Abstract
In order to meet the potential need for emergency large-scale retrospective radiation biodosimetry following an accident or attack, we have developed instrumentation and methodology for in vivo electron paramagnetic resonance spectroscopy to quantify concentrations of radiation-induced radicals within intact teeth. This technique has several very desirable characteristics for triage, including independence from confounding biologic factors, a non-invasive measurement procedure, the capability to make measurements at any time after the event, suitability for use by non-expert operators at the site of an event, and the ability to provide immediate estimates of individual doses. Throughout development there has been a particular focus on the need for a deployable system, including instrumental requirements for transport and field use, the need for high throughput, and use by minimally trained operators.
Numerous measurements have been performed using this system in clinical and other non-laboratory settings, including in vivo measurements with unexposed populations as well as patients undergoing radiation therapies. The collection and analyses of sets of three serially-acquired spectra with independent placements of the resonator, in a data collection process lasting approximately five minutes, provides dose estimates with standard errors of prediction of approximately 1 Gy. As an example, measurements were performed on incisor teeth of subjects who had either received no irradiation or 2 Gy total body irradiation for prior bone marrow transplantation; this exercise provided a direct and challenging test of our capability to identify subjects who would be in need of acute medical care.
Keywords: EPR, biodosimetry, triage, teeth
1. Definition of the Emergency Needs for Biodosimetry
Included among the National Planning Scenarios developed by United States federal and local government entities to facilitate emergency response is a scenario for the detonation of a 10 kiloton (10 kt) improvised nuclear device (IND) in a large metropolitan area (Howe 2004). Models of such an event predict that upwards of one million people will be affected and require evaluation of their personal radiation exposure levels, and hundreds of thousands of people may receive doses over 2 Gy (Grace et al. 2010, Bell and Dallas 2007, Meade and Molander 2006, Buddemeier and Dillon 2009). In addition to the exposure to prompt radiation and fallout, casualties and infrastructure damage due to over-pressure, thermal, and electromagnetic effects will be overwhelming in the 1-mile radius area surrounding the detonation site. The scale of destruction and large numbers of people affected necessitate an extraordinary and coordinated effort to minimize ongoing exposure of the surrounding population through appropriate shelter-in-place, evacuation, and decontamination actions and to maximize the impact of scarce medical resources for the treatment of significantly exposed and otherwise injured individuals. In contrast, exposures due to radiation accidents, the use of radiological dispersal devices, or the use of radiological exposure devices are expected to lead to far lower numbers of people at risk of developing acute radiation syndrome. In such cases, conventional medical resources are likely to be sufficient to provide individual care of all affected people and be effective through assessment of clinical signs and symptoms and more labor intensive and time consuming biodosimetry techniques.
The proposed architecture for assessing radiation exposure and managing medical resources in the aftermath of nuclear detonation includes the implementation of point-of-care (POC) diagnostic tools which are capable of providing rapid screening to identify individuals who have received doses > 2 Gy and who may benefit from additional evaluation and appropriate medical treatment (Grace et al. 2010). Within established medical care sites, additional biodosimetry measurements within the exposed subset of the population may be used to more precisely estimate individual exposure levels to guide immediate therapeutic or palliative measures or recommend schedules for long term monitoring. In order to effectively address these needs, a biodosimeter should meet a number of critical applicability and performance criteria (Flood et al. (this issue), Swartz et al. 2010, Alexander et al. 2007) and a number of biomarkers and diagnostic technologies have been proposed and are under development (Swartz et al. 2010, Swartz et al. (this issue)).
