Schaer et al. discuss mechanisms of immune evasions by tumors, including the recent finding that CCL2 nitrosylation prevents T cell infiltration into tumors.
Abstract
Tumors exploit many strategies to evade T cell–mediated destruction. For example, tumors can prevent T cell infiltration by modifying gene expression in the endothelial cells and pericytes that form their vasculature. New work showing that the T cell–attracting chemokine CCL2 can be posttranslationally modified in the tumor microenvironment adds another mechanism to the already formidable arsenal of immunoevasion tactics used by solid tumors.
Since the original observations of Coley (1893), and the subsequent work of Schreiber et al. (2011), it has been evident that the immune system is capable of detecting neoplastic transformation and eradicating spontaneous and experimentally induced tumors. However, despite this inherent ability, tumors escape immune destruction and cancer still remains a major cause of death. It is well known that cells of the immune system can play both protective and tumor-promoting roles during neoplastic transformation. Tumors undergo a process known as immunoediting, resulting in the selection of a tumor that has established either a favorable microenvironment that facilitates its growth or an immunosuppressive environment that enables it to avoid immune destruction (Schreiber et al., 2011). In this issue, Molon et. al. describe a novel mechanism of tumor immune escape involving the extracellular modification of the chemokine CCL2 within the tumor microenvironment, rendering it undetectable by circulating lymphocytes. In this minireview, we discuss the results of this paper and its significance in the context of our current understanding of the trafficking of antitumor T cells and tumor-induced immune suppression.
T cell infiltration correlates with prognosis
The importance of tumor-infiltrating lymphocytes (TILs) is highlighted by their prognostic value in human cancer. Using genetic and histological analysis of a large cohort of colorectal cancer patient biopsies, Galon et al. (2006) showed that both the type and location of immune cell infiltrate predicts improved patient survival. Specifically, patients whose tumor centers or invasive margins were highly infiltrated with T cells had the best predicted survival. In contrast, patients with stage I tumors containing few or no infiltrating T cells had a prognosis similar to metastatic stage IV patients, even though they originally presented with minimally invasive disease (Galon et al., 2006). Many studies examining other cancers reached similar conclusions; consequently, a more defined picture has now developed in which immune infiltrates correlate with improved prognosis or protumorigenic potential. Each infiltrating immune cell type responds differentially to various anticancer treatments (Quezada et al., 2011). Therefore, a positive balance of antitumor effector cells (M1 macrophages, CD8+ T cells, and T helper type 1 cells) versus tumor-promoting suppressive cells (M2 macrophages, myeloid derived suppressor cells [MDSCs], and regulatory T cells [T reg cells]) in the tumor predicts not only prognosis but also the therapeutic impact of chemotherapy and immunotherapy (Fridman et al., 2011; Quezada et al., 2011). Identifying the mechanisms that prevent infiltration of antitumor effector cells is therefore of the utmost importance in optimizing therapeutic benefit.
Keeping the T cells out
After the initial priming of T cells in tumor-draining lymph nodes, successful trafficking of effector cells to the tumor becomes the next goal for effective tumor immunity. Recent studies have demonstrated that the tumor vasculature itself, as a result of activation state or organization, can prevent T cell infiltration. Through the analysis of gene expression profiles of tumor endothelium from human ovarian cancers, Buckanovich et al. (2008) discovered the association of endothelin B receptor (ETBR) expression with the absence of TILs. Moreover, the ligand of ETBR, endothelin-1, is expressed by ovarian tumors in vivo (Bagnato et al., 2005). When activated by ligand binding, ETBR causes up-regulation of nitric oxide (NO) synthases, leading to NO release from the vascular endothelium (Tsukahara et al., 1994). NO in turn reduces both the expression of ICAM-1 and ICAM-1 clustering, preventing T cell adhesion to the endothelium (Buckanovich et al., 2008). Blocking ETBR with an antagonist peptide prevented the modulation of ICAM-1 and resulted in increased T cell vasculature adhesion in vitro (Buckanovich et al., 2008). Importantly, combining ETBR blockade with vaccine strategies or adoptive T cell therapy (ATC) in ovarian ID8 and HPV-expressing TC-1 tumor models enhanced their therapeutic benefit, leading to delayed tumor growth through increased T cell infiltration.
