Abstract
Background/Aims
The formation of advanced glycation end products (AGEs) is accelerated in patients with diabetic nephropathy. The aim of this study was to ascertain if the urinary excretion of proteins modified by advanced glycation can be used as biomarkers for albuminuria in individuals with type 1 or type 2 diabetes.
Methods
Community-based patients with type 1 (n = 68) or type 2 diabetes (n = 216) attending a diabetes clinic of a tertiary referral hospital were classified as having normoalbuminuria (Normo, albumin excretion rate (AER) <20 μg/min), microalbuminuria (Micro, AER 20–200 μg/min) or macroalbuminuria (Macro, AER ≥200 μg/min). Serum and urine AGE-modified proteins were measured.
Results
In patients with both type 1 diabetes and type 2 diabetes, there was a clear association between the degree of albuminuria and urinary AGE-modified proteins (p < 0.0001). Exclusive to patients with type 1 diabetes, urinary excretion of the AGE carboxymethyllysine correlated with AER, whereas patients with type 2 diabetes and macroalbuminuria had an increase in urinary methylglyoxal, an AGE intermediate. These changes were independent of isotopic glomerular filtration rate levels. Serum concentrations of AGEs or soluble receptor for AGEs were not consistently associated with albuminuria in either type 1 or type 2 diabetes.
Conclusions
Urinary excretion of proteins modified by AGEs may be useful biomarkers of albuminuria in individuals with type 1 and type 2 diabetes, warranting prospective investigation in larger diabetic cohorts.
Key Words: Advanced glycation end products, Diabetic nephropathy, Albuminuria, Carboxymethyllysine, Methylglyoxal, Urinary biomarkers
Introduction
Pro-oxidant conditions and hyperglycemia, which are characteristic of diabetes, accelerate the formation of advanced glycation end products (AGEs), which are nonenzymatic modifications to the free amino groups of proteins, by reducing sugars and reactive carbonyls [1]. The accumulation of AGEs is thought to be pivotal in mediating progressive tissue damage during the development and progression of diabetic microvascular disease and, in particular, nephropathy. The importance of decreasing AGE accumulation as a potential therapeutic target in diabetic nephropathy has been clearly demonstrated by experimental studies using a range of inhibitors of advanced glycation [2,3,4,5,6,7].
Evolutionary systems for the processing and excretion of AGEs are present within the liver [8] and kidney [9], respectively. Indeed, one of the most clinically useful biomarkers of glycemic control is the circulating AGE intermediate HbA1C. Furthermore, invasive skin collagen biopsy-associated AGE accumulation, specifically the biologically relevant carboxymethyllysine (CML), predicts the onset of nephropathy in patients with both type 1 [10] and type 2 [11] diabetes. It has been suggested that AGE accumulation within the circulation of individuals with diabetes may be a valid noninvasive biomarker of progressive diabetic nephropathy; however, previous studies investigating circulating levels of CML as a biomarker of progressive microvascular complications are conflicting, with associations reported in patients with type 2 [12] but not type 1 diabetes [13]. Moreover, urinary excretion of CML is significantly increased in individuals with type 2 diabetes and correlates with the degree of albuminuria [14]. One recent study demonstrated that increased circulating concentrations of the AGE intermediate, methylglyoxal (MGO) is an independent risk factor for microangiopathy in diabetic individuals [15]. In addition, conflicting data have been reported for circulating levels of soluble receptor for AGEs (RAGE) in progressive diabetic nephropathy [16,17,18].
Hence, in this cross-sectional study, we investigated whether circulating and urinary excretion of AGEs, including CML and the AGE intermediate MGO, are useful as biomarkers of renal injury in diabetes in a well-phenotyped cohort of 284 patients with type 1 or type 2 diabetes with nephropathy, grouped according to the degree of albuminuria.
Patients and Methods
Patient Recruitment and Sample Collection
Participating in this study were 68 individuals with type 1 diabetes and 216 individuals with type 2 diabetes drawn from a population of patients attending the diabetes clinic at the Austin and Repatriation Medical Centre, Melbourne, Australia. As our primary referral base (80%) is from general practitioners, with only 20% referred from within the hospital, the cohort is representative of patients with diabetes seen in the wider community. To be eligible for the study, patients were required to have undergone an isotopic glomerular filtration rate (GFR) measurement and to also have provided three 24-hour urine collections for the measurement of albumin excretion rate (AER) within a 24-month period. The research was carried out according to the declaration of Helsinki (2000) of the World Medical Association, and was approved by the Human Research Ethics Committee of Austin Health. All participants provided written consent.
