Abstract
Alzheimer’s disease (AD) is a common progressive neurodegenerative disorder that is not currently diagnosed until a patient reaches the stage of dementia. There is a pressing need to identify AD at an earlier stage, so that treatment, when available, can begin early. Quantitative structural MRI is sensitive to the neurodegeneration that occurs in mild and preclinical AD, and is predictive of decline to dementia in individuals with mild cognitive impairment. Objective evidence of ongoing brain atrophy will be critical for risk/benefit decisions once potentially aggressive, disease-modifying treatments become available. Recent advances have paved the way for the use of quantitative structural MRI in clinical practice, and initial clinical use has been promising. However, further experience with these measures in the relatively unselected patient populations seen in clinical practice is needed to complete translation of the recent enormous advances in scientific knowledge of AD into the clinical realm.
Keywords: amyloid, biomarker, CSF, mild cognitive impairment, prodromal Alzheimer’s disease, quantitative neuroimaging, volumetric imaging
Alzheimer’s disease (AD) is a progressive, ultimately fatal, neurodegenerative disorder. It is the most common cause of dementia, accounting for 60–80% of cases [1]. Age is the strongest risk factor for late-onset, sporadic AD, with age-specific prevalence doubling every 5 years after the age of 65 years [2]. With the increase in average life expectancy, the prevalence of AD is rising at an alarming rate. In 2005, an estimated 24 million people around the world suffered from dementia, and that number is expected to double every 20 years. By 2040, it is predicted that over 81 million people worldwide will suffer from dementia [3].
Alzheimer’s disease inflicts a terrible toll on patients, their families and society in general. If present trends continue, the cost of caring for the expected increase in the number of AD patients will bankrupt public healthcare systems [1]. Current treatments for AD offer temporary symptomatic relief, but do not affect the underlying disease process. A huge effort is underway to develop disease-modifying treatments to prevent or slow disease progression. Numerous potential treatments, aimed at different underlying pathogenic mechanisms of AD, are under development [4]. There is hope that one or more of these approaches will be effective at altering the course of AD in the coming years [4].
Most experts agree that treatment will be most beneficial if applied early, before significant, potentially irreversible neurodegeneration and functional impairment has occurred [5,6]. Several large studies around the world have been initiated to identify biomarkers to aid in earlier detection of AD [7–10]. In the USA, the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a ground-breaking publicly and privately funded, longitudinal, multisite study was launched in 2003 to examine the sensitivity of multiple biomarkers for the early detection and tracking of AD progression [11]. Data collected as part of this study has been made publicly available to the research community, resulting in a large and rapidly growing body of research on AD biomarkers. Results of many of these studies have recently been summarized in a series of articles published in a special issue of Alzheimer’s & Dementia [12–15].
The most promising biomarkers for early AD detection include cerebrospinal fluid (CSF) bio-markers, PET imaging of metabolism or cortical β-amyloid binding and structural MRI of brain atrophy. Of these, MRI is completely noninvasive and widely available. It is often used to rule out other underlying causes of impairment, and recent advances in the field have paved the way for transitioning research findings into routine clinical practice. This article will focus on recent studies that demonstrate the sensitivity of baseline and longitudinal quantitative structural MRI for the early detection of AD, and will describe the use and advantages of quantitative structural MRI in relation to emerging literature on other potential biomarkers. It will briefly discuss barriers to the adoption of quantitative MRI in clinical practice, the progress being made to surmount these barriers and results of initial clinical experience. This article is necessarily selective, as the number of articles examining MRI in AD has increased enormously in recent years. A literature search revealed a total of 586 publications with key words of “AD” and “MRI” in 1990–1999, 747 in 2000–2004, and 1515 since 2005, the period of focus for this article.
