The problem of obesity has reached epidemic proportions. Sixty-five percent of US adults are said to be overweight (body mass index, >25 kg/m2).1 Globally, the overweight population is estimated to be 1 billion people.2 This epidemic has led to a resurgence of interest in adipose tissue metabolism, physiology, and pathophysiology. The adipocyte is no longer understood merely as a repository of fat but rather is a dynamic, metabolically active factory that produces metabolic substrates, hormones, cytokines, and adipokines that exert their actions locally, systemically, and even centrally at the hypothalamus to regulate overall energy homeostasis. Increasingly, we understand that obesity is not only a problem of too much fat, per se, but also a much more far-reaching dysregulation of metabolism that affects adipose tissue and other organs, such as the liver, muscle, and pancreas.
FAT STORES
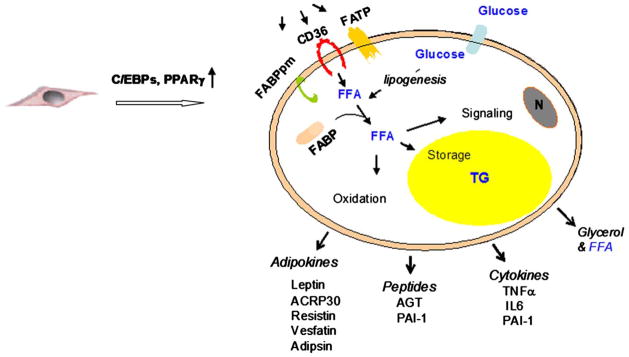
Adipose tissue is considered the natural organ for large stores of fat. A limited pool of fatty acids can be found in other organs (such as the liver, muscle, and pancreas), where they are considered “nontoxic” and are integral to cell function. The identification of non-differentiated cells (preadipocytes) confers to adipose tissue the potential to acquire new mature fat cells that appears to be permanent. The differentiation of the preadipocyte into an adipocyte is a complex event that is initiated mainly by peroxisome proliferator-activated receptor-γ and CCAAT/enhancer binding proteins.3 During this process, these nuclear factors trigger the expression of multiple genes that are involved in various steps of the adipogenesis, such as CD36 (also called fatty acid translocase), fatty acid transport proteins, fatty acid binding proteins, and lipoprotein lipase. Maturation of the adipocyte is associated with the gain of new functions that include lipogenic capacity and the appearance of cytoplasmic lipid droplets, acquisition of insulin sensitivity and increased glucose uptake, and the expression and secretion of numerous bioactive molecules (Fig 1). Transport of fatty acids through the lipid bilayer is a major step that regulates fatty acid utilization. Although passive diffusion has been described as the only mechanism of fatty acid transport through the cell membrane, the identification of new proteins in the plasma membrane added another important component in fatty acid transport. Several candidate proteins have been identified to play a role in this transport system: fatty acid translocase/CD36, fatty acid binding protein plasma membrane, and fatty acid transport protein. At the inner side of the cell membrane, fatty acid binds to fatty acid binding proteins (also designed aP2) and is transported to its destination of either oxidation, signal transduction, or esterification and triglyceride deposition.
Fig 1.
Model shows maturation of preadipocyte to adipocyte. Proteins that are implicated in fatty acid uptake and factors that are secreted by mature adipocyte are presented. FATP, Fatty acid transport protein; C/EBP, CCAAT/enhancer binding protein; PPARγ, peroxisome proliferator-activated receptor-γ; FABPpm, fatty acid binding protein plasma membrane; FFA, free fatty acids; N, nucleus; TG, triglycerides; AGT, angiotensinogen; PAI-1, plasminogen activator inhibitor type 1; IL-6, interleukin-6; ACRP30, adipocyte complement-related protein.
In obesity, adipose tissue nears its threshold in its capacity to store lipids and trap dietary fatty acids. As a result, the pool of circulating fatty acids increases and leads to an ectopic storage of fat in non-adipose tissues such as muscle, liver, pancreas, and possibly other organs. This overload of fat appears to cause an array of metabolic derangements that are associated with insulin resistance and commonly are dubbed the metabolic syndrome or “syndrome X.”4 It is now recognized that the intracellular accumulation of fatty acids and their metabolites alter insulin signaling and hinder insulin’s ability to stimulate glucose uptake.5 Furthermore, murine studies have shown that inactivation of fatty acid uptake in heart and skeletal muscle increased glucose uptake and prevented fat-induced insulin resistance.6 Numerous studies in man that were reviewed recently by Yu and Ginsberg7 show that lipid accumulation in muscle can be correlated with insulin resistance and the development of type 2 diabetes mellitus. Similarly, hepatic fatty infiltration is associated with insulin resistance and impaired suppression of hepatic glucose production by insulin. Additionally, body mass index and the waist-circumference ratio correlate positively with insulin resistance,8 which implies a specific role of central fat in the adverse obesity-related sequelae associated with the metabolic syndrome.
