Abstract
CHMP2B mutations are a rare cause of autosomal dominant frontotemporal dementia (FTD). The best studied example is frontotemporal dementia linked to chromosome 3 (FTD-3) which occurs in a large Danish family, with a further CHMP2B mutation identified in an unrelated Belgian familial FTD patient. These mutations lead to C-terminal truncations of the CHMP2B protein and we will review recent advances in our understanding of the molecular effects of these mutant truncated proteins on vesicular fusion events within the endosome-lysosome and autophagy degradation pathways. We will also review the clinical features of FTD caused by CHMP2B truncation mutations as well as new brain imaging and neuropathological findings. Finally, we collate the current data on CHMP2B missense mutations, which have been reported in FTD and motor neuron disease.
Keywords: Frontotemporal dementia, CHMP2B, endosome, lysosome, autophagy, brain imaging, neuropathology.
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) comprises a heterogeneous group of disorders which are all characterised by gross atrophy primarily of the frontal and/or temporal lobes. FTLD generally presents with either personality change, termed behavioural variant frontotemporal dementia (bvFTD) (or simply FTD) or distinct language impairments termed language variant FTD (lv-FTD), including semantic dementia (SD) and progressive non-fluent aphasia (PNFA) [1, 2]. FTLD can also be associated with parkinsonism, or with motor neuron disease, which is termed FTD-MND [3]. FTLD also has clinical and neuropathological overlap with the atypical parkinsonian movement disorders corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) [4-7].
The neuropathology of FTLD syndromes is also heterogeneous. The two major pathologies are FTLD-tau and FTLD-TDP, which are characterised by tau or TDP-43 positive inclusions respectively [4, 8]. FUS-positive inclusions have recently been described in a subset of FTLD cases [9, 10], and two rare neuropathological subtypes remain: those with ubiquitin-positive inclusions that are negative for tau, TDP-43 and FUS, termed FTLD-UPS [11] (which are primarily, CHMP2B mutation cases), and those with no discernable inclusions, termed FTLD-ni [11].
The major genetic causes of FTLD are mutations in GRN [12, 13], which encodes progranulin and MAPT [14] which encodes tau, leading to FTLD-TDP and FTLD-tau respectively. Mutations in TARDBP (which encodes TDP-43) [15, 16], VCP [17], FUS [18] and CHMP2B [19, 20] are much rarer genetic causes of FTLD. This review will focus on recent advances in the brain imaging, neuropathology, cell biology and genetics of FTLD caused by CHMP2B mutations.
C-TERMINAL TRUNCATION MUTATIONS IN FAMILIAL FTD
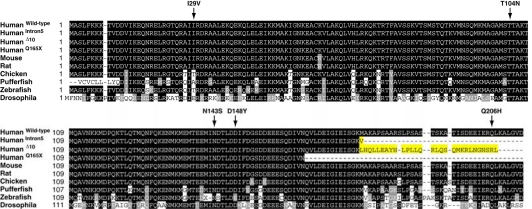
The first mutation in CHMP2B was identified in frontotemporal dementia linked to chromosome 3 (FTD-3) [20], an autosomal dominant FTD which occurs in one large kindred from Denmark [20, 21]. The mutation occurs in the splice acceptor site for the 6th and final CHMP2B exon, leading to the formation of two novel transcripts termed CHMP2BIntron5 and CHMP2BDelta10. Both transcripts are present in the brains of FTD-3 patients: CHMP2BIntron5 at ~35% and CHMP2BDelta10 at ~10% the level of the wildytpe CHMP2B transcript [22]. The resulting proteins both lose the final 36 amino acids, which are encoded by exon 6, replacing them with either a single valine residue (CHMP2BIntron5) or a 29 amino acid nonsense sequence (CHMP2BDelta10) Fig. (1). A second CHMP2B mutation was subsequently identified in an autosomal dominant Belgian FTLD pedigree [19]. This mutation changes a glutamine residue at position 165 of the CHMP2B protein sequence to a stop codon and is therefore termed CHMP2BQ165X. This leads to a protein which lacks the final 49 amino acids Fig. (1). Importantly, it therefore appears that the mutations have a common mechanism - the deletion of the C-terminus of the protein [23].
