Abstract
Stro-1 is the best-known mesenchymal stem cell marker. However, despite its bone marrow origin, its localization in bone marrow has never been demonstrated. By immunofluorescence staining, we show here that ∼0.74% of nucleated bone morrow cells expressed Stro-1. We also found that ∼8.7% of CD34-expressing cells expressed Stro-1, and more than 20% of Stro-1-expressing cells did not express CD34. In adipose tissue Stro-1 expression was identified in the endothelium of arterioles and capillaries. Stro-1 was also localized in the endothelium of some but not all adipose tissue veins. Endothelial expression of Stro-1 was also identified in blood vessels in penis and in leg muscles, but not in other tested tissues. In these other tissues, Stro-1 was scantly expressed near but not in blood vessels. These variable and endothelial expression patterns of Stro-1 point to a need to re-examine published data that relied on Stro-1 as a mesenchymal stem cell marker.
Introduction
In a 2007 review article, Kolf et al. stated: “Stro-1 is by far the best-known mesenchymal stem cell (MSC) marker” [1]. However, despite such a prominent position in MSC research and first report of the Stro-1 antibody 20 years ago [2], the Stro-1 antigen remains unidentified. In contrast, the Stro-3 and Stro-4 antigens, which are much more recently described and defined by far less-utilized Stro-3 and Stro-4 antibodies, have been identified as “tissue nonspecific alkaline phosphatase” and “heat shock protein-90b,” respectively [3,4].
Stro-1 antibody is produced by one of several hybridomas that were generated by immunizing mice intrasplenically with human CD34+ bone marrow cells [2]. These hybridomas were initially screened against T- and B-cell lines, and then further selected for reactivity with subpopulations of CD34-expressing cells. Hybridomas that fulfilled these criteria were cloned and one of the clones was named Stro-1. Additional tests identified Stro-1 antibody as of the IgM isotype and reacting with stromal cells in the adherent layer of long-term bone marrow cultures [2].
Stro-1 antibody has been used predominantly for fluorescence-activated cell sorting analysis, and, to a much less extent, immunocytochemical staining of candidate MSCs. In both situations, disagreements over whether a particular cell population is Stro-1 positive or negative are not uncommon [5–7]. Importantly, a population of human bone marrow-derived MSCs termed “size-sieved stem cells” has been found to be consistently negative for Stro-1 [8]. Thus, in regard to fluorescence-activated cell sorting and immunocytochemical staining, the utilization of Stro-1 antibody as an MSC probe is in need of a critical re-evaluation.
In addition to the above-mentioned problems, the use of Stro-1 antibody for immunohistochemical (IHC) or immunofluorescence (IF) staining has encountered difficulties as well. Importantly, despite its strong association with the bone marrow, Stro-1 antibody has been used in only 1 study to stain the bone marrow. In this study Stro-1 antibody was shown to react to blood vessel walls in frozen sections of human bone marrow [9]. However, whether Stro-1 stained the bone marrow stroma was not indicated. In another study, Stro-1 was found to localize to endothelial cells and perivascular cells of the blood vessel wall in human thymus [10]. Thus, in regard to IHC or IF staining, the presumed bone marrow stromal specificity of the Stro-1 antibody has not been demonstrated. Rather, Stro-1 antibody appears to react with endothelial cells and undefined perivascular cells.
In support of the statement that Stro-1 is the best-known MSC marker [1], we have found that more than a hundred studies depended on Stro-1 antibody for the identification and/or isolation of various types of MSCs. However, unfortunately few of these studies have demonstrated Stro-1 reactivity in the tissues from which the respective MSCs were identified or isolated. This deficiency can be problematic because Stro-1's reactivity with endothelial and perivascular cells [6,10] may lead to incorrect conclusions about the identity of the MSCs of interest. Thus, by sharing our experience with the use of Stro-1 antibody in IHC and IF staining of various tissues (including bone marrow), the present study hopes to encourage MSC researchers to consider supplementing their studies with the localization of Stro-1 in the relevant tissues.
Materials and Methods
Tissue procurement
Adipose tissue samples were obtained from patients during routine abdominoplasty after informed patient consent and according to the guidelines set by our institution's Committee on Human Research. Rat tissues were obtained from 2-month-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). All animal care and treatment procedures were approved by our Institutional Animal Care and Use Committee. Paraffin-embedded human prostate tissue sections were obtained from the tissue core of our institution's cancer center.
