Abstract
Background
It has been suggested that host matrix metalloproteinase-2 (MMP-2) present in dentin may be involved in caries progression, however, its response to caries is not known. Bone sialoprotein (BSP) has been implicated in dentin mineralization and MMP-2 modulation.
Objective
To identify and compare the distribution of MMP-2 and BSP in healthy human coronal dentin and those with early caries.
Methods
Freshly extracted 3rd molars and premolars with and without early caries were fixed, demineralized and subjected to immunohistochemistry using a monoclonal anti-MMP-2 antibody and monoclonal anti-BSP antibody with an avidin-biotin complex method. Immunoreactivity was visualized with 3,3′-diaminobenzidine substrate and observed under light microscopy.
Results
Immunohistochemical analysis revealed that MMP-2 and BSP are not detected in the tubule lumens of healthy dentin. However, intense immunoreactivity for MMP-2 and BSP was detected in association with the full length of the caries-affected dentinal tubules. The MMP-2 and BSP at the dentino-enamel junction appeared unaltered.
Conclusion
The results indicate that MMP-2 and BSP may be actively secreted by odontoblasts in response to carious insult. MMP-2 and BSP accumulation in the caries-affected dentinal tubules may indicate their potential involvement in the host defense mechanism which results in calcification of regions affected by the carious process.
Key Words: Bone sialoprotein, Dentin caries, Immunohistochemistry, Matrix metalloproteinase-2
Introduction
Sustained acidic conditions result in dissolution of dentin hydroxyapatite (HA). This HA dissolution leads to subsequent exposure of dentin matrix which is composed of mainly fibrillar type I collagen and a number of non-collagenous proteins. Degradation of the exposed collagen matrix and destruction of its structural integrity results in dentin cavitation. Host matrix metalloproteinases (MMPs) and small, integrin-binding N-linked glycoproteins (SIBLINGs) are among the non-collagenous proteins that are implicated in collagen matrix degradation [Chaussain-Miller et al., 2006]. The specific MMPs and SIBLINGs responsible for collagen degradation during the carious process have not been identified. Knowledge of such protein interactions which result in collagen degradation may allow development of strategies that prevent degradation and resultant dentin cavitation.
The MMPs in dentin which have the potential to be proteolytically active during the carious process include collagenases (MMP-1, MMP-8), gelatinases (MMP-2, MMP-9) and other matrilysins (MMP-20, MMP-3) [Tjäderhane et al., 1998; van Strijp et al., 2003; Chaussain-Miller et al., 2006; Sulkala et al., 2007; Toledano et al., 2010]. MMP-2 and MMP-9 have been found to be concentrated in the dentin immediately adjacent to the dentinoenamel junction (DEJ) and therefore have been suspect in the clinically observed, early extension of caries at the DEJ [Goldberg et al., 2003; Boushell et al., 2008].
The SIBLINGs identified in dentin include dentin sialophosphoprotein (which is proteolytically broken down into dentin sialoprotein and dentin phosphoprotein), dentin matrix protein-1 (DMP-1), osteopontin (OPN) and bone sialoprotein (BSP). These non-collagenous proteins are phosphorylated and sulfated sialoproteins that are acidic in nature. Acidic SIBLINGs are able to bind to HA [Fisher et al., 2001]. It has been found that BSP binding to HA and collagen may promote bone mineralization [Hunter and Goldberg, 1993; Baht et al., 2008]. While BSP has been identified in porcine, rat and human dentin, its role in dentin mineralization is still unknown [Chen et al., 1993; Boukpessi et al., 2008; Huang et al., 2008; Hwang et al., 2008].
The degradative activity of MMP-2 is controlled by complex formation with a tissue inhibitor of metalloproteinases (TIMP) [Brew et al., 2000]. Reactivation of the TIMP-inhibited MMP-2 can occur by binding with BSP [Fedarko et al., 2004]. Inactive pro-MMP-2 can be also activated by binding of BSP [Fedarko et al., 2004]. MMP-2 and BSP have been shown to be co-expressed in tissues with high metabolic activity [Ogbureke and Fisher, 2007]. The pulp tissue expression of MMP-2 in response to the carious process is unknown, but it has been observed that the gene expression of BSP in the pulp is upregulated more than 8 times normal in human teeth with active caries [McLachlan et al., 2005]. It may be that MMP-2 and BSP play a role in the progression of dentin caries and/or in the host defense response to dentin caries, however, this has never been investigated. Therefore, the objective of this study was to use immunohistochemistry (IHC) methods to identify the distribution of MMP-2 and BSP in human coronal dentin with and without caries. This research was approved by the UNC Biomedical Institutional Review Board.
