Abstract
Objectives
Acrolein is a toxic chemical present in tobacco, wood and coal smoke as well as automobile exhaust. Because exposure to these pollutants is associated with an increase in cardiovascular disease risk, we studied the effects of acrolein on Flk-1+/Sca-1+ cells that are involved in vascular repair.
Methods and Results
In adult male C57BL/6 mice, inhalation of acrolein (1ppm, 6h/day, 4 days or 5ppm for 2 or 6h) led to the formation of protein-acrolein adducts in the bone marrow and diminished levels of plasma NOx and circulating Flk-1+/Sca-1+ but not Sca-1+ only cells. Acrolein exposure increased the number of apoptotic Flk-1+/Sca1+ cells in circulation, and increased bone marrow-derived cells with endothelial characteristics (Dil-acLDL/UE-lectin and Flk-1+/Sca-1+) in culture. Deficits in the circulating levels of Flk-1+/Sca-1+ cells were reversed after 7 days of recovery in acrolein-free air. Exposure to acrolein blocked VEGF/AMD3100-stimulated mobilization of Flk-1+/Sca-1+ but not Sca-1+ only cells and prevented VEGF-induced phosphorylation of Akt and eNOS in the aorta.
Conclusions
Inhalation of acrolein increases apoptosis and suppresses the circulating levels of Flk-1+/Sca-1+ cells, while increasing these cells in the bone marrow and preventing their mobilization by VEGF. Exposure to acrolein-rich pollutants could impair vascular repair capacity.
Keywords: acrolein, aldehydes, endothelial dysfunction, environmental cardiology, progenitor cells, VEGF signaling
Introduction
Several epidemiological studies show that exposure to combustion products increases the risk of developing cardiovascular disease. In many large population-based studies, long-term exposure to traffic-generated combustion products has been found to be associated with an increased risk for coronary heart disease1, atherosclerosis2 and fatal myocardial infarction3, and likewise, exposure to products of wood or coal combustion is linked to increases in blood pressure and cardiovascular mortality4, 5. Moreover, individuals such as bus drivers6, chimney sweeps7 and firefighters8, who are repeatedly exposed to combustion products, have significantly higher rates of cardiovascular mortality than the general population. In addition, extensive data show that cardiovascular disease and mortality are increased by exposure to combustion products generated during smoking9 or present in secondhand tobacco smoke10. Experimental data with humans or animals exposed to automobile exhaust11 or tobacco smoke9, 10 support these epidemiologic findings. These studies suggest that combustion products induce adverse cardiovascular effects. Nonetheless, the chemicals that mediate the cardiovascular toxicity of combustion products are unidentified.
Combustion of organic material results in the generation of a complex chemical mixture. The composition of this mixture varies with the source; however, one of the chemicals common to all combustion sources is acrolein. It is produced in high amounts during combustion of organic material in any form (coal, wood, paper, cotton, gasoline, diesel or tobacco)12. Recent estimates show that high levels of acrolein (between 6–8 ppm13) are present in exhaust gases from petrol and diesel engine vehicles and that up to 50–70 ppm acrolein is generated in tobacco smoke (100–600 µg/cigarette)14. Acrolein is a highly reactive and toxic chemical,15, 16 and it appears likely that it could account, at least in part, for the cardiovascular toxicity induced by combustion products. Our previous studies show that acrolein inhalation leads to endothelial dysfunction in susceptible mice17. Endothelial injury is also an early and integral feature of the cardiovascular toxicity of automobile exhaust11, tobacco9, 10 and wood smoke18 exposures. However, the mechanisms by which these exposures affect endothelial health remain unclear. Recent work suggests that endothelial repair and regeneration depends, in part, upon circulating cells with pro-angiogenic potential19. The blood level of Flk-1+/Sca-1+ cells is a sensitive index of endothelial health and is inversely correlated with cardiovascular disease risk19. The current study was, therefore, designed to test the hypothesis that acrolein exposure adversely affects the number and mobilization of circulating Flk-1+/Sca-1+ cells.
Methods
Animals
Mice were treated according to APS’s Guiding Principles in the Care and Use of Animals and all protocols were approved by University of Louisville IACUC.
Inhalation exposure
Male C57BL/6 mice (12–16 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Acrolein atmospheres were generated from liquid acrolein (Sigma-Aldrich, St. Louis, MO, USA) and mice were exposed to acrolein (0.5, 1 or 5 ppm; for 2 h up to 4 days) using a custom vapor system (Teague Enterprises, Inc., Woodland, CA, USA; Suppl. Schematic I) as described17.