Alzheimer’s disease pathology
Although the etiology of AD is not clearly understood, the cascade of molecular changes associated with AD is becoming increasingly well delineated, opening up new avenues for potential intervention [16,17]. AD is characterized histopathologically by the presence of extracellular β-amyloid plaques and intraneuronal tangles of hyperphosphorylated tau proteins. The β-amyloid plaques first appear in basal neocortex then spread throughout the cortex [18], although neither the density nor distribution of plaques correspond to disease stage. Significant β-amyloid burden has been observed in cognitively normal individuals [19,20], but has been found to correlate with subtle cognitive dysfunction, suggesting that it may represent an early, preclinical stage of AD [20]. The role of β-amyloid in the etiology of AD is currently under sharp debate. Some suggest that it is an important contributing factor, if not the primary trigger, of the cascade of changes that lead to cell death in AD, while others contend that it is a downstream event that may even have neuroprotective benefit [21–24]. Regardless of its role, cortical β-amyloid plaque disposition is one of the defining histopathological features of AD.
The other defining histopathological feature of AD is the presence of neurofibrillary tangles (NFTs). Pathological phosphorylation of tau proteins leads to a sequence of events that results in the formation of NFTs and eventual death of the affected neuron. The tau pathology in AD correlates better with disease stage than β-amyloid burden does, and appears necessary for the clinical expression of the disease. NFTs are first observed in the transentorhinal area, then spread throughout the limbic area before appearing in widespread areas of association cortex, with sparing of primary sensory and motor areas until later disease stages [25,26]. Based on clinical diagnosis at the time of autopsy, Braak and Braak characterized the earliest, transentorhinal stage of the disease as a clinically silent period; the limbic stage, characterized by severe entorhinal and modest hippocampal damage, as clinically incipient AD; and the isocortical stage, when NFTs appear throughout association cortex, as fully developed dementia [25].
Alzheimer’s disease diagnosis
Currently, AD is clinically diagnosed as probable or possible AD based on the presence of progressive cognitive impairment of sufficient severity to interfere with activities of daily living, and by the absence of other neurological conditions that could account for the observed impairment [27]. A definitive diagnosis is available only through brain biopsy or postmortem examination, based on histopathological verification of the presence of β-amyloid plaques and NFTs. Several problems have been identified with the current criteria. One is that, given the insidious onset of AD, judgment of when functional impairment is of sufficient severity to warrant the diagnosis of dementia is subjective and somewhat arbitrary [28]. Second, reliance on the presence of dementia means that AD cannot be diagnosed until an individual has reached a relatively severe stage of the disorder, in which brain damage is widespread and likely irreversible. A third problem is the required absence of other explanatory neurological findings rather than the presence of characteristic biological features, or biomarkers, of AD [29]. Rapidly accumulating evidence indicates that AD is associated with specific patterns of cognitive impairment [30,31], abnormal levels of CSF biomarkers [32], increased cortical β-amyloid binding [33] and structural [34,35] and metabolic changes in the brain [36,37]. One or more of these biomarkers could be used to aid the earlier detection of AD. Working groups, supported by the National Institute on Aging and the Alzheimer’s Association, have been tasked with revising the diagnostic criteria for AD to reflect the strong advances in the field that have occurred since the current diagnostic criteria were introduced in 1984 [201], and their recommendations are expected to be published soon.
Mild cognitive impairment
In the quest to aid detection of AD at an earlier stage, mild cognitive impairment (MCI) has been proposed as a transitional state between normal aging and AD [38]. MCI is characterized by objective impairment in one or more cognitive domains, but is of insufficient severity to interfere with activities of daily living. When memory is one of the domains affected, MCI is associated with an increased risk of developing AD, with an annual conversion rate of approximately 10–20% per year, compared with 1–2% for the general population [38–41]. However, multiple etiologies can impair memory function. A sizeable minority of individuals with MCI develop other forms of dementia [42–44], whereas others remain cognitively stable or revert to normal cognitive function [38,45]. Thus, the MCI diagnosis by itself is not sufficient to identify individuals with a progressive neurodegenerative disorder, let alone to identify individuals whose neurodegeneration is due to AD. The addition of supporting biomarker evidence may help identify the subset of amnestic MCI individuals who are suffering from prodromal AD [5,28], and who thus represent the appropriate target for clinical trials [46] and, eventually, for early, aggressive treatment.
