Abstract
There have been major changes since the incidents of leukemia development in X-SCID patients after the treatments using retroviral gene therapy. Due to the risk of oncogenesis caused by retroviral insertional activation of host genes, most of the efforts focused on the lentiviral therapies. However, a relative clonal dominance was detected in a patient with β-thalassemia Major, two years after the subject received genetically modified hematopoietic stem cells using lentiviral vectors. This disappointing result of the recent clinical trial using lentiviral vector tells us that the current and most advanced vector systems does not have enough safety. In this review, various safety features that have been tried for the retroviral gene therapy are introduced and the possible new ways of improvements are discussed. Additional feature of chromatin insulators, co-transduction of a suicidal gene under the control of an inducible promoter, conditional expression of the transgene only in appropriate target cells, targeted transduction, cell type-specific expression, targeted local administration, splitting of the viral genome, and site specific insertion of retroviral vector are discussed here.
Keywords: Retroviral, Gene, Therapy, Safety, Insertional, Targeted, Vectors, Insulator.
INTRODUCTION
Retroviral vectors have been the most preferred gene transfer systems in clinical gene therapy until the incident of a human trial for the X-linked severe combined immunodeficiency (SCID) [1-3]. The potential risk of insertional oncogenesis was realized in the trial, infants with X-SCID were cured by retrovirus-mediated ex-vivo gene transfer, and the trial was credited as the first unequivocal success for gene therapy [4]. However, four out of nine successfully treated patients later developed leukemia, and it is generally believed that leukemeogenesis was triggered by unexpected activation of a cellular proto-oncogene as a result of retroviral integration. Since our last review about the safety issues related to the use of retroviral vectors in human clinical trials, additional leukemeogenesis were reported [5-7]. It is time to re-evaluate all the safety issues concerning the use of retroviral vectors.
Stable incorporation of retroviral viral DNA into the host genome is in itself advantageous, as long-term expression of the transgene is possible for achieving therapeutic efficacy. However, a non-specific incorporation of viral DNA throughout the host genome can either cause a disruption of a host gene at the site of incorporation or cause an abnormal expression of nearby host genes driven by the enhancer of the inserted viral DNA. An insertional interference occurs against a host gene involved in a critical cellular function such as cell cycle progression, can become a major cause of cell transformation and oncogenesis, especially in the presence of additional physiological or genetic insults.
In addition to the risk of insertional mutagenesis, another safety issue with the retroviral vector is the possibility of generating replication competent retroviruses (RCR). Although new generations of retroviral vectors are designed to reduce the production of RCR, additional efforts are required to ensure the complete elimination of the problem.
Recently developed lentiviral gene transfer systems share many features of the retroviral systems. The viral genome integrates into host chromosomes, and inserted genes are maintained in the cells permanently. In contrast to conventional retroviral vectors, which require cell division for infection, lentiviral vectors infect efficiently non-dividing cells as well as dividing cells. Therefore, lentiviral vectors can be applied for transgene expression in neuronal cells. Most of the lentiviral vectors used in gene therapy are based on the human immunodeficiency virus (HIV). The major limitations of using HIV-originated lentiviral vectors in clinical trial are the safety concerns related to their HIV origin. Recent trials of lentiviral vector systems will be described in relevant chapters.
In this review, we will discuss about safety issues relating to the use of retroviral vectors for the therapeutic gene delivery. Suggestions for the possible solutions to these issues and future directions for an overall increase in the safety and efficiency of retroviral gene therapy protocols will be provided. Other important parameters in cell-based ex-vivo retroviral gene therapy protocols are already covered elsewhere [8], including cell type and numbers, observation period, proliferation capacity, age of patients, immunity, and side effects of transgene expression.
TARGETED RETROVIRAL TRANSDUCTION
Current retroviral transfer system lacks specificity for target cell types. Non-specific infection hinders the application of retroviral system for the gene transfer to particular cell types in a mixed cell population. Tissue targeting is highly desirable and is expected to be valuable for various in vivo gene therapy protocols [9].
Infection of target cells by retroviruses is initiated by binding of the viral envelope protein to cell surface receptors [10]. Infusion of viral and cellular membranes leads to the internalization of the viral core [11]. In the past years, various retrovirus receptors, coreceptors and cofactors have been identified and studied for their role in viral entry [12], and attempts have been made to engineer viral envelope proteins and cellular receptors for attaining changes in the viral tropism [13, 14].
In an approach for a targeted transduction, envelope protein modification by attaching a peptide ligand such as epidermal growth factor (EGF) receptor binding domain to the NH2-terminus of the envelope glycoprotein (SU) was attempted [15-17]. Incorporation of the chimeric envelope protein into the viral particle allows binding of retrovirus to the receptor-positive target cells. The subsequent viral entry steps are blocked, however, because EGF receptors do not support these processes. As a protease cleavable linker was used in this chimeric protein to join the peptide ligand and SU, an appropriate cellular protease can cleave the attached ligand. Factor Xa [18, 19], plasmin [17], matrix-metallo-proteases (MMPs) [16], or intracellular protein convertases [20] were used for this purpose. Because overexpression of MMPs is frequently associated with angiogenesis, inflammation, and cancer invasion, MMPs are considered to be interesting targets for the protease-activatable gene delivery systems. Using MMP-activatable retroviral vectors, selective transduction of MMP-rich tumor cells was achieved in a heterogeneous cell population, but with somewhat reduced efficiency of transduction [17, 21]. Similarly, targeted infection for the high-molecular-weight melanoma-associated antigen (HMWMAA) expressing tumors was achieved by fusing a single chain antibody recognizing HMWMAA to the amino terminus of the surface domain of MLV with a matrix metalloprotease-2 (MMP2) cleavage site linker [22].
Matrix targeting is another approach and matrix-targeted retroviral vectors were found to be more efficient than unmodified vectors [23, 24]. A matrix-targeted retroviral vector was constructed by attaching collagen-binding polypeptide sequence to the amino-terminal region of the amphotropic 4070A envelope protein. Because tumor development and accompanied angiogenesis is associated with remodeling of extracellular matrix components, these vectors accumulate at sites of tumor development with newly exposed collagens. When the matrix-targeted retroviral vector expressing dominant mutant cyclin G1 was administered by portal vein infusion, vector particles accumulated in the angiogenic tumor vasculature within 1 hour of infusion. These vectors, Rexin-G, transduced tumor cells with high efficiency and reduced the volume of tumor [23]. In clinical studies, Rexin-G showed significant anti-tumor activities in breast, colon, lung, skin, muscle, pancreatic, and bone cancers [25, 26]. Rexin-G was granted Orphan Drug Status by the US FDA in 2008.
Targeting retroviral delivery to quiescent interleukin-2 (IL-2)-dependent cells was also reported [27]. In this report, chimeric amphotropic MLV envelope glycoprotein fused with IL-2 was used for a direct binding of the viral particles to the IL-2 receptors expressed on G0/G1 arrested cells, resulting in a transient stimulation of cell proliferation. Subsequent viral entry was mediated by unmodified envelope proteins co-expressed on the same virus particles. A 34-fold increase in transduction efficiency was observed with this method. Additionally, targeting efforts for T cells [28], and other cancer cells [29, 30] were reported. For the specific transduction of HIV-envelope expressing cells, envelope pseudotyping was used to create hybrid CD4/CXCR4 receptors for MLV retrovirus [31, 32] and lentivirus [31, 32], In order to improve transduction efficiency frequently observed to be low for the targeted retroviral vectors, binding defective but fusion competent hemagglutinin (HA) protein has also been tried [33].