Preparation of tissue sections
Frozen tissue sections were prepared by first fixing freshly dissected tissues in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetek USA, Torrance, CA) and stored at −70°C until use. Fixed frozen tissue specimens were cut at 10 μm, mounted onto Superfrost Plus charged slides (Fisher Scientific, Pittsburgh, PA), and air-dried for 5 min.
Paraffin-embedded tissue sections were prepared by first fixing freshly dissected tissue (<3 mm thick) with 10% formalin at room temperature for 48 h, followed by washing with running water for 1 h. The fixed tissue was then dehydrated through 70%, 80%, and 95% alcohol, and cleared with xylene. The tissue was then embedded in paraffin, cut at 5 μm, mounted onto Superfrost Plus charged slides, and air-dried overnight.
IHC staining
Frozen tissue sections were placed in 0.3% H2O2/methanol for 10 min, washed twice in phosphate-buffered saline (PBS) for 5 min, and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining solution from the tissue section, the tissue was incubated with mouse anti-Stro-1 antibody (R&D Systems, Inc., Minneapolis, MN). The tissue was stained with the Elite ABC kit (Vector Laboratories, Burlingame, CA), followed by hematoxylin counterstain.
Paraffin-embedded tissue sections were deparaffinized in Histo-clear (National Diagnostic, Atlanta, GA) and hydrated in graded alcoholic solutions and distilled water. Antigens were retrieved by microwave heating for 30 min in an antigen unmasking solution (Vector Laboratories). Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol for 30 min, followed by washing in PBS, pH 7.4. Normal goat serum was applied to the sections for 30 min to bind nonspecific sites. After draining solution from the tissue section, the tissue was incubated with mouse anti-Stro-1 (R&D Systems). Staining of the tissue was performed with the Elite ABC kit (Vector Laboratories), followed by hematoxylin counterstain.
IF staining
Frozen tissue sections were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min, and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining solution from the tissue section, the tissue was incubated with rabbit anti-CD34 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-Stro-1 (R&D Systems), or mouse anti-smooth muscle actin (SMA) (Sigma-Aldrich, St. Louis, MO). After rinses with PBS, the sections were incubated with FITC- or Texas Red-conjugated secondary antibody (Vector Laboratories). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole.
Image analysis and quantification
IF-stained tissues were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments, Inc., Melville, NY). For quantification of CD34 and Stro-1-positive cells in bone marrow, 5 random fields of each tissue section at 200×magnification were selected, and the numbers of nucleated cells (blue 4′,6-diamidino-2-phenylindole staining), CD34-positive cells (green stained), and Stro-1-positive cells (red stained) counted manually. To quantify CD34/Stro-1 double-positive cells, 5 random fields of each tissue section at 1000×magnification were analyzed.
Results
Stro-1 and CD34 expression in bone marrow
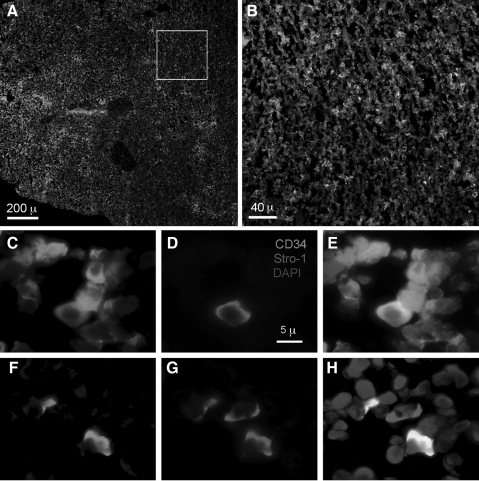
Despite the fact that Stro-1 antibody was produced by using bone marrow CD34-expressing cells as antigen [2], the relative expression of Stro-1 and CD34 in bone marrow has not been reported. In the present study we performed IF staining and identified CD34-expressing and Stro-1-expressing cells in rat femoral bone marrow (Fig. 1A). For quantification, we counted these cells and nucleated cells in 5 random microscopic fields of each tissue section at 200×magnification (Fig. 1B). The results show that there were 517±66 CD34-expressing cells, 23±14 Stro-1-expressing cells, and 3,200±146 nucleated cells per microscopic field (mean±SD). Thus, among nucleated cells 16.2±2.43% were CD34-positive, whereas 0.74±0.47% Stro-1 positive. To determine the number of cells that expressed both CD34 and Stro-1, we counted individual cells at 1000×magnification (Fig. 1C–H). The results show that among CD34-expressing cells, 8.7% expressed Stro-1, and among Stro-1-expressing cells, 78.6% expressed CD34.