Materials and Methods
Sample Preparation
Erupted human 3rd molars and premolars with caries (n = 10) and without caries (n = 6) were placed in 10% formaldehyde immediately after extraction and fixed for 72 h at 4°C. The dentin matrix was still intact in 9 of the carious teeth and represented gradually increasing caries severity but not to include initial matrix degradation. One tooth had caries which progressed to the point of disruption of the DEJ as well as denaturation, degradation and loss of collagen matrix in the outer fourth of the coronal dentin. The teeth were sectioned using a Bueler Isomet (Bueler Corp., Lake Bluff, Ill., USA) diamond impregnated slow speed saw (Isomet) at 100 rpm with ∼2°C water cooling such that 1.5-mm mesio-distal or bucco-lingual sections were obtained. The sections were then demineralized with 10% EDTA (pH 7.4) for 5 weeks and placed in phosphate-buffered saline (PBS), pH 7.4. All demineralized specimens were then paraffinized and sectioned. A microtome (Leica Jung RN 2045) was used to obtain 5-μm sections from each specimen. Two sections were placed on each glass slide and therefore represent regions of dentin approximately 5 μm apart.
Immunohistochemical Analysis
Sections from each tooth were deparaffinized with xylene, treated with 100% ethanol (EtOH) for 2 min, 50% EtOH for 2 min and deionized water (dH2O) for 2 min. To better expose the epitopes the sections were then treated with 60 μl of Proteinase K (20 μg/ml PBS) (Proteinase K, recombinant, PCR Grade, Roche Applied Science) for 20 min, and then washed with dH2O for 1 min. The sections were placed in a 0.3% H2O2/EtOH solution for 30 min and washed in dH2O.
One section on each slide was then reacted with 30 μl of a 100-μg stock solution anti-MMP-2 (α-MMP-2) (Calbiochem: IM33L: Anti-MMP-2 (Ab-3) Mouse mAb (42-5D11), EMD Biosciences, Inc., La Jolla, Calif., USA) diluted 1:25 in PBS and blocked with normal horse serum (1:66) overnight at 4°C in a humidor. The other section on the slide was treated with 30 μl of a 100-μg stock solution anti-BSP (α-BSP) (LFMb-25, Mouse mAb anti-BSP, NIDCR, NIH, Bethesda, Mass., USA) diluted (1:50) in PBS and blocked with normal horse serum (1:66) overnight at 4°C in a humidor. As a negative control, some sections were not reacted with the primary antibody (anti-MMP-2 or anti-BSP) but were incubated with PBS with normal horse serum (1:66). After incubation, sections were washed 3 times for 5 min each with PBS. A 30-μl volume biotinylated horse anti-mouse IgG immunoglobulins (α-mouse IgG)/PBS solution (1:200) blocked with normal horse serum (1:66) was prepared and incubated with the sections for 30 min.
After incubation with the biotinylated secondary antibody, the sections were washed with PBS, treated with 30 μl of an avidin DH (1:100) and biotinylated horseradish peroxidase H (1:100) complex was placed over the sections and incubated for 30 min. The reagents used were part of the Vectastain® ABC Kit (Vector Laboratories, Inc., Burlingame, Calif., USA). The sections were then washed with PBS, each section was reacted with 30 μl of a 3,3′-diaminobenzidine solution (DAB Substrate Kit®, Vector Laboratories, Inc., Burlingame, Calif., USA) for 12 min and the reaction, as visualized by the development of a brown stain, was stopped by immersion in dH2O. Initial IHC studies were counterstained with hematoxylin for 5 s before fixation. These sections were then dehydrated with 50% EtOH, 100% EtOH, xylene washes, 5 min each, and then enclosed under a slide cover with DPX (VWR International Ltd, Poole, UK) and dried.
Verification of Antibody Specificity by Western Blot Analysis
The monoclonal anti-MMP-2 (Ab-3) antibody used for these studies was generated in mouse against the sequence located in the hinge region of human MMP-2 (amino acids 468–483). The antibody recognizes MMP-2 in both its ∼72 kDa latent and ∼66 kDa active forms. Standard SDS-PAGE and Western blotting techniques using recombinant human MMP-2 (∼60.9 kDa rhMMP-2) (MMP-2, Active, Human, Recombinant, Calbiochem®, EMD Biosciences, Inc., La Jolla, Calif., USA) generated from Chinese hamster ovary cells were used to verify probe recognition of MMP-2.
The monoclonal anti-BSP (LFMb-25) antibody used for these studies was obtained from Larry Fisher (NIDCR, NIH, Bethesda, Md., USA). The antibody was generated in mouse against the amino acids located in the carboxy-terminal region of human BSP1 (amino acids 159–317). The antibody recognizes the ∼70–80 kDa human BSP. Standard SDS-PAGE and Western blotting techniques using recombinant human BSP (∼75–90 kDa rhBSP) (Sialoprotein, Bone, Recombinant, Human (BSP) United States Biological, Swampscott, Mass., USA) that was generated from Chinese hamster ovary cells was used to verify probe recognition of BSP.
Light Microscopic Evaluation
Images of the MMP-2 and BSP immunoreacted and control sections were obtained with an Olympus BX61® microscope coupled with a Q-Imaging® Retiga 4000 camera and Volocity® Software (Improvision, PerkinElmer, Waltham, Mass., USA). Pre-demineralization and post-demineralization images were oriented based on tooth-specific anatomic features and location of caries to allow assessment of areas of MMP-2 and BSP immunodetection.