LOCAL DELIVERY
If local delivery of retroviral vectors is available for an effective treatment of a disease, it will be generally safer than systemic delivery in terms of toxicology and long term side effects. A number of studies have shown the efficacy and safety of locally delivered retroviral vectors. A retroviral vector expressing antiproliferative dominant negative mutant cyclin G1 (dnG1) was successfully used for the prevention of eximer laser-induced corneal haze [34]. Biodistribution study after the treatment of surgically induced rabbits with eye drops containing dnG1 retroviral vectors showed no evidence of vector dissemination in non-target organs. Localized delivery of lentiviral vectors into the substantia nigra of adult rats has also been tried [35]. In a phase I clinical trial for direct intratumoral injection of interferon-γ retroviral vectors in advanced melanoma patients, viral injection was well tolerated and no toxicity was reported [36]. This suggests that the direct injection approach is feasible for treating solid tumors with retroviral vectors.
In terms of potential problems associated with concomitant transduction of surrounding non-target cells, ex vivo cell-based gene therapy with local delivery can be a better choice, if extra time and expenses are tolerated. As an example, an ex vivo cell-mediated gene therapy has been performed successfully for the treatment of artificially induced hyaline cartilage damage in animals, by injecting TGF-β1-retrovirus transduced fibroblasts into the knee joints [37, 38]. Cancer regressions were reported after the transfer of genetically engineered lymphocytes [39]. Autologous lymphocytes from peripheral blood using retroviruses that encode T cell receptors to specific tumor-associated antigens were transferred to patients. Transduced normal peripheral blood cells were converted into cells which specifically recognized and destroyed cells from corresponding cancers.
INTEGRATION OF RETROVIRUS INTO THE HOST CHROMOSOME
In the ex-vivo gene therapy trials to treat the rare immune deficiency disorder X-SCID, patients were treated with autologous hematopoietic stem cells transduced with a recombinant retrovirus expressing the common gamma chain (γc) of interleukin receptor [4]. Although, nine out of eleven treated children showed dramatic improvements with almost fully restored immune systems, four of the nine cured patients developed leukemia (T-cell acute lymphoblastic leukemia; T-ALL) between 3 and 6 years after the treatment in France. A copy of the vector DNA was found in the first intron of the growth-promoting LMO2 gene of the leukemic clones in patient 4, and approximately 3 kb upstream of the first exon of the same gene in patient 5 [1-3]. In patient 10, there was another insertion near the proto-oncogene BMI1 in addition to the first insertion near LMO2. In patient 7, blast cells showed an insertion near a third proto-oncogene CCND2 [40]. Another case of leukemogenesis was reported in a separate X-SCID study conducted in United Kingdom [6]. In addition to the integration of vector in the upstream of LMO2 gene, gain-of-function mutation in NOTCH1, deletion of tumor suppressor gene locus cyclin-dependent kinase 2A (CDKN2A), and translocation of the TCR-b region to the STIL-TAL1 locus were found. Two cases of myelodysplasia were reported in a clinical trial for X-linked chronic granulomatous disease (X-CGD) [7]. Both patients showed an insertional activation of ecotropic viral integration site 1 (EVI1) and monosomy 7. LMO2 is a LIM domain transcription regulator involved in hematopoiesis [41] and is reported to be activated in T cell leukemia by chromosomal translocation [42]. LMO2 is suggested to reactivate a hematopoietic stem cell (HSC) specific transcriptional program [43]. Long-term thymocyte self-renewal due to the over-expression of LMO2 could be the cause of T cell leukemia. The BMI1 gene was known to regulate stem cell proliferation [44, 45]. MMI1 is suspected to contribute the leukemic cell proliferation with LMO2. Massive and sustained expression of CCND2 was detected in the CCND2-rearranged T-ALL, compared to the down-regulation during the progression from the early stages of normal human T-cell [46]. Over-expression of CCND2 in one of the patients could be one of the oncogenic transition mechanisms to T-ALL.
Current U.S. Food and Drug Administration (FDA) perspective on X-SCID clinical trials is that gamma-retroviral vectors can be used in clinical trials to treat X-SCID under the following conditions: when previous hematopoietic stem cell/bone marrow transplantation is failed or there is no reasonable alternative therapy [47]. Although, clinical trials are allowed to proceed for other clinical indications, investigators and patients are required to be informed with strong and clear communication of risks.
The general consensus of the experts of the review boards in the US and other countries is that the benefits might outweigh the risks in most SCID-related retroviral gene therapy trials. In a Recombinant DNA Advisory Committee (RAC) meeting held on March 14, 2007, it is recommended for all integrating vectors to test for vector sequences every 6 months first 5 years and test yearly next ten years or until no vector is detected. When at least 1% of surrogate cells have detectable vector, pattern of vector integration site should be assessed. In case persistent monoclonality or vector integration near or within locus known to have oncogenic activity, additional monitoring is recommended.
TARGETING RETROVIRAL INTEGRATION; MLV VS HIV
After entering the host cell, a single-stranded retroviral RNA genome is released into the cytoplasm and converted into a double-stranded DNA by virus-encoded reverse transcriptase. The viral DNA then forms a large nucleoprotein structure, termed pre-integration complex, containing proteins necessary for nuclear localization and insertion of viral DNA into the host genome. Although the protein components and the exact mechanism of action of the complex is still not completely understood, it has been demonstrated that viral integrase (IN) catalyzes the key DNA cutting and joining reactions for inserting viral DNA into the host genome [48, 49].
Retroviral integration is not a completely random process but favors promoters and enhancer regions while lentiviral vectors integrate more randomly throughout the entire gene [50, 51]. Due to the differences of insertional preferences between two vectors, it was considered that HIV based gene therapy is safer than MLV based one. However, recent result from a gene transfer study of β-thalassemia Major and Sickle Cell anemia conducted in France suggests a different story [52]. Office of Biotechnology Activities of National Institutes of Health (NIH) published a letter in June, 2009 that a relative clonal dominance was detected in a subject with β-thalassemia Major, two years after the treatment. The vector used in the study was a self-inactivating (SIN) HIV-1 derived lentivirus which contains the gene for β-globin under the control of the β-globin promoter. Clonal populations share an integration site in HMGA2 gene. This incident raised a question about whether the use of lentiviral and modified SIN retroviral vectors containing insulators can decrease the risk of insertional mutagenesis in hematopoietic stem cells.
There are controversial reports about the function of viral proteins including integrase. Swapping the integrase between closely related viruses showed a change in the integration pattern [53, 54]. However, changing of gag, env, and pol genes of MLV with those from subgroup C Feline Leukemia Virus (FeLV-C) did not alter the basic integration profile [55]. Elucidating the mechanisms of integration and establishing the database for preferred integration sites could permit a better prediction of the integration sites of retroviral vectors. This may eventually lead to the development of retroviral vectors capable of integration site selection in the host cell chromosome, providing the ultimate solution to the problems of insertional mutagenesis.
There have been attempts to target retroviral integration to pre-selected locations of the host genome by fusing viral integrase with sequence-specific DNA binding domains obtained from phage lambda repressor, bacterial LexA, or a zinc finger protein zif268 [56-58]. However, these trials show only a limited success, as the specificity of integration was only partially altered. In a separate experiment, bovine leukemia virus (BLV) integrase was used for site-specific integration of naked DNA to the pre-integrated integrase recognition sequence of mouse genome [59]. Similarly, site-specific integration of naked DNA into human chromosome 8 has been attempted with limited success using modified phage φC-31 integrase [60]. In this study, enhanced sequence specificity and increased integrase efficiency was achieved through a directed evolution strategy. It is clear that concentrated efforts are required in defining the precise mechanism of action of the retroviral pre-integration complex and in designing modified integrases with sequence-specific integration capability. The latter may be accomplished either by rational modification of the protein or by using the directed evolution approach [61]. One example of rational modification is fusing integrase with synthetic zinc finger motifs with defined sequence specificities [58, 62, 63]. Directed evolution utilizes error-prone PCR-driven mutagenesis, recombination, or DNA shuffling, combined with a high throughput screening for the selection of modified proteins with significantly improved function. The newly developed integrases should also maintain the ability to form a pre-integration complex with a high-level of infection capability.
Generation of integration deficient lentiviral vectors was also reported [64, 65]. The vector showed durable transcription of transgenes in certain mitotic cell lineages, but non-integrated viruses were lost during cell division.