FIG. 1.
Stro-1 and CD34 staining of rat bone marrow. Frozen sections of rat femoral bone marrow were stained with CD34 (green), Stro-1 (red), and 4′, 6-diamidino-2-phenylindole (blue). Boxed area in (A) is magnified in (B). Panels (C–E) show several CD34+/Stro-1− cells (green color in C and E) and a single CD34+/Stro-1+ cell (orange color in E). (F–H) Several CD34−/Stro-1− cells (no color in F and G; blue color in H), 2 CD34+/Stro-1+ cells (orange color in H), and a single CD34−/Stro-1+ cell (red in G and H).
Stro-1 expression in adipose tissue
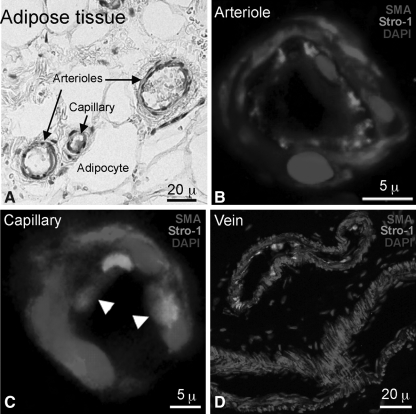
Among various types of MSCs, adipose-derived stem cells (ADSCs) have been our main interest, and in our previous studies we have discussed the controversy concerning ADSC's Stro-1 expression status [6,7]. In the present study we performed both IHC and IF staining for Stro-1 on human subcutaneous adipose tissues. As shown in the IHC image in Fig. 2A, Stro-1 reactivity was localized to the endothelium of 2 arterioles (diameters of 25 and 45 μ) and 1 capillary (10 μ). This endothelial specificity was further confirmed by IF staining for both Stro-1 and SMA (Fig. 2B, C). Interestingly, some capillary cells expressed both Stro-1 and SMA (arrowheads, Fig. 2C), and some but not all veins showed Stro-1 reactivity in the endothelium (Fig. 2D).
FIG. 2.
Stro-1 and SMA staining of human adipose tissue. Frozen sections of human subcutaneous tissue were stained for Stro-1 by IHC (A) or immunofluorescence (B–D). By immunofluorescence, Stro-1 was stained green, SMA red, and nucleus blue. White arrowheads indicate coexpression of Stro-1 and SMA. IHC, immunohistochemistry; SMA, smooth muscle actin.
Stro-1 expression in urogenital tissues
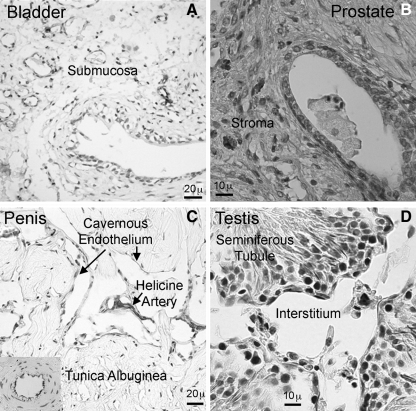
We performed IHC staining on most urogenital tissues; however, due to space limitation, we chose to display the images of 4 representative tissues. In the bladder, scant Stro-1 reactivity was seen in the submucosa and the stained cells appeared to locate near but not in blood vessels (Fig. 3A). Similar Stro-1 localization (scant submucosal expression and vascular vicinity) was seen in the urethra and vagina (data not shown). In the prostate, scant Stro-1 reactivity was localized in the stroma with unknown cellular identity (Fig. 3B). In the penis, Stro-1 reactivity was seen in helicine arteries and in some but not all cavernous endothelia (Fig. 3C). The endothelium of dorsal arteries also stained positive (Inset, Fig. 3C). In the testis, the seminiferous tubules were devoid of Stro-1 reactivity, whereas the interstitium was scantly positive (Fig. 3D).
FIG. 3.
Stro-1 staining of urogenital tissues. Paraffin-embedded tissue sections of rat bladder (A) and human prostate (B), and frozen tissue sections of rat penis (C) and testis (D) were stained for Stro-1 by IHC. Insert in the lower left corner of (C) shows a dorsal artery of the same tissue section; distinct Stro-1 expression in the endothelium is clearly visible.