Circulating progenitor cell mobilization
To stimulate Flk-1+/Sca-1+ cell mobilization, mice were injected with saline or VEGF165 daily for 4 consecutive days (Suppl. Schematic II). Immediately after the final exposure, VEGF-injected mice received the CXCR4 antagonist, AMD3100, and control mice received saline. Mice were euthanized 1h after AMD3100 or saline injection20.
Peripheral blood mononuclear cells and flow cytometry
Procedures for Flk-1+/Sca-1+ cell identification by flow cytometry were adapted from published methods21, 22 and are as described in supplement methods online. Briefly, whole blood was lysed and cells were labeled with FITC-Sca-1 and APC-Flk-1 antibodies. Cells positive for these antibodies were counted by flow cytometry (LSRII, BD BioSciences; Suppl. Fig. I) and data were analyzed using FACSDiva v6.0 (BD Biosciences).
Isolation and culture of bone marrow-derived progenitor cells
Bone marrow was aspirated from femur and tibia of both legs and mononuclear cells were separated by Ficoll gradient centrifugation. Cells (8×105) were seeded on fibronectin-coated plates, cultured 7 days, labeled with either DiI-acLDL and UE-lectin or Flk-1 and Sca-1 antibodies and analyzed by either fluorescence microscopy or flow cytometry as described in the supplement methods online.
Isolated aorta studies
Thoracic aorta were isolated and vascular reactivity assayed as described17. To test for intact VEGF signaling, isolated aortas were incubated in autologous plasma and stimulated with VEGF165 (20ng/mL) for 15 min.
Western blot analyses
Standard protocols were used for Western blotting of Akt, eNOS, ERK (total and phosphorylated forms), VEGFR-2, MMP-9 and protein-acrolein adducts. Additional procedures and details are described in the supplement methods online.
Statistical analysis
Data are reported as mean ± SEM. For comparing two groups, unpaired Student’s t-test was used. Multiple groups were compared using One Way ANOVA with Bonferroni post-hoc test where appropriate (SigmaStat, SPSS, Chicago, IL, USA). Statistical significance was accepted at p<0.05.
Results
Characterization of circulating Flk-1+/Sca-1+ cells
Analysis of the mononuclear cell population in peripheral blood showed that Flk-1+/Sca-1+ cells (6.6±0.9 cells/µl whole blood; n=12) account for 14–16% of cells within the relatively small (3–5 µm) lymphocytic subpopulation of the gated region (Figs. 1A, 2A). These cells also stained positive for CXCR4 (97±1%, Fig. 1C), CD45 (92.6±1.7%, Fig. 1D), CD31 (i.e., PECAM, endothelial cell marker, >99%) and progenitor cell markers (e.g., CD133, 65.7±1.9%) as well as several lymphocytic markers (Suppl. Fig. I). The majority of Flk-1+/Sca-1+ cells stained positive for the transcription factor inhibitor of DNA binding 1, Id1 (98%; Fig. 1E), which is a selective endothelial progenitor cell (EPC) marker23.
Figure 1. Characterization of Flk-1+/Sca-1+ cells by flow cytometry and microscopy.
A, Representative dot plots of forward- (FSC) and side-scatter (SSC) of circulating cells and size beads indicating an approximate size range of Flk-1+/Sca-1+ cells (3–5 µm). B, Brightfield and fluorescence images of Flk-1+/Sca-1+ cells sorted by MoFlo. Sca-1 and Flk-1 positive immunoreactivity indicated by green and red staining, respectively (merged image in far right panel). C, Dot plots of FSC and PE-CXCR4 fluorescence of Flk-1+/Sca-1+ cells that were also CXCR4+. D, Dot plots of SSC and PerCP-CD45 fluorescence of Flk-1+/Sca-1+ cells representing CD45+/− cells. E, Confocal image of sorted Flk-1+/Sca-1+ cells stained with Id1 antibody. Nuclei were stained with DAPI.
Figure 2. Effects of acrolein on circulating Flk-1+/Sca-1+ cells.
A, Flow cytometry analysis of forward- (FSC) and side-scatter (SSC) of peripheral blood mononuclear cells of mice breathing filtered air or acrolein (1ppm, 6h/day, 4 days). B, Representative dot plots of circulating Flk-1+/Sca-1+ cells in peripheral blood of mice after 4 days of exposure to filtered air or acrolein. Levels of C, Flk-1+/Sca-1+ cells and D, Sca-1+ only cells in peripheral blood after breathing filtered air or acrolein for indicated days (n=8–12). E, Flk-1+/Sca-1+ cells in blood obtained from mice breathing filtered air or 5 ppm acrolein for 2 or 6h. F, Flk-1+/Sca-1+ cells in blood of mice breathing air or 1 ppm acrolein (6h/d, 4d) and after 7 days recovery (* p<0.05; n=4). G, Representative dot plots of SSC/FSC and staining for 7-AAD and Annexin V within the Flk-1+/Sca-1+ cell population in the blood of mice inhaling air or acrolein (1 ppm; 6h). The bottom panel shows group data (n=5); * p<0.05).