Fig. (1).
Multiple alignment of CHMP2B proteins. Wildtype CHMP2B and C-terminal truncations CHMP2BIntron5, CHMP2BDelta10 and CHMP2BQ165X were aligned with CHMP2B homologues from other species. The novel C-termini of CHMP2BIntron5 and CHMP2BDelta10 are highlighted in yellow. CHMP2B missense mutations identified in FTD-MND spectrum disorders are arrowed.
CLINICAL FINDINGS
The most comprehensive clinical picture of FTLD caused by CHMP2B mutation comes from the description of 22 FTD-3 patients [24] (of the 33 FTD-3 cases identified to date). Early personality change is the most common feature which can include less concern for others, an unkempt appearance, disinhibition, inappropriate emotional responses and restlessness which later can be accompanied by aggression. Apathy can also occur as the disease progresses. Hyperorality is also common, encompassing mouthing of non-food objects, over-eating of sweet foods and chain smoking. Early dyscalculia was observed in 8 of the 22 patients and was the presenting symptom in one case. Sterotyped behavioural routines were observed in 11 of the 22 patients. For example, one patient continuously walked round the outside of her house and another repeatedly turned objects upside down to inspect their bases. Apraxia is also common with patients unable to copy gestures. A progressive aphasia develops during the course of the disease, although normally not with features of PNFA or SD. It is characterised by reduced spontaneous speech with relative preservation of reading and repetition, most consistent with a dynamic aphasia. An overt motor syndrome develops late in the disease course which can include parkinsonian features, dystonia, pyramidal signs and myoclonus, ultimately leaving the patients bedridden. None of the patients have had any clinical signs of either upper or lower motor neuron impairment, and no neurophysiological EMG studies have been done so far in the Danish FTD-3 patients. A new branch of the FTD-3 family was recently identified and clinical descriptions of a further two cases were similar to those previously reported [21]. The average age of onset for FTD-3 is 58 years of age and the mean duration is 10 years. Ongoing neuropsychological studies indicate that some of the patients can have impaired memory function in the earlier phases of the disease, but this is not a prominent feature and most often not a subjective complaint by the patients.
The clinical picture of the Belgian CHMP2B mutation patient has some similarities to the FTD-3 cases including loss of decorum, mild disinhibtion and severe dyscalculia early in the disease course [19]. The presenting symptom was dysgraphia [19] which has not been reported in any FTD-3 case.
BRAIN IMAGING IN FTD-3
Structural Imaging
In patients with clinical FTD-3 disease computerised tomography (CT) scans have been performed in 9 cases [21, 24] and MRI has been reported in one case [24], with scans performed on 3 further patients (our unpublished data). At diagnosis the scans shows a generalised central and cortical atrophy. There is no anterior or lateral preponderance. The posterior central atrophy might be more pronounced although the number of patients studied has been limited [24]. MRI on one patient has shown some non-specific white matter hyper intensities on T2 and Flair Imaging, which were considered most likely not directly related to FTD-3. A generalised atrophy was observed which was most marked in the frontal parietal and occipital lobes [24].
MRI in presymptomatic CHMP2B mutation carriers compared to first degree relatives without the mutation indicated that a global [25] as well as focal cortical atrophy [26] can occur before onset of symptoms.
Two CT scans have been performed on the Belgian CHMP2B mutation positive case [19]. The first scan was performed 2 years after symptom onset and showed mild frontal atrophy. A second CT scan 6 years into the disease course showed a generalised atrophy.