Stro-1 expression in heart, skeletal muscle, aorta, and rectum
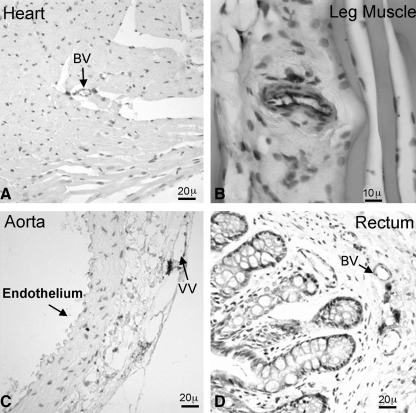
In the heart, the cardiac muscle and endothelium were devoid of Stro-1 expression, whereas scant Stro-1 reactivity was seen in the vicinity of blood vessels (Fig. 4A). In leg muscle, Stro-1 reactivity was seen only in the endothelium of blood vessels (Fig. 4B). In the aorta, the endothelium and smooth muscle were devoid of Stro-1 expression; the vasa vasorum was negative as well although in its vicinity strong Stro-1 expression was seen (Fig. 4C). In the rectum, scant Stro-1 reactivity was seen in the submucosa and the stained cells were located near but not in blood vessels (Fig. 4D).
FIG. 4.
Stro-1 staining of various tissues. Frozen tissue sections of rat heart (A), leg muscle (B), aorta (C), and rectum (D) were stained for Stro-1 by IHC. BV, blood vessels; VV, vasa vasorum.
Discussion
Numerous studies have considered Stro-1 an important marker for MSCs, including ADSCs. However, in our effort to identify ADSCs in adipose tissue, we were surprised by Stro-1's exclusive expression in the endothelium of the adipose vasculature [6]. We later learned that endothelial expression of Stro-1 has been reported in the thymus [10] and disagreements over whether ADSCs express Stro-1 are commonplace. Although there has been no explanation for Stro-1's endothelial localization, differences in Stro-1 antibody sources and detection methods have been offered as explanations for the controversy concerning ADSC's Stro-1 expression status [5]. In regard to different Stro-1 antibody sources, we have found that all 5 commercially and academically available Stro-1 antibody preparations produced similar results in IHC and flow cytometric analysis (data not shown). As such, alternative reasons are needed to possibly resolve controversies surrounding Stro-1; to begin this process, it is important that we understand what the Stro-1 antigen really is.
The immunogen by which Stro-1 antibody was generated is a cell preparation of CD34-postive bone marrow cells [2]. Further screening procedures and analyses suggested that the Stro-1 antigen is likely located in the bone marrow stroma [2]. However, neither the CD34 connection nor the bone marrow stoma localization has been previously demonstrated within bone marrow. In the present study, attempts to resolve these 2 important issues were carried out as we demonstrated the localization of CD34 and Stro-1 antigens in femoral bone marrow (Fig. 1). Further, we determined that Stro-1 was expressed by ∼8.7% of CD34-expressing cells. Interestingly, we found that not all Stro-1-expressing cells expressed CD34 — as one would have expected because CD34+ cells were the immunogen for Stro-1 antibody. Instead, we found that more than 20% of Stro-1-expressing cells did not express CD34, and this may be related to the fact that CD34 expression is reversible [11]. In any event, our studies have provided the basis for future experiments that may lead to the identification of the Stro-1 antigen.
In a 2001 review article, Bianco et al. [10] stressed the importance of establishing in vivo–in vitro relationship by using Stro-1 antibody for not only cytemetric analysis but also IHC. The authors went on to imply why in vivo localization of Stro-1 had not been done was due to “Stro-1 reagent has limited application in fixed and paraffin-embedded tissue.” However, in the present study we demonstrated that Stro-1 could be localized in frozen bone marrow sections, and we also conducted IHC localization of Stro-1 in paraffin-embedded tissues. Importantly, it should be pointed out that at least 5 published studies have demonstrated the applicability of Stro-1 antibody for paraffin-embedded tissues [12–16].
In frozen adipose tissues Stro-1 was localized to the endothelium of arterioles and capillaries. Interestingly, while Stro-1 expression in arterioles was independent of SMA expression, colocalization of Stro-1 and SMA was identifiable in the capillaries (Fig. 2B, C). One possibility is that the Stro-1/SMA double-positive cells in capillaries are vascular progenitor cells that are yet to differentiate into separate endothelial and smooth muscle cells. In any case, another interesting finding in adipose tissue was the appearance of both Stro-1-positive and -negative veins (Fig. 2D). Whether this is an indication of differences in these veins' physiological, vasculogenic, or other biological states remains to be elucidated.