INSULATORS TO PREVENT POSITIONAL EFFECTS AND INSERTIONAL ONCOGENE ACTIVATION
Retroviruses are often susceptible to positional effects and transcriptional silencing depending on the site of integration in the chromosome [66]. In order to overcome positional silencing effect, chromatin insulators have been used in retroviral vectors. Chromatin insulators are believed to form expression boundaries [67, 68] and can block positive and negative positional effects at the site of integration when they flank a transgene [69-71]. They prevent interferences between promoters and enhancers of adjacent genes [72]. As an example, when a 1.2 kb chromatin insulator obtained from the chicken β-globin locus control region hypersensitive site 4 (cHS4) was inserted in the retrovirus 3’ LTR, protection of the positional effects was observed either from transduced cultured cells and from mice transplanted with transduced marrow cells [73]. Similarly, cHS4 insulator used with gamma-globin expression cassette increased the likelihood of stable gamma-globin expression nearly 10-fold, allowing for the expression at the therapeutic range for treating sickle cell anemia and beta thalassemia in mouse bone marrow transplantation models [74]. A part of full length cHS4 (650 bp) was confirmed to work for the practical purpose [75]. Insertion of a cHS4 in SIN lentiviral vectors resulted in higher and less variable expression of human ß-globin [76, 77].
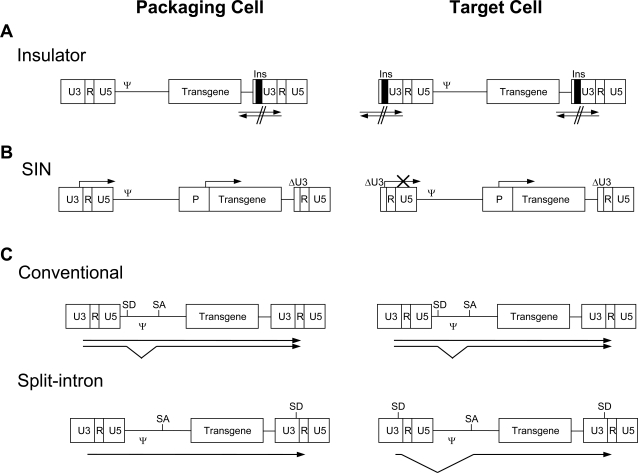
Because sequences in the 3’ LTR of retroviral vectors are copied to the 5’ LTR during the processing of viral genome into the provirus, if an insulator is inserted in the 3’ LTR of the recombinant vector, a barrier of insulators will be formed surrounding the transgene Fig. (1A). In addition, insulators are inserted in the place of the U3 region of the 3’ LTR, which is the viral enhancer region. These vectors will become SIN in the proviral form, as there is no viral enhancer required to produce replication competent retrovirus. Therefore, insulator containing retroviral vectors will be less prone to silencing of the transgene expression as a result of chromosome positional effect [73], and at the same time, will have less chance of causing aberrant induction of host genes near the site of incorporation as it has no viral enhancer. Boundaries formed by insulators will also prevent the influence of an internal heterologous enhancer used to drive transgene expression on the transcription of nearby host genes, although it has yet to be experimentally proven.
Fig. (1).
Designs of the improved retroviral vectors. (A) Insulator located in U3 of 3’LTR is copied to 5’LTR in target cell. Insulators located in the both ends of vector DNA blocks the cross activation between retroviral DNA and the chromosomal DNA. (B) Due to the duplication of U3-deleted LTR of the SIN vector in target cell, the proviral form does not contain viral enhancer any more, and thus require an internal promoter for the expression of the transgene. Arrows show the transcription start. P, internal promoter; ΔU3, deletion of U3 sequence. (C) Comparison of splicing in conventional and split-intron vectors. Arrows show transcripts from the vector DNA. SD, Splice Donor site; SA, Splice Acceptor site.
Although retroviral insertion can cause either a disruption or an abnormal activation of host genes, the latter is a primary concern in terms of oncogenesis. This is because, in most cases, retroviral insertion will occur in only one allele of the host genome leaving the other locus intact, and insertional disruption of the host gene will become problematic only in rare cases where haplo-insufficiency is phenotypically relevant for oncogenic transformation. Thus, although chromatin insulators cannot prevent host gene disruption by retroviral insertion, the benefits associated with the use of insulators preventing unwanted activation of host genes will be rather significant.
The efficiency of insulator function is, however, dependent on several factors including topological constraints, cell types, and the state of cell differentiation [78, 79]. Also the size limitations of retroviral vectors should be considered. A 265 bp sea urchin insulator termed sns (silencing nucleoprotein structure) was found to be effective for insulator function in human cells, and thus may be useful in retroviral vectors [80, 81]. In a recent report, anti-repressor elements were identified by screening a library of human genomic DNA fragments between 500 and 2,000 bp, based on their ability to relieve LexA-dependent transcription repression [82]. These elements can confer high and stable transgene expression in mammalian cells when they were used to flank the transgene, suggesting that they play a similar role as insulators.
Scaffold (or matrix) attachment region (SAR) is another DNA sequence element believed to play an important role in defining boundaries of independent chromatin domains [83, 84]. SARs bind to the nuclear scaffold or nuclear matrix with high affinity and are proposed to form chromosomal loops [85]. SARs have been used in retroviral vectors with an enhancement of transgene expression in several different cell types [86, 87]. A report shows that a high-level transgene expression can be achieved from a SIN lentiviral vector containing both the human interferon-beta scaffold attachment region and the chicken β-globin insulator [88]. The proviral form of this vector does not contain HIV-1 U3 region transcriptional regulatory elements and is flanked by the enhancer-blocking β-globin insulators. These observations indicate that the usage of SARs in addition to insulators could significantly improve transgene expression and lower the risk of uncontrolled activation of cellular proto-oncogenes at or near the site of incorporation.
Activation of proto-oncogene may also arise due to the retroviral RNA processing. A strong internal splice acceptor (SA) is recommended after the splice donor (SD) of retroviral vector to reduce the positional effects of inserted retroviral RNA processing on the expression of the transgene as well as the disrupted host gene. Combination of a strong SA, deleting cryptic SD in the transgene [89], using an improved polyadenylation signal [90], the removal of LTR promoter, and the insulator will largely prevent the interactions of the retroviral splice donor with downstream chromosome sequences.
TRANSCRIPTIONAL TARGETING
Transcriptional targeting using cell type-specific promoters and enhancers can be applied either alone or in combination with targeted transduction to minimize the expression of transduced genes in non-target cells and thereby reducingpotential side effects. Table 2 summarizes examples of cell type-specific promoters and enhancers used for transcriptional targeting in retroviral gene therapy. Promoters of oncogenes overexpressed in the tumor cells can be the targets for tumor specific promoters (e.g. c-erbB2 and c-myc). Tyrosinase promoter was used for the expression of HSV-tk or IL-2 for the treatment of malignant melanomas [91]. Tyrosinase is rate-limiting enzyme for melanin production, which is highly expressed in melanomas. In addition to cell type-specific promoters, inducible or regulatable expression systems can also be used for safety and efficacy. In the case for mammary tumor and prostate cancer, the growth of tumor is hormone dependent. Therefore, using a combined steroid hormone-responsive and cell type specific promoters is an attractive approach for retroviral gene therapy [92]. Additionally, genes that are induced by cancer therapies such as gamma-irradiation or chemotherapy can also be the targets for the regulatory elements [93, 94].
Table 2.