The techniques employing electron paramagnetic resonance (EPR) spectroscopy to detect radiation-induced radicals in teeth and fingernails are distinguished from most other proposed methods due to the precise physical basis of the measured quantity, as opposed to the characterization of processes that are integral to or affected by biological activity. In tooth enamel, carbonate radical centers are created within the hydroxyapatite matrix following irradiation in proportion to the absorbed dose and remain stable for an indefinite period of time (See Fattibene and Callens 2010 for a comprehensive review). EPR is a form of magnetic resonance spectroscopy that is specifically sensitive to the presence of radical species within measured samples, whose concentrations and molecular environments can be quantitatively interrogated and characterized. Using this technology and as consequences of this underlying physical phenomenon, the measured quantity is not affected by the existence of simultaneous injury or physiologic factors, measurements are not time dependent and can be made and repeated at any time after the event, the results are available immediately, measurements provide dose estimates at well-defined locations in the body, and measurements are unaffected by dose rate. These capabilities make in vivo EPR tooth dosimetry a particularly attractive approach for initial triage, as well as an option for use in dose estimation to guide subsequent treatment (Swartz et al. (this issue), Flood et al. (this issue), Flood et al. 2007, Swartz et al. 2010). In order to enable practical and efficient use of this effect for large-scale emergency radiation biodosimetry, we have developed a deployable EPR spectrometer and procedures that facilitate rapid and reliable measurements of the relative radiation-induced radical concentrations present in the enamel of intact teeth.
2. Development of Transportable EPR Dosimeter
A great deal of progress toward a fully deployable in vivo EPR tooth dosimeter has been achieved over the last years of concerted effort toward this goal (Williams et al. 2010, Swartz et al. 2006, Swartz et al. 2005, Swartz et al. 2007, Iwasaki et al. 2005a, Iwasaki et al. 2005b, Williams et al. 2007, Demidenko et al. 2007). Initial in vivo tooth dosimetry measurements were performed using the whole-body clinical EPR spectrometer developed at Dartmouth primarily for use as a clinical EPR oximetry system (Williams et al. 2007, Williams et al. 2010). This system is comprised of a 3000 lb permanent magnet with a 50 cm gap, a stretcher for measurements of subjects in a supine position, and EPR detection using surface-loop resonators and a manually-controlled RF bridge (Figure 1, left). Measurements required expert spectroscopists, a considerable amount of cooperation on the part of the subjects, and support of dedicated engineers for operation and maintenance. Using this system, we demonstrated the ability to perform accurate non-invasive in vivo EPR tooth dosimetry measurements in unirradiated subjects, volunteers with inserted irradiated teeth, and patients undergoing radiation therapy for head and neck cancers (Iwasaki et al. 2005b, Williams et al. 2007, Swartz et al. 2007, Williams et al. 2010). However, due to the emergency operational situation that EPR dosimetry systems will be deployed within, a number of instrumental refinements are required, including miniaturization of the device, increased mechanical robustness, and automation of measurement procedures. The automation needs to occur on multiple levels of the overall dosimetry system, limiting the requirements of the operator to assistance in the placement of the resonator in the mouth and initiation of data acquisition. Considerations include replacement of mechanical components with electronically adjustable analogues and realization of complete computer control of the instrument. These developments will increase robustness of the system and allow the implementation of rigorous quality control and fail-safe operation, which will help to facilitate effective use of this technology with minimally trained operators in an emergency setting. With these goals, and building upon the engineering concepts inherent within the earlier instrument and the established capability to perform in vivo EPR measurements with human subjects, we have designed and fabricated a deployable EPR spectrometer with features optimized for the performance of tooth dosimetry in emergency environments with minimally trained operators.
Figure 1.
(left) The original laboratory instrument (left) and recently developed deployable dosimeter (center) are shown, demonstrating the evolution of the design and miniaturization of components. One concept for the future is a compact kiosk design (right) with fully integrated components and comprehensive automation of resonator positioning and data.
The current dosimeter is composed of an array of custom-built and commercial instruments contained within a rolling shock-resistant container and a dipole magnet with a separate shipping container (Figure 1, center). This system is not yet optimized for weight reduction and is intended for vehicular transportation. The RF instruments weigh a total of 10 kg; the existing power supply for magnetic field sweeping weighs 20 kg; and the magnet weighs 30 kg. The current container for transportation weighs approximately 70 kg and an additional base for the magnet is being used during initial development.