Although the previous example demonstrates how ovarian tumors can modulate the endothelial barrier through tumor-derived factors, the inherently disorganized and leaky tumor vasculature itself can also act as a major barrier to T cell infiltration. Investigating markers of pathological angiogenesis, Berger et al. (2005) found that regulator of G-protein signaling-5 (RGS-5) was overexpressed in pericytes of tumor neovasculature. Normally, early tumors in the RIP1-Tag5 model of pancreatic islet cell cancer are characterized by a disorganized and leaky vasculature that creates a hypoxic environment devoid of TILs. RGS5-expressing pericytes displaying an immature phenotype were found to preferentially associate with this highly angiogenic neovasculature (Manzur et al., 2009). Deletion of RGS-5 in RIP1-Tag5 mice resulted in pericyte maturation and normalization of vasculature inside the tumor, thereby removing the barrier to infiltrating lymphocytes (Hamzah et al., 2008). ATC therapy with tumor-specific T cells or vaccination with tumor antigens substantially increased the survival of tumor-bearing RIP1-Tag5 Rgs5−/− mice, but had no effect in RIP1-Tag5 Rgs5+/+ mice.
Modifying the directions
Although the aforementioned examples show how trans-migration through the vascular endothelium into the tumor can represent a formidable barrier to tumor immunity, there are many cases where T cells are recruited to the tumor, yet remain in its periphery (Galon et al., 2006; Mrass et al., 2006; Boissonnas et al., 2007). Determining the cause of this phenotype is the focus of the study by Molon et al. (2011). Previous studies from the same group documented that reactive nitrogen species (RNS) are produced inside various tumors through metabolism of l-arginine by arginase and NO synthase (Bronte et al., 2005; De Santo et al., 2005). Arginase lowers l-arginine levels to the point that iNOS makes a mixture of NO and O2-. These react with each other to form the RNS peroxynitrite, whose rapidly arising breakdown product, the radical NO2, is a potent nitrosylating agent, leading to nitrotyrosinylation of proteins inside the tumor microenvironment (Bronte and Zanovello, 2005; Nathan and Ding, 2010). The direct nitrotyrosinylation of important signaling proteins in the T cell receptor (TCR) cascade is believed to block TCR signaling in TILs (Bronte and Zanovello, 2005; Nagaraj et al., 2010). However, while investigating the pattern of nitrotyrosine (n-Tyr) in human colorectal tumors, Molon et al. (2011) found an inverse distribution of n-Tyr and TILs. Staining for n-Tyr was observed mainly within the tumor core, whereas T cells accumulated in the periphery of the tumor. Given the considerable production of n-Tyr inside various tumors, they hypothesized that other proteins such as the chemokine CCL2, an important chemoattractant for TIL, could also be nitrotyrosinalated. After developing an antibody that could distinguish between nitrotyrosinalated CCL2 (n-CCL2) and unmodified CCL2, Molon et al. (2011) demonstrated that n-CCL2 was present in human prostate and colon carcinomas. The significance of intratumoral n-CCL2 was highlighted by experiments showing that neither human nor mouse T cells were able to migrate toward n-CCL2. Conversely, myeloid cells, which express higher levels of the CCL2 receptor (CCR2), were still able to detect and migrate toward n-CCL2. This is an important finding, as it is believed that immature myeloid cells, also known as MDSCs, are responsible for producing the intratumoral RNS that could lead to the production of n-CCL2. In support of this concept, we have recently found that ablation of CCR2-expressing myeloid cells directly enhances activated T cell entry into the tumor site, implying a critical role for CCR2+ myeloid cells in limiting T cell entry into the tumor (unpublished data).