Functional CBF Imaging in FTD-3
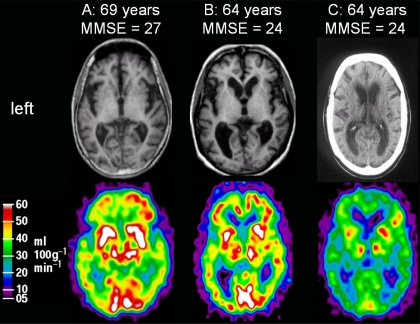
Measurements of regional cerebral blood flow (rCBF) with H215O positron emission tomography (PET) has been performed in 3 FTD-3 patients with MMSE ranging from 0-24 points [21, 24]. The rCBF was normal in the primary visual cortex, thalami, basal ganglia, cerebellum, and in the more mild patients also in a small area of the right lateral frontal cortex. The rCBF of all other cortical regions was severely impaired with the most prominent flow deficits in the parietal, anterior-frontal, and lateral temporal cortices. Representative MRI and PET scans are shown in Fig. (2).
Fig. (2).
Representative brain scans in FTD-3 patients. Upper row: structural scans in FTD-3 with MRI for patients A and B, and a CT-scan for patient C. Lower row shows cerebral blood flow as measured by with H215O -PET scanning for the same 3 patients.
NEUROPATHOLOGY
FTD-3 brains show a global cortical atrophy, which is most marked in the frontal cortex but also prominent in the temporal cortex; the parietal lobe can also be affected but the cerebellum is spared [24, 27]. There is no obvious atrophy of the hippocampus, amygdala, basal ganglia or substantia nigra [24, 27]. Cortical neuronal loss is accompanied by gliosis and microvacuolisation [24, 27]. Ubiquitin- and p62-positive neuronal cytoplasmic inclusions are observed in FTD-3 brains in the dentate granule cell layer of the hippocampus and to a lesser extent in the frontal cortex [27]. These inclusions are negative for both TDP-43 [27] and FUS [28], classifying FTD-3 as FTLD-UPS. We recently reported that two familial FTLD-UPS cases were negative for CHMP2B mutation [29], suggesting that although FTLD-UPS is a rare FTLD pathology it has more than one genetic cause.
The neuropathology of FTD-3 has been further refined by the discovery of enlarged vacuoles in cortical neurons [22]. The vacuoles were present in the frontal, temporal, parietal and occipital cortices, but not in the cerebellar cortex, a pattern generally reflecting the atrophy observed by neuropathology and brain imaging. The vacuoles were positive for a marker to the late endosome, suggesting they could be aberrant enlarged late endosomes. Enlarged endosomes have not been described in other FTLD cases to date, although enlarged early and late endosomes have been observed in cortical neurons in Down’s syndrome and Alzheimer’s disease brains [30-32].
FTD-3 brains have no abnormal aβ, α-synuclein, prion protein or neurofilament staining [27]. Some tau pathology has been observed, but at insufficient levels for a diagnosis of FTLD-tau. Tau positive inclusions were identified in the frontal cortex of three FTD-3 brains, but at levels that could be explained by normal aging [33]. Tau staining in further brain regions of the same cases, as well as one additional case, revealed neurofibrillary tangles, in the absence of amyloid deposits, in the hippocampus, entorhinal cortex and transentorhinal cortex consistent with Braak stage IV [27]. This distribution of neurofibrilliary tangles in the absence of amyloid pathology is typical of tangle predominant dementia [34, 35], but such cases are clinically distinct from FTD-3, presenting with an Alzheimer’s disease-like dementia with very late onset (80-90 years of age) [34, 35].
FUNCTIONAL STUDIES
CHMP2B is part of the multi-protein ESCRT-III complex (endosomal sorting complex required for transport-III). ESCRT-III has a role in two protein degradation pathways that converge on the lysosome: the endosome-lysosome pathway and autophagy. The CHMP2B C-terminal truncation mutants can impair both of these pathways and each will be discussed.