2.1. Magnet
The magnet is a critical component of the spectrometer, whose characteristics have a strong impact on the overall sensitivity and uncertainty of tooth measurements, as well as clear roles in defining the composite weight of the system, applicability across the general population, and power requirements. In order to minimize the weight of the system, reduce power consumption, and alleviate possible needs for cooling, our dosimeter designs have relied on permanent magnets, with additional smaller electromagnets for field sweeping and modulation (Swartz et al. 2007, Salikhov et al 2005). The deployable spectrometer uses a dipole magnet which was designed and fabricated by Resonance Research Inc. (Billerica, MA). This magnet operates at 41.5 mT weighs 30 kg, has a 17 cm gap, and magnetic field homogeneity of ±500 ppm within a 2 cm central measurement volume. Integrated field sweep and modulation coils provide 4 mT sweep range and 0.4 mT modulation at 20 kHz.
2.2. RF Bridge
An essential difference in bridge construction for laboratory use and for field-deployable operation is that the latter needs to be operated by a non-experienced person. Therefore, especially considering the needs for reliability and high throughput, the bridge has to be fully automated via computer control and integrated circuitry. A number of modifications in the bridge design have been implemented in order to meet this need and optimize performance for in vivo tooth dosimetry. Earlier bridges, built primarily for oximetry, were equipped with RF oscillators with wide frequency ranges to cover the changes in resonator frequency that result from loading with different tissues, which may be as large as 80 MHz. While these cavity-stabilized, mechanically tuned oscillators do provide the desired low phase noise, they are vulnerable to mechanical vibration and are not appropriate for electronic adjustment of frequency. The requirement for RF bandwidth is greatly reduced in a spectrometer for tooth dosimetry applications, where the loading results in frequency changes of <10 MHz and Voltage-Controlled Oscillators (VCO) can readily be used. The new bridge specifically built for the transportable dosimeter employs a VCO which is electronically tunable from 1130 to 1170 MHz and followed by a power amplifier to provide up to 100 mW of incident power. Another new component used in this bridge is an electronically tunable phase shifter that enables automated adjustment of the RF phase for the homodyne detection. Furthermore, as discussed further below, an electronic remote coupling circuit has been incorporated within the transmission line to provide automated compensation of small mismatches which may occur during measurements. Following detection at the RF carrier frequency, the signal is fed to a lock-in amplifier (Signal Recovery SR7265, Oak Ridge, TN) for detection of the 1st-harmonic EPR signal at the 20 kHz modulation frequency.
2.3. Resonator
The microwave resonator is one of the key elements of the EPR dosimeter for achieving high sensitivity for detection of the radiation-induced radicals. Surface-coil resonators with optimized transmission line lengths and loop sizes for measurements on molar and incisor teeth have been especially productive, providing both high sensitivity and geometries that facilitate localized measurements on teeth in situ (Walczak et al. 2005, Salikhov et al. 2003, Salikhov et al. 2005, Swartz et al. 2005, Pollack et al. 2010). In the deployable dosimetry system, electronic adjustment of the resonator coupling is provided by a newly designed remote coupling unit. This unit is comprised of a variable-intensity light emitting diode and nonmagnetic photo sensitive resistor placed across the central conductor and ground of the RF transmission line that connects the bridge to the resonator. The diode is computer-controlled based on a feedback signal which reflects the balance of the bridge. The remote coupling system provides a range of control that is sufficient to automatically compensate for differences between installations of the resonator on different subjects and it does not produce any significant change of resonant frequency of resonator. Additionally, this unit is small, works with all existing resonators, and does not decrease the SNR or generate additional baseline distortion.
In the current configuration of the dosimeter, each resonator incorporates a built-in reference standard, whose characteristic signal appears in each recorded EPR spectrum. A small ampoule of 15N-perdeuterated tempone (15N-PDT) is permanently affixed to the transmission line of each resonator and results in a set of stable EPR signals offset in magnetic field from the tooth signals. In order to increase the stability of these samples and provide a narrow EPR linewidth, ampoules are prepared using glass tubing, which is nitrogen purged prior to sealing, and deuterated water. We have observed that the amplitudes and other spectral characteristics of these samples are stable for periods of at least months. The PDT signal serves many quality control purposes, including continuous overall verification that the spectrometer is operating correctly, physical recording of the applied modulation field amplitude and magnetic field scan width, and absolute physical magnetic field calibration for each of the recorded spectra for use in data analysis. The PDT signal, or that of another paramagnetic material such as the Bruker Marker (Bruker Bio-Spin, Billerica, MA), could also be applied as an amplitude standard, though this is not implemented in the current configuration of the dosimeter and supplementary quality control of reference material stability may be needed. The reliable use of these signals as amplitude standards is currently limited by the physical construction of the existing resonators, where the detection loop is allowed to flex slightly when under pressure. Future resonators will be designed with increased rigidity or with incorporation of these reference materials directly on the loops to facilitate use of these materials as reliable amplitude standards, providing an additional level of quality control.