Cell Type-Specific Promoters and Enhancers for Transcriptional Targeting in Retroviral Gene Therapy
| Promoter | Target Cell/Tissue | Transgene | References |
|---|---|---|---|
| PEPCK promoter | Hepatocyte | Neo, bovine growth hormone | [99] |
| hAAT promoter | Hepatocyte | Alpha I antitrypsin | [100] |
| MMTV-LTR | Mammary gland | TNF-α | [92] |
| MCK promoter | Muscle | β-galactosidase, dystrophin minigene | [101] |
| AFP promoter | Cancer: Hepatocellular carcinomas | HSV-tk, VZV-tk | [102] |
| Tyrosine promoter | Cancer: Melanomas | HSV-tk, IL-2 | [91] |
| Col1a1 promoter | Bone | β-geo (β-gal, neo fusion) | [103] |
| HSP70 promoter | Cancer | Dominant negative IGF-IR | [104] |
| WAP promoter | Cancer: Mammary | β-galactosidase | [105] |
| ppET1 promoter | Cancer: Endothelium | β-galactosidase | [106] |
| AFP enhancer; PGK promoter | Cancer: Hepatocellular carcinomas | HSV-tk | [96] |
| HRE, PGK-1 enhancer; E-selectin, KDR promoter | Cancer: Endothelium | TNF-α, luciferase | [95] |
| HRE enhancer; AFP promoter | Cancer: Hepatocellular carcinomas | HSV-tk, luciferase | [107] |
| Rat alpha-fetoprotein | Human hepatocarcinoma cell | HSV-tk, luciferase | [97] |
| HS2 of erythroid-specific GATA-1 gene; HIV-1 promoter | Mature erythroblasts | GFP | [98] |
Although a number of cell type specific promoters were tested in many trials, the overall efficiency of transcription achieved from cell type specific promoters is relatively weak compared to viral promoters generally used. In a report, hypoxic and cytokine-inducible enhancers, both of which are active in some tumor environments, are combined with endothelial cell-specific E-selectin and VEGF receptor 2 promoters [95] to achieve a maximum possible tumor endothelium-specific transcription. In another report, human α-fetoprotein (AFP) enhancer was combined with a housekeeping gene phosphoglycerate kinase-1 (PGK-1) promoter, to augment the activity of the weak tumor-selective AFP promoter [96].
Rat alpha-fetoprotein promoter was used as a cell type-specific promoter for a lentivirally transduced expression in human hepatocarcinoma cells [97]. Replacement of U3 region of the lentiviral LTR with an upstream enhancer (HS2) of the erythroid-specific GATA-1 gene and HIV-1 promoter showed a high level of transgene expression specifically in mature erythroblasts [98].
In many cases, the size constraints of retroviral vector limit the use of enhancers, which are generally long in size. Therefore, construction of minimum enhancer/promoter cassettes with strategic combinations of different sequence elements will be required to facilitate the efficacy of gene therapy trials.
COEXPRESSION OF A SUICIDAL GENE
Herpes simplex virus thymidine kinase (HSV-tk) has been used for selective destruction of cells in several different settings. When anti-viral prodrug nucleobase analogue ganciclovir (GCV) is applied to HSV-tk expressing cells, GCV is efficiently converted into monophosphate form by HSV-tk, and then into cytotoxic triphosphate derivatives by cellular kinases. Actively dividing cells will be killed as they incorporate the nucleotide derivatives into their genome. In allogeneic bone marrow transplantation (BMT), donor T cells are able to mediate anti-leukemic effects but they can also induce graft-vs-host disease (GvHD), which is often fatal. In an attempt to reduce GvHD while maintaining anti-leukemic effect, scientists have retrovirally transduced HSV-tk to donor T-cells before being used in animal myeloablative BMT trials [108]. At first, the donor T-cells helped to eliminate residual malignant leukemic cells, but when signs of GvHD development were noticed, proliferating donor T-cells were rapidly destroyed by treating the animals with ganciclovir. When this strategy was used in a human trial, three out of eight patients treated with donor lymphocytes transduced with HSV-TK gene could be effectively controlled by ganciclovir-induced elimination of the transduced cells when they developed GvHD 12 months after transduction [109].
Similarly, retroviral vectors can be designed to co-express HSV-tk suicide gene to be used as a safety switch, in addition to a therapeutic gene. If abnormal growth of transduced cells is observed such as the cases in the X-SCID, treatment with ganciclovir can eliminate all the transduced cells theoretically. However, constitutive expression of HSV-tk can also induce the death of neighboring uninfected cells by the bystander effects when ganciclovir is administered. In order to minimize unwanted side effects due to bystander effects, the use of cell type-specific or inducible promoter for the expression of HSV-tk or the use of other pro-apoptotic genes with a minimum bystander effect may be advantageous. As an example, lentivirally transduced expression unit containing the rat alpha-fetoprotein promoter was used to restrict the HSV-tk induced GCV sensitivity to human hepatocarcinoma cells [97].
On the other hand, retroviral vectors expressing HSV-tk have been used in antitumor treatment trials [110-112]. In this case, maximum “bystander effect” is required to kill neighboring uninfected cells as well as infected cells. The results of tumor treatment with HSV-tk expressing retroviral vectors were, however, not fully successful due to low infection efficiency and weak bystander effects.
One potential obstacle for co-expressing HSV-tk suicide gene as a safety switch in addition to the therapeutic gene however, is the limited insert size constraint of the retroviral vector. In cell-based ex-vivo gene therapy using the clonally-derived cells, selection of single clones, transduced with two separate retroviral vectors harboring the HSV-tk gene and the therapeutic gene in each vector, could be a solution for the problem of size restriction.
AVOIDING REPLICATION COMPETENT RETROVIRUS (RCR)
Generation of RCR remains as a potential safety issue in retroviral gene therapy. Retroviral vectors transfected into a packaging cell line can produce RCR by recombination processes between homologous sequences of the retroviral vector DNA and the gag, pol, and env coding sequences in the packaging systems. In order to lower the chances for recombination, both minimizing the homologous sequences and physically separating genes for gag, pol and env into two different expression cassettes, have become standard practices. However, residual gag, pol, and env coding sequences are frequently included in these vectors in an attempt to increase transduction efficiency and viral titer. Therefore, remaining part of gag, pol, and env gene is raising a concern for RCR generation. There is a report that a complete removal of residual coding sequences for gag, pol, and env genes did not show any detrimental effect on viral transduction efficiency, and it also reduced the chance of RCR generation [113]. The same concept was also applied to lentiviral vectors. To avoid the production of replication competent lentivirus (RCL), the components required for the production of lentivirus were divided into at least three parts: vector plasmid which contains the gene of interest and the minimal cis-acting element of HIV; packaging plasmid which has all HIV viral genes except the env gene; envelope proteins which were provided from a plasmid containing the envelope gene via co-transfection. Typically, the Glycoprotein from vesicular stomatitis virus (VSV-G) is used as an envelope gene [114]. One of the safety concerns specific to HIV virus is the recombination between vector sequences and endogenous HIV sequences in HIV positive patients. In spite of the large deletion of endogenous retroviral sequences, the possibility of recombination and mobilization cannot be overlooked. Stable lentivirus producer cell line has been tried due to the higher probability of recombination during the packaging process through transient transfection is much higher than that of the stable producer cell lines [115, 116]. Due to the concerns of using HIV in humans, researchers are developing non-human lentiviral vector systems. Simian immunodeficiency virus (SIV), Feline immunodeficiency virus (FIV), and Equine immunodeficiency virus (EIAV) are some of them [117-119]. However, the safety features of non-primate lentiviruses in humans have yet to be determined [120].
SIN retroviral vector was developed by introducing a deletion in the U3 region of the 3’ LTR which contains all the enhancer and promoter activities of the viral vector Fig. (1B) [121]. No active viral particle is arising from SIN vectors, because the 5’LTR carrying the same deletion in the chromosome is not capable of inducing transcription for the production of packagable RNAs. An additional advantage of the SIN vector is the minimal chance of LTR-mediated insertional activation of proto-oncogene near the site of insertion. SIN vector approach has been tested more extensively in lentiviruses [122]. Although SIN vector was considered as an ideal vector, a SIN vector mobilization was detected with a very low level [116]. Additionally, one of the drawbacks of SIN retroviral vectors is the low transcriptional activity of the internal promoter compared to the viral LTR in a number of different cell types. Improvements in the design of the internal promoter/enhancer are required to overcome this obstacle.