Height and weight were measured for determination of BMI, and body surface area was measured using the formula (weight0.425) × (height0.725) × 71.84/10,000. AER was estimated by immunoturbidimetry (Dade-Behring, Marburg GmbH, Marburg, Germany). The level of albuminuria was defined categorically according to standard guidelines. Patients were classified as having normoalbuminuria (Normo, AER <20 μg/min), microalbuminuria (Micro, AER 20–200 μg/min) or macroalbuminuria (Macro, AER ≥200 μg/min). GFR was measured isotopically using the plasma disappearance of 99m-technetium-diethylene-triamine-penta-acetic-acid (99Tc-DTPA) and expressed per 1.73 m2 of body surface area. Serum was collected by centrifuging blood at 3,500 rpm at 4°C for 15 min and storing at –20°C. Same day 24-hour urine was collected and stored at –20°C. Systolic and diastolic blood pressures were measured after 5 min of recumbency using the appearance and disappearance of the Korotkoff sounds. Urine and plasma electrolytes were measured on a Hitachi 911 automatic analyzer (Roche Diagnostics, Basel, Switzerland) and HbA1c (glycated hemoglobin) was measured by automated HPLC (Bio-Rad Laboratories, Hercules, Calif., USA). Urinary creatinine excretion was measured with a Beckman Coulter DXC800 Synchron using the Jaffe rate method. Fasting lipids and total cholesterol were measured by enzymatic colorimetry and LDL (low-density lipoprotein)-cholesterol was calculated using the Friedewald equation.
Measurement of AGE Modifications by ELISA (AGE) and Assay Validation
AGE concentrations in urine and serum from patients with diabetes were measured by an in-house indirect ELISA as previously described [19]. Urinary AGEs were standardized to urinary creatinine concentration. The limit of detection of the assay was 0.2 nmol/mol lysine. The intra- and interassay coefficients of variation were 5.4 and 7.9%, respectively. The linearity of dilution of the assay was r2 = 0.93.
To assess if the ELISA measured free or protein-bound AGE modifications, serum was pooled from 3 patients and subject to trichloroacetic acid (TCA) precipitation as follows. Urine (150 μl) was added to an equal volume of ice cold 20% TCA for 15 min. Samples were centrifuged at 12,000 g for 10 min at 4°C. The pellet was dissolved in 10 μl 1.0 M NaOH, followed by 200 μl 0.5 M carbonate buffer, pH 9.6. After checking the pH, and neutralizing if necessary, the nonfractioned (native) serum, protein fraction and the nonprotein fraction were assayed by ELISA.
Measurement of CML
A competitive enzyme immunoassay specific to CML was performed as per the manufacturer's instructions (CircuLex CML ELISA kit, CycLex, Nagano, Japan). This ELISA employs the anti-CML-adduct monoclonal antibody KM-2A9. Urinary CML content was standardized to urinary creatinine concentration. The limit of detection of the assay was 0.126 ng/ml. The intra- and interassay coefficients of variation were 6.8 and 7.9%, respectively. The linearity of dilution of the assay was r2 = 0.95.
Urinary MGO Assessment
Methylglyoxal was measured by a commercially available sandwich ELISA (Human Methylglyoxal ELISA kit, Cusabio, Wuhan, China). The urinary MGO concentration was standardized to the urinary creatinine concentration. The limit of detection of the assay was 0.8 ng/ml. The intra- and interassay coefficients of variation were 6.9 and 8.3%, respectively. The linearity of dilution of the assay was r2 = 0.91.
Soluble RAGE
Soluble RAGE was measured in serum by a sandwich ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn., USA). The limit of detection of the assay was 4.1 pg/ml. The intra- and interassay coefficients of variation were 6.2 and 8.2%, respectively. The linearity of dilution of the assay was r2 = 0.90.
Urinary 8-Hydroxy-2-Deoxy-Guanosine
Urinary 8-hydroxy-2-deoxy-guanosine (8-OHdG) excretion was measured using a commercially available enzyme immunoassay from Cayman Chemical Company (Ann Arbor, Mich., USA) according to the manufacturer's instructions. Urinary 8-OHdG concentrations were standardized to urinary creatinine concentration.
Statistical Analyses
All statistical computations were performed using IBM SPSS version 19 (IBM Corp., USA) and GraphPad Prism version 5.0 for Mac OS X (GraphPad Software, San Diego, Calif., USA). Values of experimental groups are shown as means, with bars showing the standard error of the mean (SEM), unless otherwise stated. One-way ANOVA with Tukey's post-test analysis was used to determine statistical significance. Where appropriate, a two-tailed t test was performed. Variables were correlated using Pearson's coefficient. Relationships between categorical variables were assessed by χ2 analyses. Data for AER were not normally distributed and therefore analyzed after logarithmic transformation. Thus, the results are reported as the geometric mean (antilog of arithmetic mean) multiplied and/or divided by the tolerance factor (antilog of SD of log-transformed data). Other data not normally distributed were analysed after logarithmic transformation. A probability of p < 0.05 was considered to be statistically significant.
Results
Clinical Characteristics of Patients Studied
The clinical and biochemical characteristics of individuals with type 1 diabetes are displayed in table 1. A total of 68 patients with type 1 diabetes (57% men; mean 48 years) were analyzed. Patients were grouped according to albuminuria status: normoalbuminuria, microalbuminuria or macroalbuminuria. There were no differences in age, diabetes duration, BMI, systolic blood pressure, fasting glucose, isotopic GFR or cholesterol among the groups studied. Patients with macroalbuminuria had increased HbA1c and triglycerides compared to patients with normoalbuminuria. Microalbuminuric patients had a greater use of ACE inhibitors compared to normoalbuminuric patients.
Table 1.