2.4. Computer Control and Automated Spectral Analysis
In the deployable dosimeter, all aspects of instruments necessary for tuning, data collection, and data analysis are automated through the use of dedicated software developed using LabVIEW (National Instruments, Austin, TX). The data acquisition computers are PCI based with digital acquisition cards (Measurement Computing DAS6036, Norton, MA) to perform data acquisition, control of the magnetic field sweep, and monitoring of the bridge. Currently several instruments in each system are controlled by GPIB or USB/GPIB, including the lock-in amplifier (Signal Recovery SR7265, Oak Ridge, TN) and a frequency counter (Agilent 5318A, Santa Clara, CA). Automatic tuning of the spectrometer is performed by sweeping the frequency of the VCO while monitoring the balance of the bridge, providing a capability similar to a simplified network analyzer, while iteratively optimizing the coupling and RF phase.
One of the signature features of in vivo EPR tooth dosimetry is the ability to provide an immediate estimate of dose at the time of data acquisition. The data acquisition software performs online spectral fitting using repeated scans which are median-filtered to improve SNR and automatically exclude outlying data. The peak-to-peak amplitude of this signal is estimated and related to the absorbed dose using a tooth-type and resonator-shape dependent calibration curve (Williams et al.2010). Spectral fitting is performed using the Levenberg-Marquadt non-linear least squares method with a simple overmodulated Lorentzian model of the tooth spectrum, as spectral features due to g-anisotropy are not readily apparent at L-band frequencies (Demidenko et al. 2007). The linewidth of the tooth signal is fixed based on empirical data, the center of the tooth spectrum is fixed relative to that of the PDT reference standard, and the phases of these signals are locked. Uncertainties in each of the fit parameters are given by the non-linear least square fitting algorithm and allow an estimate of the uncertainty in the absorbed dose for a single individual. Additional measurements can be made to lower the uncertainty as needed (Demidenko et al. 2007).
2.5. Positioning and Ergonomic Considerations
The successful acquisition of data to reliably estimate absorbed dose relies critically upon the ability to immobilize the subject in a comfortable position with their tooth located centrally within the magnet and the resonator detection loop fixed against the tooth surface. Improper positioning of the resonator and motion during the measurements are sources of systematic error and noise which reduce the quality of dose prediction. In the current system, the subjects’ heads are positioned, supported, and immobilizing using an adjustable bar placed against the forehead and a U-shaped bite platform that is attached to the magnet and guides subjects to position themselves so the tooth of interest at the center of the magnet within the volume of magnetic field homogeneity. The system is designed to support the weight of the head and avoid discomfort in the neck due compression of the cervical vertebrae, making it easier for the subject to remain still during measurement. The resonator is mounted to a non-magnetic lockable articulating arm which is adjusted to position and hold the detection loop of the resonator against the tooth surface. This system requires an expert operator for proper positioning and does not adapt automatically if the subject moves after the position has been locked. To overcome these deficiencies, a refined resonator positioning system is under development which provides only two rotational and two translational limited degrees of freedom, incorporates a spring-loading mechanism to ensure proper pressure of the loop against the tooth throughout the measurement, and includes clear reference marks and locking mechanisms for easy operability.
3. Initial Results
3.1. Field Deployment Exercises
In the course of development a number of exercises were conducted where the existing system was deployed to a remote site to perform feasibility testing and conduct benchmark tests of technical improvements of the field deployable tooth EPR dosimeter (Nicolalde et al. 2010). These exercises included deployments of the dosimeter to the local firehouse for measurements of normal and irradiated subjects, the grounds of a local middle school for measurements of volunteers participating in a cancer center fundraiser, and shipment of the dosimeter to the EPR 2010 conference in San Juan, Puerto Rico and the Cutting-Edge Biomedical EPR Methods workshop in Milwaukee, WI, USA.