Split-intron retroviral vector has been shown to enhance expression of an inserted gene and safety was improved [90]. A strong synthetic splice donor (SD) site and a splice acceptor (SA) site were inserted between U3 and R of the 3’ LTR and downstream of the packaging signal, respectively. During the reverse transcription process, the strong synthetic splice donor site introduced in the 3’ LTR is copied into 5’LTR, and theoretically all the transcripts made in the transduced cells are spliced and the packaging signal is removed Fig. (1C). Therefore, the possibility of producing RCR is greatly reduced.
Finally, activation of proto-oncogene may arise due to aberrant retroviral RNA processing. A strong internal SA is recommended after SD site of retroviral vector to prevent a generation of aberrant read through transcripts containing both the transgene and a portion of the disrupted host gene. Combination of strong SA, deleting cryptic SD in the transgene [89], an improved polyadenylation signal [123], removal of LTR promoter, and the presence of an insulator will largely prevent the interactions of the retroviral splice donor with downstream chromosome sequences.
CONCLUDING REMARKS
Applications of gene therapy protocols have been continuously expanded to wide variety of acquired and inherited diseases, such as cancer, SCID, and other life threatening diseases. Retroviral gene therapy approaches for the treatment of these diseases have to address safety issues. Targeted infection, local delivery, targeted retroviral insertion, insulators, transcriptional targeting, co-transduction with a suicidal gene, and SIN vectors were suggested as possible solutions for the risks of retroviral gene therapy. Some of these precautions can also be applied to gene therapy protocols using other viral and non-viral vector systems. One major immediate concern in terms of retroviral gene therapy, as revealed by the X-SCID case, is insertional oncogenesis. Several approaches to decrease the possibility of insertional oncogenesis were considered in depth. In addition to the widely used retroviral systems that were discussed above, foamy viruses may be used as a safe and efficient means of targeting non-dividing cells [124, 125]. Foamy viruses are known to have a broad host range, without causing any disease and persist in infected humans [126-128]. In the case of ex vivo cell-based gene therapy, transduced cells could be pre-screened to select for clones with the insertion of the transgene only at a desirable site of the chromosome, which can minimize the chances for insertional oncogenesis. Orthopedic indication is one of the most promising areas of gene therapy in spite of the non-lethal conditions [37, 129-135].
The incident from a gene transfer study of β-thalassemia Major in France showed us that there is no guaranteed way to safe gene therapy. Pursuing prudent designing of vectors and monitoring adverse effects in patients are proper directions. Additionally, selection of patient specific method is recommended for each patient based on the risk versus benefit.
Table 1.
Retroviral Vectors for Targeted Infection
| Modification | Target Cell | References |
|---|---|---|
| EGF-MMP cleavable linker chimeric env | Cancer invasion, angiogenesis, inflammation | [16, 17, 20] |
| IL-2 chimeric env | IL-2 R | [27] |
| EGF chimeric env | EGFR | [15] |
| SCF-Factor Xa chimeric env | Stem cell (Kit) | [19] |
| vWF (collagen binding) chimeric env | Cancer (collagen expressing); vascular lesion | [23, 24] |
| Single-chain variable fragmented antibody (scFv) for EGFRvIII | Cancer (brain, breast, lung, ovary) | [29] |
| scFv for HMWMAA | Cancer | [22] |
| scFv from phage display | T cell | [28] |
| scFv for Carcino embryonic antigen (CEA) | Cancer | [30] |
| Receptor pseudotyping (CD4 and CXCR4) | HIV-1 infected cell | [31, 32] |
ACKNOWLEDGEMENT
We thank Vivian Yip, and Sally Hwang for proofreading this manuscript.
REFERENCES
- 1.Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003;299:495. doi: 10.1126/science.299.5606.495. [DOI] [PubMed] [Google Scholar]
- 2.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–88. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 3.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. The New England journal of medicine. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 5.Yi Y, Hahm SH, Lee KH. Retroviral gene therapy: safety issues and possible solutions. Current gene therapy. 2005;5:25–35. doi: 10.2174/1566523052997514. [DOI] [PubMed] [Google Scholar]
- 6.Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. The Journal of clinical investigation. 2008;118:3143–50. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein S, Ott MG, Schultze-Strasser S, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nature medicine. 2010;6:98–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 8.Baum C, Dullmann J, Li Z, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 9.Frecha C, Szecsi J, Cosset FL, Verhoeyen E. Strategies for targeting lentiviral vectors. Current gene therapy. 2008;8:449–60. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- 10.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 11.White JM. Membrane fusion. Science. 1992;258:917–24. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 12.Overbaugh J, Miller AD, Eiden MV. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol Mol Biol Rev. 2001;65:371–89. doi: 10.1128/MMBR.65.3.371-389.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ting YT, Wilson CA, Farrell KB, Chaudry GJ, Eiden MV. Simian sarcoma-associated virus fails to infect Chinese hamster cells despite the presence of functional gibbon ape leukemia virus receptors. J Virol. 1998;72:9453–8. doi: 10.1128/jvi.72.12.9453-9458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudry GJ, Farrell KB, Ting YT, et al. Gibbon ape leukemia virus receptor functions of type III phosphate transporters from CHOK1 cells are disrupted by two distinct mechanisms. J Virol. 1999;73:2916–20. doi: 10.1128/jvi.73.4.2916-2920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosset FL, Morling FJ, Takeuchi Y, Weiss RA, Collins MK, Russell SJ. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–22. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng KW, Morling FJ, Cosset FL, Murphy G, Russell SJ. A gene delivery system activatable by disease-associated matrix metalloproteinases. Hum Gene Ther. 1997;8:729–38. doi: 10.1089/hum.1997.8.6-729. [DOI] [PubMed] [Google Scholar]
- 17.Peng KW, Vile R, Cosset FL, Russell S. Selective transduction of protease-rich tumors by matrix-metalloproteinase-targeted retroviral vectors. Gene Ther. 1999;6:1552–7. doi: 10.1038/sj.gt.3300982. [DOI] [PubMed] [Google Scholar]
- 18.Nilson BH, Morling FJ, Cosset FL, Russell SJ. Targeting of retroviral vectors through protease-substrate interactions. Gene Ther. 1996;3:280–6. [PubMed] [Google Scholar]
- 19.Fielding AK, Maurice M, Morling FJ, Cosset FL, Russell SJ. Inverse targeting of retroviral vectors: selective gene transfer in a mixed population of hematopoietic and nonhematopoietic cells. Blood. 1998;91:1802–9. [PubMed] [Google Scholar]
- 20.Buchholz CJ, Peng KW, Morling FJ, Zhang J, Cosset FL, Russell SJ. In vivo selection of protease cleavage sites from retrovirus display libraries. Nat Biotechnol. 1998;16:951–4. doi: 10.1038/nbt1098-951. [DOI] [PubMed] [Google Scholar]
- 21.Schneider RM, Medvedovska Y, Hartl I, et al. Directed evolution of retroviruses activatable by tumour-associated matrix metalloproteases. Gene Ther. 2003;10:1370–80. doi: 10.1038/sj.gt.3302007. [DOI] [PubMed] [Google Scholar]
- 22.Martin F, Chowdhury S, Neil S, Phillipps N, Collins MK. Envelope-targeted retrovirus vectors transduce melanoma xenografts but not spleen or liver. Mol Ther. 2002;5:269–74. doi: 10.1006/mthe.2002.0550. [DOI] [PubMed] [Google Scholar]