Statistics
Fisher's exact test (p = 0.05) was chosen as the statistical design based on the observation that in control teeth there was no concentration of immunoreactivity associated with the dentinal tubules in any one area of the coronal dentin. The statistical software used was StatXact version 8 (Cytel, Inc., Cambridge, Mass., USA).
Results
MMP-2 and BSP Recognition by Antibody Probes
rhMMP-2 was detected as a ∼61-kDa band by Western blotting with Ab-3 (fig. 1). rhBSP was detected as a ∼75-kDa band by Western blotting with LFMb-25 (fig. 2).
Fig. 1.
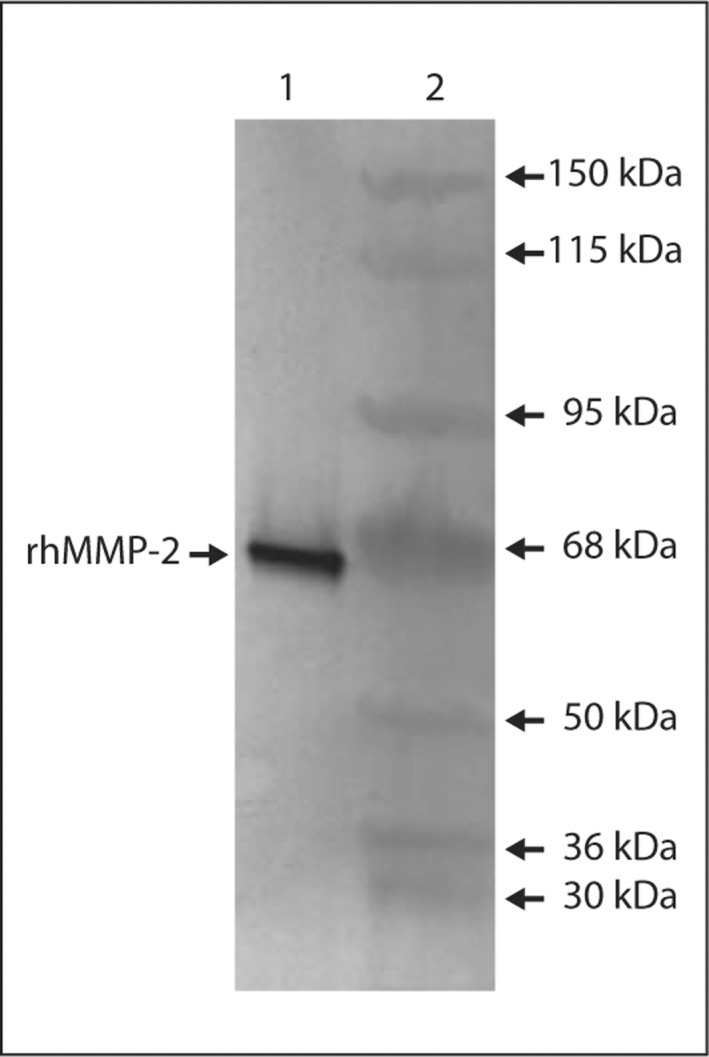
Western blot analysis using Ab-3 (anti-MMP-2) to detect rhMMP-2. Lane 1 = rhMMP-2 (∼61 kDa); lane 2 = apparent MW standards.
Fig. 2.
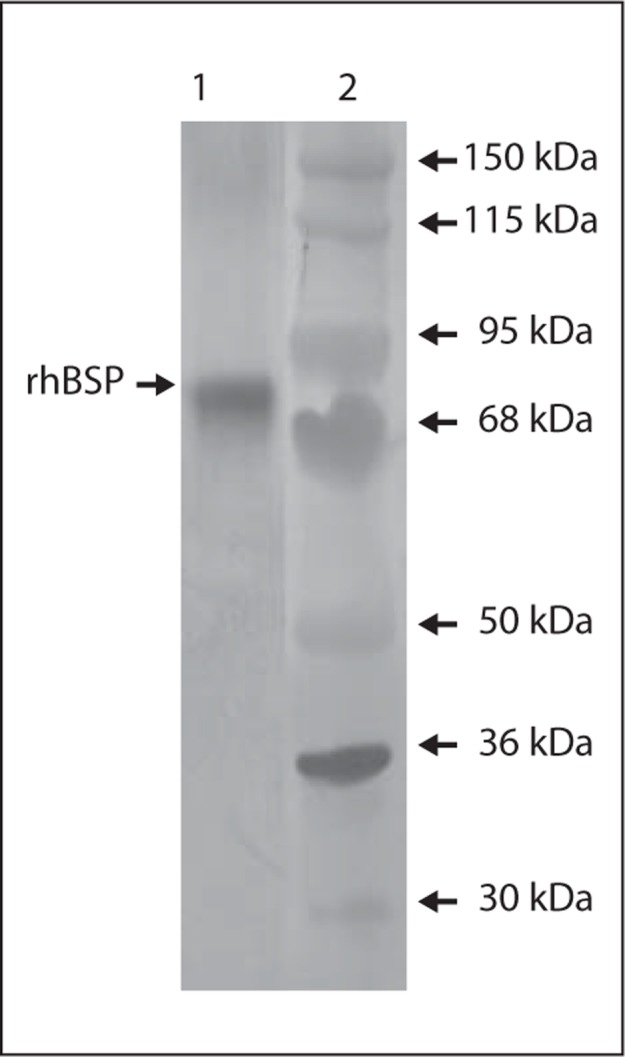
Western blot analysis using LFMb-25 (anti-BSP) to detect rhBSP. Lane 1 = rhBSP (∼75–90 kDa); lane 2 = apparent MW standards.