CENTRAL VERSUS PERIPHERAL ADIPOSITY
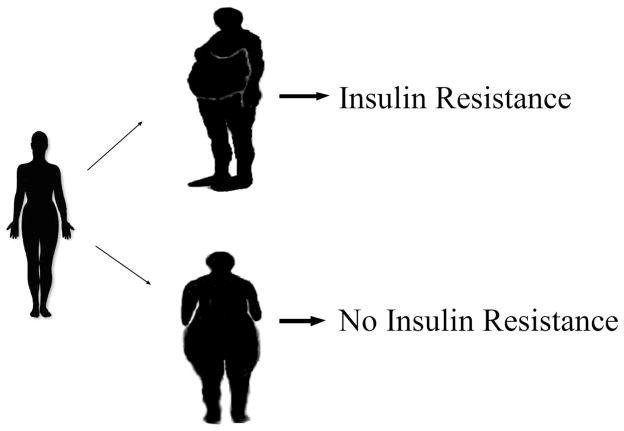
The current paradigm is that obese phenotypes can be classified as either android (truncal/central fat deposition or the “apple” shape) or gynoid (predominance of gluteofemoral fat deposition or the “pear” shape) with the android phenotype being linked to a greater prevalence of the comorbidities of hypertension, dyslipidemia, and type 2 diabetes mellitus, the sum of which encompasses the metabolic syndrome (Fig 2). Accordingly, the International Diabetes Federation’s newly released definition of the metabolic syndrome is predicated on racial- and gender-specific criteria for central obesity.9
Fig 2.
Development of an android phenotype (truncal/central obesity) leads to insulin resistance. A gynoid habitus (gluteofemoral obesity) does not confer the same risk of insulin resistance.
Regional differences in fat depots are intriguing and far-reaching and involve processes such as lipolysis, lipogenesis, fatty acid uptake, and the expression and secretion of hormones and inflammatory factors. Relative to visceral adipose tissue, subcutaneous fat, for example, exhibits a higher basal rate of lipolysis, hormone-sensitive lipase activity, anti-lipolytic action of insulin, expression of insulin receptor substrate 1 expression, lipoprotein lipase activity, and fatty uptake by preadipocytes.10 Additionally, expression of leptin (a pleiotropic hormone that, among other actions, regulates satiety through its hypothalamic action) correlates positively with the area of subcutaneous adipose tissue. Conversely, visceral adiposity exhibits a greater lipolytic response to catecholamines10 and a blunted response to insulin’s anti-lipolytic effect with increasing doses of insulin.11 This response may account for the hypertriglyceridemia seen in the metabolic syndrome.
Visceral fat predominates in the production of certain adipokines (eg, resistin, adiponectin), inflammatory cytokines (eg, interleukin 6), and plasminogen activator inhibitor 1.10 This observation provides a link between the low-grade, chronic inflammatory state of obesity and insulin resistance, in that resistin can induce insulin resistance in mice, and tumor necrosis factor-α (TNF-α) and interleukin-6 interfere with insulin activity directly by altering intracellular signaling and indirectly by influencing leptin concentration. Furthermore, the increase in glucose and non-esterified fatty acid concentrations seen with diabetes mellitus can lead to β-cell loss, which further facilitates the onset of diabetes mellitus.12 Some investigators have postulated that the increase in visceral fat-derived, portally delivered non-esterified fatty acids to the liver is a primary mechanism in the development of impaired hepatic glucose metabolism. Ironically, visceral fat secretes the newly identified hormone, visfatin that exhibits insulin-like properties, which is a fact that, at best, appears to mitigate the role of visceral fat in the development of insulin resistance.13 Visceral adipose tissue also expresses the angiotensinogen gene,14 which provides a potential mechanism for the hypertension that is seen in the metabolic syndrome.
Visceral and subcutaneous fats differ also in their morphologic condition and adipogenic capacity. In obese premenopausal women, the gynoid habitus is associated with smaller and more numerous fat cells than the android habitus, and visceral adipocytes are smaller than subcutaneous adipocytes.15 Fat cells that are harvested from obese patients who are undergoing gastric bypass exhibit 2 distinct populations of preadipocytes, rapidly and slowly replicating subtypes, both of which were identified in subcutaneous, mesenteric, and omental fat biopsy specimens, albeit in differing proportions by depot.16 Relative to mesenteric or subcutaneous fat, the slowly replicating subtype comprises a statistically greater proportion of preadipocytes, has a lower lipid-accumulating capacity, is more susceptible to TNF-α–induced apoptosis, and expresses less CCAAT/enhancer binding proteins α (adipogenic transcription factor). In fact, both preadipocyte subtypes accumulate less fat in omental tissue than in subcutaneous tissue. Although not proved, it seems a logical conclusion that visceral adipose tissue is “over-loaded” more readily, thereby leading to untoward sequelae.