Quantitative structural MRI for detection of neurodegeneration in AD
Baseline atrophy in AD
The clinical symptoms of AD arise from progressive neuron and synapse loss, with the resulting tissue atrophy visible on high-resolution structural MRIs. As expected from the pathology and clinical expression of AD, significant atrophy is observed in early disease stages in the memory-related structures of the medial temporal lobe, particularly the hippocampus and entorhinal cortex [47,48], with the degree of atrophy correlating with memory impairment [48–50].
The initial use of MRI for the detection of atrophy in AD used manual tracing methods, necessitating focus on a few selected regions, such as the hippocampus or entorhinal cortex, or on global measures of atrophy, such as reduced global brain volumes and increased ventricular volumes. The advent of computer-based methods for quantitative MRI has allowed for efficient quantification of AD-related atrophy across the brain [51–55]. This revealed that even mild AD is associated with widespread atrophy of cortical association areas, with significant involvement of medial and lateral temporal, inferior parietal, posterior cingulate and prefrontal cortices (Figure 1) [47,56,57]. The degree of clinical impairment is associated with the severity of regional atrophy observed [57,58].
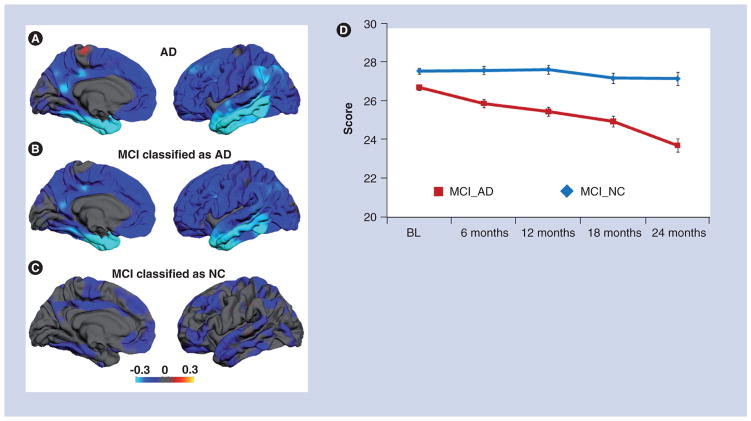
Figure 1. Regional atrophy patterns in mild Alzheimer’s disease and mild cognitive impairment.
(A) The average difference in thickness (mm) for 84 patients with mild AD relative to 139 controls are shown on medial (left) and lateral (right) views of the pial surface of the left hemisphere. Greatest thinning is observed in medial and lateral temporal areas, but significant thinning also appears across association cortices. (B) Average thickness difference for MCI subjects who were classified as ‘AD’ by a discriminant and control data. Although less severe, the atrophy pattern is very similar to that of AD subjects. (C) MCI subjects classified as ‘normal control’ by the discriminant model. These subjects show significantly less atrophy, particularly in medial temporal regions, subjects classified as ’AD’. For all brain images, the scale reflects thickness differences ranging from −0.3 (yellow)
(D) Average Mini-Mental State Examination score over time for MCI subjects classified as ‘AD’ and those classified with phenotypic AD atrophy showed significant, steady cognitive decline over a 2-year period, whereas those without the phenotypic AD atrophy pattern remained cognitively stable.
Brain images are reprinted with permission from [60].
AD: Alzheimer’s disease; BL: Baseline; MCI: Mild cognitive impairment; NC: Normal control.
Structural MRI measures can discriminate AD from healthy control data with high sensitivity and specificity, with the accuracy of discrimination improved when measures of atrophy beyond the medial temporal lobe are included in the analysis [59–61]. Importantly, it has been demonstrated that the degree of atrophy correlates well with disease stage determined from histopathology [62], and that the topographical pattern of atrophy is relatively consistent with the distribution of NFTs in later disease stages [63]. Regional atrophy patterns have also been found to differ between AD and other dementing disorders, such as frontotemporal dementia and dementia with Lewy bodies [64–67]. Thus, quantitative measures of atrophy from structural MRIs are sensitive to the neurodegeneration that occurs in AD, and although atrophy itself is nonspecific to AD, the topographical pattern of atrophy may be a sensitive and specific surrogate marker of AD pathology.