MMP-2 and BSP in Dentin without Caries
MMP-2 was detected throughout the caries-free dentin with increased staining of the odontoblastic processes in the inner (pre-dentin side) ∼100–200 μm of dentin as well as intense staining of the matrix of the region adjacent to the DEJ, likely mantle dentin. There was no discernible immunoreactivity of MMP-2 in the dentinal tubules between these regions (fig. 3).
Fig. 3.
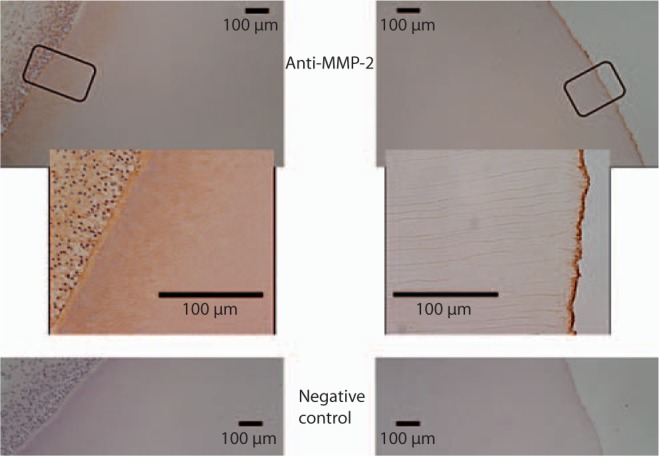
Immunodetection of MMP-2 in demineralized sections of sound coronal dentin. Increased immunoreactivity of MMP-2 was observed in association with the odontoblastic processes and mantle dentin (adjacent to the DEJ). No MMP-2 was detected in the dentinal tubules. Approximate region of higher magnification indicated by outline on the image above. Sections were counterstained with hematoxylin.
BSP was detected throughout the caries-free dentin with increased staining of the odontoblastic processes in the inner ∼100–200 μm of dentin. There was slightly increased immunodetection of BSP in the area of the mantle dentin (adjacent to the DEJ). There was no discernible immunoreactivity of BSP in the dentinal tubules between these regions (fig. 4).
Fig. 4.
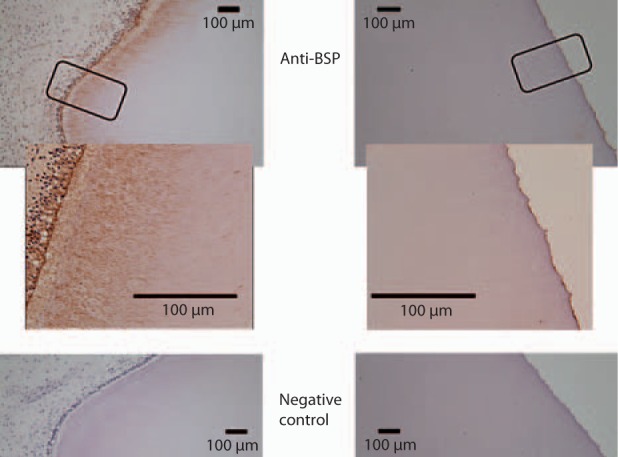
Immunodetection of BSP in demineralized sections of healthy coronal dentin. Increased immunoreactivity of BSP was observed in association with the odontoblastic processes. No BSP was detected in the dentinal tubules. A slight increase in BSP immunoreactivity was identified in the mantle dentin immediately adjacent to the DEJ. Sections were counterstained with hematoxylin.
MMP-2 and BSP in Dentin with Caries
There was visible change in the light reflection characteristics of the mineralized dentin in the area associated with caries (fig. 5a). In the demineralized sections the level of MMP-2 immunodetection in the inner dentin associated with odontoblastic processes was similar in carious and non-carious teeth. Intense MMP-2 immunoreactivity was detected at the DEJ and there was no change in immunostaining in the DEJ region associated with the carious lesion (fig. 5b). A statistically significant increase in the level of MMP-2 immunoreactivity was detected in the caries-affected tubules in all teeth with caries (p = 0.0001) (fig. 5, 6, 7). The level of MMP-2 detection did not change with the level of caries severity identified in the various teeth studied.