Effects of acrolein on circulating Flk-1+/ Sca-1+ cells
Exposure to acrolein (1ppm, 6h/day) resulted in a progressive decline in the level of circulating Flk-1+/Sca-1+ cells (Fig. 2C). After 4 consecutive days of exposure, the number of Flk-1+/Sca-1+ cells in acrolein-exposed mice were <25% of those in mice breathing filtered air (p<0.05). No changes in Sca-1+ only cell levels were observed (control: 41.6±7.7 cells/µl whole blood; n=12; Fig. 2D). Exposure to a lower level of acrolein (0.5ppm, 6h/day) for 4 days did not significantly affect the number of circulating Flk-1+/Sca-1+ or Sca-1+ only cells (data not shown); however, exposure to higher (5ppm) acrolein concentration led to a rapid (within 6h) 43% decrease in Flk-1+/Sca-1+ cell level (Fig. 2E). Although 1ppm acrolein (6h/d, 4d) did not alter plasma lipids (Suppl. Table I) or blood leukocyte levels, exposure to 5ppm acrolein (6h) significantly decreased the number of circulating leukocytes (Suppl. Table II). These results suggest that acrolein inhalation induces a progressive loss of circulating Flk-1+/Sca-1+ cells without inducing overt toxicity at least at low acrolein concentration. The effects of acrolein were reversible. Recovery for 7 days in filtered air after 4 days of exposure to 1ppm acrolein led to a complete reversal of the effect, as shown by a similar number of Flk-1+/Sca-1+ cells in exposed and unexposed mice (Fig. 2F). This indicates that removal of acrolein from the atmosphere allows full recovery. Acrolein exposure (1ppm, 2d, 6h/d) significantly increased the percentage of Flk-1+/Sca-1+ cells that stained positive for both necrosis/late-apoptosis (7AAD) and early apoptosis (Annexin-V) markers (Fig. 2G). However, no significant changes in Sca-1+ cell population were observed.
Effects of acrolein on Flk-1+/ Sca-1+ cells in the bone marrow
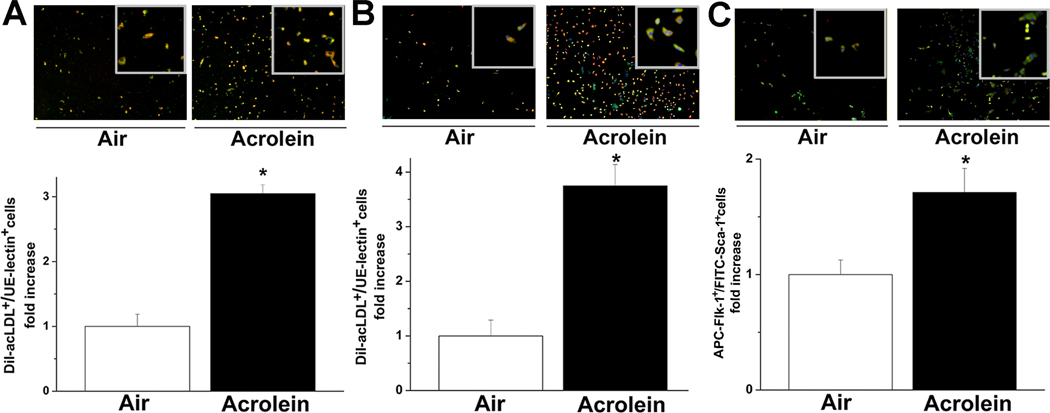
To examine changes in Flk-1+/Sca-1+ cells in the bone marrow, mononuclear cells were isolated from the bone marrow of mice exposed to filtered air or acrolein (1 or 5ppm) and cultured on fibronectin-coated plates. Cells were cultured for 7 days, incubated with Dil-acLDL and stained with UE-lectin (Suppl. Fig. IIA, B) or immunolabeled for Flk-1/Sca-1. The majority of Flk-1+/Sca-1+ cells isolated from bone marrow also stained positive for Dil-acLDL as measured by flow cytometry (Suppl. Fig. IIC). Additionally, culturing these cells on Matrigel™ resulted in the formation of tube-like structures (data not shown), indicating that these cells isolated from the bone marrow had endothelial cell characteristics. The number of Dil-acLDL+ and UE-lectin+ cells growing out of the bone marrow of mice inhaling 5ppm acrolein for 6h was 3.1±0.1-fold higher than the number of cells growing from mice breathing filtered air (Fig. 3A). Similarly, the number of dual-positive cells in bone marrow isolated from mice exposed to 1ppm acrolein for 4 days was 3.8±0.4-fold higher than in air-breathing controls (Fig. 3B). Exposure to 1ppm acrolein for 4 days also led to a 1.7±0.2-fold increase in bone marrow-derived Flk-1+/Sca-1+ cells (Fig. 3C). Taken together, these data show that acrolein inhalation increases the population of bone marrow cells that differentiate into endothelial cells.