Baseline atrophy in preclinical AD
Consistent with the finding that histopathological changes occur prior to the clinical diagnosis of AD, significant brain atrophy is visible prior to the onset of dementia. Amnestic MCI, with its hallmark memory impairment, is associated with entorhinal and hippocampal atrophy, with thickness or volume measures intermediate between those of healthy controls and patients with mild AD [47,48,68,69]. However, even at this pre-dementia stage of the disorder, atrophy is not restricted to medial temporal areas, but extends to widespread areas of association cortex [47,57,68–70]. MCI individuals with isolated memory impairments, which may represent an even earlier stage of the disorder, show significant atrophy in areas beyond the medial temporal lobe, including thinning of lateral temporal, posterior cingulate, inferior parietal, precuneus and caudal middle frontal cortex [47].
Elderly individuals who do not meet objective criteria for memory impairment, but who report subjective memory complaints, are also at increased risk for developing dementia [71,72]. Hippocampal volumes and thickness of frontal cortex in these individuals are intermediate between those of elderly individuals without cognitive complaints and those with MCI [73]. These findings suggest that structural MRIs are sensitive not only to the neurodegeneration that occurs in MCI, but also to the neurodegeneration that occurs prior to the ability to reliably document cognitive changes with standard neuropsychological tests [73].
Prediction of conversion to AD
The atrophy in medial temporal lobe structures correlates with clinical decline and conversion to AD in individuals with MCI [74–76]. Nevertheless, retrospective comparisons between MCI patients who remained cognitively stable and those who progressed to a clinical diagnosis of AD, as well as prospective studies of MCI patients, have highlighted the importance of atrophy beyond medial temporal areas in predicting conversion to AD [50,59,60,68,70,77–79].
Recently, several groups have used multivariate analysis techniques to reduce the pattern of regional atrophy that best discriminates AD from healthy control data to a single numeric index [59–61,80]. These studies have demonstrated that the degree to which individuals with amnestic MCI express the characteristic AD atrophy pattern is predictive of a decline in cognitive function, progressive structural brain loss and conversion to AD [50,59,60,79]. Figure 1 shows an example of this approach. This study used semi-automated, high-throughput methods to derive individually specific measures of regional brain thickness and volumes from 139 healthy controls, 175 individuals with MCI and 84 with mild AD from the ADNI. Discriminant analyses applied to healthy control and AD data revealed that atrophy in medial temporal (hippocampus and entorhinal cortex), lateral temporal, orbitofrontal and isthmus cingulate cortex best differentiated AD from control data. Application of this model to MCI data demonstrated that the degree to which an MCI individual expressed the regional AD atrophy pattern was predictive of clinical decline and progressive structural brain loss [46,60]. MCI subjects whose data most resembled the AD atrophy pattern also experienced a significantly higher rate of conversion to AD (Figure 1) [60].
Recently, it was shown that this index can be converted into an estimate of an individual patient’s risk of imminent conversion to AD [81]. The 1-year risk of conversion to AD among 317 MCI patients from ADNI ranged from 3 to 41%. Individuals with the lowest scores showed a similar level of risk as that of healthy controls (1–2%/year), whereas those with the highest scores had more than double the risk of conversion (41%) associated with the diagnosis of MCI (which typically ranges from 10 to 20% per year [38–41]). These results demonstrate that information derived from quantitative structural MRI can provide useful information, not just for discriminating groups of subjects, but also for predicting individual patient prognosis [81].
Longitudinal MRI measures
Information to aid in the early clinical diagnosis of AD should ideally be derived from a single time point baseline MRI. However, due to the progressive nature of the disease and to interindividual variability in baseline brain structural measures, measures of brain change over time provide more sensitive detection of AD-related neurodegeneration and improved prediction of the conversion to AD in MCI [81–83]. Longitudinal measures are also likely to provide a valuable index for tracking disease progression and monitoring efficacy of disease-modifying therapies.