Clinical and biochemical characteristics of participants with type 1 diabetes
| Normo | Micro | Macro | P | |
|---|---|---|---|---|
| n | 34 | 21 | 13 | |
| Age, years | 47±16 | 48±17 | 48±12 | 0.9 |
| Male, % | 54 | 62 | 56 | |
| Diabetes duration, years | 20±11 | 24±13 | 27±6 | 0.14 |
| BMI | 28±5 | 27±4 | 28±7 | 0.72 |
| Systolic blood pressure mm Hg | 129±20 | 135±16 | 140±19 | 0.17 |
| Fasting glucose, mmol/1 | 10.0±4.4 | 9.5±2.7 | 9.4±3.8 | 0.89 |
| HbA1c, % | 7.8±0.8 | 8.2±1.0 | 9.3±1.8« | 0.001 |
| AER, μg/min | 6.1 ×/÷2.7 | 43.2×/÷1.6 | 827.8×/÷2.5 | |
| iGFR, ml/min/1.73 m2 | 81±11 | 89±24 | 84±23 | 0.34 |
| Total cholesterol, mmol/1 | 4.6±0.9 | 4.4±1.2 | 4.4±1.2 | 0.80 |
| LDL-cholesterol, mmol/1 | 2.8±0.8 | 2.9±1.5 | 2.9±1.2 | 0.90 |
| HDL-cholesterol, mmol/1 | 1.4±0.7 | 1.5±0.6 | 1.4±0.5 | 0.90 |
| Triglycérides, mmol/1 | 0.8±0.4 | 1.2±0.8 | 1.4±0.8∗ | 0.01 |
| Medication use, % | ||||
| ACE inhibitors | 17 | 61 | 50 | 0.013 |
| ARBs | 13 | 33 | 25 | 0.29 |
| β-Blockers | 13 | 17 | 38 | 0.29 |
| Diuretics | 13 | 6 | 38 | 0.09 |
| Statins | 34 | 52 | 36 | 0.39 |
Data are means 8 SD except for AER, which is shown as the geometric mean ×/÷ tolerance factor and medication use, which is displayed as % use. ACE = Angiotensin-converting enzyme; ARB = angiotensin receptor blocker; HbA1c = glycated hemoglobin; iGFR = isotopic GFR.
p < 0.001 vs. Normo.
Characteristics of patients with type 2 diabetes are listed in table 2. A total of 216 patients with type 2 diabetes (61% men; mean age of 66 years) were studied. Patients were grouped according to albuminuria status: normoalbuminuria, microalbuminuria or macroalbuminuria. There were no differences in diabetes duration, BMI, systolic blood pressure, fasting glucose, HbA1c, GFR, fasting lipids or medication use among the three groups.
Table 2.
Clinical and biochemical characteristics of participants with type 2 diabetes
| Normo | Micro | Macro | P | |
|---|---|---|---|---|
| n | 112 | 70 | 34 | |
| Age, years | 66±11 | 67±14 | 66±9 | 0.78 |
| Male, % | 52 | 65 | 68 | |
| Diabetes duration, years | 13±8 | 14±10 | 13±8 | 0.41 |
| BMI | 31±6 | 32±6 | 35±9 | 0.06 |
| Systolic blood pressure mm Hg | 137±18 | 136±17 | 139±17 | 0.64 |
| Fasting glucose, mmol/1 | 8.7±2.3 | 8.2±2.2 | 9.6±4.0 | 0.42 |
| HbA1c, % | 7.5±1.0 | 7.8±1.6 | 8.0±1.6 | 0.19 |
| AER, μg/min | 6.9×/÷1.9 | 51.0×/÷2.0 | 688.1×/÷2.1 | |
| iGFR, ml/min/1.73m2 | 66.8±18.4 | 67.2±21.0 | 58.7±20.9 | 0.09 |
| Total cholesterol, mmol/l | 4.3±1.1 | 4.3±1.1 | 4.2±1.1 | 0.87 |
| LDL-cholesterol, mmol/l | 2.5±0.9 | 2.6±1.2 | 2.5±0.9 | 0.67 |
| HDL-cholesterol, mmol/l | 1.1±0.4 | 1.0±0.3 | 1.1±0.5 | 0.22 |
| Triglycérides, mmol/1 | 1.5±0.9 | 1.8±1.2 | 1.7±1.0 | 0.13 |
| Medication use, % | ||||
| ACE inhibitors | 52 | 60 | 60 | 0.77 |
| ARBs | 39 | 46 | 67 | 0.18 |
| β-Blockers | 36 | 28 | 53 | 0.27 |
| Diuretics | 41 | 50 | 54 | 0.69 |
| Statins | 74 | 88 | 86 | 0.39 |
Data are means 8 SD except for AER, which is shown as the geometric mean ×/÷ tolerance factor and medication use, which is displayed as % use. ACE = Angiotensin-converting enzyme; ARB = angiotensin receptor blocker; HbA1c = glycated hemoglobin; iGFR = isotopic GFR.