Fig. 5.
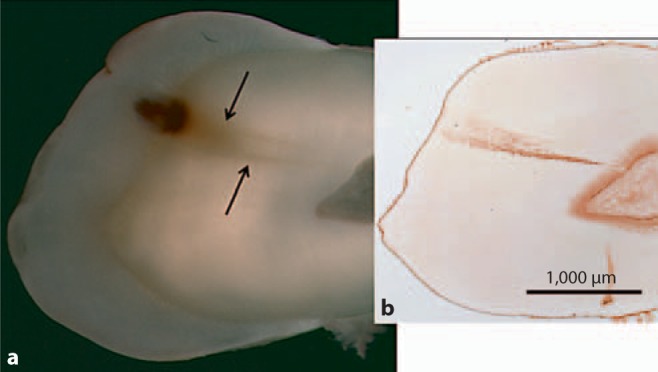
a Image of mineralized section revealing caries and changes in mineralized dentin (arrows). b Image of anatomically oriented, demineralized section of the same tooth that had been probed for MMP-2. Intense immunoreactivity for MMP-2 was identified in association with the dentinal tubules in the area of caries-affected dentin.
Fig. 6.
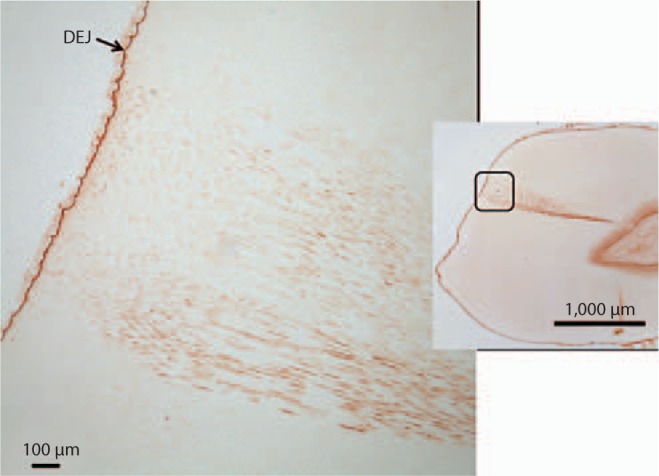
Image of carious tooth section probed with anti-MMP-2 antibody revealing an intact DEJ and staining within the caries-associated dentinal tubules. Region of higher magnification indicated by outline on the right image.
Fig. 7.
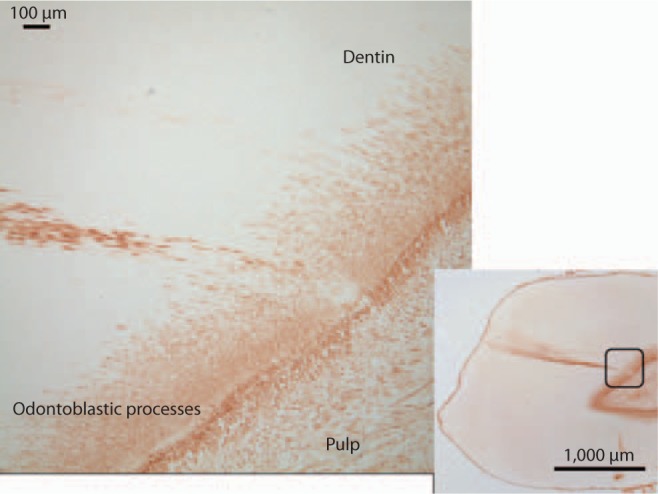
Image of carious tooth section probed with anti-MMP-2 antibody revealing generalized typical staining of odontoblastic processes in the inner 200 μm of the dentin and staining within the caries-associated dentinal tubules. Region of higher magnification indicated by outline on the right image.
In the demineralized sections the level of BSP immunoreactivity in the inner dentin associated with odontoblastic processes was similar in carious and non-carious teeth. Slightly increased BSP immunoreactivity was detected at the DEJ and there was no change in immunoreactivity in the DEJ region associated with the carious lesion. However, a significant increase in the level of BSP immunoreactivity was detected in the caries-affected tubules in 8 out of the 9 teeth with caries and intact dentin matrices. The carious tooth with dentin matrix degradation demonstrated BSP immunoreactivity in the caries-affected tubules pulpal to and associated with the area of collagen destruction. The increase in BSP immunodetection was statistically significant (p = 0.0009) (fig. 8, 9, 10). The level of BSP detection did not change with the level of caries severity identified in the various teeth studied.
Fig. 8.