Figure 3. Acrolein increases proliferation of bone marrow-derived cells.
Representative fluorescence images (upper panels) and number of of DiI-acLDL+/FITC-UE-lectin+ (lower panels) cells isolated from bone marrow of mice exposed to air or A, 5 ppm acrolein for 6h, B, or 1 ppm acrolein (6h/d, 4 days). Mononuclear cells were isolated from the bone marrow and grown in culture for 7 days. Cells were labeled with DiI-acLDL and FITC-UE-lectin and nuclei were stained with DAPI. Merged images show co-localization of both markers in orange (i.e., double-positive cells). C, Representative fluorescence images (upper panel) and analysis (lower panel) of Flk-1+/Sca-1+-cells from mice exposed to air or 1 ppm acrolein (6h/d, 4d) after day 7 of culture. Cells were labeled with FITC-Flk-1 and APC-Sca-1 and with DAPI for nuclear staining. Merged images show co-localization of the markers. Changes in the number of DiI-acLDL+/FITC-UE-lectin+ and Flk-1+/Sca-1+ cells are presented as fold increase in double positive cells compared with controls (* p<0.05, air vs. acrolein exposure; n=4).
Protein-acrolein-adducts
Inhalation exposure to acrolein could directly affect circulating and bone marrow resident Flk-1+/Sca-1+ cells by delivering acrolein into blood and bone marrow or it could affect this cell population indirectly. To distinguish between these possibilities, we examined the systemic delivery of inhaled acrolein by measuring the abundance of protein-acrolein adducts in the plasma and the bone marrow using an acrolein-protein adduct-specific antibody. Acrolein is an unsaturated aldehyde that readily forms covalent adducts with nucleophilic sites in proteins, DNA and lipids24. Yet, it also is rapidly metabolized by oxidases, reductases and conjugative enzymes25. Hence, because of its short biological half-life, it is difficult to obtain reliable measurements of free acrolein in biological systems. Results shown in Fig. 4 demonstrate that inhalation of acrolein (1ppm, 6h/d, 4d) induced an increase in the abundance of acrolein-protein adducts in the plasma and bone marrow.
Figure 4. Formation of protein-acrolein adducts.
Western blot analyses of protein-acrolein adducts in A, plasma or B, bone marrow extracts obtained from mice breathing air or 1 ppm acrolein for 4 days (6h/d). Amido-black stain (plasma) and actin detection (bone marrow) were used as loading controls. Data are presented as fold increase of protein-acrolein adducts after acrolein exposure compared with air-exposed mice (* p<0.05; n=4–5).
Several protein-acrolein adducts were detected in the plasma (see 250 and 150 kDa bands; Fig. 4A) and the bone marrow (250, 37, 18, 17 kDa bands; Fig. 4B) of mice inhaling filtered air and because acrolein is also an end product of several metabolic processes, we attribute these bands to endogenously generated acrolein. However, in mice inhaling acrolein there was a 2–2.5-fold increase in the intensity of a 150 kDa Mr band in the plasma and of a 250 kDa Mr band in the bone marrow. Acrolein inhalation was also associated with an increase in the immunoreactivity of the anti-acrolein antibody with sections of the pelvic bone (Suppl. Fig. III). Spatial distribution of adducts was heterogeneous, and distinct, intense positive staining of protein-acrolein adducts was observed in blood vessels within the surrounding connective tissue as well as in the bone trabeculae (black arrows; Suppl. Fig. III). The bone marrow matrix, which is hematopoietic tissue, showed more diffuse staining (yellow arrows); however, some focal, intense staining was localized in the largest bone marrow cells within the endosteum at the border between the bone marrow cavity and compact bone (yellow arrow; Suppl. Fig. III). Taken together these results indicate that inhalation delivers acrolein from the lung to the plasma and the bone marrow, and that some of the effects of acrolein might be induced by direct interactions of acrolein (or protein-acrolein adducts) with cells in the bone marrow.