Association of Circulating and Urinary AGEs with Albuminuria
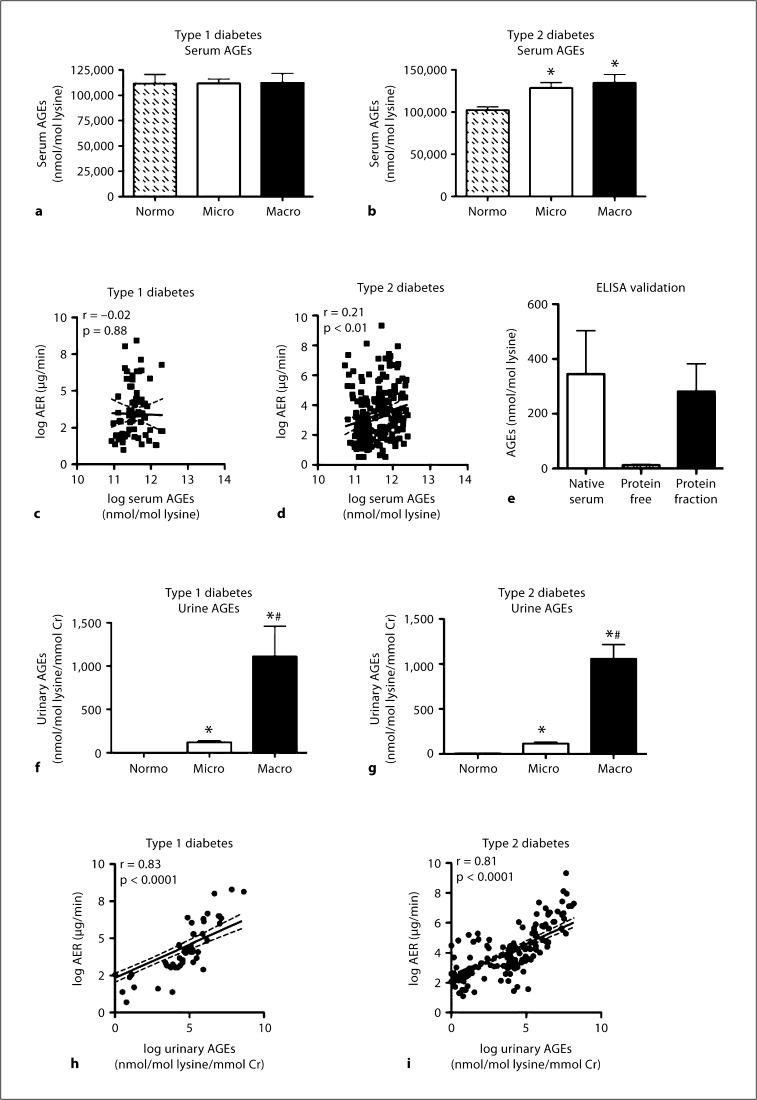
In patients with type 1 diabetes (fig. 1a), serum AGE concentrations were not different among patients with normo-, micro- and macroalbuminuria. In patients with type 2 diabetes (fig. 1b), serum AGE concentrations were increased in patients with micro- and macroalbuminuria to a similar degree when compared to patients with normoalbuminuria. A weak significant correlation (r = 0.21, p < 0.01) was observed between logarithmically transformed serum AGE concentrations and log urinary AER in patients with type 2 diabetes (fig. 1d), which was not present in individuals with type 1 diabetes (fig. 1c). The in-house ELISA used in this study measures predominantly protein-associated AGEs as assessed by pooled serum fractions measured following TCA precipitation (fig. 1e). This was confirmed by the observation that there was negligible AGE immunoreactivity in protein-free fractions of pooled sera.
Fig. 1.
Serum AGEs were measured in patients with type 1 diabetes (a) and type 2 diabetes (b), grouped according to renal function. Normo, Micro and Macro describe patients with normoalbuminuria, microalbuminuria and macroalbuminuria, respectively. c Correlation between log-transformed urinary AER (log AER) and log-transformed serum AGEs in patients with type 1 diabetes; r = –0.02, NS, n = 68. d Correlation between urinary log AER and serum log AGE concentration in patients with type 2 diabetes; r = 0.21, p < 0.01, n = 216. e Pooled serum (from 3 patients) was subjected to TCA precipitation and the concentration of AGEs contained within intact (native) serum, the protein-free serum fraction (free AGEs) and the protein fraction (protein-bound AGEs) were tested by in-house ELISA, assayed in triplicate. Urinary excretion of AGEs was measured in patients with type 1 diabetes (f) and type 2 diabetes (g), grouped according to renal function. h Correlation between log AER and urinary log AGE excretion in patients with type 1 diabetes; r = 0.83, p < 0.0001, n = 68. i Correlation between log AER and urinary log AGE excretion in patients with type 2 diabetes; r = 0.81, p < 0.0001, n = 222. Bars represent the mean ± SEM; n = 13–112 patients per group. ∗ p < 0.05 compared to Normo; # p < 0.05 compared to Micro.
