A new genetic mouse model demonstrates the necessity of Foxp3+ T reg cells for infectious tolerance.
Abstract
A paradigm shift in immunology has been the recent discovery of regulatory T cells (T reg cells), of which CD4+Foxp3+ cells are proven as essential to self-tolerance. Using transgenic B6.Foxp3hCD2 mice to isolate and ablate Foxp3+ T reg cells with an anti-hCD2 antibody, we show for the first time that CD4+Foxp3+ cells are crucial for infectious tolerance induced by nonablative anti–T cell antibodies. In tolerant animals, Foxp3+ T reg cells are constantly required to suppress effector T cells still capable of causing tissue damage. Tolerated tissue contains T cells that are capable of rejecting it, but are prevented from doing so by therapeutically induced Foxp3+ T reg cells. Finally, Foxp3+ cells have been confirmed as the critical missing link through which infectious tolerance operates in vivo. Peripherally induced Foxp3+ cells sustain tolerance by converting naive T cells into the next generation of Foxp3+ cells. Empowering Foxp3+ regulatory T cells in vivo offers a tractable route to avoid and correct tissue immunopathology.
An enduring goal of therapeutic immunosuppression is to exploit tolerance mechanisms so that short-term therapy can provide long-lived benefit (Kendal and Waldmann, 2010). The discovery that short pulses of blocking antibodies to co-receptors and co-stimulatory molecules could initiate the self-sustaining process of infectious transplantation tolerance, and that this was dependent on CD4+ regulatory T cells, provided the impetus to harness regulatory T cells as a therapeutic strategy (Qin et al., 1993). Such approaches would benefit from the knowledge of exactly which regulatory cells are responsible, and where and when they act. Until now, much has been assumed in the absence of definitive proof. For example, despite the identification of many CD4+ regulatory T cell subsets, including Foxp3+ T cells (Bennett et al., 2001; Fontenot et al., 2003; Hori et al., 2003), IL-10–secreting Tr1 cells (Levings et al., 2002), TGF-β–secreting Th3 cells (Weiner, 2001), and most recently Foxp3neg iT(R)35 cells (Collison et al., 2010), Foxp3+ T reg cells have been championed over the rest as essential for graft tolerance by largely descriptive data. This circumstantial evidence typically falls into three categories: (1) the enhancement of graft survival by adoptive transfer of Foxp3+-enriched but impure (Graca et al., 2002b) CD25+CD4+ populations (Feng et al., 2008; Xia et al., 2009); (2) the peripheral induction of Foxp3+ T reg cells as a result of tolerogenic protocols (Cobbold et al., 2004; Battaglia et al., 2006; Turnquist et al., 2007); and (3) the detection of small numbers of Foxp3+ T reg cells in tolerated grafts (Lee et al., 2005; Fan et al., 2010; Semiletova et al., 2010). However, simply demonstrating their presence, regardless of how well it is performed, fails to confirm a genuine operational role for Foxp3+ T reg cells. Achieving that conclusive evidence has been hampered by the lack of a natural cell surface marker by which Foxp3+ T reg cells can be specifically manipulated in vivo. In addition, although diphtheria toxin ablation of Foxp3+ cells in DEREG models (depletion of regulatory T cells) has dramatically demonstrated the importance of Foxp3+ T reg cells in self-tolerance, the resultant systemic immunopathology is so severe that it limits the investigation of long-term induced tolerance to foreign antigen (Kim et al., 2007). Few mice survive beyond 3 wk, and any destruction of foreign graft tissue within that time period would have to be interpreted in the context of global tissue disruption (whether foreign or host) by an unregulated immune system (Kim et al., 2007; Lahl et al., 2007). Using a different approach, in this paper we provide the first definitive evidence of an essential role for Foxp3+ T reg cells in therapeutic transplant tolerance in otherwise healthy mice. We use a transgenic reporter mouse containing a sequence coding for a GPI-linked human CD2_CD52 fusion protein in the 3′UTR of the X-linked foxp3 gene (B6.Foxp3hCD2) such that all Foxp3-expressing cells coexpress the fusion protein on their cell surface (Komatsu et al., 2009). Using ablative anti–human CD2 antibodies, we show that Foxp3+ T reg cells are necessary for therapeutic tolerance induced by co-receptor/co-stimulatory blockade across full MHC, multiple minors, and single minor histocompatibility barriers. T cells capable of rejecting the tolerated graft remain quiescent in a tolerant host as a result of constant suppression by Foxp3+ T reg cells. A key site of this suppression turns out to be the graft tissue itself, which contains T cells otherwise capable of rejection. Finally, the ability of antigen-specific induced Foxp3+ (i)T reg cells to recruit new Foxp3+ T reg cells from naive CD4+ cells in vivo provides a long awaited explanation for infectious tolerance.
RESULTS
Ablation of Foxp3+ T reg cells in B6.Foxp3hCD2 mice
Except for the knocked-in sequence, B6.Foxp3hCD2 mice are genetically identical to WT C57BL/6 (B6) mice, have an identical phenotype, and, importantly, do not develop any wasting immunopathology indicating normal Foxp3 regulatory function (Fig. S1). Analysis of splenic, lymph node, and blood compartments reveals similar CD4+, CD8+, and CD19+ lymphocyte composition between knock-in and WT B6 mice (Fig. S1). The absolute number, as well as the percentage, of CD4+ cells that are Foxp3+ is also similar between the two strains (Fig. S1, C and D). In B6.Foxp3hCD2 mice, the hCD2_CD52 fusion protein appears to be a highly accurate reporter of Foxp3+CD4+ cells (>98% of Foxp3+ cells are hCD2+; Fig. S1 B), allowing reliable isolation of live Foxp3negative and Foxp3positive T cell populations by flow cytometry and cell sorting.
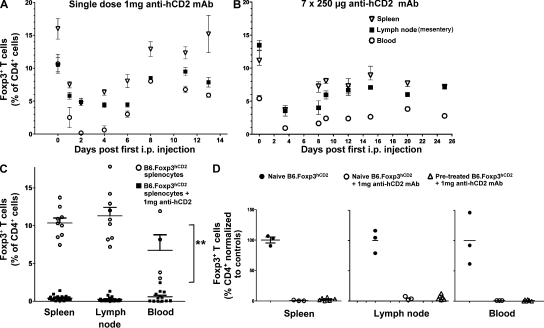
The high sensitivity of the hCD2_CD52 fusion protein for Foxp3 expression also allows targeted ablation of Foxp3+ T reg cells by anti–human CD2 (YTH655) rat IgG2b mAb (Friend et al., 1987; Qin et al., 1993). A single dose of 1 mg of depleting anti-hCD2 mAb removed >95% of circulating Foxp3+ T cells in euthymic B6.Foxp3hCD2 mice within 3 d—at least 10 d before the peak anti-globulin response is detected (Fig. 1 A and Fig. S2). However, between 30 and 40% residual Foxp3+ T cells were still detected in the spleen and lymph nodes, potentially contributing to the rapid recovery of pretreatment Foxp3+ T reg cells levels after 6 d. The use of multiple smaller doses of depleting anti-hCD2 mAb (e.g., 7 d of 250 µg) improved the degree of Foxp3+ T cell ablation in splenic and lymph compartments and further delayed T reg cell recovery until at least 25 d after the first dose (Fig. 1 B). Our finding that circulating Foxp3+ T cells were more susceptible to ablation than those resident in lymphoid tissue allowed us to exploit adoptive cell transfer protocols to deplete intravenously infused circulating cells. When splenocytes from B6.Foxp3hCD2 mice were intravenously injected into lymphocyte-deficient (RAG−/−) recipients, 1 mg anti-hCD2 mAb i.p./i.v. proved far more ablative, with <2% of residual Foxp3+ T reg cells detectable in splenic, lymphatic, and blood compartments of recipient RAG−/− mice 4 wk later (Fig. 1 C). Even Foxp3+ splenocytes from B6.Foxp3hCD2 reporter mice that appeared refractory to the initial round of systemic depletion were efficiently ablated on subsequent adoptive transfer in the presence of anti-hCD2 mAb (Fig. 1 D).