Endothelial dysfunction and NO signaling
The steady-state levels of Flk-1+/Sca-1+ cells in peripheral blood are maintained by ongoing processes of mobilization from resident niches and recruitment to sites of injury. To determine whether the decrease in circulating Flk-1+/Sca-1+ cells upon acrolein inhalation was due to increased recruitment to an injured endothelium, we measured endothelial function. For this, aortic rings were prepared from air- or acrolein-exposed mice and acetylcholine-induced (ACh) relaxation was measured ex vivo. Consistent with previous observations17, no evidence of frank endothelial or smooth muscle cell dysfunction was found in comparisons of mice inhaling air or 1 ppm acrolein (6h/d, 4d; Suppl. Table III). However, aortic rings from mice exposed to 5 ppm acrolein for 6h had a modest but consistent decrease in their ACh-induced relaxation compared with air controls (Fig. 5A; Suppl. Table III). These observations suggest that acrolein exposure induces endothelial dysfunction, yet, acrolein at 1ppm decreased the number of Flk-1+/Sca-1+ cells in the absence of frank endothelial dysfunction (i.e., no change in ACh-induced relaxation), indicating that Flk-1+/Sca-1+ cells were more sensitive targets of acrolein than the mature endothelium.
Figure 5. Endothelial response to acrolein exposure.
A, Relaxation of aortas isolated from mice breathing filtered air or 5 ppm acrolein for 6h. The sensitivity of aorta to acetylcholine (ACh) was measured to assess endothelium-dependent relaxation (* p<0.05, n=8 mice/group). B, NOx levels in plasma of mice breathing filtered air or acrolein (* p<0.05; n=8). C, Western blot analyses of eNOS or VEGFR2 in lysates of lung, heart, aorta or bone marrow of mice breathing filtered air or 1 ppm acrolein (6h/d, 4d).
To examine more subtle endothelium changes, NO bioavailability was measured. As shown in Fig. 5B, inhalation of acrolein (1ppm for 6h/d, 4d) or 5ppm (6h) was associated with a significant (15%) decrease in plasma NOx levels. This decrease in NOx levels was reversed after 7 days of recovery in air (Fig. 5B). Recovery of NOx level coincided with the return of circulating Flk-1+/Sca-1+ cell level (Fig. 2F), indicating that the two events could be interrelated. The abundance of eNOS in the lung, heart or aorta or of VEGFR-2 in the bone marrow was unaltered by acrolein exposure (Fig. 5C). Similarly, the basal level of aortic Akt and eNOS (total or phosphorylated) and bone marrow Akt abundance was not different between air and acrolein-exposed mice (5ppm, 6h; Suppl. Fig. IVA, B); however, bone marrow content of active MMP9 (73 kDa) was significantly decreased in acrolein-exposed mice (Suppl. Fig. IVC). Collectively, these data indicate that acrolein decreased plasma NOx levels independent of a change in eNOS protein abundance.
Acrolein prevents mobilization of Flk-1+/Sca-1+ cells
To investigate the functional consequence of acrolein-induced suppression of circulating Flk-1+/Sca-1+ cells, we examined the mobilization of these cells in response to VEGF/AMD3100 treatment. In agreement with previous findings of Pitchford et al.,20 treatment with VEGF each day for 4 days followed by a single injection of AMD3100 led to a 3-fold increase in the circulating levels of Flk-1+/Sca-1+ cells. VEGF/AMD3100 significantly increased Flk-1+/Sca-1+ cells expressing a variety of stem and B-/T-cell antigenic markers, especially cells co-expressing CD31/CD45R(B220)/CD133 and CD31/CD45R(B220)/CD19 (Suppl. Fig. V). In contrast, in mice inhaling acrolein (1ppm, 4d) VEGF/AMD3100 treatment did not increase the circulating level of Flk-1+/Sca-1+ cells (Fig. 6A). However, acrolein inhalation did not affect VEGF/AMD3100-induced increase in Sca-1+ only cells in the blood (Fig. 6A) or the overall leukocyte blood cell count (Suppl. Table IV). These data further demonstrate that the suppressive effect of acrolein was selective for the Flk-1+/Sca-1+ cell population.
Figure 6. Effect of acrolein on the mobilization of Flk-1+/Sca-1+ cells.
A, Representative two color (Flk-1 and Sca-1) flow cytometry dot plots (upper panel) of circulating blood cells of mice breathing filtered air or 1 ppm acrolein (6h/d, 4 d) after combination of VEGF/AMD3100 treatment. Levels of Flk-1+/Sca-1+ cells per µl blood (left y-axis) and of Sca-1+ cells (right y-axis; fold-change relative to air control) of mice breathing filtered air or 1 ppm acrolein (6h/d, 4d) after treatment with VEGF/AMD3100 (* p<0.05, n=8). Representative Western blots and densitometric analysis of B, Akt and C, eNOS in lysates of aortas obtained from mice breathing air or acrolein (1 ppm, 6h/d, 4d) or after saline or VEGF/AMD3100 treatment (n=4). D–F, Analysis of VEGF signaling in aortas isolated from mice breathing air or acrolein (1 ppm, 6h/d, 4d) and treated with VEGF for 15 min in autologous plasma. Representative Western blots and analysis of membranes probed for D, phospho-Akt (Ser473)/total Akt; E, phospho-eNOS (Ser1177)/total eNOS; and F, phospho-ERK (Thr202/Tyr204)/total ERK (* p<0.05; n=3–7).