Previously, imaging studies of mouse EG.7 thymomas undergoing rejection have demonstrated that intratumoral T cells engage in a random walk pattern, suggesting that they lack a specific chemoattractant signal to direct intratumor migration (Mrass et al., 2006). Further studies have shown that not only do T cells congregate in the periphery of the tumor, but in examples of ATC therapy, T cells must first kill tumor cells in the periphery before working their way into the tumor itself (Boissonnas et al., 2007; Breart et al., 2008). Using this same model, Molon et al. (2011) showed that these tumors contained a significant amount of n-CCL2, which could explain the distribution and migration phenotype of TIL previously described for EG.7 tumors.
Putting T cells back on track
As mentioned earlier, the normalization of vasculature in Rgs5−/− mice or the blocking ETBR with antagonist peptides restored T cell tumor trafficking and enhanced the therapeutic benefit of ATC therapy. In prior research, De Santo et al. (2005) showed that blocking production of RNS with nitroaspirin prevented the generation of n-Tyr inside tumors, leading to improved antitumor immunotherapy with vaccines. In the current study, nitroaspirin was not found to be an effective adjuvant for ATC therapy; Molon et al. (2011) therefore developed a new compound (AT38) that efficiently blocked the production of peroxynitrite. AT38 reduced the generation of n-Tyr and prevented nitrotyrosinylation of CCL2 in TRAMP, C26GM, and EG.7 tumors. This treatment reversed the block in T cell trafficking and enabled adoptively transferred OT-1 TILs to migrate into the core of EG.7 tumors leading to significantly enhanced tumor rejection and long-term protection in both EG.7 and MCA-203 fibrosarcoma models. Because AT38 was unable to improve T cell infiltration when tumors were grown in Ccr2−/− mice, and because the effects of AT38 could be mimicked by the direct intratumoral injection of unmodified CCL2 in wild-type mice, the effects of AT38 were likely directly related to the unmasking of CCL2.
Getting there may not always be the problem
The extent to which chemokine nitrotyrosinylation and subsequent masking of TIL chemoattractant signals plays a role in other tumors remains to be determined. Although it is tempting to consider this pathway a ubiquitous mechanism of suppression, it is likely that excess MDSC and a paucity of CD8+ T effector cells are one of multiple suppressive mechanisms (Gajewski et al., 2011; Schreiber et al., 2011). As Molon et al. (2011) point out, imaging studies using the same OVA-expressing EG.7 thymoma tumor model have suggested that there is a barrier to deep tumor infiltration by TIL. However, we have recently reported using intravital imaging that the same barrier did not exist in a different tumor model (Schaer et al., 2011). Specifically, during the early stages of the anti-B16 melanoma immune response, gp100 self-antigen–specific Pmel-1 CD8+ TILs were found throughout the tumor (Schaer et al., 2011). Nonspecific, OVA-reactive OT-1 T cells also infiltrated the tumor to the same degree without exhibiting tumor recognition, demonstrating that in the case of B16 tumors, activated T cells are recruited regardless of antigen specificity. Furthermore, B16 tumors grow despite the fact that intratumoral Pmel-1 T cells recognize cognate tumor antigens, similar to what has been described by prior imaging studies of OVA-expressing thymomas (Mrass et al., 2006; Boissonnas et al., 2007; Schaer et al., 2011). These observations suggest ongoing intratumoral inhibition of the antitumor immune response. Possible suppressive mediators could be the same MDSCs that remain responsive to n-CCL2 and have been shown to inhibit intratumoral T cell function (Marigo et al., 2008; Rodríguez and Ochoa, 2008). T reg cells have also been known to play an important role in suppressing tumor immunity, and in a manner somewhat synonymous with the data of Molon et al. (2011), tumors can preferentially recruit T reg cells by secreting CCL22 (Nishikawa and Sakaguchi, 2010; Faget et al., 2011). In fact, in our own studies, we observed that T reg cell are in proximity to and interact with Pmel-1 TIL (unpublished data). Thus, although it is clear in the models presented by Molon et al. (2011) that n-CCL2 represents a major barrier to tumor immunity, it is possible that not all tumor models use this same mechanism of immune evasion.