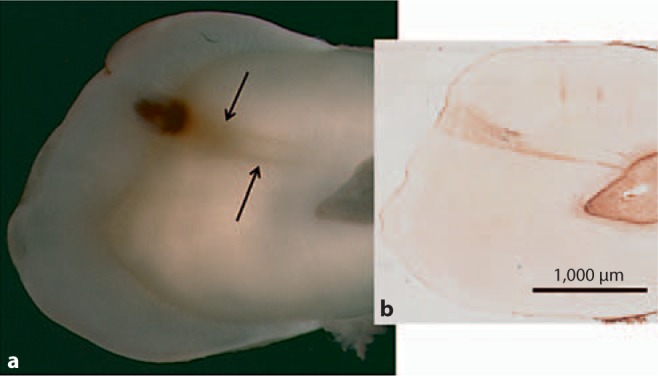
a Image of mineralized section revealing caries and changes in mineralized dentin (arrows). b Image of anatomically oriented, demineralized section of the same tooth that had been probed for BSP. Intense immunoreactivity for BSP was identified in association with the dentinal tubules in the area of caries-affected dentin.
Fig. 9.
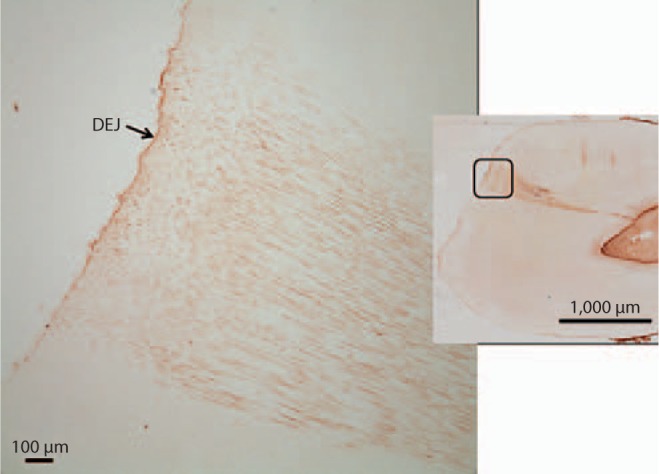
Image of carious tooth section probed with anti-BSP antibody revealing an intact DEJ and staining within the caries-associated dentinal tubules. Region of higher magnification indicated by outline on the right image.
Fig. 10.
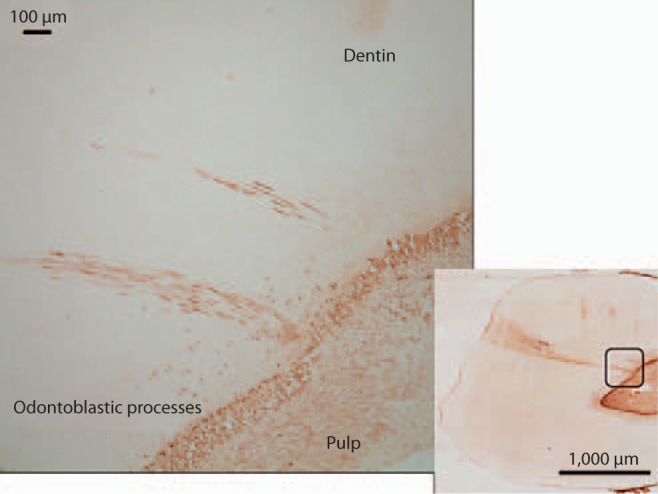
Image of carious tooth section probed with anti-BSP antibody revealing intense staining within the caries-associated dentinal tubules. Region of higher magnification indicated by outline on the right image.
Discussion
A previous study of healthy human coronal dentin utilized IHC methodologies to identify MMP-2 with the odontoblasts, odontoblastic processes, demineralized pre-dentin/dentin matrix (intertubular dentin matrix) but not within the dentinal tubules [Boushell et al., 2008]. This study confirms the presence of MMP-2 and identifies BSP in association with odontoblasts, odontoblastic processes near pre-dentin and demineralized pre-dentin/dentin matrix of healthy human coronal dentin. Similar to MMP-2, BSP was not detected in the dentinal tubules between the end of the odontoblastic processes and the DEJ of healthy demineralized dentin.
The host response to a slowly progressing carious insult results in an area, within the zone of carious affected dentin, which has a transparent appearance. The transparent zone of carious dentin is thought to be caused by HA precipitation within the dentinal tubules in the affected region [Arnold et al., 2001, 2003; Zavgorodniy et al., 2008a, b]. The mineral which precipitates in and occludes the tubules during the carious process may be supplied by the dental pulp or may be from localized intertubular/peritubular dentin mineral dissolved by bacterial acids [Arnold et al., 2003; Zavgorodniy et al., 2008a, b]. The intratubular and intertubular mineral phases of transparent dentin have been found to be chemically similar [Zavgorodniy et al., 2008b]. The mechanism by which intratubular mineral precipitation occurs is currently unknown. Analysis of the organization and orientation of the intratubular mineral crystals suggests that non-collagenous proteins present in the tubule lumens had determined the growth and final shape of the mineral crystals that had formed and occluded the lumens [Fisher and Termine, 1985; Zavgorodniy et al., 2008b].