- 23.Gordon EM, Liu PX, Chen ZH, et al. Inhibition of metastatic tumor growth in nude mice by portal vein infusions of matrix-targeted retroviral vectors bearing a cytocidal cyclin G1 construct. Cancer Res. 2000;60:3343–7. [PubMed] [Google Scholar]
- 24.Hall FL, Liu L, Zhu NL, et al. Molecular engineering of matrix-targeted retroviral vectors incorporating a surveillance function inherent in von Willebrand factor. Hum Gene Ther. 2000;11:983–93. doi: 10.1089/10430340050015293. [DOI] [PubMed] [Google Scholar]
- 25.Chawla SP, Chua VS, Fernandez L, et al. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther. 2009;17:1651–7. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla SP, Chua VS, Fernandez L, et al. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol Ther. 2010;18:435–41. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice M, Mazur S, Bullough FJ, et al. Efficient gene delivery to quiescent interleukin-2 (IL-2)-dependent cells by murine leukemia virus-derived vectors harboring IL-2 chimeric envelope glycoproteins. Blood. 1999;94:401–10. [PubMed] [Google Scholar]
- 28.Engelstadter M, Bobkova M, Baier M, et al. Targeting human T cells by retroviral vectors displaying antibody domains selected from a phage display library. Hum Gene. Ther. 2000;11:293–303. doi: 10.1089/10430340050016030. [DOI] [PubMed] [Google Scholar]
- 29.Lorimer IA, Lavictoire SJ. Targeting retrovirus to cancer cells expressing a mutant EGF receptor by insertion of a single chain antibody variable domain in the envelope glycoprotein receptor binding lobe. J Immunol Methods. 2000;237:147–57. doi: 10.1016/s0022-1759(99)00219-7. [DOI] [PubMed] [Google Scholar]
- 30.Khare PD, Shao-Xi L, Kuroki M, et al. Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res. 2001;61:370–5. [PubMed] [Google Scholar]
- 31.Somia NV, Miyoshi H, Schmitt MJ, Verma IM. Retroviral vector targeting to human immunodeficiency virus type 1-infected cells by receptor pseudotyping. J Virol. 2000;74:4420–4. doi: 10.1128/jvi.74.9.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bittner A, Mitnacht-Kraus R, Schnierle BS. Specific transduction of HIV-1 envelope expressing cells by retroviral vectors pseudotyped with hybrid CD4/CXCR4 receptors. J Virol Methods. 2002;104:83–92. doi: 10.1016/s0166-0934(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin AH, Kasahara N, Wu W, et al. Receptor-specific targeting mediated by the coexpression of a targeted murine leukemia virus envelope protein and a binding-defective influenza hemagglutinin protein. Hum Gene Ther. 2001;12:323–32. doi: 10.1089/10430340150503957. [DOI] [PubMed] [Google Scholar]
- 34.Behrens A, Gordon EM, Li L, et al. Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze. Invest Ophthalmol Vis Sci. 2002;43:968–77. [PubMed] [Google Scholar]
- 35.Deglon N, Tseng JL, Bensadoun JC, et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Human gene therapy. 2000;11:179–90. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- 36.Nemunaitis J, Fong T, Robbins JM, et al. Phase I trial of interferon-gamma (IFN-gamma) retroviral vector administered intratumorally to patients with metastatic melanoma. Cancer Gene Ther. 1999;6:322–30. doi: 10.1038/sj.cgt.7700019. [DOI] [PubMed] [Google Scholar]
- 37.Lee KH, Song SU, Hwang TS, et al. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum Gene Ther. 2001;12:1805–13. doi: 10.1089/104303401750476294. [DOI] [PubMed] [Google Scholar]
- 38.Noh MJ, Copeland RO, Yi Y, et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C) Cytotherapy. 2010;12:384–93. doi: 10.3109/14653240903470639. [DOI] [PubMed] [Google Scholar]
- 39.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. The Journal of clinical investigation. 2008;118:3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R, Rabbitts TH. The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci U S A. 1998;95:3890–5. doi: 10.1073/pnas.95.7.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royer-Pokora B, Loos U, Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11) Oncogene. 1991;6:1887–93. [PubMed] [Google Scholar]
- 43.McCormack MP, Young LF, Vasudevan S, et al. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–83. doi: 10.1126/science.1182378. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes & development. 1999;13:2678–90. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–60. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 46.Clappier E, Cuccuini W, Cayuela JM, et al. Cyclin D2 dysregulation by chromosomal translocations to TCR loci in T-cell acute lymphoblastic leukemias. Leukemia. 2006;20:82–6. doi: 10.1038/sj.leu.2404008. [DOI] [PubMed] [Google Scholar]
- 47.Cellular, Tissue, and Gene Therapies. Advisory Committee meeting. 2005. Mar 4,
- 48.Katz RA, Skalka AM. The retroviral enzymes. Annu Rev Biochem. 1994;63:133–73. doi: 10.1146/annurev.bi.63.070194.001025. [DOI] [PubMed] [Google Scholar]
- 49.Wei SQ, Mizuuchi K, Craigie R. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. Embo J. 1997;16:7511–20. doi: 10.1093/emboj/16.24.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–9. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–51. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 52.Persons DA. Lentiviral vector gene therapy: effective and safe? Mol Ther. 2010;18:861–2. doi: 10.1038/mt.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibagaki Y, Chow SA. Central core domain of retroviral integrase is responsible for target site selection. The Journal of biological chemistry. 1997;272:8361–9. doi: 10.1074/jbc.272.13.8361. [DOI] [PubMed] [Google Scholar]
- 54.Harper AL, Sudol M, Katzman M. An amino acid in the central catalytic domain of three retroviral integrases that affects target site selection in nonviral DNA. Journal of virology. 2003;77:3838–45. doi: 10.1128/JVI.77.6.3838-3845.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metais JY, Topp S, Doty RT, et al. Feline leukemia virus integrase and capsid packaging functions do not change the insertion profile of standard Moloney retroviral vectors. Gene therapy. 2010;17:799–804. doi: 10.1038/gt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goulaouic H, Chow SA. Directed integration of viral DNA mediated by fusion proteins consisting of human immunodeficiency virus type 1 integrase and Escherichia coli LexA protein. J Virol. 1996;70:37–46. doi: 10.1128/jvi.70.1.37-46.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz RA, Merkel G, Skalka AM. Targeting of retroviral integrase by fusion to a heterologous DNA binding domain: in vitro activities and incorporation of a fusion protein into viral particles. Virology. 1996;217:178–90. doi: 10.1006/viro.1996.0105. [DOI] [PubMed] [Google Scholar]
- 58.Bushman FD, Miller MD. Tethering human immunodeficiency virus type 1 preintegration complexes to target DNA promotes integration at nearby sites. J Virol. 1997;71:458–64. doi: 10.1128/jvi.71.1.458-464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka AS, Tanaka M, Komuro K. A highly efficient method for the site-specific integration of transfected plasmids into the genome of mammalian cells using purified retroviral integrase. Gene. 1998;216:67–76. doi: 10.1016/s0378-1119(98)00312-6. [DOI] [PubMed] [Google Scholar]
- 60.Sclimenti CR, Thyagarajan B, Calos MP. Directed evolution of a recombinase for improved genomic integration at a native human sequence. Nucleic Acids Res. 2001;29:5044–51. doi: 10.1093/nar/29.24.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen R. Enzyme engineering: rational redesign versus directed evolution. Trends Biotechnol. 2001;19:13–4. doi: 10.1016/s0167-7799(00)01522-5. [DOI] [PubMed] [Google Scholar]