Endosome-Lysosome Pathway
The endosome-lysosome pathway is responsible for the degradation of endocytosed proteins such as transmembrane proteins and cell surface receptors. The proteins are endocytosed into early endosomes which mature into late endosomes and then fuse with lysosomes, allowing protein degradation to occur. Over-expression of CHMP2BIntron5, CHMP2BDelta10 or CHMP2BQ165X in human neuroblastoma cells leads to the formation of enlarged late endosomes [19], suggesting an impairment of this pathway. The relevance of this cell culture data was confirmed by the observation of aberrant enlarged late endosomes in fibroblasts from both FTD-3 patients and the Belgian CHMP2B mutation patient, as well as the identification of enlarged endosomes in cortical neurons in FTD-3 patient brain [22]. Further cell culture data indicates that this defect is due to the C-terminal CHMP2B mutants impairing the fusion of endosomes with lysosomes [22]. The late endosomes in CHMP2B mutant cells were impaired in their ability to recruit Rab7 [22], a key member of the endosome-lysosome fusion machinery [36]. RAB7 mutations have been identified in the rare motor and sensory neuropathy Charcot-Marie-Tooth type 2B [37, 38], suggesting altering Rab7 function can lead to neurodegenerative disease.
How impairment of endosome-lysosome fusion leads to disease is still not clear. The ESCRT complex mediates the lysosomal degradation of growth factor receptors [39] and neurotransmitter receptors [40, 41], both of which are essential for neuronal maintenance and function. CHMP2BIntron5 expression can also activate the Toll-like receptor pathway [42], which has been implicated in neurodegeneration [43]. Further research, including the development of mouse models, will be required to determine how these different pathways contribute to the neuronal cell death caused by CHMP2B mutation.
Autophagy
Autophagy is a cellular system that can degrade damaged organelles, long-lived proteins and protein aggregates [44]. The proteins or organelles to be degraded are encapsulated by autophagosomes, which either fuse directly with lysosomes, or first with endosomes forming a hybrid organelle termed an amphisome, which then fuses with the lysosome [45, 46]. Expression of CHMP2B C-terminal truncation mutants leads to an accumlation of autophagosomes, due to their impaired fusion with endosomes and/or lysosomes [47, 48]. Rab7 is also required for autophagosome-lysosome fusion [49], suggesting a similar mechanism could be responsible for impairing vesicular fusion events in both the autophagy and endosome-lysosome pathways. An accumulation of autophagosomes in affected neurons has been observed in Alzheimer’s disease [50, 51], Parkinson’s disease [52], and prion diseases [53-57], and deletion of key autophagy genes leads to neurodegeneration in mice [58, 59]. Deciphering the precise mechanism by which mutant CHMP2B affects autophagy may therefore have broad relevance for understanding neurodegenerative disease mechanisms.
MISSENSE MUTATIONS IN FTD-MND SPECTRUM DISORDERS
Five missense mutations Fig. (1) in eight individuals have been identified in CHMP2B in a range of FTD-MND spectrum disorders (Table 1). A recent study screened 433 MND patients for CHMP2B mutations and identified 3 distinct CHMP2B missense mutations in four patients. Interestingly, all 4 patients suffered from primary muscular atrophy (PMA), a form of motor neuron disease which predominantly affects the lower motor neurons [60]. 40 of the 433 MND cohort were PMA variant cases suggesting that CHMP2B mutations could account for 10% of such cases. A previous study found no CHMP2B mutations in 538 MND cases [61], although it is unclear how many PMA cases were present in this cohort. Two of the four PMA cases had the same mutation, I29V, which has previously been identified in one case of FTD-MND [62] one case of FTD [63], but also in one control [64]. The final two PMA mutations, T104N and Q206H [60], have not been described in any other FTD or MND case. A further two missense mutations have been described, N143S, in a case of corticobasal degeneration [19], and D148Y in a case of semantic dementia [20].