Treatment with VEGF/AMD3100 mobilizes bone marrow cells without altering bone marrow cell function20. Analysis of bone marrow Flk-1+/Sca-1+ cell numbers after 7 days in culture showed that VEGF/AMD3100 treatment alone had no effect (data not shown). Similarly, no changes in aortic Akt or eNOS protein level were observed in any treatment group (Fig. 6B, C). Based on these results, we conclude that acrolein inhalation blocked mobilization of Flk-1+/Sca-1+ cell population in response to stimulation of the VEGF/SDF-1 signaling axis.
To determine whether acrolein interferes with VEGF signaling, aortas from mice breathing filtered air or acrolein (1 ppm, 4d) were incubated in autologous plasma ex vivo with or without VEGF, and signaling events downstream of VEGFR-2 were examined. In aorta from mice breathing filtered air, treatment with VEGF led to a nearly 2-fold increase in phosphorylation of Akt and eNOS (Fig. 6D, E). However, treatment with VEGF did not stimulate phosphorylation of these proteins in aorta from acrolein-exposed mice (Fig. 6D, E). Moreover, the basal level of phospho-ERK in the aorta of acrolein-exposed mice was significantly elevated over that in the aorta from air control mice (Fig. 6F). Taken together, these findings indicate that inhalation of acrolein impairs VEGF signaling.
Discussion
Results of the current study show that inhalation of acrolein suppresses the level of Flk-1+/Sca-1+ cells in the peripheral blood and prevents their mobilization by VEGF/SDF-1. The effects of acrolein were specific because acrolein decreased Flk-1+/Sca-1+ cells without affecting the level of Sca-1+ cells, indicating a unique sensitivity of this heterogenous cell population to acrolein. The effects of acrolein inhalation were, however, transient, and recovery in an acrolein-free environment for 7 days led to complete restoration of the level of these cells in the peripheral blood. Moreover, the decrease in Flk-1+/Sca-1+ cells is associated with decreased plasma NOx level, prevention of VEGF/CXCR4-induced mobilization of Flk-1+/Sca-1+ cells and inhibition of VEGF-induced Akt and eNOS phosphorylation – a constellation of changes impinging on vascular function and repair.
Our results also indicate that the bone marrow could be a specific locus of acrolein action. We found that acrolein inhalation increases accumulation of protein-acrolein adducts in the plasma and the bone marrow, suggesting that despite its high reactivity and rapid metabolism, acrolein is delivered from the lung into the systemic circulation and to distal vascular sites. Our previous studies also show that exposure to tobacco smoke or acrolein results in the formation of protein-acrolein adducts in non-pulmonary sites17, 26. Thus, acrolein appears to cross from the lungs and directly induce bone marrow toxicity.
Although acrolein inhalation decreases the levels of circulating Flk-1+/Sca-1+ cells, it increases the number of bone marrow-derived Flk-1+/Sca-1+ cells that accumulated acLDL and were positive for Ulex lectin binding in culture. Because the number of cells that grow out of the bone marrow on fibronectin-coated dishes is indicative of their population in the bone marrow20, it appears likely that exposure to acrolein increases proliferation of Flk-1+/Sca-1+cells in the bone marrow, while preventing their egress into the circulation. Alternatively, decreased mobilization by acrolein could indirectly result in the expansion of the bone marrow population of these cells. Nevertheless, this increase in levels of Flk-1+/Sca-1+ cells in the bone marrow indicates that acute acrolein exposure does not permanently impair the growth or viability of these cells but that it prevents their mobilization from the bone marrow. We suggest that this defect could be due to alterations in VEGF/SDF-1 signaling, but it also may be related to other specific processes such as cell release from the bone marrow stroma by matrix metalloproteases (MMPs), which is a NO-dependent process. Indeed, our measurements show that exposure to acrolein is associated with a decreased level of active MMP-9 (73 kDa) in bone marrow lysate (Suppl. Fig. IVC; Schematic III).