- 62.Kim JS, Pabo CO. Getting a handhold on DNA: design of poly-zinc finger proteins with femtomolar dissociation constants. Proc Natl Acad Sci U S A. 1998;95:2812–7. doi: 10.1073/pnas.95.6.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jamieson AC, Miller JC, Pabo CO. Drug discovery with engineered zinc-finger proteins. Nat Rev Drug Discov. 2003;2:361–8. doi: 10.1038/nrd1087. [DOI] [PubMed] [Google Scholar]
- 64.Sarkis C, Philippe S, Mallet J, Serguera C. Non-integrating lentiviral vectors. Current gene therapy. 2008;8:430–7. doi: 10.2174/156652308786848012. [DOI] [PubMed] [Google Scholar]
- 65.Qasim W, Vink CA, Thrasher AJ. Hybrid lentiviral vectors. Mol Ther. 2010;18:1263–7. doi: 10.1038/mt.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pannell D, Ellis J. Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol. 2001;11:205–17. doi: 10.1002/rmv.316. [DOI] [PubMed] [Google Scholar]
- 67.Burgess-Beusse B, Farrell C, Gaszner M, et al. The insulation of genes from external enhancers and silencing chromatin. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16433–7. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun FL, Elgin SC. Putting boundaries on silence. Cell. 1999;99:459–62. doi: 10.1016/s0092-8674(00)81534-2. [DOI] [PubMed] [Google Scholar]
- 69.Chung JH, Bell AC, Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc Natl Acad Sci USA. 1997;94:575–80. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung JH, Whiteley M, Felsenfeld G. A 5' element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–14. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 71.Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–50. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- 72.Labrador M, Corces VG. Setting the boundaries of chromatin domains and nuclear organization. Cell. 2002;11:51–4. doi: 10.1016/s0092-8674(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 73.Emery DW, Yannaki E, Tubb J, Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc Natl Acad Sci U S A. 2000;97:9150–5. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emery DW, Yannaki E, Tubb J, Nishino T, Li Q, Stamatoyannopoulos G. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100:2012–9. doi: 10.1182/blood-2002-01-0219. [DOI] [PubMed] [Google Scholar]
- 75.Arumugam PI, Urbinati F, Velu CS, Higashimoto T, Grimes HL, Malik P. The 3' region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PloS one. 2009;4:e6995. doi: 10.1371/journal.pone.0006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puthenveetil G, Scholes J, Carbonell D, et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;04:3445–53. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- 77.Malik P, Arumugam PI. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology; 2005. Gene Therapy for beta-thalassemia; pp. 45–50. [DOI] [PubMed] [Google Scholar]
- 78.Yannaki E, Tubb J, Aker M, Stamatoyannopoulos G, Emery DW. Topological constraints governing the use of the chicken HS4 chromatin insulator in oncoretrovirus vectors. Mol Ther. 2002;5:589–98. doi: 10.1006/mthe.2002.0582. [DOI] [PubMed] [Google Scholar]
- 79.Rivella S, Callegari JA, May C, Tan CW, Sadelain M. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. J Virol. 2000;74:4679–87. doi: 10.1128/jvi.74.10.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Simone P, Di Leonardo A, Costanzo G, Melfi R, Spinelli G. The sea urchin sns insulator blocks CMV enhancer following integration in human cells. Biochem Biophys Res Commun. 2001;284:987–92. doi: 10.1006/bbrc.2001.5082. [DOI] [PubMed] [Google Scholar]
- 81.D'Apolito D, Baiamonte E, Bagliesi M, et al. The sea urchin sns5 insulator protects retroviral vectors from chromosomal position effects by maintaining active chromatin structure. Mol Ther. 2009;17:1434–41. doi: 10.1038/mt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwaks TH, Barnett P, Hemrika W, et al. Identification of anti-repressor elements that confer high and stable protein production in mammalian cells. Nat Biotechnol. 2003;21:553–8. doi: 10.1038/nbt814. [DOI] [PubMed] [Google Scholar]
- 83.Bode J, Kohwi Y, Dickinson L, et al. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992;255:195–7. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- 84.Namciu SJ, Blochlinger KB, Fournier RE. Human matrix attachment regions insulate transgene expression from chromosomal position effects in Drosophila melanogaster. Mol Cell Biol. 1998;18:2382–91. doi: 10.1128/mcb.18.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bode J, Benham C, Knopp A, Mielke C. Transcriptional augmentation: modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements) Crit Rev Eukaryot Gene Expr. 2000;10:73–90. [PubMed] [Google Scholar]
- 86.Agarwal M, Austin TW, Morel F, Chen J, Bohnlein E, Plavec I. Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells. J Virol. 1998;72:3720–8. doi: 10.1128/jvi.72.5.3720-3728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurre P, Morris J, Thomasson B, Kohn DB, Kiem HP. Scaffold attachment region-containing retrovirus vectors improve long-term proviral expression after transplantation of GFP-modified CD34+ baboon repopulating cells. Blood. 2003;102:3117–9. doi: 10.1182/blood-2003-03-0962. [DOI] [PubMed] [Google Scholar]
- 88.Ramezani A, Hawley RG. Human immunodeficiency virus type 1-based vectors for gene delivery to human hematopoietic stem cells. Methods Mol Med. 2003;76:467–92. doi: 10.1385/1-59259-304-6:467. [DOI] [PubMed] [Google Scholar]
- 89.Knipper R, Kuehlcke K, Schiedlmeier B, et al. Improved post-transcriptional processing of an MDR1 retrovirus elevates expression of multidrug resistance in primary human hematopoietic cells. Gene Ther. 2001;8:239–46. doi: 10.1038/sj.gt.3301384. [DOI] [PubMed] [Google Scholar]
- 90.Ismail SI, Kingsman SM, Kingsman AJ, Uden M. Split-intron retroviral vectors: enhanced expression with improved safety. Journal of virology. 2000;74:2365–71. doi: 10.1128/jvi.74.5.2365-2371.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vile R, Miller N, Chernajovsky Y, Hart I. A comparison of the properties of different retroviral vectors containing the murine tyrosinase promoter to achieve transcriptionally targeted expression of the HSVtk or IL-2 genes. Gene Ther. 1994;1:307–16. [PubMed] [Google Scholar]
- 92.Sparmann G, Walther W, Gunzburg WH, Uckert W, Salmons B. Conditional expression of human TNF-alpha: a system for inducible cytotoxicity. Int J Cancer. 1994;59:103–7. doi: 10.1002/ijc.2910590119. [DOI] [PubMed] [Google Scholar]
- 93.Walther W, Wendt J, Stein U. Employment of the mdr1 promoter for the chemotherapy-inducible expression of therapeutic genes in cancer gene therapy. Gene Ther. 1997;4:544–52. doi: 10.1038/sj.gt.3300451. [DOI] [PubMed] [Google Scholar]
- 94.Walther W, Stein U, Fichtner I, Alexander M, Shoemaker RH, Schlag PM. Mdr1 promoter-driven tumor necrosis factor-alpha expression for a chemotherapy-controllable combined in vivo gene therapy and chemotherapy of tumors. Cancer Gene Ther. 2000;7:893–900. doi: 10.1038/sj.cgt.7700196. [DOI] [PubMed] [Google Scholar]
- 95.Modlich U, Pugh CW, Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- 96.Cao G, Kuriyama S, Gao J, et al. Gene therapy for hepatocellular carcinoma based on tumour-selective suicide gene expression using the alpha-fetoprotein (AFP) enhancer and a housekeeping gene promoter. Eur J Cancer. 2001;37:140–7. doi: 10.1016/s0959-8049(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 97.Uch R, Gerolami R, Faivre J, et al. Hepatoma cell-specific ganciclovir-mediated toxicity of a lentivirally transduced HSV-TkEGFP fusion protein gene placed under the control of rat alpha-fetoprotein gene regulatory sequences. Cancer Gene Ther. 2003;10:689–95. doi: 10.1038/sj.cgt.7700621. [DOI] [PubMed] [Google Scholar]
- 98.Lotti F, Mavilio F. The choice of a suitable lentivirus vector: transcriptional targeting. Methods Mol Biol. 2003;229:17–27. doi: 10.1385/1-59259-393-3:17. [DOI] [PubMed] [Google Scholar]