Table 1.
Cases Identified with CHMP2B Missense Mutations.
| DNA Change | Protein Change | Disease | Family History | Controls Analysed in Study | Reference |
|---|---|---|---|---|---|
| c.85A>G | I29V | FTD | Y | 190 | [63] |
| c.85A>G | I29V | FTD-MND | Possible | 640 | [62] |
| c.85A>G | I29V | PMA | N | 500 | [60] |
| c.85A>G | I29V | PMA | N | 500 | [60] |
| c.311C>A | T104N | PMA | Y | 500 | [60] |
| c.428A>G | N143S | CBD | Y | 459 | [19] |
| c.442G>T | D148Y | SD | N | 100 | [20] |
| c.618A>C | Q206H | PMA | N | 500 | [60] |
FTD-MND – Frontotemporal Dementia with Motor Neuron Disease. PMA – Primary Muscular Atrophy. CBD – Corticobasal Degeneration. SD – Semantic Dementia.
Three of the eight missense mutation cases have a family history (Table 1) but unfortunately samples of family members were not available to confirm segregation of the mutations. In order to confirm pathogenicity of the missense mutations it will be important to show that they segregate with disease in familial cases. Further clarification is also needed on the penetrance of the I29V mutation and the pathogenic mechanism of the missense mutations.
It is also intriguing that the PMA missense cases have TDP-43 pathology [60], whereas FTD-3 cases do not [27]. This could be explained by the missense mutations having a different effect to the gain of function C-terminal truncation mutations. Indirect evidence to support this possibility comes from an experiment where loss of function of the ESCRT-III complex due to knockdown of CHMP3 (also termed VPS24) led to the formation of cytoplasmic TDP-43 aggregates in cell culture, whereas expression of CHMP2BIntron5 did not [47]. The generation of knockout and missense mouse models may shed further light on this issue. In summary, further work is required to fully assess the role and importance of CHMP2B missense mutations in FTD and MND.
CONCLUSIONS
The early clinical picture of FTD caused by CHMP2B mutations is similar to other behavioural variant FTD cases while the brain imaging and neuropathological findings are somewhat distinct. Despite being a rare cause of dementia, recent advances in our understanding of the molecular basis of CHMP2B mutations indicate that the mechanisms involved may be broadly relevant to neurodegenerative processes.
ACKNOWLEDGEMENTS
Our work is funded by the UK Medical Research Council and Danish MRC grant 22-04-0458.
ABBREVIATIONS
- CBD
= Corticobasal degeneration
- CHMP2B
= Charged multivesicular body protein 2B
- CT
= Computerised tomography
- ESCRT-III
= Endosmal sorting complex required for transport-III
- FTD-3
= Frontotemporal dementia linked to chromosome three
- FTLD
= Frontotemporal lobar degeneration
- GRN
= Progranulin
- Vps24
= Vacuolar protein for sorting 24
- MAPT
= Microtubule associated protein tau
- MND
= Motor neuron disease
- MRI
= Magnetic resonance imaging
- PET
= Positron emission tomography
- PMA
= Primary muscular atrophy
- PNFA
= Progressive non-fluent aphasia
- SD
= Semantic dementia
- TDP-43
= TAR DNA binding protein-43
REFERENCES
- 1.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 3.Lillo P, Hodges JR. Frontotemporal dementia and motor neurone disease: overlapping clinic-pathological disorders. J Clin Neurosci. 2009;16:1131–1135. doi: 10.1016/j.jocn.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 6.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 7.Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dement Geriatr Cogn Disord. 2004;17:282–286. doi: 10.1159/000077155. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seelaar H, Klijnsma KY, de KI, van der LA, Chiu WZ, Azmani A, et al. Frequency of ubiquitin and FUS-positive. TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;25(5):747–53. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;19:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;42:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 13.Cruts M, Gijselinck I, van der ZJ, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 14.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 15.Benajiba L, Le B I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–473. doi: 10.1002/ana.21612. [DOI] [PubMed] [Google Scholar]