Figure 1.
Ablation of FoxP3+ T cells with an anti–human CD2 antibody. (A) Ablation of Foxp3+ cells after B6.Foxp3hCD2 mice received 1 mg YTH655 (at anti-hCD2 IgG2b mAb) i.p. in indicated organs. Three mice/group/time point are shown. (B) Foxp3+ cells after seven daily i.p. injections of 250 µg YTH655. P < 0.001. Three mice/group/time point are shown. (C) Flow cytometry analysis of spleen, lymph, and blood cells 4 wk after 2–10 million B6.Foxp3hCD2 splenocytes were injected i.v. into lymphocyte-deficient B6.RAG−/− recipients with 1 mg anti-hCD2 (squares; n = 16 composite of four experiments). **, P < 0.01 for the ablative effect of antibody compared to controls. (D) Splenocytes from B6.Foxp3hCD2 mice pretreated (open triangles, n = 6) or not (open circles, n = 3) with 7 × 250 µg anti-hCD2 mAb (30–40% of Foxp3+ T cells survived) were harvested and transferred into B6 RAG−/− mice with 1 mg anti-hCD2. P < 0.0001 compared with control (circles, n = 3). In all cases, horizontal bars represent the arithmetic mean and error bars represent SEM.
Adoptive transfer of tolerance requires Foxp3+ T reg cells
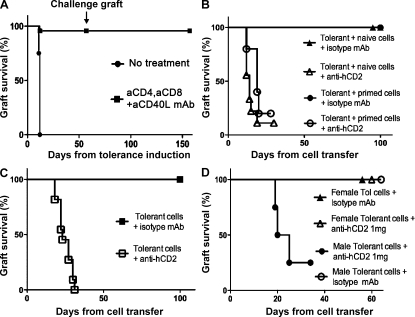
It has long been known that short-term co-receptor/co-stimulatory blockade can induce long-term tolerance to foreign tissue and that regulatory elements within the CD4+ population carry that tolerance with them when adoptively transferred into secondary hosts (Scully et al., 1994). We investigated whether Foxp3+ T reg cells, in particular, were the critical CD4+ regulatory cells responsible for this suppression. B6.Foxp3hCD2 were crossed with CBA/Ca mice to generate hybrid offspring known to be susceptible to our tolerance-inducing protocols (Daley et al., 2007). Because the foxp3 gene is X linked, all the Foxp3+ T cells in male (CBAxB6hCD2)F1 progeny would be susceptible to anti-hCD2 mAb–mediated ablation. CBAxB6hCD2 mice are haplotype H-2kxH-2b and rapidly rejected skin grafts mismatched by multiple minor antigens from H-2k Balb/K mice (Fig. 2 A). They did, however, accept Balb/K skin long term when the grafts were transplanted under the cover of nondepleting anti-CD4, anti-CD8, and anti-CD40L mAb blockade (P < 0.0001). Once therapeutic antibody levels had naturally decayed, a challenge Balb/K graft failed to break tolerance to the first graft and was itself accepted long term, indicating that tolerance remains active and self-sustaining (Fig. 2 A and Fig. S3). Classical adoptive transfer studies have previously demonstrated that tolerant CD4+ cells, from mice tolerized in this way, can suppress both naive and primed lymphocytes when transferred into lymphocyte deficient hosts (Marshall et al., 1996). Using a similar cell transfer protocol where we could guarantee >95% removal of Foxp3+ T cells (Fig. 1, C and D), we revisited these pioneering studies to investigate the exclusive roles of Foxp3+ T reg cells. 20 million tolerant splenocytes were harvested from male (CBAxB6hCD2)F1 mice that had accepted a Balb/K graft for over 60 d. These were then transferred into lymphocyte-deficient RAG−/− recipient mice that were transplanted on the same day with a challenge Balb/K skin graft. All such grafts were accepted despite co-transfer of five million naive splenocytes (Fig. 2 B, triangles) or one million primed splenocytes (Fig. 2 B, circles). Suppression by tolerant cells was abrogated by injecting the ablative anti-hCD2 mAb to target Foxp3+ T reg cells (Fig. 2 B, open triangles [P = 0.0026] and open circles [P = 0.0168]). This demonstrated unequivocally, and for the first time, that Foxp3+ T reg cells are essential for the suppression found in therapeutic transplant tolerance.
Figure 2.
Loss of transplant tolerance and suppression on adoptive transfer after ablation of FoxP3+ T cells. (A) (CBAxB6hCD2)F1 mice can be made tolerant to multiple minor mismatched Balb/K skin grafts with anti-CD4/CD8/CD40L blocking mAb (squares, n = 23; P < 0.0001 compared with control; circles, n = 4). The skin grafts were accepted for >150 d and were not rejected despite challenge grafting at day 60 (arrow). (B) Tolerant spleen cells were harvested from another cohort of (CBAxB6hCD2)F1 mice that had accepted a Balb/K graft for at least 60 d and transferred into RAG−/− recipients in conjunction with a challenge Balb/K graft. 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice suppressed the addition of five million naive splenocytes (triangles, n = 6) and one million primed splenocytes (circles, n = 5). This suppression was dependent on Foxp3+ T cells because anti-hCD2 mAb led to rapid rejection of the skin grafts. P = 0.0026 (n = 9) and P = 0.0168 (n = 5), respectively (one mouse in each group was euthanized). (C) 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice that accepted the first Balb/K graft also accepted a Balb/K skin graft when adoptively transferred into lymphocyte deplete (RAG−/−) recipients. When 1 mg anti-hCD2 was co-administered, the grafts were rejected. P = 0.0008 (n = 11). (D) 20 million tolerant splenocytes from female (CBAxB6hCD2)F1 mice that accepted the first Balb/K graft also accepted a Balb/K skin graft when adoptively transferred into lymphocyte deplete (RAG−/−) recipients with (open triangles, n = 8) or without (closed triangles, n = 3) anti-hCD2 mAb. In the same experiment, 20 million tolerant splenocytes from male (CBAxB6hCD2)F1 mice rejected the second Balb/K graft when co-administered with anti-hCD2 mAb (circles, n = 4; P = 0.0047 one mouse was euthanized).