In patients with type 1 diabetes, urinary excretion of AGEs increased with worsening albuminuria (fig. 1f). Microalbuminuric patients had a 100-fold increase in urinary AGEs when compared to normoalbuminuric patients, whereas those with macroalbuminuria had a 1,000-fold increase when compared to normoalbuminuric patients. Similarly, urinary AGE excretion was increased in type 2 diabetes with increasing albuminuria (fig. 1g). Microalbuminuric patients had an 18-fold increase in urinary AGEs compared to normoalbuminuric patients, and those with macroalbuminuria had a 170-fold increase compared to normoalbuminuric patients. There was a robust positive correlation between logarithmically transformed urinary AGEs and log AER in patients with both type 1 (fig. 1h; r = 0.83, p < 0.0001) and type 2 (fig. 1i; r = 0.81, p < 0.0001) diabetes.
Association of Circulating and Urinary CML with Albuminuria
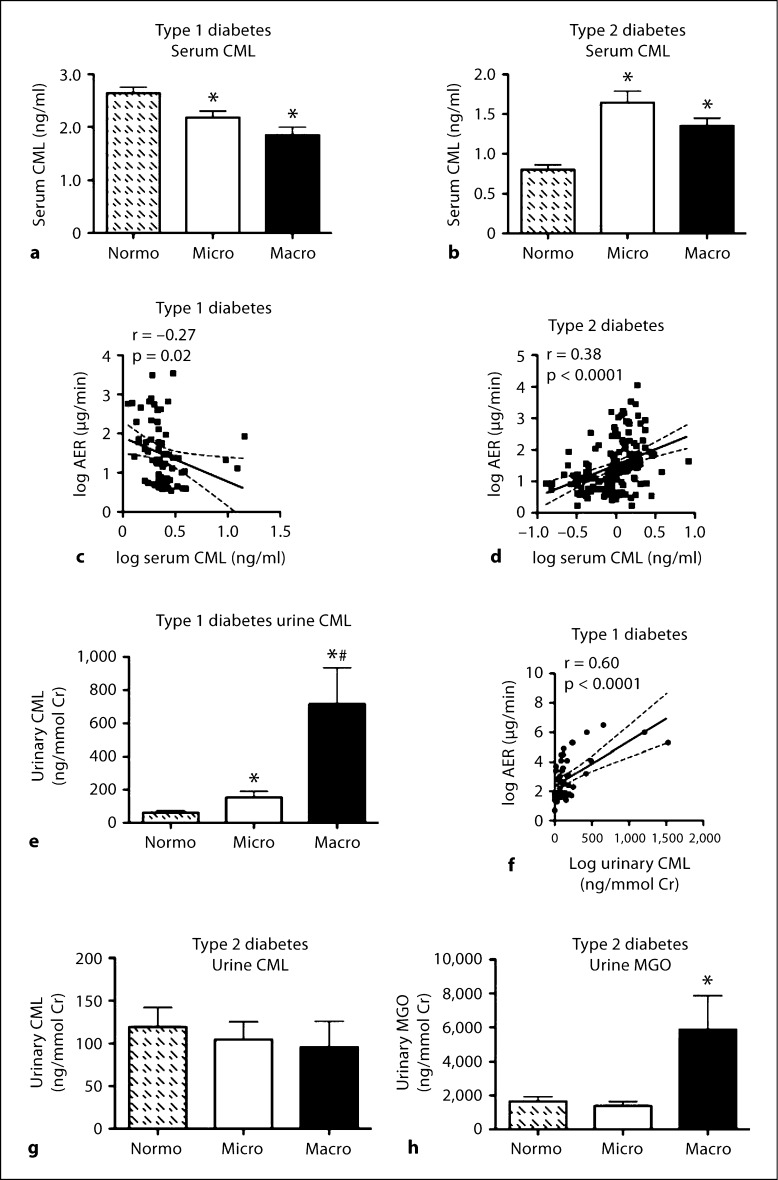
Serum CML was next measured by a commercially available competitive ELISA. Both individuals with type 1 diabetes and microalbuminuria or macroalbuminuria had reduced serum CML concentrations compared with normoalbuminuric patients (fig. 2a). In contrast, patients with type 2 diabetes and micro- or macroalbuminuria had increased serum CML concentrations compared to normoalbuminuric patients (fig. 2b). A negative correlation was observed between log serum CML in patients with type 1 diabetes and log AER (r = –0.27, p < 0.05; fig. 2c), while the same parameters in patients with type 2 diabetes demonstrated a positive correlation (r = 0.38, p < 0.0001; fig. 2d).
Fig. 2.
Serum CML was measured in patients with type 1 diabetes (a) and type 2 diabetes (b), grouped according to renal function. c Correlation between log AER and log-transformed serum CML in patients with type 1 diabetes; r = –0.27, p = 0.02, n = 68. d Correlation between log AER and log serum CML concentration in patients with type 2 diabetes; r = 0.38, p < 0.0001, n = 216. e Urinary excretion of CML was measured in patients with type 1 diabetes grouped according to renal function. f Correlation between log urinary AER and log urinary CML excretion in patients with type 1 diabetes; r = 0.60, p < 0.0001, n = 68. g Urinary excretion of CML was measured in patients with type 2 diabetes grouped according to renal function. h Urinary excretion of MGO was measured in a subset of patients with type 2 diabetes grouped according to renal function (n = 10–18 per group). Bars represent the mean ± SEM; n = 13–112 patients per group. ∗ p < 0.05 compared to Normo; # p < 0.05 compared to Micro.