- 16.Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–E983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 17.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 18.Van Langenhove T, van der ZJ, Sleegers K, Engelborghs S, Vandenberghe R, Gijselinck I, et al. Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology. 2010;74:366–371. doi: 10.1212/WNL.0b013e3181ccc732. [DOI] [PubMed] [Google Scholar]
- 19.van der Zee J, Urwin H, Engelborghs S, Bruyland M, Vandenberghe R, Dermaut B, et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum Mol Genet. 2008;17:313–322. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- 20.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist SG, Braedgaard H, Svenstrup K, Isaacs AM, Nielsen JE. Frontotemporal dementia linked to chromosome 3 (FTD-3)--current concepts and the detection of a previously unknown branch of the Danish FTD-3 family. Eur J Neurol. 2008;15:667–670. doi: 10.1111/j.1468-1331.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 22.Urwin H, Authier A, Nielsen JE, Metcalf D, Powell C, Froud K, et al. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum Mol Genet. 2010;19:2228–38. doi: 10.1093/hmg/ddq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urwin H, Ghazi-Noori S, Collinge J, Isaacs A. The role of CHMP2B in frontotemporal dementia. Biochem Soc Trans. 2009;37:208–212. doi: 10.1042/BST0370208. [DOI] [PubMed] [Google Scholar]
- 24.Gydesen S, Brown JM, Brun A, Chakrabarti L, Gade A, Johannsen P, et al. Chromosome 3 linked frontotemporal dementia (FTD-3) Neurology. 2002;59:1585–1594. doi: 10.1212/01.wnl.0000034763.54161.1f. [DOI] [PubMed] [Google Scholar]
- 25.Rohrer JD, Ahsan RL, Isaacs AM, Nielsen JE, Ostergaard L, Scahill R, et al. Presymptomatic generalized brain atrophy in frontotemporal dementia caused by CHMP2B mutation. Dement Geriatr Cogn Disord. 2009;27:182–186. doi: 10.1159/000200466. [DOI] [PubMed] [Google Scholar]
- 26.Eskildsen SF, Ostergaard LR, Rodell AB, Ostergaard L, Nielsen JE, Isaacs AM, et al. Cortical volumes and atrophy rates in FTD-3 CHMP2B mutation carriers and related non-carriers. Neuroimage. 2009;45:713–721. doi: 10.1016/j.neuroimage.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Holm IE, Englund E, Mackenzie IR, Johannsen P, Isaacs AM. A Reassessment of the Neuropathology of Frontotemporal Dementia Linked to Chromosome 3. J Neuropathol Exp Neurol. 2007;66:884–891. doi: 10.1097/nen.0b013e3181567f02. [DOI] [PubMed] [Google Scholar]
- 28.Holm IE, Isaacs AM, Mackenzie IR. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol. 2009;118:719–720. doi: 10.1007/s00401-009-0593-1. [DOI] [PubMed] [Google Scholar]
- 29.Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neurophathol. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cataldo AM, Mathews PM, Boiteau AB, Hassinger LC, Peterhoff CM, Jiang Y, et al. Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects. Am J Pathol. 2008;173:370–384. doi: 10.2353/ajpath.2008.071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer's disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancopoulou D, Crowther RA, Chakrabarti L, Gydesen S, Brown JM, Spillantini MG. Tau protein in frontotemporal dementia linked to chromosome 3 (FTD-3) J Neuropathol Exp Neurol. 2003;62:878–882. doi: 10.1093/jnen/62.8.878. [DOI] [PubMed] [Google Scholar]