The collagen and non-collagenous proteins of the teeth used in this study were fixed with formalin prior to sectioning to minimize protein loss during demineralization and processing. Gross evaluation of the sectioned teeth revealed increased translucency of the dentin beneath the caries which was suggestive of increased intratubular mineralization. Subsequent IHC analysis revealed that MMP-2 and BSP were retained in the caries-affected tubules during the demineralization, paraffinization, sectioning and immunohistochemical processing. Further study is required to identify whether MMP-2 and BSP were complexed with each other and/or intratubular collagen in the caries-affected dentinal tubules. A potential alternative explanation may be that MMP-2 and BSP were actually associated with peritubular dentin matrix collagen that had been exposed due to acidic demineralization of the peritubular dentin and were subsequently instrumental in formation of transparent dentin [Chen et al., 1993]. Ultrastructural studies of transparent dentin reveal that the intratubular HA is predominantly in association with the tubule walls and not in the center of the tubules [Zavgorodniy et al., 2008b]. Dentinogenesis begins with the formation of collagen fibrils that are subsequently associated with several non-collagenous proteins such that mineralization will occur [Butler et al., 2003; Waddington et al., 2003]. Mineralization within the dentinal tubule lumens similarly may require the presence of collagen to act as a structural template prior to intratubular mineralization. Collagen fibrils have been found in over 60% of coronal dentin tubules regardless of age or tooth type [Dai et al., 1991]. Collagen has been identified in transparent (sclerotic) dentin [Suppa et al., 2006]. Early studies of carious dentin collagen suggested that MMPs were present and potentially complexed with the collagen and TIMPs [Dayan et al., 1983]. Later it was found that collagen modification/degradation requires the formation of specific complexes with MMPs [Ottl et al., 2000]. In this study, MMP-2 and BSP, which have been reported to have a unique protein/protein interaction, were detected in the caries-affected tubules [Fedarko et al., 2004].
The finding that MMP-2 and BSP are specific binding partners and the detection of both in the similar dentinal area impacted by caries suggests a potential functional relationship of these proteins in the early host response to the carious process [Fedarko et al., 2004]. It has been identified that BSP (and other SIBLINGs) are able to bind to HA. Other studies suggest that these non-collagenous proteins play a role in dentin mineralization [Chen et al., 1993; Huang et al., 2008]. Indeed, BSP has been implicated in the initiation of calcification in mineralized tissues [Hunter and Goldberg, 1993; Baht et al., 2008]. Some have postulated that BSP and MMP-2 may be involved in the protein processing and matrix maturation process that occurs before mineralization [Ogbureke and Fisher, 2007].
The carious teeth investigated in this study had various levels of caries that ranged from early to initial matrix denaturation and degradation. However, the level of MMP-2 and BSP detection in the caries-affected tubules remained the same regardless of the level of caries progression. The presence of intratubular MMP-2 and BSP prior to the development of matrix degradation may be part of the mechanism, with probable modulation by various other proteins such as TIMPs, by which intratubular mineralization (transparent dentin) occurs during dentin caries. We question whether MMP-2 and BSP upregulation/secretion by odontoblasts, in response to bacterial acids/byproducts, and MMP-2 and BSP release from the demineralized dentin matrix may be simultaneously occurring. Is it possible that MMP-2/BSP complex formation with collagen fibrils develops in the caries-affected tubules followed by HA binding and subsequent intratubular mineralization? Identification of MMP-2 and BSP does not mean the proteins were in association with each other. The anti-MMP-2 antibody used in this study was able to recognize both pro-MMP-2 and MMP-2 and as such it was impossible to determine which form(s) of the gelatinase were present. In addition to the specific interactions between MMP-2 and BSP, it has been shown that MMP-3 and MMP-9 have the unique SIBLING binding partners OPN and DMP-1, respectively [Fedarko et al., 2004]. MMP-3, MMP-9, as well as MMP-8 have been implicated in dental caries and further research should seek to identify the various roles these proteinases and/or protein complexes may play in the carious process.
It should be noted that the sectioning process utilized in this study was technique-sensitive with regard to the orientation of the sectioning plane. It was found that multiple sections which parallel the overall dentinal tubule orientation in the center of the caries-affected dentin were most useful for immunodetection of MMP-2 and BSP within the tubules.
The statistical power study revealed that the number of teeth analyzed was adequate to establish statistical significance. However, in some cases more than one carious tooth came from an individual subject. Further research should seek to increase the number of subjects.
Conclusions
The presence of MMP-2 and BSP throughout the healthy demineralized matrix suggests their involvement in dentinogenesis. Concentrated MMP-2 and BSP detection with the odontoblast processes that extend into the pre-dentin and dentin may represent an active role in ongoing dentin matrix mineralization. BSP concentration appears somewhat increased in the area of the DEJ but not nearly as highly concentrated as MMP-2. Increased immunodetection of BSP at the DEJ is in support of the notion that initiation of dentinogenesis is a unique stage in tooth development and that BSP may play an important role. The detection of MMP-2 and BSP in the full length of caries-affected dentinal tubules suggests dentin-odontoblast communication via the tubule network and that the odontoblasts secrete MMP-2 and BSP possibly as part of the host defense mechanism. Insight into this protective, host defense mechanism may lead to more effective anti-caries strategies.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
We greatly appreciate the insights and services of the UNC Histology and Microscopy Services Laboratories. We sincerely thank Dr. Larry Fisher of the National Institute of Dental and Craniofacial Research at the National Institutes of Health, Bethesda, Md., for the development and use of the monoclonal anti-BSP. This work was supported by NIH grant DE019569 and the UNC School of Dentistry.