In contrast, urinary CML excretion was increased in both micro- and macroalbuminuric type 1 diabetic patients compared to normoalbuminuric patients (fig. 2e). There was a strong positive correlation between log urinary CML and log AER in patients with type 1 diabetes (r = 0.60, p < 0.0001; fig. 2f). There were, however, no changes in urinary CML excretion associated with albuminuria observed in individuals with type 2 diabetes (fig. 2g). In addition, there was a positive correlation between urinary CML and urinary AGE levels in patients with type 1 diabetes (r = 0.66, p < 0.0001, n = 48), but not in patients with type 2 diabetes (r = –0.02, p = 0.8, n = 102). Further, urinary concentrations of MGO were increased in individuals with type 2 diabetes and macroalbuminuria (fig. 2h), but did not correlate with urinary AGE excretion (r = 0.07, p = 0.52, n = 76).
Association of Soluble RAGE with Albuminuria
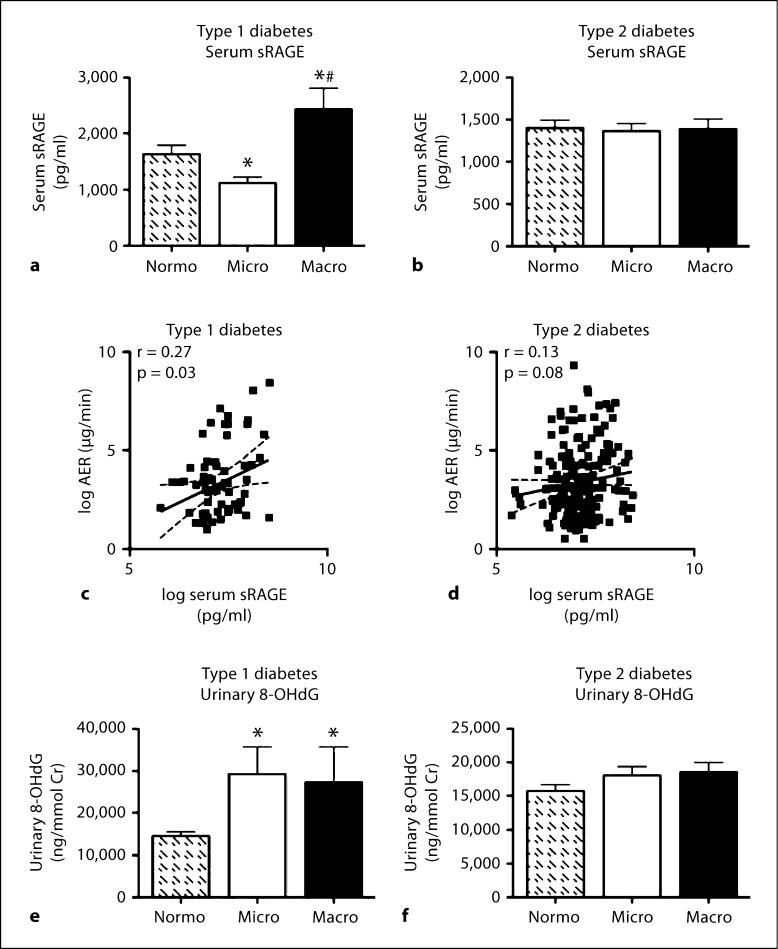
There was a decrease in the concentrations of soluble RAGE in patients with type 1 diabetes and microalbuminuria (fig. 3a) compared to normoalbuminuric subjects. Conversely, an increase in soluble RAGE was observed in serum samples taken from individuals with type 1 diabetes and macroalbuminuria (fig. 3a). Overall, this translated to a weak but significant correlation with urinary albumin excretion (r = 0.27, p < 0.05; fig. 3c). In type 2 diabetes, circulating soluble RAGE was unchanged (fig. 3b), and consequently did not correlate with the degree of albuminuria (fig. 3d).
Fig. 3.
Serum soluble RAGE (sRAGE) was measured in patients with type 1 diabetes (a) and type 2 diabetes (b), grouped according to renal function. Bars represent the mean ± SEM; n = 12–62 patients per group. ∗ p < 0.05 compared to Normo; # p < 0.05 compared to Micro. c Correlation between log urinary AER and log serum soluble RAGE concentration in patients with type 1 diabetes; r = 0.27, p < 0.05, n = 68. d Correlation between log urinary AER and log serum soluble RAGE concentration in patients with type 2 diabetes; r = 0.13, p = 0.08, n = 174. Urinary excretion of 8-OHdG was measured in patients with type 1 diabetes (e) or type 2 diabetes (f), grouped according to renal function. Bars represent the mean ± SEM; n = 6–20 patients per group. ∗ p < 0.05 compared to Normo.
Oxidative Stress Status
Urinary excretion of 8-OHdG as a marker of oxidative stress was determined. Urinary 8-OHdG excretion was increased in patients with type 1 diabetes and microalbuminuria, and in patients with type 1 diabetes and macroalbuminuria compared to patients with type 1 diabetes and normoalbuminuria. There was no difference in urinary 8-OHdG excretion in patients with type 2 diabetes and varying albuminuria status.
