50 values demonstrate significant binding of all three compounds to Bcl-B, making it more plausible that our other measurements were in fact displaying competitive binding between F-Bim and the compounds to Bcl-B. The ITC demonstration of binding is particularly instructive, for the flow cytometry and FP measurements are prone to similar optical artifacts. The compound CID 650929 appears particularly strong with respect to Bcl-B binding. Control experiments showed no binding of the compounds to Bcl-XL (data not shown), as a demonstration of specificity. The structures of these three active compounds are shown in Figure 4. PubChem reports many comparisons of the screening and follow-up data, including the number of assays in which a compound has been declared active and the number of assays in which it has been tested: for CID650929, these numbers are 9/513 (active/tested); for CID1243212, these are 3/371; and for CID666339, these are 11/488, making all three compounds attractive with respect to specificity of interaction.
Fig. 4.
Structures and compound identifier numbers of the three inhibitors of the binding of F-Bim to Bcl-B.
Discussion
This report demonstrates a multiplexed, bead-based flow cytometry screen that covers a small family of proteins, requiring <1 mg of each GST-fusion protein per family member, a fluorescent probe, and the GSH bead sets described herein. In general, the capture and display of molecules on microspheres has the potential of reducing the quantities of the captured reagent (the GST-fusion protein) and allows the less-expensive fluorescent reagent (the F-Bim peptide) to be used near its Kd. By this approach, a screen of a small library of compounds is within the reach of an individual laboratory with a single flow cytometer, but a larger screen would require either a longer duration commitment or multiple flow cytometers operating in parallel. This screen was conducted with two people using one cytometer at a rate of 24 plates per day including analysis and took 26 days to screen the ∼195,000 compounds in the library at that time. Biotinylated proteins can also be used on differentially fluorescent streptavidin bead sets for multiplexing.
Our purpose was to find modulators for the Bcl-2 family of proteins, which is a step toward finding small molecules that modulate protein–protein interactions in general. Thus far, small molecule modulators of Bcl-2 family of proteins have been identified through FP assays or NMR-based methods. In this study, we have employed a different type of assay and screening approach, namely high-throughput flow cytometry, to identify potent and selective modulators of antiapoptotic members of Bcl-2 family. A multiplex HTS assay was developed and the MLSMR library (194,920 samples and 194,829 unique compounds) was screened against the six antiapoptotic proteins. A strategy was developed with the aim of identifying new chemotypes that could mimic the interactions between the binding groove of Bcl-2 proteins and BH3 domain of the proapoptotic proteins. The activities of the compounds were confirmed using FP and ITC.
Our approach involved multiple distinct steps, namely the screening of compounds in a primary HTS assay at a single concentration, the confirmation of the primary screening hits in a dose–response assay, run on cherry-picked samples and reordered powders, and follow-up secondary assays. The primary screening assay provided a large amount of data, and the processing of these data to discover active compounds was a challenging task. For this reason, a classification schema was developed to select the compounds from primary screening data to be confirmed in the dose–response analysis. The main idea was to prioritize relevant chemical scaffolds, or chemotypes, taking into account biological and chemical information. The confirmatory assay was aided by a counter screen, whose purpose was to identify potential “false hits,” compounds that could interact with the binding of GST to GSH and give a false biological signal, PubChem Bioassay AID 1324. In this counter screen, the bead sets were coated with 50 nM GST-GFP and assayed for loss of fluorescence when compounds were incubated with them; a loss of >50% fluorescence was defined as a false hit.