Conclusion
Recent advances in immune checkpoint blockade and ATC have brought therapeutic options to patients with advanced cancers where there had previously been none (Hodi et al., 2010; Robert et al., 2011; Rosenberg et al., 2011). Yet, these successes are still limited to a subset of patients. Thus, further investigation of the underlying causes of tumor immune escape is needed to extend clinical benefit to more patients. Modification of chemokines by nitrosylation represents a significant new paradigm in tumor-induced immune suppression (Fig. 1). Most importantly, Molon et al. (2011) demonstrate that this adverse modification can be therapeutically targeted, resulting in enhancement of both ATC and endogenous antitumor T cell responses. However, as noted in this study, commercial reagents are unable to detect n-CCL2, so its current involvement in human cancer and preclinical tumor models may not yet be fully appreciated. Barriers to T cell tumor infiltration, such as chemokine nitrosylation, should be considered another mechanism of tumor-induced immunosuppression. The combination of current immunotherapies with inhibition of n-CCL2 is a promising venue and could lead to better outcomes for cancer patients.
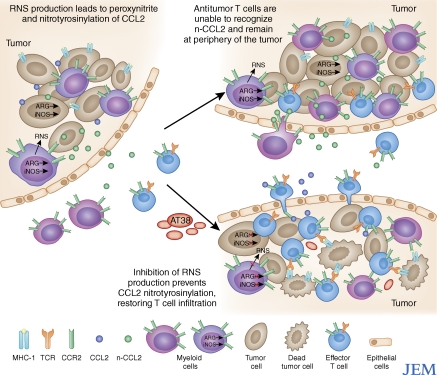
Figure 1.
Masking of chemokine signals through intratumoral production of RNS. Metabolism of l-Arginine in the tumor by arginase and iNOS from myeloid or tumor cells leads to the generation of RNS, such as peroxynitrite, inside of the tumor microenvironment (left). This results in the nitrotyrosinylation of proteins, including the chemokine CCL2 (n-CCL2), in the tumor microenvironment. Because n-CCL2 binds to its receptor (CCR2) with much lower affinity than the unmodified version, it prevents n-CCL2 from acting as a strong chemoattractant signal for antitumor T cells (top right). However, myeloid cells express higher levels of CCR2 receptor and are still able to migrate toward n-CCL2 gradients. When the small molecule inhibitor of RNS production AT38 is administered, it blocks peroxynitrite formation and subsequent nitrotyrosinylation of CCL2. This restores deep T cell infiltration into the tumor, enhancing the effectiveness of both adoptive T cell therapy and endogenous antitumor responses (bottom right).
Acknowledgments
We would like to thank Dr. Taha Merghoub and Dr. Sadna Budhu for critical reading of this review.
This work was supported by National Institutes of Health grants R01CA56821 and P01CA59350, Swim Across America, the Melanoma Research Alliance, the Cancer Research Institute, and the Commonwealth Cancer Research Foundation/MSKCC Experimental Therapeutics Center (J.D. Wolchok); a National Institutes of Health Clinical Training for Scholar grant K12 CA120121-01, T32 CA09149-30, and a John D. Proctor Foundation: Margaret A. Cunningham Immune Mechanisms in Cancer Research Fellowship Award (D.A. Schaer); and an American Cancer Society Career Development Award and the Melanoma Research Alliance (A.M. Lesokhin).