- 34.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology. 2003;23:311–317. doi: 10.1046/j.1440-1789.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 36.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houlden H, King RH, Muddle JR, Warner TT, Reilly MM, Orrell RW, et al. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 38.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 40.Kantamneni S, Holman D, Wilkinson KA, Nishimune A, Henley JM. GISP increases neurotransmitter receptor stability by down-regulating ESCRT-mediated lysosomal degradation. Neurosci Lett. 2009;452:106–110. doi: 10.1016/j.neulet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantamneni S, Holman D, Wilkinson KA, Correa SA, Feligioni M, Ogden S, et al. GISP binding to TSG101 increases GABA receptor stability by down-regulating ESCRT-mediated lysosomal degradation. J Neurochem. 2008;107:86–95. doi: 10.1111/j.1471-4159.2008.05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad ST, Sweeney ST, Lee JA, Sweeney NT, Gao FB. Genetic screen identifies serpin5 as a regulator of the toll pathway and CHMP2B toxicity associated with frontotemporal dementia. Proc Natl Acad Sci USA. 2009;106:12168–12173. doi: 10.1073/pnas.0903134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun. 1988;151:40–47. doi: 10.1016/0006-291x(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 46.Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, Fisher EM, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol. 2007;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 50.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 51.Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 53.Boellaard JW, Schlote W, Tateishi J. Neuronal autophagy in experimental Creutzfeldt-Jakob's disease. Acta Neuropathol (Berl) 1989;78:410–418. doi: 10.1007/BF00688178. [DOI] [PubMed] [Google Scholar]
- 54.Boellaard JW, Kao M, Schlote W, Diringer H. Neuronal autophagy in experimental scrapie. Acta Neuropathol (Berl) 1991;82:225–228. doi: 10.1007/BF00294449. [DOI] [PubMed] [Google Scholar]
- 55.Sikorska B, Liberski PP, Giraud P, Kopp N, Brown P. Autophagy is a part of ultrastructural synaptic pathology in Creutzfeldt-Jakob disease: a brain biopsy study. Int J Biochem Cell Biol. 2004;36:2563–2573. doi: 10.1016/j.biocel.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Sikorska B, Liberski PP, Brown P. Neuronal autophagy and aggresomes constitute a consistent part of neurodegeneration in experimental scrapie. Folia Neuropathol. 2007;45:170–178. [PubMed] [Google Scholar]
- 57.Liberski PP, Yanagihara R, Wells GA, Gibbs CJ, Jr, Gajdusek DC. Comparative ultrastructural neuropathology of naturally occurring bovine spongiform encephalopathy and experimentally induced scrapie and Creutzfeldt-Jakob disease. J Comp Pathol. 1992;106:361–381. doi: 10.1016/0021-9975(92)90022-m. [DOI] [PubMed] [Google Scholar]
- 58.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 59.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 60.Cox LE, Ferraiuolo L, Goodall EF, Heath PR, Higginbottom A, Mortiboys H, et al. Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS) PLoS One. 2010;5:e9872. doi: 10.1371/journal.pone.0009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair IP, Vance C, Durnall JC, Williams KL, Thoeng A, Shaw CE, et al. CHMP2B mutations are not a common cause of familial or sporadic amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:849–850. doi: 10.1136/jnnp.2007.140541. [DOI] [PubMed] [Google Scholar]
- 62.Parkinson N, Ince PG, Smith MO, Highley R, Skibinski G, Andersen PM, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- 63.Rizzu P, van Mil SE, Anar B, Rosso SM, Kaat LD, Heutink P, et al. CHMP2B mutations are not a cause of dementia in Dutch patients with familial and sporadic frontotemporal dementia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:944–946. doi: 10.1002/ajmg.b.30410. [DOI] [PubMed] [Google Scholar]
- 64.Cannon A, Baker M, Boeve B, Josephs K, Knopman D, Petersen R, et al. CHMP2B mutations are not a common cause of frontotemporal lobar degeneration. Neurosci Lett. 2006;398:83–84. doi: 10.1016/j.neulet.2005.12.056. [DOI] [PubMed] [Google Scholar]