Foxp3+ T reg cells constantly suppress effector T cells in a tolerant host
Remarkably, mice that received just the 20 million tolerant splenocytes with ablative anti-hCD2 mAb also rapidly rejected the Balb/K graft in comparison with control mice (Fig. 2 C, open vs. closed squares; P = 0.0008), albeit at a slower rate than when naive cells were co-transferred (Fig. 2, B and C; median survival time [MST] = 23 vs. 14 d, respectively; P = 0.0019). Importantly, anti-hCD2 mAb ablation of female (CBAxB6hCD2)F1 splenocytes (in which only approximately half of all Foxp3+ cells would be hCD2+ through random X inactivation) failed to result in graft rejection compared with male positive controls (Fig. 2 D; P = 0.0047). The finding that a tolerant population, when stripped of its Foxp3+ T reg cells, could nonetheless reject grafts demonstrated that rejection-competent T cells were still present despite the state of operational tolerance. This challenges preconceptions that regulatory cells are at their most active during tolerance induction by rendering any potential rejecting T effector cells anergic (Alters et al., 1991; Wekerle et al., 2002). It is also consistent with our previous findings that CD8+ cells from primed, yet tolerized, hosts retained the ability to reject foreign grafts once all CD4+ cells (including regulators) were removed (Marshall et al., 1996). Collectively, these experiments suggest that T cells fully capable of rejection remain within tolerant hosts and are constantly kept in check by Foxp3+ T reg cells.
A major limitation of cell transfer studies, such as those in the previous paragraph, is that homeostatic expansion may artificially magnify the potency of an otherwise insignificant population of effector T cells. In addition, adoptive transfer of lymphocytes in the presence of anti-hCD2 mAb led to such efficient ablation of Foxp3+ cells (Fig. 1, C and D) that the recipient mice developed immunopathology within 4 wk and three mice had to be euthanized (Fig. 2, B and D). In comparison, mice treated systemically with anti-hCD2 mAb, which spares up to a third of splenic Foxp3+ cells (Fig. 1, A and B), do not exhibit signs of disease, remain well, and gain weight for >150 d. For these reasons, we investigated whether residual effector function could also be revealed when Foxp3+ T reg cells were ablated in the more physiologically relevant model of a healthy naive mouse with a replete immune system.
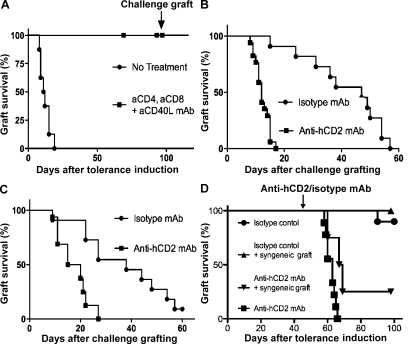
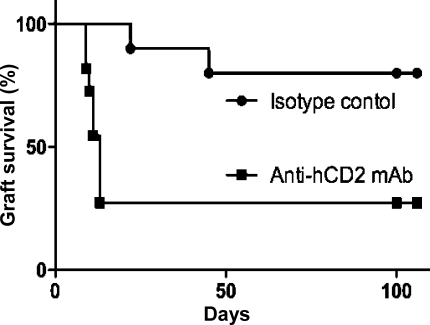
CBAxB6hCD2 (H-2kxH-2b) accepted MHC mismatched skin grafts from Balb/C (H-2d) mice long term when those grafts were transplanted under the cover of nondepleting anti-CD4, anti-CD8, and anti-CD40L mAb blockade (Fig. 3 A; P < 0.0001). Once therapeutic antibody levels had naturally decayed, the mice were able to accept second donor challenge grafts long term. In this stringent MHC mismatched graft model, we could again show that Foxp3+ T reg cells were necessary for long-term graft survival as ablative anti-hCD2 mAb therapy resulted in prompt rejection of secondary challenge grafts compared with control mice (Fig. 3 B; MST 12 vs. 47 d of isotype control mAb; P < 0.0001). Moreover, we observed that the original tolerated graft was also rejected, despite appearing well healed for >100 d (Fig. 3 C; MST [+100d] 17 vs. 38 d of isotype control; P = 0.0012). Rejection of the original tolerizing grafts did not require any inflammatory antigenic stimulus from a second challenge graft because treatment with anti-hCD2 mAb alone 50 d after tolerance induction again resulted in prompt rejection (Fig. 3 D; MST 16, P < 0.0001). That rejection can occur without reexposing the immune system to antigen under inflammatory conditions (i.e., challenge grafting) or an inflammatory insult from a syngeneic graft (Fig. 3 D) demonstrates the existence of a residual population of effector T cells that remain capable of rejecting a graft were it not for their continuous restraint by Foxp3+ T reg cells. These results indicate that short-term antibody-mediated immune blockade achieves long-term tolerance through promoting constant immune vigilance by Foxp3+ T reg cells, not simply by incapacitating effector T cells at the outset.
Figure 3.
Loss of transplant tolerance after ablation of Foxp3+ T cells in tolerant hosts. (A) Male (CBAxB6hCD2)F1 mice treated with anti-CD4/CD8/CD40L blocking mAb (3 × 3 mg doses over 7 d) accepted an MHC mismatched Balb/C skin (n = 27; P < 0.0001). (B) After 100 d, a challenge Balb/C skin graft was transplanted. Mice that received ablative anti-hCD2 mAb to deplete Foxp3+ T cells (squares, n = 16) rapidly rejected the challenge graft. MST 12.0 versus 47.0 d of control group (circles, n = 11), P < 0.0001. (C) The original tolerizing Balb/C graft was also rapidly rejected when mice received anti-hCD2 mAb (squares, n = 16). MST (+100d) 17.5 versus 38.0 d of control group (circles, n = 11), P = 0.0012. (D) Even without a challenge graft, mice that received anti-hCD2 mAb to ablate Foxp3+ T cells (downward triangles, n = 4; squares, n = 9) rejected a Balb/C graft that had been well tolerated for 50 d, with (P = 0.045) or without (P < 0.0001) a co-transplanted syngeneic graft.