From 960 selected compounds, 834 were obtained and actually tested in a dose–response assay against all six proteins. Applying a prioritizing algorithm that took into account the biological responses (EC50 and efficacy) on each particular target, the signal on the GST to GSH interaction (if any), the statistical relevance of data, and the number of members for each family, we identified 19 classes of putatively active compounds and 94 singletons. Because of the problem with the Bfl-1 preparation used in the confirmatory dose response, 385 unique compounds were tested on the other five proteins and reported. The more stringent criteria for confirmation resulted in only 27 of the 385 being declared “active” in the dose–response assay or 7% confirmation of “hits.” This post-HTS analysis revealed an overall “hit” frequency in screening this library of ∼0.06% for hits with potency values less than 10 μM, and 0.1% for hits with potency values less than 100 μM. An assay with a Z′ score greater than 0.5 is considered an excellent assay for screening,17 and our Z′ values were Bcl-XL, 0.79±0.11; Bcl-2, 0.81±0.10; Bcl-W, 0.84±0.07; Bcl-B, 0.81±0.08; Bfl-1, 0.81±0.11; and Mcl-1, 0.82±0.12, consistent over the course of the screen. Overall, three selective compounds for Bcl-B were discovered using high-throughput flow cytometry and confirmed via FP and ITC. Although these compounds do not form a chemical series, they nevertheless may serve as a promising starting point for further optimization toward creating potent and biologically active selective inhibitors of the antiapoptotic protein Bcl-B.
The gene encoding Bcl-B was first recognized as the human genome project moved toward completion, with the first publication appearing in 2001. It has no clear ortholog in mice and thus represents an evolutionary progression of the Bcl-2 family in higher organisms. The Bcl-B protein shows selectivity for the BH3-containing proteins with which it interacts. For example, unlike Bcl-2, the Bcl-B protein binds to and neutralizes the proapoptotic protein Bax, but not Bak.18,19 In normal tissues, Bcl-B is highly expressed in B-lymphocytes and especially abundant in plasma cells.20 Prior experiments have suggested that Bcl-B is important for the survival of some plasma cell malignancies.9 Several types of solid tumors show pathological elevation of Bcl-B protein, sometimes correlating with poor patient prognosis.19 Selective inhibitors of Bcl-B, therefore, might find applications for autoimmune diseases, perhaps either by impairing the survival of long-lived autoantibody-producing cells or as a complement to chemical inhibitors of Bcl-2/Bcl-XL currently in clinical development for cancer.
In summary, this study illustrates a novel approach leading to the identification of chemical modulators of antiapoptotic Bcl-2 family proteins, in which flow cytometry was employed as a platform for simultaneous HTS of six antiapoptotic proteins Bcl-2, Bcl-B, Bcl-W, Bcl-XL, Bfl-1, and Mcl-1 against a large collection of compounds. Several future applications of multiplex flow cytometry can be envisioned for targets in the field of apoptosis, including assays using the BIR domains of IAP family proteins (there are eight IAP family members in humans), many of which are known to bind a tetrapeptide ligand derived from endogenous antagonists (second mitochondria-derived activator of caspase, SMAC, and high temperature requirement A 2, HtrA2, also named OMI) that suppresses their antiapoptotic activity. This multiplex assay method offers the advantage of assessing upfront the differential binding activities of chemicals for members of these multigene families, thus efficiently creating opportunities for identifying starting points for optimization of chemical inhibitors with unique spectrums of activity that may translate into distinct efficacy and toxicity profiles for different clinical indications.
Glossary
Abbreviations
- BH3
Bcl-2 homology region 3
- CID
PubChem compound accession identifier number
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- F-Bim
fluoresceinated Bim
- FP
fluorescence polarization
- GSH
glutathione
- GST
glutathione-S-transferase
- HTS
high-throughput screening
- ITC
isothermal titration calorimetry
- MLSMR
Molecular Libraries' Small Molecule Repository
- NIH
National Institutes of Health
- SMCC
succinimidyl 4-[N-maleimidomethyl]-cyclohexane-1-carboxylate.
Acknowledgment
This research was supported by an NIH grant (No. U54MH074425).
Author Disclosure Statement
L.A.S. and B.S.E. declare competing financial interests as co-inventors of HyperCyt and cofounders of Intellicyt.