Studies that used animal models have shown that the association of visceral fat with the metabolic syndrome is not absolute, however.17,18 Transgenic expression of human growth hormone in the hypothalamus of the rat17 induced late onset obesity in the male rat that is mostly visceral in nature and due to adipocyte hyperplasia and not hypertrophy, which suggests that the visceral fat in these animals has a greater storage potential because of a greater number of adipocytes. However, the animals have normal fasting blood glucose, enhanced insulin sensitivity, and a lack of intrahepatocellular or intramyocellular fatty deposits. This evidence indicates that abdominal obesity cannot be the sole, fundamental cause of the metabolic syndrome. Moreover, Korach-Andre et al18 questioned the causal relationship between visceral adiposity and insulin resistance in the muscle of rats under dietary and pharmacologic treatments.
Conversely, selective over-expression of 11β–hydroxysteroid dehydrogenase type 1 (the enzyme responsible for the formation of active glucocorticoids) in mouse adipose tissue increased the level of corticosterone and induced visceral obesity that was associated with pronounced insulin-resistance and hyperlipidemia.19 In humans and mice, elevation of serum retinol binding protein 4 is seen in obesity. Serum retinol binding protein 4 impairs insulin signaling in muscle and increases hepatic glucose production, likely through an effect on phosphoenolpyruvate kinase.20 Taken together, these observations suggest that abdominal obesity may not be the sole, fundamental cause of the metabolic syndrome. The onset of metabolic syndrome is most likely the result of biochemical factors either secreted or regulated by visceral fat that acts on vulnerable target organs.
LIPODYSTROPHY
The various forms of lipodystrophy (acquired and congenital) provide further support for the notion that the obesity-related metabolic syndrome is perhaps more an issue of storage capacity than fat quantity or location (lipodystrophy), in general, is a condition whereby a pathologic loss of fat leads to a constellation of symptoms like the metabolic syndrome. Despite the heterogeneity of the various lipodystrophies, the resultant impairment in insulin sensitivity common to all forms leads to type 2 diabetes mellitus.
STRESS AND INFLAMMATION
The question now is how this putative triglyceride overload leads to the constellation of symptoms seen in the metabolic syndrome. The answer may lie in the body’s stress response. Weisberg et al21 and Xu et al22 showed that, in obese humans, macrophages can account for up to 40% of the tissue mass. Accumulation of macrophages in the subcutaneous tissue appeared to plateau at an earlier level than that in visceral fat; these macrophages account for most of the overall secretion of TNF-α. These results lead to a reconsideration of the role of the adipocytes (and preadipocytes) alone and to take into account the role of macrophage infiltration into adipose tissue as a source of the inflammatory molecules.23
In 1990, Daniel et al24 discovered a group of molecules termed F2-isoprostanes, which are derived from free radical–catalyzed peroxidation of arachidonic acid. These investigators documented a very strong correlation between increased F2-isoprostanes and body mass index,25 which are findings that were reproduced by Keany et al26 in 2800 subjects who were enrolled in the Framing-ham study. Additional indirect support is obtained from the very strong association of obesity with platelet-activation, with more than a 3-fold increase in the excretion of platelet-derived thromboxanes in the urine of obese women.27 Furthermore, Ozcan et al28 studied murine models of obesity (diet-induced and genetic [obese mice]) and demonstrated a link between the stress of the endoplasmic reticulum and the inhibition of insulin action that is mediated by c-Jun N-terminal kinase and inositol-requiring kinase-1α.
TARGETED LIPECTOMY
When the evidence for the role of “ectopic fat” is considered and especially the differences in various fat depots and the particularly detrimental sequelae of central obesity in regard to coronary and cerebrovascular disease, it seems logical to focus on the ectopic visceral fat stores in the setting of obesity as a means of mitigating risk factors for stroke, heart attack, and even diabetes mellitus. Although the lipodystrophic model would suggest that excess fat removal could lead to a state of insulin resistance, perhaps there is a role for the selective removal of adipose tissue in the setting of obesity-associated insulin resistance.