Endothelial expression of Stro-1 was also identified in blood vessels in penis (Fig. 3C) and in leg muscles (Fig. 4B). In regard to leg muscles, a previous study has shown that skeletal muscle-derived cells expressed Stro-1 abundantly [17], and a more recent study contended that skeletal stem cells could be enriched by Stro-1 selection [18]. However, since neither study has demonstrated the localization of Stro-1 in skeletal muscles, the identity and tissue origin of their skeletal-derived cells need further confirmation. Likewise, while we found no Stro-1 immunoreactivity in seminiferous tubules (Fig. 3D), a recent study described the isolation of Stro-1-positive gonadal stem cells without confirmation of their tissue origin [19]. Such a lack of in vivo confirmation of Stro-1 expression is by no means unique to the above-mentioned studies but widespread among MSC studies that relied upon Stro-1 as marker. In any case, we have found that endothelial Stro-1 expression was limited in tissue distribution, as most tissues, including heart and aorta, that have strong endothelial representation did not display such feature. Instead, these tissues expressed very little Stro-1 and only in the vicinity of blood vessels. However, this vascular vicinity is different from what have been described as perivascular by Bianco et al. for Stro-1 expression [10]. Thus, although similar results have been obtained from frozen and paraffin-embedded tissues, the cellular identity of Stro-1 expression in these tissues remains unknown and must be further investigated.
Acknowledgments
This work was supported by the Rock Foundation and the National Institutes of Health (DK64538, DK045370, and DK069655).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kolf CM. Cho E. Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons PJ. Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 3.Gronthos S. Fitter S. Diamond P. Simmons PJ. Itescu S. Zannettino AC. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007;16:953–963. doi: 10.1089/scd.2007.0069. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S. McCarty R. Mrozik K. Fitter S. Paton S. Menicanin D. Itescu S. Bartold PM. Xian C. Zannettino AC. Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev. 2009;18:1253–1262. doi: 10.1089/scd.2008.0400. [DOI] [PubMed] [Google Scholar]
- 5.Gimble JM. Katz AJ. Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin G. Garcia M. Ning H. Banie L. Guo YL. Lue TF. Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CS. Xin ZC. Deng CH. Ning H. Lin G. Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 8.Hung SC. Chen NJ. Hsieh SL. Li H. Ma HL. Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- 9.Shi S. Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 10.Bianco P. Riminucci M. Gronthos S. Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 11.Goodell MA. Introduction: focus on hematology. CD34(+) or CD34(-): does it really matter? Blood. 1999;94:2545–2547. [PubMed] [Google Scholar]
- 12.Kemoun P. Laurencin-Dalicieux S. Rue J. Vaysse F. Romeas A. Arzate H. Conte-Auriol F. Farges JC. Salles JP. Brunel G. Localization of STRO-1, BMP-2/-3/-7, BMP receptors and phosphorylated Smad-1 during the formation of mouse periodontium. Tissue Cell. 2007;39:257–266. doi: 10.1016/j.tice.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Rolf HJ. Kierdorf U. Kierdorf H. Schulz J. Seymour N. Schliephake H. Napp J. Niebert S. Wolfel H. Wiese KG. Localization and characterization of STRO-1 cells in the deer pedicle and regenerating antler. PLoS One. 2008;3:e2064. doi: 10.1371/journal.pone.0002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grogan SP. Miyaki S. Asahara H. D'Lima DD. Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko R. Akita H. Shimauchi H. Sasano Y. Immunohistochemical localization of the STRO-1 antigen in developing rat teeth by light microscopy and electron microscopy. J Electron Microsc (Tokyo) 2009;58:363–373. doi: 10.1093/jmicro/dfp029. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki S. Grogan SP. Miyaki S. Kinoshita M. Asahara H. Lotz MK. Tissue neogenesis and STRO-1 expression in immature and mature articular cartilage. J Orthop Res. 2010;28:96–102. doi: 10.1002/jor.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claros S. Alonso M. Becerra J. Andrades JA. Selection and induction of rat skeletal muscle-derived cells to the chondro-osteogenic lineage. Cell Mol Biol (Noisy-le-grand) 2008;54:1–10. [PubMed] [Google Scholar]
- 18.Biggs MJ. Richards RG. Gadegaard N. Wilkinson CD. Oreffo RO. Dalby MJ. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials. 2009;30:5094–5103. doi: 10.1016/j.biomaterials.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez R. Griparic L. Vargas V. Burgee K. Santacruz P. Anderson R. Schiewe M. Silva F. Patel A. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun. 2009;385:570–575. doi: 10.1016/j.bbrc.2009.05.103. [DOI] [PubMed] [Google Scholar]