De novo–induced Foxp3+ (i)T reg cells can also suppress rejection
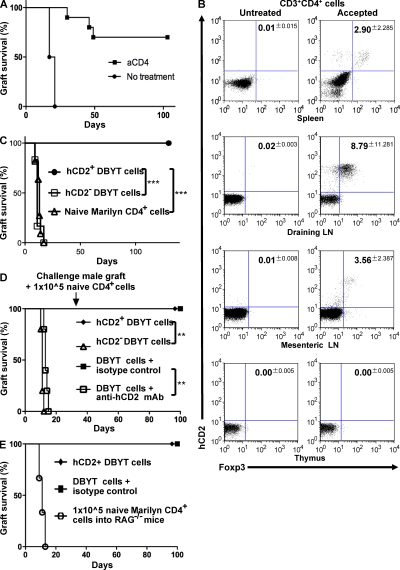
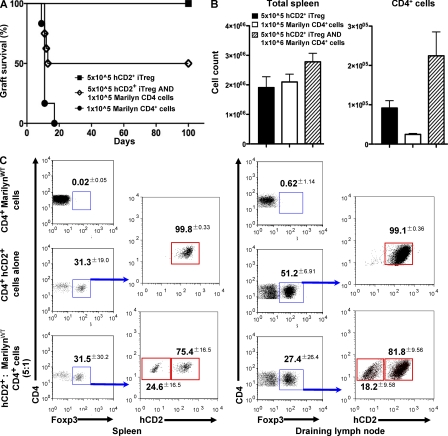
Having shown that Foxp3+ T reg cells are critical to therapeutic transplant tolerance in conventional mice, we next used a single TCR transgenic Foxp3 reporter model to investigate the role, if any, of antigen-specific peripherally induced Foxp3+ T reg cells (iT reg cells). The original B6.Foxp3hCD2 mice were backcrossed onto transgenic RAG−/− Marilyn mice to generate RAG−/− Marilyn.Foxp3hCD2 female mice containing only monoclonal CD4+ lymphocytes recognizing the male Dby peptide. Because these mice do not have any natural thymic-derived T reg cells, any detectable Foxp3+ cells will be induced (iT reg cells), should express cell surface hCD2, and consequently should be susceptible to ablation by anti-hCD2 mAb. As expected, female RAG−/− Marilyn.Foxp3hCD2 recipients rejected male B6 RAG−/− skin grafts but could be made tolerant by short-term treatment with anti-CD4 blocking mAb (Fig. 4 A; P = 0.0001). This induced tolerance was associated with the appearance of hCD2+Foxp3+ iT reg cells in the periphery, whereas no hCD2+Foxp3+ T reg cells were found in untreated female RAG−/− Marilyn.Foxp3hCD2 that had rejected the male graft (Fig. 4 B). As previously discussed, such correlative data does not prove a necessary role for hCD2+Foxp3+ iT reg cells in therapeutic tolerance. Their presence could, for example, be a bystander consequence and not the driving force of tolerance induction. Past work has shown that TGF-β–conditioned CD4+ T cells could suppress graft rejection in vivo (Cobbold et al., 2004), but because such populations are mixtures of Foxp3+ and Foxp3− T cells it has not yet been established which are the active components. To investigate whether Foxp3+ iT reg cells are the suppressive agents, we derived antigen-specific Foxp3+ iT reg cells from RAG−/− Marilyn.Foxp3hCD2 splenocytes cultured in vitro with dendritic cells, TGF-β, and the cognate Dby peptide for 7 d (DBYT cells; Fig. S4). Female RAG−/− mice that received either unsorted DBYT cells or purified hCD2+Foxp3+ T reg cells accepted a male graft and exhibited dominant tolerance by resisting a later challenge graft given together with 1 × 105 monoclonal naive T cells (Fig. 4, D and E), a cell number at least 10-fold in excess of the minimum required to reject male skin (unpublished data). In comparison, female RAG−/− mice that received MoFlow-sorted hCD2negative cells, devoid of Foxp3+ T reg cells, rejected the male RAG−/− grafts (Fig. 4 C; P = 0.0003). The hCD2− group (Fig. 4 C, squares) initially included four mice that received hCD2− MoFlow-sorted DBYT cells combined with further ablative anti-hCD2− to ensure that removal of Foxp3+ cells was optimized. In retrospect, cell sorting of hCD2− cells alone turned out to be sufficient to expose rejection, and this precautionary maneuver was not performed in subsequent assays (Fig. 4, C and D).
Figure 4.
Foxp3+ iTreg cells are induced in vivo by co-receptor blockade, and FoxP3+ iTreg cells induced by TGF in vitro are suppressive in vivo. (A) Female transgenic RAG−/− Marilyn.Foxp3hCD2 mice can be tolerized to male B6 RAG−/− skin by anti-CD4 blocking mAb (squares, n = 10; P = 0.0001). (B) De novo hCD2+Foxp3+ T reg cells were found in the spleen, draining, and mesenteric lymph nodes, but not the thymus, of Marilyn.Foxp3hCD2 mice that accepted a male graft for at least 60 d but not in untreated rejecting RAG−/− Marilyn.Foxp3hCD2 mice (P < 0.0001). (C) 5 × 105 TGF-β–conditioned male H-Y antigen-specific hCD2+ CD4+ RAG−/− Marilyn.Foxp3hCD2 (DBYT) cells failed to reject a male CBA.Ca RAG−/− graft (circles, n = 7) when injected into B6 RAG−/− recipients compared with 5 × 105 hCD2− DBYT cells (open squares, n = 11; P = 0.0003) or 1 × 105 naive CD4+ RAG−/− Marilyn.Foxp3hCD2 cells (open triangles, n = 6; P = 0.0006). The hCD2− DBYT group consists of mice injected with MoFlow-sorted hCD2− DBYT cells. Alone, n = 3; plus anti-hCD2 mAb, n = 4; plus isotype control mAb, n = 4. (D) 5 × 105 unsorted (DBYT) cells (squares, n = 4) and 5 × 105 hCD2+ cells (diamond, n = 5) fail to reject a male CBA.Ca RAG−/− graft when injected into B6 RAG−/− recipients. Elimination of hCD2+Foxp3+ T cells, either by anti-hCD2 mAb (open squares, n = 5; P = 0.0095) or MoFlow cell sorting of hCD2− cells (open triangles, n = 5; P = 0.0039), results in graft rejection. A challenge graft co-transplanted with 1 × 105 naive RAG−/− MarilynWT CD4+ cells after 30 d (arrow) fails to break tolerance and is itself accepted long term. (E) A positive control group is included to demonstrate that 1 × 105 naive RAG−/− MarilynWT CD4+ cells normally reject male skin when injected into naive RAG−/− mice (open circles, n = 3; P = 0.0062). **, P < 0.01; ***, P < 0.001.
Tolerated grafts harbor rejecting T cells
We have previously shown that T cells found in tolerated skin can recolonize a lymphocyte-deficient mouse and suppress graft rejection (Graca et al., 2002a). We have also shown that tolerated grafts are enriched with peripherally induced Foxp3+ iT reg cells in RAG−/− TCR transgenic mice (Cobbold et al., 2004). Indeed, CD25+ cells within grafts accepted by RAG−/− TCR transgenic tolerance can carry tolerance over to naive lymphocyte deficient mice. In comparison, spleens cells from tolerant RAG−/− TCR transgenic mice were still able to reject the same donor skin on adoptive transfer (Cobbold et al., 2006). Consistent with these early findings, lymphocytes harvested from the whole animal, but not the graft, of anti-CD4–treated female RAG−/− Marilyn.Foxp3hCD2 that had accepted a male graft long term rejected a subsequent male graft when adoptively transferred into female B6.RAG−/− mice (Fig. S5). Ablation of Foxp3+ T reg cells with anti-hCD2 mAb did result in significant increase in the rate of graft rejection, revealing a regulatory, albeit minimal, role for these cells at the whole animal level (Fig. S5; P = 0.0313). Collectively, it seems that, compared with conventional mice, transferable regulation in TCR transgenic mice is best seen within accepted grafts, making graft transfers a useful model to investigate the role of Foxp3+ iT reg cells in tissue specific transplantation tolerance.