- 99.Hatzoglou M, Lamers W, Bosch F, Wynshaw-Boris A, Clapp DW, Hanson RW. Hepatic gene transfer in animals using retroviruses containing the promoter from the gene for phosphoenolpyruvate carboxykinase. J Biol Chem. 1990;265:17285–93. [PubMed] [Google Scholar]
- 100.Hafenrichter DG, Ponder KP, Rettinger SD, et al. Liver-directed gene therapy: evaluation of liver specific promoter elements. J Surg Res. 1994;56:510–7. doi: 10.1006/jsre.1994.1082. [DOI] [PubMed] [Google Scholar]
- 101.Ferrari G, Salvatori G, Rossi C, Cossu G, Mavilio F. A retroviral vector containing a muscle-specific enhancer drives gene expression only in differentiated muscle fibers. Hum Gene Ther. 1995;6:733–42. doi: 10.1089/hum.1995.6.6-733. [DOI] [PubMed] [Google Scholar]
- 102.Ido A, Nakata K, Kato Y, et al. Gene therapy for hepatoma cells using a retrovirus vector carrying herpes simplex virus thymidine kinase gene under the control of human alpha-fetoprotein gene promoter. Cancer Res. 1995;55:3105–9. [PubMed] [Google Scholar]
- 103.Stover ML, Wang CK, McKinstry MB, et al. Bone-directed expression of Col1a1 promoter-driven self-inactivating retroviral vector in bone marrow cells and transgenic mice. Mol Ther. 2001;3:543–50. doi: 10.1006/mthe.2001.0293. [DOI] [PubMed] [Google Scholar]
- 104.Romano G, Reiss K, Tu X, et al. Efficient in vitro and in vivo gene regulation of a retrovirally delivered pro-apoptotic factor under the control of the Drosophila HSP70 promoter. Gene Ther. 2001;8:600–7. doi: 10.1038/sj.gt.3301441. [DOI] [PubMed] [Google Scholar]
- 105.Ozturk-Winder F, Renner M, Klein D, Muller M, Salmons B, Gunzburg WH. The murine whey acidic protein promoter directs expression to human mammary tumors after retroviral transduction. Cancer Gene Ther. 2002;9:421–31. doi: 10.1038/sj.cgt.7700456. [DOI] [PubMed] [Google Scholar]
- 106.Mavria G, Jager U, Porter CD. Generation of a high titre retroviral vector for endothelial cell-specific gene expression in vivo. Gene Ther. 2000;7:368–76. doi: 10.1038/sj.gt.3301093. [DOI] [PubMed] [Google Scholar]
- 107.Ido A, Uto H, Moriuchi A, et al. Gene therapy targeting for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human alpha-fetoprotein promoter. Cancer Res. 2001;61:3016–21. [PubMed] [Google Scholar]
- 108.Drobyski WR, Gendelman M, Vodanovic-Jankovic S, Gorski J. Elimination of leukemia in the absence of lethal graft-versus-host disease after allogenic bone marrow transplantation. J Immunol. 2003;170:3046–53. doi: 10.4049/jimmunol.170.6.3046. [DOI] [PubMed] [Google Scholar]
- 109.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–24. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 110.Barzon L, Bonaguro R, Castagliuolo I, et al. Gene therapy of thyroid cancer via retrovirally-driven combined expression of human interleukin-2 and herpes simplex virus thymidine kinase. Eur J Endocrinol. 2003;148:73–80. doi: 10.1530/eje.0.1480073. [DOI] [PubMed] [Google Scholar]
- 111.Orchard PJ, Blazar BR, Burger S, et al. Clinical-scale selection of anti-CD3/CD28-activated T cells after transduction with a retroviral vector expressing herpes simplex virus thymidine kinase and truncated nerve growth factor receptor. Hum Gene Ther. 2002;13:979–88. doi: 10.1089/10430340252939087. [DOI] [PubMed] [Google Scholar]
- 112.Greco E, Fogar P, Basso D, et al. Retrovirus-mediated herpes simplex virus thymidine kinase gene transfer in pancreatic cancer cell lines: an incomplete antitumor effect. Pancreas. 2002;25:e21–9. doi: 10.1097/00006676-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 113.Yu SS, Kim JM, Kim S. High efficiency retroviral vectors that contain no viral coding sequences. Gene Ther. 2000;7:797–804. doi: 10.1038/sj.gt.3301164. [DOI] [PubMed] [Google Scholar]
- 114.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farson D, Witt R, McGuinness R, et al. A new-generation stable inducible packaging cell line for lentiviral vectors. Hum Gene Ther. 2001;12:981–97. doi: 10.1089/104303401750195935. [DOI] [PubMed] [Google Scholar]
- 116.Xu K, Ma H, McCown TJ, Verma IM, Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol Ther. 2001;3:97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- 117.Bienemann AS, Martin-Rendon E, Cosgrave AS, et al. Long-term replacement of a mutated nonfunctional CNS gene: reversal of hypothalamic diabetes insipidus using an EIAV-based lentiviral vector expressing arginine vasopressin. Mol Ther. 2003;7:588–96. doi: 10.1016/s1525-0016(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 118.Pandya S, Boris-Lawrie K, Leung NJ, Akkina R, Planelles V. Development of an Rev-independent, minimal simian immunodeficiency virus-derived vector system. Hum Gene Ther. 2001;12:847–57. doi: 10.1089/104303401750148847. [DOI] [PubMed] [Google Scholar]
- 119.Poeschla EM, Wong-Staal F, Looney DJ. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–7. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 120.Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730–4. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- 121.Yu SF, von Ruden T, Kantoff PW, et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–8. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–80. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ismail SI, Rohll JB, Kingsman SM, Kingsman AJ, Uden M. Use of intron-disrupted polyadenylation sites to enhance expression and safety of retroviral vectors. J Virol. 2001;75:199–204. doi: 10.1128/JVI.75.1.199-204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rethwilm A. Foamy virus vectors: an awaited alternative to gam-maretro- and lentiviral vectors. Current gene therapy. 2007;7:261–71. doi: 10.2174/156652307781369092. [DOI] [PubMed] [Google Scholar]
- 125.Mergia A, Chari S, Kolson DL, Goodenow MM, Ciccarone T. The efficiency of simian foamy virus vector type-1 (SFV-1) in nondividing cells and in human PBLs. Virology. 2001;280:243–52. doi: 10.1006/viro.2000.0773. [DOI] [PubMed] [Google Scholar]
- 126.Callahan ME, Switzer WM, Matthews AL, et al. Persistent zoonotic infection of a human with simian foamy virus in the absence of an intact orf-2 accessory gene. J Virol. 1999;73:9619–24. doi: 10.1128/jvi.73.11.9619-9624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heneine W, Switzer WM, Sandstrom P, et al. Identification of a human population infected with simian foamy viruses. Nat Med. 1998;4:403–7. doi: 10.1038/nm0498-403. [DOI] [PubMed] [Google Scholar]
- 128.Schweizer M, Falcone V, Gange J, Turek R, Neumann-Haefelin D. Simian foamy virus isolated from an accidentally infected human individual. J Virol. 1997;71:4821–4. doi: 10.1128/jvi.71.6.4821-4824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evans CH, Scully SP. Orthopaedic gene therapy. Clin Orthop. 2000;379(Suppl):S2. [PubMed] [Google Scholar]
- 130.Robbins PD, Evans CH, Chernajovsky Y. Gene therapy for arthritis. Gene Ther. 2003;10:902–11. doi: 10.1038/sj.gt.3302040. [DOI] [PubMed] [Google Scholar]
- 131.Lieberman JR, Ghivizzani SC, Evans CH. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141–7. doi: 10.1006/mthe.2000.0663. [DOI] [PubMed] [Google Scholar]
- 132.Palmer G, Pascher A, Gouze E, et al. Development of gene-based therapies for cartilage repair. Crit Rev Eukaryot Gene Expr. 2002;12:259–73. doi: 10.1615/critreveukaryotgeneexpr.v12.i4.20. [DOI] [PubMed] [Google Scholar]
- 133.Nussenbaum B, Rutherford RB, Teknos TN, Dornfeld KJ, Krebsbach PH. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003;14:1107–15. doi: 10.1089/104303403322124819. [DOI] [PubMed] [Google Scholar]
- 134.Evans CH, Ghivizzani SC, Herndon JH, et al. Clinical trials in the gene therapy of arthritis. Clin Orthop. 2000;379(Suppl):S300–7. doi: 10.1097/00003086-200010001-00039. [DOI] [PubMed] [Google Scholar]
- 135.Lee CW, Martinek V, Usas A, et al. Muscle-based gene therapy and tissue engineering for treatment of growth plate injuries. J Pediatr Orthop. 2002;22:565–72. [PubMed] [Google Scholar]