The exercise conducted at the Hanover, NH firehouse during the summer of 2009, included measurements of canine teeth in 8 normal subjects and 2 patients who had received treatment for head and neck cancers. Measurements were performed using the 1st generation prototype of the deployable dipole magnet, a version of the RF bridge which did not include features for automation, and a resonator configured for measurements of canine teeth. The system was powered via the municipal 110V supply and the firehouse communications and operation were unabated during measurements. Measurements were successfully performed for all subjects without physical complications, though data quality was affected by significant heating of the magnet which introduced noise and a considerable magnetic field shift in later measurements. As expected, radiation induced signals were not observed for the unirradiated subjects while signals were observable for the irradiated subjects. Figure 2 shows a spectrum recorded from one irradiated patient whose tooth had received an estimated dose of 17.6 Gy, based on the median of 24 three-second scans. This spectrum can be compared to a measurement made of the same tooth, using 60 three-second scans, with the same resonator and detection system in the laboratory with the whole-body magnet. It should be noted that the detection sensitivity for canine teeth and the resonator used in these experiments, which was the best available at that time, is markedly lower than that for molar teeth and incisor teeth using our current resonators. In these spectra, the amplitudes of the radiation-induced tooth signals are similar, while the SNR of the spectrum acquired with the deployed system is improved by a factor of 3.5, presumably due to improved immobilization with the new magnet geometry, bite platform, and with seated subjects.
Figure 2.
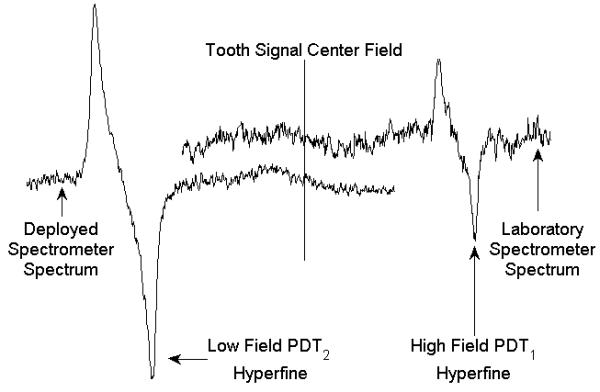
EPR spectra from a natural canine tooth which had been irradiated to an estimated 17.6 Gy acquired using the fixed laboratory spectrometer and an early version of the deployable dosimeter are compared. Different PDT reference standards and sweep ranges were used for these measurements, as is reflected in the plot, though the EPR amplitudes are on the same scale.
More recently the fully automated dosimeter, including a second generation dipole magnet with improved heat dissipation, was used to measure isolated incisor teeth at the EPR 2010 conference in San Juan, Puerto Rico. The spectrometer was set up in the hall outside the main conference room and powered by the municipal 110V supply. The spectrometer was shipped with the integrated detection system and power supplies contained within the rugged instrument rack and the magnet in a separate magnetically shielded box. Spectra are shown for an unirradiated tooth and teeth irradiated to doses of 2 Gy and 10 Gy (Figure 3). The increase observed in the radiation induced signal is consistent with results collected in the laboratory, with a linear dose response and comparable SNR.
Figure 3.
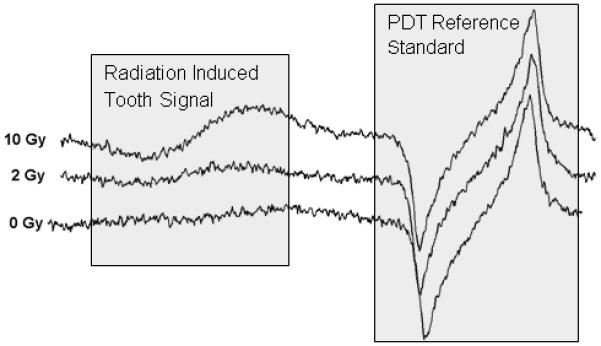
Data collected using the fully automated dosimeter at the EPR 2010 conference in San Juan, Puerto Rico of isolated incisor teeth which had been irradiated to 0, 2, or 10 Gy. The dose dependence of the radiation signal signal (to the left) is apparent and consistent with laboratory results.