By adapting a two-step transplantation study, we now ask whether specifically Foxp3+ iT reg cells in the graft tissue are innocent bystanders or contributing to tolerance by protecting the tissue from infiltrating effector cells. Lymphocyte-deficient male B6 RAG−/− skin grafts were first placed on female RAG−/− Marilyn.Foxp3hCD2 mice and tolerance was induced through short-term anti-CD4 mAb blockade (Fig. 4 A). After 2 mo, the well tolerated male skin graft was then immediately retransplanted onto a female lymphocyte-deficient host. This process ensured that any functional T cells in the new host (whether effector or regulator) would be from the original female RAG−/− Marilyn.Foxp3hCD2 mice and could only have been transferred within the graft tissue itself (Fig. 5). Remarkably, injection of ablative hCD2 antibody into recipient mice resulted in significant rejection of these previously tolerated grafts compared with control mice (Fig. 5; P = 0.0040). Collectively, these data establish several key findings. First, de novo antigen-specific Foxp3+ iT reg cells are produced in the periphery during tolerance induction through co-receptor blockade. Second, these peripheral Foxp3+ iT reg cells are suppressive and prevent potential effectors from rejecting grafts. Third, because the tolerated graft tissue itself contains CD4+ effector T cells fully capable of rejection, its survival depends on constant suppression by Foxp3+ iT reg cells generated de novo in response to the tolerizing protocol.
Figure 5.
FoxP3+ T cells within grafted tissue contributed to the survival of tolerated skin grafts transferred onto lymphopenic recipients. Male B6 RAG−/− grafts that were tolerated by anti-CD4 mAb–treated female RAG−/− Marilyn.Foxp3hCD2 mice (Fig. 4 A) were then retransplanted onto lymphocyte-deficient female B6 RAG−/− recipients. Ablation with anti-hCD2 mAb (squares, n = 11) resulted in rapid graft rejection compared with isotype control mAb (circles, n = 10; P = 0.0040).
Infectious tolerance requires and generates Foxp3+ T reg cells
Infectious transplant tolerance describes a self-sustaining process by which a population of tolerant immune cells can pass on that tolerance to naive cells (Qin et al., 1993). We investigated whether antigen-specific Foxp3+ (i)T reg cells were the secondary cohort of T cells generated by infectious tolerance. This required reliable distinction between the original tolerant population and the naive population that might be converted, as well as the absence of Foxp3+ cells in the naive population at the outset of the experiment. In vitro–generated TGF-β–induced hCD2+CD4+ antigen-specific Foxp3+ (i) T reg cells were isolated (Fig. S4) and infused into lymphocyte-deficient female B6.RAG−/− recipient mice (Fig. 6). A second group received naive CD4+ cells from RAG−/− MarilynWT mice that lacked natural Foxp3+ T reg cells. The third group received a mixture of 5:1 hCD2+CD4+ cells and naive CD4+ cells. All groups received a male CBA.RAG−/− skin graft after 24 h. None of the mice that received hCD2+ cells rejected their male grafts. In comparison, all the mice receiving naive RAG−/− MarilynWT CD4+ cells (alone) rejected their grafts (Fig. 6 A; P = 0.0066). Half of the mice that received a mixture of hCD2+ cells (iT reg cells) and naive CD4+ cells rejected the male graft (Fig. 6 A; P = 0.0247). Those mice that accepted the male skin for >100 d were analyzed for Foxp3+ expression. Although the total splenic white cell count was similar in all three groups, there were more CD4+ cells in the group that received a mixture of tolerant + naive cells (Fig. 6 B). Very few, if any, Foxp3+ cells were found in mice that had received 100,000 naive CD4+ cells alone and rejected the male skin. In contrast, Foxp3+ cells were found in the spleen and draining lymph nodes of mice that had accepted the male graft for >100 d (Fig. 6 C). In those mice that received a 5:1 mixture of hCD2+/naive CD4+ cells, 18% of Foxp3+ cells in draining lymph nodes and 24% in the spleen were hCD2 negative, whereas <2% of Foxp3+ cells in mice that received hCD2+ cells alone were hCD2neg (Fig. 6 C; P = 0.0009). The presence of hCD2negFoxp3+ cells in only those mice that received the 5:1 mixture demonstrated that some of the naive CD4+ cells from RAG−/− MarilynWT mice developed into Foxp3+ T reg cells as a result of coexistence with tolerant hCD2+ cells from RAG−/− Marilyn.Foxp3hCD2 mice. This finding that Foxp3+ T reg cells not only suppress but also facilitate conversion of naive T cells into new Foxp3+ (i)T reg cells provides one mechanism by which infectious tolerance may occur in vivo.
Figure 6.
In vitro–generated Foxp3+ iTreg cells prevented graft rejection by naive CD4 T cells and allowed some naive T cells to become Foxp3+ themselves. Female B6.RAG−/− received a male CBA.RAG−/− skin graft in conjunction with either 5 × 105 in vitro–cultured female hCD2+ RAG−/− Marilyn.Foxp3hCD2 DBYT cells (squares, n = 6), 1 × 105 naive CD4+ cells from RAG−/− MarilynWT mice (circles, n = 6), or a mixture of both (diamonds, n = 8). (A) All of the mice that received 1 × 105 naive CD4+ cells rejected the male skin compared with none of those that received 5 × 105 hCD2+ cells (P = 0.0066) and half that received a mixture (P = 0.0247). After 100 d, the mice were killed and Foxp3+ cell composition was analyzed. (B) Total white cell spleen counts were similar between the three groups, but there were more CD4+ cells in mice that received a mixture of hCD2+ and naive CD4+ cells. (C) FACS analysis demonstrated conversion of naive CD4+ cells into Foxp3+ T cells. Over 98% of Foxp3+ cells in mice that received 5 × 105 isolated hCD2+ RAG−/− Marilyn.Foxp3hCD2 cells alone were hCD2+, whereas 18% of Foxp3+ cells in the draining lymph and 24% in the spleen of mice that received a 5:1 mixture (and had accepted the male graft) were hCD2 negative (P = 0.0009). Error bars represent SEM.
DISCUSSION
Foxp3+ T reg cells are known to be essential for self-tolerance with a severe wasting immunopathology developing in their absence (Kim et al., 2007). Similarly, they are necessary in certain mouse models that can naturally accept foreign tissue without the need for additional immunosuppression (Benghiat et al., 2005; Robertson et al., 2007; Miyajima et al., 2011). We have now, for the first time, been able to demonstrate that Foxp3+ cells are also critical to long-term therapeutic tolerance to foreign antigen induced by clinically relevant protocols of antibody-mediated co-receptor and co-stimulatory blockade. Foxp3+ T reg cells are required for tolerance induction across all three levels of histocompatibility mismatch: full MHC, multiple minor, and single minor antigen.
Adoptive transfer of spleen cells from tolerant mice into naive lymphocyte-deficient recipients demonstrated that once tolerance is established, Foxp3+ T reg cells maintain that state despite challenges from naive and primed lymphocytes (Fig. 2). A recent study has demonstrated that nonspecific cell lysis may release extracellular factors (e.g., NAD+) that could themselves override suppression (Scheuplein et al., 2009). Given that all adoptively transferred Foxp3+hCD2+ cells from male (CBAxB6hCD2)F1 mice were susceptible to ablation by anti-hCD2 mAb, it was conceivable that the resulting cell destruction was a major contributor to the loss of tolerance, independent of specifically targeting Foxp3+ cells. To exclude this, spleen cells from tolerant female (CBAxB6hCD2)F1 mice were adoptively transferred into lymphocyte-deficient hosts, with and without anti-hCD2 mAb. Because both the natural and knocked in foxp3 gene are X linked, female (CBAxB6hCD2)F1 mice would inherit both copies and random X inactivation would ensure that approximately half the Foxp3+ cells would be hCD2+, and so lysed by anti-hCD2 antibody, whereas the rest would express the WT foxp3 gene and survive (Fig. 2 D). The failure of tolerant female (CBAxB6hCD2)F1 spleen cells plus ablative anti-hCD2 to reject any grafts demonstrated that cell lysis alone was unlikely to contribute significantly to the abrogation of tolerance in this model.