Discussion
Within the present study we explored the accumulation of AGEs, specifically CML, MGO and total AGEs as noninvasive biomarkers for diabetic nephropathy. Although the relationship of serum AGE concentrations to diabetic microvascular complications has been frequently explored, the studies have been conflicting. Our data clearly indicate that the most informative biomarker of albuminuria appears to be the urinary excretion of protein-linked AGEs.
For the first time we saw concordance for a biomarker of diabetic nephropathy that closely associates with severity of albuminuria in both type 1 and type 2 diabetic cohorts, and is independent of GFR. Although serum protein-linked AGEs were increased in patients with type 2 diabetes, there was only a weak correlation with AER. This was not observed in patients with type 1 diabetes. In contrast, urinary excretion of protein-linked AGEs was closely associated with AER in patients with both type 1 and type 2 diabetes, and to the same extent. Importantly, the isotopic GFR levels were similar for normo-, micro- and macroalbuminuric patients with type 1 diabetes. There was also no significant difference in isotopic GFR levels for different levels of albuminuria in subjects with type 2 diabetes.
To further investigate the possible AGE moieties present in patients with diabetic nephropathy, we then measured the well-characterized AGE, CML. Serum CML was negatively associated with AER in patients with type 1 diabetes, but this parameter was positively associated with AER in patients with type 2 diabetes. In the urine, CML was increased in patients with type 1 diabetes and positively correlated with AER; however, in patients with type 2 diabetes, there was no change. Interestingly, when we measured urinary excretion of methylglyoxal in these patients, an increase was observed in patients with type 2 diabetes and macroalbuminuria. Since MGO is a key intermediate in the generation of AGEs, this could be a factor in the increased total AGEs observed in this group of diabetic subjects.
Total urinary protein-bound CML, but not nonprotein-bound CML, has been shown previously to be increased in diabetic versus nondiabetic individuals although complications were not assessed in that study [20]. In the context of our own findings, this suggests that post-translational modifications of larger proteins may be more indicative of progressive renal dysfunction than smaller molecular weight AGEs. Indeed, our group has demonstrated previously that ligands that activate the RAGE present in the circulation of patients with type 2 diabetes and nephropathy are predominantly of high molecular weight [21], indicating that they are protein-associated.
AGEs present within the circulation may become bound to soluble RAGE, which may account for some of the discrepancies seen in circulating AGE concentrations. In the present study, soluble RAGE positively correlated with AER in type 1 diabetes, but not type 2 diabetes. Furthermore, it should be noted that hyperlipidemia is a potential confounding factor that can influence serum AGE levels since these post-translational modifications can also be derived via lipoxidation reactions [22].
An association was not observed between serum AGEs and albuminuria in type 1 diabetes. Indeed, in patients with type 1 diabetes, the serum AGE concentration was not different among patients with normo-, micro- and macroalbuminuria. In support of this, other studies in type 1 diabetes have found that circulating CML concentrations did not correlate with urinary albumin excretion [23,24]. Furthermore, a recent prospective study demonstrated that increased circulating non-CML AGE concentrations, specifically pentosidine and carboxyethyllysine, are associated with cardiovascular disease as well as all-cause mortality in individuals with type 1 diabetes [25]. In fact, in our study, serum CML from patients with type 1 diabetes decreased with albuminuria severity, demonstrating a negative correlation between circulating and urinary CML concentrations in type 1 diabetes.
Previously, there have been conflicting data reported for CML concentrations in the circulation and the urine of patients with type 2 diabetes. A study by Wagner et al. [26] found increased CML levels in the serum of patients with type 2 diabetes when compared to healthy individuals in association with a decrease in urinary CML with progression of renal impairment. In contrast, and consistent with our observations, Morcos et al. [14] found that urinary CML excretion correlated highly with the degree of albuminuria in patients with type 2 diabetes. Our data have identified key discrepancies between circulating AGEs and circulating CML amongst type 1 and type 2 diabetic subjects, indicating that circulating levels of AGEs cannot be considered appropriate biomarkers of diabetic nephropathy. In contrast, urinary proteins which have been modified post-translationally by AGEs appear to be accurate biomarkers of albuminuria in patients with diabetic nephropathy. However, AGEs are a diverse group of chemical moieties, and testing of such substances needs to be standardized if such assays are to be applied to samples from diabetic subjects.
In summary, urinary protein-bound AGE concentrations closely paralleled changes in albuminuria, reflecting the severity of diabetic nephropathy. It is now critical to examine this urinary biomarker as a predictive tool by performing careful prospective studies to assess if changes in urinary protein-bound AGE concentrations precede the rise in urinary albumin excretion, thereby strengthening the possibility of developing this method into an inexpensive high-throughput screening assay for use in the diabetic population.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to thank Trudy Smith and Aysel Akdeniz for the collection of the serum samples and Amy Morley, Felicia Yap and Karey Cheong for their technical support. This research was funded by a Juvenile Diabetes Research Foundation (JDRF) Centre Grant (4-2004-804 Proj 4) and a grant from the National Institutes of Health (ROI-HLO83452-01). M.T.C. is supported by an Australian Diabetes Society Early Career Fellowship. J.F. is in receipt of an NHMRC Fellowship. Supported in part by the Victorian Government's Operational Infrastructure Support Program.