3.2. In vivo Measurements with Unirradiated Subjects and TBI Patients
The performance of the deployable system was evaluated in the laboratory setting using 10 unirradiated subjects and 6 patients who had received total body irradiation (TBI) to a prescribed dose of 2 Gy. With an eye toward rapidly identifying people who have been exposed to doses >2Gy, measurements were performed for the labial surface of the upper incisor teeth. Variability in the EPR signal due to UV light exposure is expected to have minimal impact on screening accuracy (Ivannikov et al. 1997, Sholom et al. 2000). For each subject, three independent sets of data were collected, where data collection for each set required 80 seconds and the results from each set were averaged to provide a single dose estimate. A statistically significant 75% increase in the radiation induced EPR signal was observed (p=0.001, one-tailed Student’s t-test with equal variance), the dose response was consistent with similar measurements performed in a mouth model system, and the standard error of dose prediction was measured to be 0.9 ± 0.3 Gy (Figure 4). Efforts to further reduce variability in the estimated doses include improvement in the resonator positioning system to ensure consistent positioning of the detection loop on the tooth surface and instrumental refinements to decrease the impact of baseline distortion.
Figure 4.
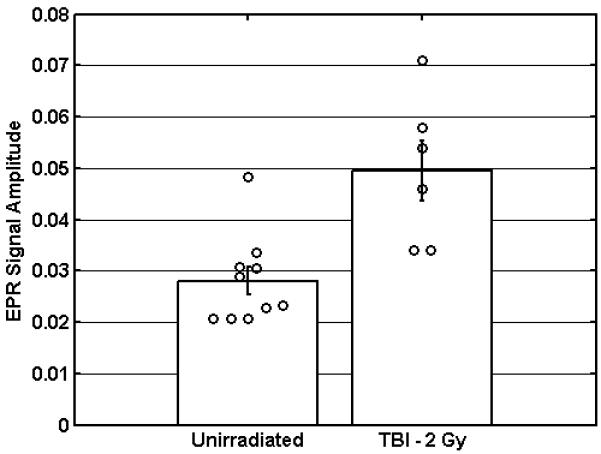
In vivo measurements were performed for unirradiated subjects (n=10) and patients who had received TBI to a prescribed dose of 2 Gy (n=6). Along with the individual dose estimates, the mean EPR amplitudes are shown which demonstrate a 75% increase in amplitude. (Error bars represent the standard error of the mean).
4. Discussion and Conclusion
A number of technical and procedural refinements have been implemented which enable the execution of in vivo EPR tooth dosimetry at remote sites, increase the level of automation for reliable operation by untrained operators, and provide the ability to perform dose estimation with a standard error of dose prediction near 1 Gy based on a data acquisition period of 5 minutes. These advances are crucial improvements upon the previous laboratory device and, with these capabilities, the prototype device is close to meeting the requirements for use during triage. However, a number of additional refinements are necessary for optimal incorporation of the EPR tooth dosimeter into a national plan to respond to an IND detonation or other massive radiation exposure. These refinements include further miniaturization of the instrument and improved robustness to facilitate operation in the field. Significant reduction in the size and weight of the instrument can readily be achieved through the elimination of several commercial instruments within the system, including the lock-in amplifier, frequency counter, and power supplies, in favor of specialized integrated components. A number of alternate, smaller magnet designs are under development, including an already operational flat magnet which weighs only 10 kg (Resonance Research Inc, Billerica MA). Significant advances in the construction of the surface loop resonators are expected to increase the mechanical reliability of these devices. In addition to these physical refinements, it will be necessary to design the dosimeters to be manufacturable in significant numbers and approved by the FDA for use in the United States or by other regulatory agencies for international use. Significant procedural refinements are also necessary to ensure fail-safe operation, including further improvements of the resonator and subject positioning systems and the incorporation of automated quality assurance processes.
We envision that with these improvements, reliable throughput of 5 min per subject will be achieved. We have already performed proof-of-concept measurements using 5 minutes of scan time on our current system. With additional advances in patient and resonator positioning, as well as increases in signal detection sensitivity, we expect the average total measurement time to be reduced to 5 minutes or less, including subject transfers and positioning. Assuming that each machine can be operated 23 hours a day with 1 hour allowed each day for maintenance, approximately 275 people could be measured per machine-system per day. If the goal then is to perform triage for 500,000 people using EPR biodosimetry as rapidly as possible, using the above assumptions, 1000 concurrently operated systems could accomplish this task in a 48 hour period.