References
- 1.Carson RT. Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 2.Tessema M. Simons PC. Cimino DF. Sanchez L. Waller A. Posner RG. Wandinger-Ness A. Prossnitz ER. Sklar LA. Glutathione-S-transferase-green fluorescent protein fusion protein reveals slow dissociation from high site density beads and measures free GSH. Cytometry A. 2006;69:326–334. doi: 10.1002/cyto.a.20259. [DOI] [PubMed] [Google Scholar]
- 3.Zuck P. Lao Z. Skwish S. Glickman JF. Yang K. Burbaum J. Inglese J. Ligand-receptor binding measured by laser-scanning imaging. Proc Natl Acad Sci U S A. 1999;96:11122–11127. doi: 10.1073/pnas.96.20.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JM. Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oltersdorf T. Elmore SW. Shoemaker AR. Armstrong RC. Augeri DJ. Belli BA. Bruncko M. Deckwerth TL. Dinges J. Hajduk PJ. Joseph MK. Kitada S. Korsmeyer SJ. Kunzer AR. Letai A. Li C. Mitten MJ. Nettesheim DG. Ng S. Nimmer PM. O'Connor JM. Oleksijew A. Petros AM. Reed JC. Shen W. Tahir SK. Thompson CB. Tomaselli KJ. Wang B. Wendt MD. Zhang H. Fesik SW. Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 6.Reed JC. Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 7.Chen L. Willis SN. Wei A. Smith BJ. Fletcher JI. Hinds MG. Colman PM. Day CL. Adams JM. Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 8.van Delft MF. Wei AH. Mason KD. Vandenberg CJ. Chen L. Czabotar PE. Willis SN. Scott CL. Day CL. Cory S. Adams JM. Roberts AW. Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax If Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luciano F. Krajewska M. Ortiz-Rubio P. Krajewski S. Zhai D. Faustin B. Bruey JM. Bailly-Maitre B. Lichtenstein A. Kolluri SK. Satterthwait AC. Zhang XK. Reed JC. Nur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myeloma. Blood. 2007;109:3849–3855. doi: 10.1182/blood-2006-11-056879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipinski CA. Lombardo F. Dominy BW. Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhai D. Jin C. Satterthwait AC. Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 12.Edwards BS. Young SM. Oprea TI. Bologa CG. Prossnitz ER. Sklar LA. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nat Protoc. 2006;1:59–66. doi: 10.1038/nprot.2006.9. [DOI] [PubMed] [Google Scholar]
- 13.Black CB. Duensing TD. Trinkle LS. Dunlay RT. Cell-based screening using high-throughput flow cytometry. Assay Drug Dev Technol. 2010;9:13–20. doi: 10.1089/adt.2010.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons PC. Young SM. Carter MB. Waller A. Zhai D. Reed JC. Edwards BS. Sklar LA. Simultaneous in vitro molecular screening of protein–peptide interactions by flow cytometry using six Bcl-2 family proteins as examples. Nature Protocols. doi: 10.1038/nprot.2011.339. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labi V. Grespi F. Baumgartner F. Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H. Nimmer P. Rosenberg SH. Ng SC. Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L) Anal Biochem. 2002;307:70–75. doi: 10.1016/s0003-2697(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JH. Chung TD. Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 18.Zhai D. Ke N. Zhang H. Ladror U. Joseph M. Eichinger A. Godzik A. Ng SC. Reed JC. Characterization of the anti-apoptotic mechanism of Bcl-B. Biochem J. 2003;376:229–236. doi: 10.1042/BJ20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai D. Jin C. Huang Z. Satterthwait AC. Reed JC. Differential regulation of Bax and Bak by anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem. 2008;283:9580–9586. doi: 10.1074/jbc.M708426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krajewska M. Kitada S. Winter JN. Variakojis D. Lichtenstein A. Zhai D. Cuddy M. Huang X. Luciano F. Baker CH. Kim H. Shin E. Kennedy S. Olson AH. Badzio A. Jassem J. Meinhold-Heerlein I. Duffy MJ. Schimmer AD. Tsao M. Brown E. Sawyers A. Andreeff M. Mercola D. Krajewski S. Reed JC. Bcl-B expression in human epithelial and nonepithelial malignancies. Clin Cancer Res. 2008;14:3011–3021. doi: 10.1158/1078-0432.CCR-07-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]