A surprising finding was that even a population of tolerant cells, without the addition of naive or primed cells, could still reject a graft if stripped of its Foxp3+ T reg cells (Fig. 2 D). This implied, first, that tolerance induction fails to incapacitate all cells capable of rejection at the outset, as was previously assumed (Alters et al., 1991; Wekerle et al., 2002), and second, compensates for this inadequacy by promoting sustained regulation by Foxp3+ T reg cells. An important limitation of adoptive transfer studies of this kind is that homeostatic expansion of newly transferred cells may lead to false rejection by artificially magnifying the effect of an otherwise insignificant population of effector T cells. A second concern is that the lympho-deficient RAG−/− recipients developed a wasting immunopathology ∼3–4 wk after transfer of spleen cells devoid of Foxp3+ T reg cells (Fig. 2, B and D). As previously stated, the onset of systemic immune dysregulation not only shortens the time period during which graft experiments can be conducted but also means that graft tolerance cannot be assessed independently of global immune attack. For these reasons, we turned to conventional lymphocyte-replete (CBAxB6hCD2)F1 mice, which did not develop wasting immunopathology, remained healthy for >150 d, and continued to gain weight despite anti-hCD2 mAb treatment, presumably because sufficient T reg cells are spared to prevent autoimmunity yet insufficient to maintain transplantation tolerance (Fig. 1, A and B). Once again, Foxp3+ T reg cells were found to be responsible for maintaining transplant tolerance by suppressing effector cells (Fig. 3). Indeed, short-term ablation of Foxp3+ cells 50 d after the induction of tolerance to a foreign skin graft resulted in rejection. In this setting, the rapid destruction of healthy, well healed, and well integrated skin dramatically demonstrated the rejection potential of residual effector T cells otherwise suppressed by Foxp3+ T reg cells (Fig. 3 D).
One critical zone of engagement is the graft tissue itself, which has been shown in this study to harbor T cells capable of causing its own rejection but which are held in check by Foxp3+ iT reg cells. Using a transgenic system in which female mice have monoclonal CD4 cells recognizing only the cognate male peptide, we were able to investigate the role of antigen-specific Foxp3+ iT reg cells produced in response to antibody-mediated transplant tolerance. De novo, Foxp3+ iT reg cells were found in the periphery (and, importantly, not the thymus; Fig. 4 B) of those female transgenic mice that had accepted a male graft. These generated iT reg cells were shown to be active participants in transplant tolerance and not merely coincidental bystanders because purified (hCD2− sorted) Foxp3−, but not (hCD2+ sorted) Foxp3+, cell populations resulted in male graft rejection when transferred into lymphocyte-deficient hosts (Fig. 4, C–E). Finally, male B6 RAG−/− skin grafts, which had been well tolerated by female RAG−/− Marilyn.Foxp3hCD2 for >60 d, were surgically removed and immediately retransplanted onto the flank of fresh lymphocyte-deficient female B6 RAG−/− mice. This process ensured that any functional T cells in the new host (whether effector or regulator) would be from the original female RAG−/− Marilyn.Foxp3hCD2 mice and could only have been transferred within the graft tissue itself (Fig. 5). Ablation of Foxp3+ T reg cells resulted in rejection of the graft, demonstrating that a fully tolerated graft can still harbor T cells capable of causing its own rejection. Graft acceptance therefore requires constant suppression of these potential rejecter cells by resident Foxp3+ iT reg cells—originally generated by the tolerizing protocol.
Several studies have previously shown de novo induction of Foxp3+ iT reg cells in response to tolerogenic conditions, but their active role in maintaining a state of transplant tolerance has not been established (Cobbold et al., 2004; Ochando et al., 2006; Fan et al., 2010; Farquhar et al., 2010). Our generation and subsequent ablation of Foxp3+ (i)T reg cells in TCR transgenic RAG−/− Marilyn.Foxp3hCD2 mice is the first direct evidence that peripherally generated new Foxp3+ (i)T reg cells are genuinely involved in therapeutic tolerance (Figs. 4 and 5). Furthermore, once Foxp3+ (i)T reg cells are induced, they maintain their numbers through the conversion of naive CD4+ cells into new Foxp3+ cells—i.e., infectious tolerance (Fig. 6). Early transplant studies explored the conditions required for infectious tolerance (Qin et al., 1993; Graca et al., 2000; Cobbold et al., 2009), whereas in vitro assays suggested a role for Foxp3+ (i)T reg cells (Jonuleit et al., 2002; Chen et al., 2003; Andersson et al., 2008). We have been able to build on these to demonstrate in vivo that infectious tolerance both requires and generates Foxp3+ cells. The necessity for persistent antigenic stimuli to maintain the new Foxp3+ converts has been suggested in previous studies in which tolerance is lost when the accepted graft is removed prematurely (Scully et al., 1994; Marshall et al., 1996). Consistent with this, very few new Foxp3+ converts from the naive CD4+ population were seen in those RAG−/− mice that rejected their male graft despite co-transfer of hCD2+ iT reg cells (Fig. S6). Collectively, these findings demonstrate that short-term immune blockade initiates the generation of new Foxp3+ cells that thereafter maintain transplant tolerance through self-replenishment.
Our findings support the emerging importance of the local tissue microenvironment in determining the degree of immune regulation within a tissue. At extremes, such tissue-dependent regulation can manifest as sites of immune privilege, such as the eye, which accept grafts without additional immunosuppression. Indeed, local tolerance of alloantigen injected into the anterior chamber of the eye can spread beyond the local environment to influence systemic tolerance, the so-called anterior chamber–associated immune deviation (Streilein, 2003). In a similar fashion, gastrointestinal mucosal immunity is tightly regulated at a local level to balance immunity (against colonizing pathogens) with tolerance to commensal bacterial flora and self-tissue (Coombes et al., 2005). The physiological role for induced regulatory T cells still remains unclear. Certainly gut microbiota seem to influence the numbers of Foxp3+ T reg cells within intestinal tissue and can safeguard against inflammatory bowel disease (Round and Mazmanian, 2010; Atarashi et al., 2011). It is possible that, unwittingly, our short-term therapeutic immune blockade has harnessed this otherwise gut-exclusive mechanism to enable exploitation of the antiinflammatory activities of iT reg cells in other tissues. Decentralizing a substantial part of immune regulation to the local tissue in this way perhaps has the advantage of promoting a more rapid, efficient, and appropriate immune response to a given tissue stress. Microenvironments of immune regulation are not restricted to normal healthy tissue, as demonstrated by their discovery in many tumor models. Indeed, local amplification of tumor-associated regulatory cells may prove a significant obstruction to cancer therapy (Colombo and Piconese, 2007). We would argue that part of the process of generating lasting transplant tolerance also involves establishing a local tissue microenvironment favoring graft immune regulation. Foxp3+ T reg cells are crucial to this process and indeed their presence within the demilitarized zone of a tolerated tissue may be necessary to keep in check resident T cells otherwise capable of insurgency.