The promise of in vivo EPR tooth dosimetry as a technique for large-scale biodosimetry has been widely recognized due to the unique stability of the radiation induced signal, the ability to provide immediate results, and the independence from confounding biologic interactions. Earlier laboratory studies established that the technique is feasible and that adequate accuracy and precision of dose estimation could be achieved. However, the design, fabrication and testing of a deployable instrument remained to be done. With the newly existing deployable prototype, we have now demonstrated that in vivo EPR tooth dosimetry can be performed at remote locations. These developments represent the next step toward production of a fully functional biodosimeter suitable for performing triage after catastrophic radiation exposure of a large population.
Acknowledgements
Supported by NIH U19AI067733 and R43AI081495.
Abbreviations
- VCO
Voltage Controlled Oscillator
- IND
Improvised Nuclear Device
- SEP
Standard Error of Prediction
- PDT
Perdeuterated Tempone
- TBI
Total Body Irradiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GA, Swartz HM, Amundson SA, Blakely WF, Buddemeier B, Gallez B, et al. BiodosEPR-2006 Meeting, 2007. Acute dosimetry consensus committee recommendations on biodosimetry applications in events involving uses of radiation by terrorists and radiation accidents. Radiation Measurements. 2007;42:972–996. [Google Scholar]
- Bell WC, Dallas CE. Vulnerability of populations and the urban health care systems to nuclear weapon attack – examples from four American cities. International Journal of Health Geographics. 2007;6(5) doi: 10.1186/1476-072X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddemeier BR, Dillon MB. Key Response Planning Factors for the Aftermath of Nuclear Terrorism. 2009. LLNL-TR-410067.
- Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, Yeskey K, Knebel A. Medical Response to a Radiologic/Nuclear Event: Integrated Plan From the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Annals of Emergency Medicine. 2009;53(2) doi: 10.1016/j.annemergmed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Demidenko E, Williams BB, Sucheta A, Dong R, Swartz HM. Radiation dose reconstruction from L-band in vivo EPR spectroscopy of intact teeth: Comparison of methods. Radiation Measurements. 2007;42:1089–1093. doi: 10.1016/j.radmeas.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattibene P, Callens F. EPR dosimetry with tooth enamel: A review. Applied Radiation and Isotopes. 2010;68(11):2033–116. doi: 10.1016/j.apradiso.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Flood AB, Bhattacharyya S, Nicolalde RJ, Swartz HM. Implementing EPR Dosimetry for Life-Threatening Incidents: Factors Beyond Technical Performance. Radiat Meas. 2007;42:1099–1109. doi: 10.1016/j.radmeas.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood AB, Nicolalde RJ, Demidenko E, Williams BB, Shapiro A, Wiley AL, Jr., Swartz HM. A Framework for Comparative Evaluation of Dosimetric Methods to Triage a Large Population Following a Radiological Event. Radiation Measurements. doi: 10.1016/j.radmeas.2011.02.019. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, Coleman N, Manning RG, Maidment BW, Hatchett R. Rapid Radiation Dose Assessment for Radiological Public Health Emergencies: Roles of NIAID and BARDA. Health Physics. 2010;98(2):172–178. doi: 10.1097/01.HP.0000348001.60905.c0. [DOI] [PubMed] [Google Scholar]
- Howe D. National planning scenarios executive summaries. The Homeland Security Council; 2004. [Google Scholar]
- Ivannikov AA, Skvortzov VG, Stepanenko VF, Tikunov DD, Fedosov IM, Romanyukha AA, Wieser A. Wide Scale EPR Retrospective Dosimetry: Results and Problems. Radiation Protection Dosimetry. 1997;71:175–180. [Google Scholar]