Depletion of Flk-1+/Sca-1+ cells in the peripheral blood in acrolein-exposed mice could also be due induction of cell death. Our measurements show that even brief exposure (2h) to acrolein results in a significant increase in the markers of cell death within the circulating Flk-1+/Sca-1+ but not the Sca-1+ cell population (Fig. 1). These observations reinforce the view that the Flk-1+/Sca-1+ population is uniquely sensitive to acrolein; however, persistent suppression of the steady-state levels of circulating Flk-1+/Sca-1+ cells (Fig. 2) implies that the primary defect is in the bone marrow because of which the depletion of these cells, due to increased cell death or increased recruitment to sites of injury, is not adequately compensated via mobilization from the bone marrow.
Because acrolein is one of the most reactive and toxic components of tobacco smoke, it is not surprising that many of the effects we observed with acrolein inhalation are consistent with findings of tobacco smoke exposure. Our observation that acrolein induces endothelial injury is consistent with evidence showing that exposure to combustion products generated by automobile exhaust11 or tobacco smoke9, 10 impairs endothelial function. Previous studies have shown that chronic smoking decreases the number of endothelial progenitor cells (EPCs) and that smoking cessation restores EPC levels in human subjects27. The reversible effects of smoking are similar to the reversible effects of acrolein inhalation (see Fig. 2F), as well as particulate matter (PM2.5) exposure28. In contrast to chronic smoking, brief exposure to secondhand smoke has been reported to increase EPC levels29. Although we studied low-dose (0.5ppm) and brief (2h) exposures, we did not observe an increase in Flk-1+/Sca-1+ cells in acrolein-exposed mice. We speculate that the increase in EPCs upon brief exposure to secondhand smoke may be related to other combustion products such as CO30, which by inducing transient or pseudo-hypoxia could increase EPC mobilization. Yet, inhibition of VEGF signaling by secondhand smoke exposure31 is similar to the effect of acrolein (Fig. 6) indicating that some of the pathological effects of secondhand smoke could be mediated by acrolein.
The levels of acrolein used in the current mouse exposure studies are relevant to those encountered by passive and active smokers as well as humans with occupations that include high level or chronic exposure to vehicle exhaust or smoke (e.g., bus drivers, bartenders, firefighters). Given the obvious lack of systemic toxicity (Suppl. Tables I, II) in mice, our observations suggest that acrolein at environmentally relevant levels could suppress circulating Flk-1+/Sca-1+ cell numbers without inducing overt toxicity. Moreover, it is important to point out that humans are exposed to acrolein not only from combustion, but also from foods and beverages, which contain high levels of acrolein32. In addition, acrolein is generated endogenously during lipid peroxidation and via myeloperoxidase activity at sites of inflammation33. Hence acrolein generated endogenously from distal inflamed tissues could also suppress Flk-1+/Sca-1+ cell mobilization from bone marrow (and other sites) and decrease the circulating levels of these cells. Nevertheless, the pathophysiological significance of acrolein-induced changes in Flk-1+/Sca-1+ cells remains unclear and deserves additional assessment. Previous studies have shown that the circulating levels of these cells are increased acutely in response to injury and that chronic suppression of similar progenitor cells is associated with an elevated CVD risk in humans17. In our analyses, we measured considerable antigenic diversity within the Flk-1+/Sca-1+ population, which has characteristics of monocytes, T- and B-cells. However, most of these cells were CXCR4+ (Fig. 1) and they were mobilized by VEGF/AMD3100 (Suppl. Fig. V), indicating that this population is likely to be recruited to the site of hypoxic or traumatic tissue injury. Moreover, the cells were also Id1+ (Fig. 1). Recent work suggests that Id1 is a selective marker of EPCs because ablation of 1d1 in bone marrow-derived cells reduces circulating EPC levels and induces significant defects in angiogenesis as Id1+ cells are incorporated in tumor neo-vessels23. Thus, the Flk-1/Sca-1 population affected by acrolein could be important for wound healing and angiogenesis.
Wound healing is a complex process. It involves the clearance of cell debris, regeneration of injured tissue and the growth of new blood vessels. This process requires the recruitment of a diverse set of progenitor cells containing both monocytic34 and endothelial23 characteristics, which ultimately turn into macrophages and vascular cells to secrete proteases, cytokines and growth factors that promote growth of tissue-resident cells. Therefore, we speculate that suppression of this recruitable and pro-angiogenic cell population by acrolein could lead to deficits in wound healing capacity. Future work is required to identify the effects of acrolein on specific cell populations and how these interfere with individual steps in the wound healing response.
Supplementary Material
Acknowledgments
We thank D. Bolanowski, L. Guo, G. Hunt, D. Mosley, E. Steinmetz and D. Young for technical assistance.