Although we have established a crucial role for Foxp3+ T reg cells, we cannot exclude other regulatory cell subsets from playing a part in transplant tolerance. It may be that Foxp3+ T reg cells are the dominant suppressors, but in their absence and with the right kind of encouragement other regulatory mechanisms could fill that void. These findings should serve as encouragement for fledgling therapeutic strategies aimed at selectively enhancing Foxp3+ T reg cells in transplant tolerance. A note of caution though: the persistence of rejecting lymphocytes within the graft risks a loss of tolerance if restraint by Foxp3+ T reg cells is disrupted by subsequent life-time events.
MATERIALS AND METHODS
Experimental mice.
B6.Foxp3hCD2 knock-in, (CBAxB6hCD2)F1, (CBA.RAG1−/− × B6.RAG1−/−), B6.RAG1−/−, and RAG−/− Marilyn.Foxp3hCD2 knock-in mice were bred and maintained under specific pathogen-free conditions in the animal facility of the Sir William Dunn School of Pathology. B6.Foxp3hCD2 reporter mice were generated by homologous recombination in a B6-derived ES cell line using a targeting construct in which cDNA encoding a human CD2_CD52 fusion protein, along with an intra-ribosomal entry site, was inserted into the 3′ untranslated region of the endogenous foxp3 locus.
Skin grafting, tolerance induction, and ablation of hCD2+ cells.
All procedures were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986. Skin grafting was conducted by grafting full thickness tail skin (1 × 1 cm) on the lateral flank as previously described (Qin et al., 1990). Grafts were observed daily after the removal of the cast at day 8 and considered rejected when no viable donor skin was present. Statistical analysis of graft survival was made by the log-rank method. Tolerance across MHC and multiple minor histocompatibility barriers was achieved using three doses of 1 mg anti-CD4 (YTS 177.9), anti-CD8 (YTS 105.18), and anti-CD154 (MR1) nondepleting mAbs over 7 d. Tolerance of female RAG−/− Marilyn.Foxp3hCD2 knock-in mice to male B6.RAG1−/− skin was achieved using a single injection on day 0 of 250 µg anti-CD4 mAb (YTS 177.9; Qin et al., 1990).
YTH655 was prepared in house via purification of integra supernatant. An anti–dog rat IgG2b mAb (YKIX 337.217.1) prepared under the same conditions was used in all in vivo experiments as an isotype control mAb. Unless otherwise stated, Foxp3+hCD2+ T cell ablation was achieved using seven daily doses of 250 µg of rat IgG2b anti–human CD2 mAb (YTH655). Adoptive cell transfer was achieved by tail vein injection of an appropriate volume of washed cell suspension in sterile 1× PBS. 1 mg anti-hCD2 mAb (YTH655) or isotype control mAb per mouse was added to the cell suspension immediately before intravenous infusion. All transplant experiments were performed at least twice and results were pooled.
Immunofluorescence analysis and mAbs.
Spleen, lymph node, and blood cells were labeled directly with fluorescently conjugated mAb. For FACS staining, anti-hCD2 (RPA-2.10) PE-conjugated Mouse IgG1, anti–mouse CD4 (H129.19) FITC-conjugated mAb, anti-CD3 (145-2C11) PerCp-conjugated mAb (BD), anti–mouse CD8 (53–6.7) PE-conjugated mAb, anti–mouse CD19 (1D3) APC-conjugated mAb, and APC-conjugated anti–mouse Foxp3 (JFK-16s) mAb (eBioscience) were used to label live T cells in PBS containing 0.1% NaN3, 1% BSA, 5% heat-inactivated normal rabbit serum at 4°C. When required, the cells were then washed in PBS containing 0.1% NaN3 and 0.1% BSA. For Foxp3 intracellular staining, cells were fixed, permeabilized, and stained with anti-Foxp3 mAb as per the manufacturer’s protocol (eBioscience). Four-color analysis was performed using a FACSCalibur (BD) with dual laser (488 nm and 633 nm) excitation. The analysis gate was set on the forward and side scatters to eliminate cell debris and dead cells.
In vitro culture of iT reg cells.
RAG−/− Marilyn.Foxp3hCD2 spleen cells were harvested and prepared, including red blood cell lysis, under sterile conditions. 5 × 105 RAG−/− Marilyn.Foxp3hCD2 cells were cultured at 37°C and 6% CO2 in 2 ml R10 medium (RPMI 1640 medium [Lonza] + 10% vol/vol FCS [Invitrogen] + 50 µg/ml penicillin + streptomycin [Invitrogen] + 2 mM l-glutamine [Invitrogen] + 50 µM 2-mercapto-ethanol), 2 ng/ml TGF-β, 1 × 105 B6 WT bone marrow dendritic cells, and 100 nM of male H-Y peptide (Dby H-2Ab; NAGFNSNRANSSRSS).
MoFlow cell separation of cultured iT reg cells.
All steps were performed using sterile polypropylene materials where relevant to minimize cell loss. Cell samples were passed through a 70-µm cell strainer and suspended in R-10 medium, centrifuged for 6 min at 1,200 rpm, and resuspended in R-10 medium. The resultant cell suspension was counted, washed, and 10 µl/150 million cells of fluorescently conjugated antibodies were added in running buffer (1× PBS + 2 mM EDTA + 0.5% wt/vol BSA) before incubation for a further 45 min at 4°C. Single stain samples were also prepared for MoFlow calibration before cell sorting. After two washes with running buffer, the cell suspension was slowly passed through a 70-µm sieve and MoFlow cell sorted as per manufacturer’s guidelines.
Online supplemental material.
Fig. S1 shows the nature and cellular expression of the CD52-hCD2 reporter system for Foxp3 expression. Fig. S2 shows that ablation occurred independently of the host anti-globulin response. Fig. S3 shows that transplantation tolerance in mice tolerating grafts mismatched for multiple minor antigens was abrogated by ablation of Foxp3+ T cells in vivo. Fig. S4 shows induction and isolation of Foxp3+ iTreg in vitro. Fig. S5 shows evidence for a small but significant suppressive effect within central lymphoid cells derived from tolerant TCR transgenic mice. Fig. S6 shows that few newly converted Foxp3+ T cells were found in draining lymph nodes of mice where hCD2+ (Foxp3+) T cells had failed to suppress rejection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110767/DC1.
Acknowledgments
Kindly supported by the Medical Research Council and the Wellcome Trust.