We have collected preliminary data in the dog model of the role of visceral fat on glucose metabolism. Catheters in the hepatic vein, portal vein, and femoral artery were used to sample blood during hyperinsulinemic-euglycemic clamps. We studied mongrel dogs under a stepwise hyperinsulinemic-euglycemic clamp and achieved 4- and 10-fold basal levels of insulin, respectively, before and after removal of visceral fat (“omentectomy”). Low-dose insulin infusion is meant to shut off hepatic glucose production; higher insulin infusion maximizes peripheral (skeletal muscle) glucose uptake. The removal of visceral fat resulted in a reduction in basal hepatic glucose production by nearly 40%. Furthermore, during the high insulin infusion, omentectomy resulted in a greater than 2-fold increase from basal in peripheral glucose use by insulin-dependent tissue (predominantly skeletal muscle). In like fashion, Barzilai et al29 showed that in Zucker Diabetic Fatty rats, removing visceral fat delayed the onset of diabetes mellitus. Conversely, Klein et al30 found no improvement in insulin resistance, glucose, blood pressure, or other cardiovascular risk factors in 15 obese women before and after liposuction removal of subcutaneous abdominal fat in amounts equivalent to 9% of total body mass or 18% of total fat mass.
CONCLUSION
The proposition therefore is that fat, in and of itself, may not be the fundamental problem, but rather it may be the out-stripping of the adipocytes ability to handle a given triglyceride load that results in “ectopic fat storage”31 and subsequent inflammation that lead to the metabolic syndrome.32 Obesity, and specifically visceral obesity, could be considered a problem of excess triglycerides that leads to ectopic fat deposition, which could potentially initiate a stress response that leads to inflammation, excess circulating triglycerides, the under production of beneficial adipokines such as adiponectin, and the over production of deleterious adipokines such as resistin, the sum of which leads to insulin resistance and ultimately the metabolic syndrome. Understanding the significant role that visceral fat plays in the metabolic syndrome, either because of its more limited capacity to handle an increasing triglyceride load or its preferential production of deleterious cytokines, in conjunction with our own animal data combined with that of Barzilai et al,29 shows that targeted lipectomy can mitigate the detrimental effects of ectopic fat positively. To that end, we feel compelled to ask whether it would not make sense to offer omentectomy alone as treatment for type 2 diabetes mellitus. This approach effectively would remove a major source of portally delivered non-esterified fatty acids and possibly cytokines and adipokines and result in a reduction in basal glucose production and a concomitant improvement in glucose use by skeletal muscle, thus offering a potential cure for some of the major metabolic derangements of type 2 diabetes mellitus and the metabolic syndrome. The procedure could be performed easily by trained laparoscopic surgeons as an outpatient procedure and would obviate the need for the chronic medical treatment of diabetes mellitus.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Obesity and Overweight. Diet, nutrition, and the prevention of chronic diseases. Geneva/Rome: World Health Organization; 2003. World Health Report TRS916. [PubMed] [Google Scholar]
- 3.Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29(suppl):S13–6. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 5.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–9. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042–52. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 8.Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, et al. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians: analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest. 1996;98:1195–209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The IDF consensus worldwide definition of the metabolic syndrome. 2005. [Google Scholar]
- 10.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–83. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 11.Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–4. doi: 10.2337/diabetes.48.1.10. [DOI] [PubMed] [Google Scholar]
- 12.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 14.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19:457–66. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 15.Garaulet M, Perex-Llamas F, Fuente T, Zamora S, Tebar FJ. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol. 2000;143:657–66. doi: 10.1530/eje.0.1430657. [DOI] [PubMed] [Google Scholar]
- 16.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–77. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 17.Bains RK, Wells SE, Flavell DM, Fairhall KM, Strom M, Le Tissier P, et al. Visceral obesity without insulin resistance in late-onset obesity rats. Endocrinology. 2004;145:2666–79. doi: 10.1210/en.2003-1608. [DOI] [PubMed] [Google Scholar]
- 18.Korach-Andre M, Gao J, Gounarides JS, Deacon R, Islam A, Laurent D. Relationship between visceral adiposity and intramyocellular lipid content in two rat models of insulin resistance. Am J Physiol Endocrinol Metab. 2005;288:E106–16. doi: 10.1152/ajpendo.00089.2004. [DOI] [PubMed] [Google Scholar]
- 19.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 21.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel VC, Minton TA, Brown NJ, Nadeau JH, Morrow JD. Simplified assay for the quantification of 2,3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl. 1994;653:117–22. doi: 10.1016/0378-4347(93)e0432-p. [DOI] [PubMed] [Google Scholar]
- 25.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, et al. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 26.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 27.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–14. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 29.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–8. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- 30.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–57. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 31.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J ObesRelat Metab Disord. 2004;28(suppl):S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 32.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–34. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]