References
- Arnold WH, Konopka S, Gaengler P. Qualitative and quantitative assessment of intratubular dentin formation in human natural carious lesions. Calcif Tissue Int. 2001;69:268–273. doi: 10.1007/s002230020023. [DOI] [PubMed] [Google Scholar]
- Arnold WH, Konopka S, Kriwalsky MS, Gaengler P. Morphological analysis and chemical content of natural dentin carious lesion zones. Ann Anat. 2003;185:419–424. doi: 10.1016/S0940-9602(03)80099-7. [DOI] [PubMed] [Google Scholar]
- Baht GS, Hunter GK, Goldberg HA. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27:600–608. doi: 10.1016/j.matbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Boukpessi T, Menashi S, Camoin L, Ten Cate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–4373. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Arch Oral Biol. 2008;53:109–116. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Butler WT, Brunn JC, Qin C. Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res. 2003;44(suppl 1):171–178. [PubMed] [Google Scholar]
- Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- Chen J, McCulloch CA, Sodek J. Bone sialoprotein in developing porcine dental tissues: cellular expression and comparison of tissue localization with osteopontin and osteonectin. Arch Oral Biol. 1993;38:241–249. doi: 10.1016/0003-9969(93)90034-j. [DOI] [PubMed] [Google Scholar]
- Dai XF, Ten Cate AR, Limeback H. The extent and distribution of intratubular collagen fibrils in human dentine. Arch Oral Biol. 1991;36:775–778. doi: 10.1016/0003-9969(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Dayan D, Binderman I, Mechanic GL. A preliminary study of activation of collagenase in carious human dentine matrix. Arch Oral Biol. 1983;28:185–187. doi: 10.1016/0003-9969(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin-binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–736. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Termine JD. Noncollagenous proteins influencing the local mechanisms of calcification. Clin Orthop Relat Res. 1985;200:362–385. [PubMed] [Google Scholar]
- Fisher LW, Torchida DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Septier D, Bourd K, Hall R, George A, Goldberg H, Menashi S. Immunohistochemical localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the forming rat incisor. Connect Tissue Res. 2003;44:143–153. doi: 10.1080/03008200390223927. [DOI] [PubMed] [Google Scholar]
- Huang B, Sun Y, Maciejewska I, et al. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008;116:104–112. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci USA. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YC, Hwang IN, Oh WM, Park JC, Lee DS, Son HH. Influence of TGF-β1 on the expression of BSP, DSP, TGF-β1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol. 2008;39:153–160. doi: 10.1007/s10735-007-9148-8. [DOI] [PubMed] [Google Scholar]
- McLachlan JL, Smith AJ, Bujalska IJ, Cooper PR. Gene expression profiling of pulpal tissue reveals the molecular complexity of dental caries. Biochim Biophys Acta. 2005;1741:271–281. doi: 10.1016/j.bbadis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–409. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- Ottl J, Gabriel D, Murphy G, et al. Recognition and catabolism of synthetic heterotrimeric collagen peptides by matrix metalloproteinases. Chem Biol. 2000;7:119–132. doi: 10.1016/s1074-5521(00)00077-6. [DOI] [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Suppa P, Ruggeri A, Tay FR, et al. Reduced antigenicity of type 1 collagen and proteoglycans in sclerotic dentin. J Dent Res. 2006;85:133–137. doi: 10.1177/154405910608500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- Toledano M, Nieto-Aguilar R, Osorio R, Campos A, Osorio E, Tay FR, Alaminos M. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentin. J Dent. 2010;38:635–640. doi: 10.1016/j.jdent.2010.05.001. [DOI] [PubMed] [Google Scholar]
- van Strijp AJP, Jansen DC, DeGroot J, ten Cate JM, Everts V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003;37:58–65. doi: 10.1159/000068223. [DOI] [PubMed] [Google Scholar]
- Waddington RJ, Hall RC, Embery G, Lloyd DM. Changing profiles of proteoglycans in the transition of predentine to dentine. Matrix Biol. 2003;22:153–161. doi: 10.1016/s0945-053x(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Zavgorodniy AV, Rohanizadeh R, Bulcock S, Swain MV. Ultrastructural observations and growth of occluding crystals in carious dentin. Acta Biomater. 2008a;4:1427–1439. doi: 10.1016/j.actbio.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zavgorodniy AV, Rohanizadeh R, Swain MV. Ultrastructure of dentin carious lesions. Arch Oral Biol. 2008b;53:124–132. doi: 10.1016/j.archoralbio.2007.08.007. [DOI] [PubMed] [Google Scholar]