Footnotes
Abbreviation used:
- MST
- median survival time
References
- Alters S.E., Shizuru J.A., Ackerman J., Grossman D., Seydel K.B., Fathman C.G. 1991. Anti-CD4 mediates clonal anergy during transplantation tolerance induction. J. Exp. Med. 173:491–494 10.1084/jem.173.2.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Tran D.Q., Pesu M., Davidson T.S., Ramsey H., O’Shea J.J., Shevach E.M. 2008. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β–dependent manner. J. Exp. Med. 205:1975–1981 10.1084/jem.20080308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 331:337–341 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M.G. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177:8338–8347 [DOI] [PubMed] [Google Scholar]
- Benghiat F.S., Graca L., Braun M.Y., Detienne S., Moore F., Buonocore S., Flamand V., Waldmann H., Goldman M., Le Moine A. 2005. Critical influence of natural regulatory CD25+ T cells on the fate of allografts in the absence of immunosuppression. Transplantation. 79:648–654 10.1097/01.TP.0000155179.61445.78 [DOI] [PubMed] [Google Scholar]
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P., Rebello P.R.U.B., Davies H.F.S., Friend P.J., Clark M.R. 1990. A simple method for measuring patient anti-globulin responses against isotypic or idiotypic determinants. J. Immunol. Methods. 127:19–24 10.1016/0022-1759(90)90335-S [DOI] [PubMed] [Google Scholar]
- Cobbold S.P., Castejon R., Adams E., Zelenika D., Graca L., Humm S., Waldmann H. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010 [DOI] [PubMed] [Google Scholar]
- Cobbold S.P., Adams E., Graca L., Daley S., Yates S., Paterson A., Robertson N.J., Nolan K.F., Fairchild P.J., Waldmann H. 2006. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol. Rev. 213:239–255 10.1111/j.1600-065X.2006.00428.x [DOI] [PubMed] [Google Scholar]
- Cobbold S.P., Adams E., Farquhar C.A., Nolan K.F., Howie D., Lui K.O., Fairchild P.J., Mellor A.L., Ron D., Waldmann H. 2009. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA. 106:12055–12060 10.1073/pnas.0903919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A., et al. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 11:1093–1101 10.1038/ni.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M.P., Piconese S. 2007. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer. 7:880–887 10.1038/nrc2250 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Robinson N.J., Maloy K.J., Uhlig H.H., Powrie F. 2005. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204:184–194 10.1111/j.0105-2896.2005.00250.x [DOI] [PubMed] [Google Scholar]
- Daley S.R., Ma J., Adams E., Cobbold S.P., Waldmann H. 2007. A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J. Immunol. 179:3648–3654 [DOI] [PubMed] [Google Scholar]
- Fan Z.G., Spencer J.A., Lu Y., Pitsillides C.M., Singh G., Kim P., Yun S.H., Toxavidis V., Strom T.B., Lin C.P., Koulmanda M. 2010. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat. Med. 16:718–722 10.1038/nm.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C.A., Paterson A.M., Cobbold S.P., Garcia Rueda H., Fairchild P.J., Yates S.F., Adams E., Saunders N.J., Waldmann H., Nolan K.F. 2010. Tolerogenicity is not an absolute property of a dendritic cell. Eur. J. Immunol. 40:1728–1737 10.1002/eji.200939974 [DOI] [PubMed] [Google Scholar]
- Feng G., Wood K.J., Bushell A. 2008. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 86:578–589 10.1097/TP.0b013e3181806a60 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Friend P.J., Tighe H., Lim S., Collier D.S., Decurtins M., Gilliland L.K., Thiru S., Calne R., Waldmann H. 1987. The use of monoclonal antibodies against activated human T cells following renal allografting in the baboon. Transplant. Proc. 19:4317–4318 [PubMed] [Google Scholar]
- Graca L., Honey K., Adams E., Cobbold S.P., Waldmann H. 2000. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J. Immunol. 165:4783–4786 [DOI] [PubMed] [Google Scholar]
- Graca L., Cobbold S.P., Waldmann H. 2002a. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 195:1641–1646 10.1084/jem.20012097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca L., Thompson S., Lin C.Y., Adams E., Cobbold S.P., Waldmann H. 2002b. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J. Immunol. 168:5558–5565 [DOI] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Kakirman H., Stassen M., Knop J., Enk A.H. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196:255–260 10.1084/jem.20020394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A.R., Waldmann H. 2010. Infectious tolerance: therapeutic potential. Curr. Opin. Immunol. 22:560–565 10.1016/j.coi.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Komatsu N., Mariotti-Ferrandiz M.E., Wang Y., Malissen B., Waldmann H., Hori S. 2009. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA. 106:1903–1908 10.1073/pnas.0811556106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Wang L.Q., Wells A.D., Dorf M.E., Ozkaynak E., Hancock W.W. 2005. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201:1037–1044 10.1084/jem.20041709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings M.K., Sangregorio R., Sartirana C., Moschin A.L., Battaglia M., Orban P.C., Roncarolo M.G. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335–1346 10.1084/jem.20021139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S.E., Cobbold S.P., Davies J.D., Martin G.M., Phillips J.M., Waldmann H. 1996. Tolerance and suppression in a primed immune system. Transplantation. 62:1614–1621 10.1097/00007890-199612150-00015 [DOI] [PubMed] [Google Scholar]
- Miyajima M., Chase C.M., Alessandrini A., Farkash E.A., Della Pelle P., Benichou G., Graham J.A., Madsen J.C., Russell P.S., Colvin R.B. 2011. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am. J. Pathol. 178:1635–1645 10.1016/j.ajpath.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochando J.C., Homma C., Yang Y., Hidalgo A., Garin A., Tacke F., Angeli V., Li Y., Boros P., Ding Y., et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662 10.1038/ni1333 [DOI] [PubMed] [Google Scholar]
- Qin S.X., Wise M., Cobbold S.P., Leong L., Kong Y.C.M., Parnes J.R., Waldmann H. 1990. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur. J. Immunol. 20:2737–2745 10.1002/eji.1830201231 [DOI] [PubMed] [Google Scholar]
- Qin S.X., Cobbold S.P., Pope H., Elliott J., Kioussis D., Davies J., Waldmann H. 1993. “Infectious” transplantation tolerance. Science. 259:974–977 10.1126/science.8094901 [DOI] [PubMed] [Google Scholar]
- Robertson N.J., Chai J.G., Millrain M., Scott D., Hashim F., Manktelow E., Lemonnier F., Simpson E., Dyson J. 2007. Natural regulation of immunity to minor histocompatibility antigens. J. Immunol. 178:3558–3565 [DOI] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 107:12204–12209 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuplein F., Schwarz N., Adriouch S., Krebs C., Bannas P., Rissiek B., Seman M., Haag F., Koch-Nolte F. 2009. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J. Immunol. 182:2898–2908 10.4049/jimmunol.0801711 [DOI] [PubMed] [Google Scholar]
- Scully R., Qin S.X., Cobbold S., Waldmann H. 1994. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur. J. Immunol. 24:2383–2392 10.1002/eji.1830241019 [DOI] [PubMed] [Google Scholar]
- Semiletova N.V., Shen X.D., Baibakov B., Andakyan A. 2010. Intensity of transplant chronic rejection correlates with level of graft-infiltrating regulatory cells. J. Heart Lung Transplant. 29:335–341 10.1016/j.healun.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Streilein J.W. 2003. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 74:179–185 10.1189/jlb.1102574 [DOI] [PubMed] [Google Scholar]
- Turnquist H.R., Raimondi G., Zahorchak A.F., Fischer R.T., Wang Z., Thomson A.W. 2007. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 178:7018–7031 [DOI] [PubMed] [Google Scholar]
- Weiner H.L. 2001. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 182:207–214 10.1034/j.1600-065X.2001.1820117.x [DOI] [PubMed] [Google Scholar]
- Wekerle T., Kurtz J., Bigenzahn S., Takeuchi Y., Sykes M. 2002. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr. Opin. Immunol. 14:592–600 10.1016/S0952-7915(02)00378-3 [DOI] [PubMed] [Google Scholar]
- Xia G.L., Shah M., Luo X.R. 2009. Prevention of allograft rejection by amplification of Foxp3(+)CD4(+)CD25(+) regulatory T cells. Transl. Res. 153:60–70 10.1016/j.trsl.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]