References
- 1.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 2.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 3.Coughlan MT, Thallas-Bonke V, Pete J, Long DM, Gasser A, Tong DC, Arnstein M, Thorpe SR, Cooper ME, Forbes JM. Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: synergy or redundancy? Endocrinology. 2007;148:886–895. doi: 10.1210/en.2006-1300. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 5.Forbes JM, Soulis T, Thallas V, Panagiotopoulos S, Long DM, Vasan S, Wagle D, Jerums G, Cooper ME. Renoprotective effects of a novel inhibitor of advanced glycation. Diabetologia. 2001;44:108–114. doi: 10.1007/s001250051587. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes. 1997;46:895–899. doi: 10.2337/diab.46.5.895. [DOI] [PubMed] [Google Scholar]
- 7.Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40:1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- 8.Smedsrod B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. 1997;322:567–573. doi: 10.1042/bj3220567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyata T, Ueda Y, Horie K, Nangaku M, Tanaka S, van Ypersele de Strihou C, Kurokawa K. Renal catabolism of advanced glycation end products: the fate of pentosidine. Kidney Int. 1998;53:416–422. doi: 10.1046/j.1523-1755.1998.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 12.Wautier MP, Massin P, Guillausseau PJ, Huijberts M, Levy B, Boulanger E, Laloi-Michelin M, Wautier JL. N(carboxymethyl)lysine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Metab. 2003;29:44–52. doi: 10.1016/s1262-3636(07)70006-x. [DOI] [PubMed] [Google Scholar]
- 13.Galler A, Muller G, Schinzel R, Kratzsch J, Kiess W, Munch G. Impact of metabolic control and serum lipids on the concentration of advanced glycation end products in the serum of children and adolescents with type 1 diabetes, as determined by fluorescence spectroscopy and nepsilon-(carboxymethyl)lysine ELISA. Diabetes Care. 2003;26:2609–2615. doi: 10.2337/diacare.26.9.2609. [DOI] [PubMed] [Google Scholar]
- 14.Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, Kloeting I, Schleicher E, Mentz S, Abd el Baki RF, Tritschler H, Kasper M, Schwenger V, Hamann A, Dugi KA, Schmidt AM, Stern D, Ziegler R, Haering HU, Andrassy M, van der Woude F, Nawroth PP. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Nakayama K, Nakayama M, Mori T, Matsushima M, Okamura M, Senda M, Nako K, Miyata T, Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension. 2010;56:471–476. doi: 10.1161/HYPERTENSIONAHA.110.156786. [DOI] [PubMed] [Google Scholar]
- 16.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 17.Humpert PM, Djuric Z, Kopf S, Rudofsky G, Morcos M, Nawroth PP, Bierhaus A. Soluble rage but not endogenous secretory rage is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol. 2007;6:9. doi: 10.1186/1475-2840-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49:2756–2762. doi: 10.1007/s00125-006-0394-1. [DOI] [PubMed] [Google Scholar]
- 19.Norman PE, Davis WA, Coughlan MT, Forbes JM, Golledge J, Davis TM. Serum carboxymethyllysine concentrations are reduced in diabetic men with abdominal aortic aneurysms: Health in Men Study. J Vasc Surg. 2009;50:626–631. doi: 10.1016/j.jvs.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 20.Friess U, Waldner M, Wahl HG, Lehmann R, Haring HU, Voelter W, Schleicher E. Liquid chromatography-based determination of urinary free and total N(epsilon)-(carboxymethyl)lysine excretion in normal and diabetic subjects. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:273–280. doi: 10.1016/s1570-0232(03)00472-0. [DOI] [PubMed] [Google Scholar]
- 21.Penfold SA, Coughlan MT, Patel SK, Srivastava PM, Sourris KC, Steer D, Webster DE, Thomas MC, MacIsaac RJ, Jerums G, Burrell LM, Cooper ME, Forbes JM. Circulating high-molecular-weight rage ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010;78:287–295. doi: 10.1038/ki.2010.134. [DOI] [PubMed] [Google Scholar]
- 22.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11(suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 23.Miura J, Yamagishi S, Uchigata Y, Takeuchi M, Yamamoto H, Makita Z, Iwamoto Y. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with type 1 diabetes. J Diabetes Complications. 2003;17:16–21. doi: 10.1016/s1056-8727(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 24.Berg TJ, Snorgaard O, Faber J, Torjesen PA, Hildebrandt P, Mehlsen J, Hanssen KF. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22:1186–1190. doi: 10.2337/diacare.22.7.1186. [DOI] [PubMed] [Google Scholar]
- 25.Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care 34:442–447. [DOI] [PMC free article] [PubMed]
- 26.Wagner Z, Wittmann I, Mazak I, Schinzel R, Heidland A, Kientsch-Engel R, Nagy J. N(epsilon)-(carboxymethyl)lysine levels in patients with type 2 diabetes: role of renal function. Am J Kidney Dis. 2001;38:785–791. doi: 10.1053/ajkd.2001.27695. [DOI] [PubMed] [Google Scholar]