- Iwasaki A, Walczak T, Grinberg O, Swartz HM. Differentiation of the observed low frequency (1200MHz) EPR signals in whole human teeth. Applied Radiation and Isotopes. 2005a;62(2):133–9. doi: 10.1016/j.apradiso.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Grinberg O, Walczak T, Swartz HM. In vivo measurements of EPR signals in whole human teeth. Applied Radiation and Isotopes. 2005b;62(2):187–90. doi: 10.1016/j.apradiso.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Meade C, Molander RC. Technical Report of the RAND Center for Terrorism Risk Management Policy. RAND Corporation; Santa Monica, CA: 2006. Considering the effects of a catastrophic terrorist attack. [Google Scholar]
- Nicolalde RJ, Gougelet RM, Rea M, Williams BB, Dong R, Kmiec MM, Lesniewski PN, Swartz HM. The view from the trenches: Part 2-technical considerations for EPR screening. Health Physics. 2010;98(2):128–35. doi: 10.1097/01.HP.0000348461.00071.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J, Williams B, Sidabras J, Grinberg O, Salikhov I, Lesniewski P, Kmiec M, Swartz HM. Surface Loop Resonator Design for in vivo Epr Tooth Dosimetry Using Finite Element Analysis. Health Physics. 2010;98(2):339–344. doi: 10.1097/HP.0b013e3181a6dd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids. I. Theory and data analysis. Journal of Magnetic Resonance. 1999;138:199–209. doi: 10.1006/jmre.1999.1737. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Hirata H, Walczak T, Swartz HM. An improved external loop resonator for in vivo L-band EPR spectroscopy. J Magn Reson. 2003;164(1):54–59. doi: 10.1016/s1090-7807(03)00175-7. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM. EPR spectrometer for clinical applications. Magnetic Resonance in Medicine. 2005;54(5):1317–1320. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- Sholom SV, Chumak VV, Pasalskaja LF. Some aspects of EPR dosimetry of liquidators. Appl. Radiat. Isot. 2000;52(5):1283–1286. doi: 10.1016/s0969-8043(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB. A Critical Assessment of Biodosimetry Methods for Large-Scale Incidents. Health Physics. 2010;98(2):95–108. doi: 10.1097/HP.0b013e3181b8cffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Burke G, Coey M, Demidenko E, Dong R, Grinberg O, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo K-M, Nicolalde RJ, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell CA, Romanyukha A, Schauer DA. In vivo EPR for dosimetry. Radiation Measurements. 2007;42(6-7):1075–1084. doi: 10.1016/j.radmeas.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikhov I, Khan N, Lesniewski P, Thomas J, Romanyukha A, Schauer D, Starewicz P. In vivo EPR dosimetry to quantify exposures to clinically significant doses of ionizing radiation. Radiation Protection Dosimetry. 2006;120(1-4):163–70. doi: 10.1093/rpd/nci554. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikov I, Lesniewski P, Starewicz P, Schauer D, Romanyukha A. Measurements of clinically significant doses of ionizing radiation using non-invasive in vivo EPR spectroscopy of teeth in situ. Applied Radiation and Isotopes. 2005;62(2):293–9. doi: 10.1016/j.apradiso.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Williams BB, Nicolalde RJ, Demidenko E, Flood AB. Overview of Biodosimetry for Management of Unplanned Exposures to Ionizing Radiation. Radiation Measurements. (this issue) [Google Scholar]
- Walczak T, Lesniewski P, Salikhov I, Sucheta A, Szybinski K, Swartz HM. L-band electron paramagnetic resonance spectrometer for use in vivo and in studies of aqueous biological samples. Review of Scientific Instruments. 2005;76(1):013107–6. [Google Scholar]
- Williams BB, Dong R, Kmiec M, Burke G, Demidenko E, Gladstone D, Nicolalde RJ, Sucheta A, Lesniewski P, Swartz HM. Development of in vivo tooth EPR for individual radiation dose estimation and screening. Health Physics. 2010;98(2):327–38. doi: 10.1097/HP.0b013e3181a6de5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Sucheta A, Dong R, Sakata Y, Iwasaki A, Burke G, Grinberg O, Lesniewski P, Kmiec M, Swartz HM. Experimental procedures for sensitive and reproducible in situ EPR tooth dosimetry. Radiation Measurements. 2007;42(6):1094–1098. doi: 10.1016/j.radmeas.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]