References
- Bagnato A., Spinella F., Rosanò L. 2005. Emerging role of the endothelin axis in ovarian tumor progression. Endocr. Relat. Cancer. 12:761–772 10.1677/erc.1.01077 [DOI] [PubMed] [Google Scholar]
- Berger M., Bergers G., Arnold B., Hämmerling G.J., Ganss R. 2005. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 105:1094–1101 10.1182/blood-2004-06-2315 [DOI] [PubMed] [Google Scholar]
- Boissonnas A., Fetler L., Zeelenberg I.S., Hugues S., Amigorena S. 2007. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J. Exp. Med. 204:345–356 10.1084/jem.20061890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breart B., Lemaître F., Celli S., Bousso P. 2008. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 118:1390–1397 10.1172/JCI34388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Zanovello P. 2005. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5:641–654 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- Bronte V., Kasic T., Gri G., Gallana K., Borsellino G., Marigo I., Battistini L., Iafrate M., Prayer-Galetti T., Pagano F., Viola A. 2005. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201:1257–1268 10.1084/jem.20042028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckanovich R.J., Facciabene A., Kim S., Benencia F., Sasaroli D., Balint K., Katsaros D., O’Brien-Jenkins A., Gimotty P.A., Coukos G. 2008. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14:28–36 10.1038/nm1699 [DOI] [PubMed] [Google Scholar]
- Coley W. 1893. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am. J. Med. Sci. 105:487–511 10.1097/00000441-189305000-00001 [DOI] [PubMed] [Google Scholar]
- De Santo C., Serafini P., Marigo I., Dolcetti L., Bolla M., Del Soldato P., Melani C., Guiducci C., Colombo M.P., Iezzi M., et al. 2005. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. USA. 102:4185–4190 10.1073/pnas.0409783102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget J., Biota C., Bachelot T., Gobert M., Treilleux I., Goutagny N., Durand I., Leon-Goddard S., Blay J.-Y., Caux C., Menetrier-Caux C. 2011. Early detection of tumor cells by innate immune cells leads to Treg recruitment through CCL22 production by tumor cells. Cancer Res. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Fridman W.H., Galon J., Pagès F., Tartour E., Sautès-Fridman C., Kroemer G. 2011. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 71:5601–5605 10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- Gajewski T.F., Fuertes M., Spaapen R., Zheng Y., Kline J. 2011. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 23:286–292 10.1016/j.coi.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- Hamzah J., Jugold M., Kiessling F., Rigby P., Manzur M., Marti H.H., Rabie T., Kaden S., Gröne H.J., Hämmerling G.J., et al. 2008. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 453:410–414 10.1038/nature06868 [DOI] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur M., Hamzah J., Ganss R. 2009. Modulation of G protein signaling normalizes tumor vessels. Cancer Res. 69:396–399 10.1158/0008-5472.CAN-08-2842 [DOI] [PubMed] [Google Scholar]
- Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. 2008. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222:162–179 10.1111/j.1600-065X.2008.00602.x [DOI] [PubMed] [Google Scholar]
- Molon B., Ugel Stefano, Del Pozzo Federica, Soldani Cristiana, Zilio Serena, Avella Debora, De Palma Antonella, Mauri PierLuigi, Monegal Ana, Rescigno Maria, et al. 2011. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208:1949–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrass P., Takano H., Ng L.G., Daxini S., Lasaro M.O., Iparraguirre A., Cavanagh L.L., von Andrian U.H., Ertl H.C., Haydon P.G., Weninger W. 2006. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J. Exp. Med. 203:2749–2761 10.1084/jem.20060710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S., Schrum A.G., Cho H.I., Celis E., Gabrilovich D.I. 2010. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 184:3106–3116 10.4049/jimmunol.0902661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Ding A. 2010. SnapShot: Reactive Oxygen Intermediates (ROI). Cell. 140:951–951: e2 10.1016/j.cell.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Nishikawa H., Sakaguchi S. 2010. Regulatory T cells in tumor immunity. Int. J. Cancer. 127:759–767 [DOI] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Simpson T.R., Allison J.P. 2011. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol. Rev. 241:104–118 10.1111/j.1600-065X.2011.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., M D J.W., Garbe C., Lebbe C., Baurain J.F., Testori A., Grob J.J., et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Rodríguez P.C., Ochoa A.C. 2008. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222:180–191 10.1111/j.1600-065X.2008.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R., et al. 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17:4550–4557 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer D.A., Li Y., Merghoub T., Rizzuto G.A., Shemesh A., Cohen A.D., Li Y., Avogadri F., Toledo-Crow R., Houghton A.N., Wolchok J.D. 2011. Detection of intra-tumor self antigen recognition during melanoma tumor progression in mice using advanced multimode confocal/two photon microscope. PLoS ONE. 6:e21214 10.1371/journal.pone.0021214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R.D., Old L.J., Smyth M.J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 331:1565–1570 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- Tsukahara H., Ende H., Magazine H.I., Bahou W.F., Goligorsky M.S. 1994. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J. Biol. Chem. 269:21778–21785 [PubMed] [Google Scholar]