Funding: This work supported in part by funding from U.S. DOD, U.S. EPA, Philip Morris International and NIH grants (RR024489, HL89380, HL89380-S1, HL55477 and HL59378) and NIEHS T35 Training Grant (ES014559).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Kan H, Heiss G, Rose KM, Whitsel EA, Lurmann F, London SJ. Prospective analysis of traffic exposure as a risk factor for incident coronary heart disease: The atherosclerosis risk in communities (aric) study. Environ Health Perspect. 2008;116:1463–1468. doi: 10.1289/ehp.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 3.Rosenlund M, Bellander T, Nordquist T, Alfredsson L. Traffic-generated air pollution and myocardial infarction. Epidemiology. 2009;20:265–271. doi: 10.1097/EDE.0b013e318190ea68. [DOI] [PubMed] [Google Scholar]
- 4.Clancy L, Goodman P, Sinclair H, Dockery DW. Effect of air-pollution control on death rates in dublin, ireland: An intervention study. Lancet. 2002;360:1210–1214. doi: 10.1016/S0140-6736(02)11281-5. [DOI] [PubMed] [Google Scholar]
- 5.McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfredsson L, Hammar N, Hogstedt C. Incidence of myocardial infarction and mortality from specific causes among bus drivers in sweden. Int J Epidemiol. 1993;22:57–61. doi: 10.1093/ije/22.1.57. [DOI] [PubMed] [Google Scholar]
- 7.Hansen ES. Mortality from cancer and ischemic heart disease in danish chimney sweeps: A five-year follow-up. Am J Epidemiol. 1983;117:160–164. doi: 10.1093/oxfordjournals.aje.a113526. [DOI] [PubMed] [Google Scholar]
- 8.Choi BC. A technique to re-assess epidemiologic evidence in light of the healthy worker effect: The case of firefighting and heart disease. J Occup Environ Med. 2000;42:1021–1034. doi: 10.1097/00043764-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 11.Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular mmp-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 13.Smith D, Cheng P, Spanel P. Analysis of petrol and diesel vapour and vehicle engine exhaust gases using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:1124–1134. doi: 10.1002/rcm.691. [DOI] [PubMed] [Google Scholar]
- 14.Dong JZ, Moldoveanu SC. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J Chromatogr A. 2004;1027:25–35. doi: 10.1016/j.chroma.2003.08.104. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida M, Tomitori H, Machi Y, Hagihara M, Higashi K, Goda H, Ohya T, Niitsu M, Kashiwagi K, Igarashi K. Acrolein toxicity: Comparison with reactive oxygen species. Biochem Biophys Res Commun. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp RO, Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck HD. A critical review of the literature on acrolein toxicity. Crit Rev Toxicol. 1985;14:309–380. doi: 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- 17.Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-s-transferase p protects against endothelial dysfunction induced by exposure to tobacco smoke. AmJPhysiol Heart CircPhysiol. 2009;296:H1586–H1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campen MJ, Lund AK, Doyle-Eisele ML, McDonald JD, Knuckles TL, Rohr AC, Knipping EM, Mauderly JL. A comparison of vascular effects from complex and individual air pollutants indicates a role for monoxide gases and volatile hydrocarbons. Environ Health Perspect. 2010;118:921–927. doi: 10.1289/ehp.0901207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells: An expanding universe. Hypertension. 2010;55:593–599. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 20.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 22.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: Effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 23.Mellick AS, Plummer PN, Nolan DJ, Gao D, Bambino K, Hahn M, Catena R, Turner V, McDonnell K, Benezra R, Brink R, Swarbrick A, Mittal V. Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth. Cancer Res. 2010;70:7273–7282. doi: 10.1158/0008-5472.CAN-10-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauchamp RO, Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck HD. A critical review of the literature on acrolein toxicity. Crit RevToxicol. 1985;14:309–380. doi: 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- 25.Conklin D, Prough R, Bhatanagar A. Aldehyde metabolism in the cardiovascular system. MolBiosyst. 2007;3:136–150. doi: 10.1039/b612702a. [DOI] [PubMed] [Google Scholar]
- 26.Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O'Toole TE, Bhatnagar A, D'Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol. 2010;248:100–110. doi: 10.1016/j.taap.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A, Pope CA., 3rd Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–203. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost. 2009;7:1401–1408. doi: 10.1111/j.1538-7836.2009.03478.x. [DOI] [PubMed] [Google Scholar]
- 31.Marwick JA, Edirisinghe I, Arunachalam G, Stevenson CS, Macnee W, Kirkham PA, Rahman I. Cigarette smoke regulates vegfr2-mediated survival signaling in rat lungs. J Inflamm (Lond) 7:11. doi: 10.1186/1476-9255-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced pkcepsilon signaling and cardioprotection. JMolCell Cardiol. 2008;44:1016–1022. doi: 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. JClinInvest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. Vegfr-1-selective vegf homologue plgf is arteriogenic: Evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.