Studies comparing rates of whole-brain atrophy or ventricular expansion from serial MRIs have demonstrated that AD and MCI are characterized by significantly higher atrophy rates than that observed in healthy controls [84]. Despite the known regional specificity of AD pathology, several studies have found that these nonspecific measures of brain shrinkage over time were more sensitive to AD progression than atrophy rates in regions most strongly affected in AD [82,85,86]. However, recently developed semi-automated methods for quantifying regional change across the brain in individual subjects have indicated that atrophy rates in MCI and mild AD are highest in medial temporal areas, particularly the entorhinal cortex [58,87].
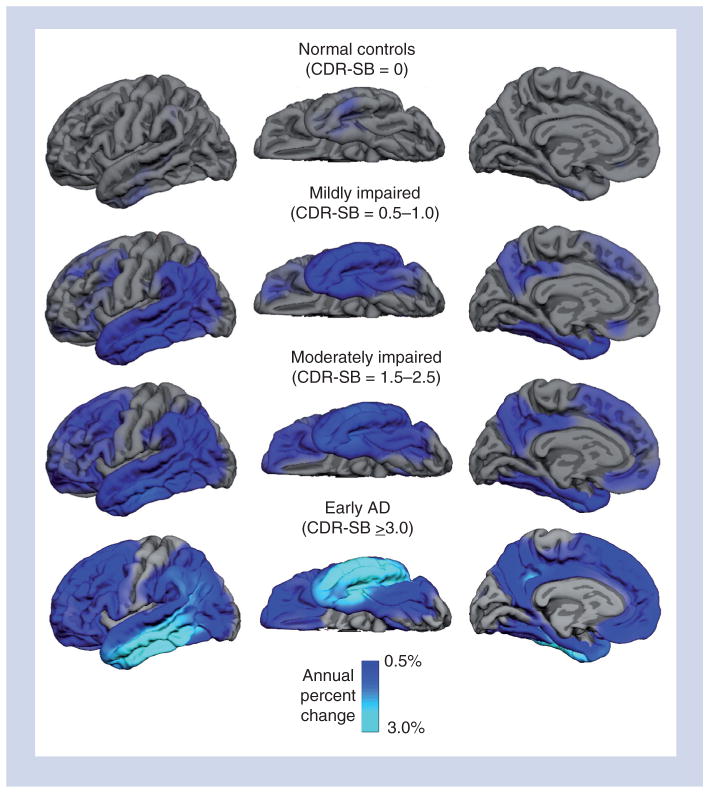
The ability to monitor region-specific atrophy rates is important since the rate of neurodegeneration in different brain regions is likely to impact different cognitive abilities [88] and to reflect different stages of disease severity [58]. For example, a study of 472 participants from ADNI found that atrophy rates varied as a function of brain region and disease severity, as characterized by Clinical Dementia Rating Scale Sum of Boxes score (CDR-SB) (Figure 2) [58]. At the mildest stages of functional impairment (CDR-SB scores of 0.5–1.0), significantly elevated atrophy rates, relative to the unimpaired elderly, were observed in medial and inferolateral temporal, inferior parietal and posterior cingulate regions. With increasing functional impairment (CDR-SB 1.5–2.5), elevated atrophy rates were observed in superior temporal, superior parietal, lateral occipital and widespread frontal regions. At the mild AD stage (CDR-SB > 2.5), elevated atrophy rates were observed across the cortex, with the exception of primary visual and auditory areas. The finding of higher atrophy rates with greater functional impairment is consistent with other studies that have demonstrated accelerated atrophy rates with disease progression [89,90]. However, the increase in atrophy rate with disease severity was not always linear. Atrophy rates increased monotonically with disease severity in hippocampal and lateral temporal areas. Greater acceleration in atrophy rates with disease severity was observed in prefrontal, parietal and anterior cingulate regions, whereas entorhinal cortex showed less acceleration with increased disease severity. These results are important for several reasons. First, they show that atrophy rates continue to accelerate with disease progression. No evidence was found in this, or other studies [89,90], that atrophy rates stabilize or decrease with increasing disease severity, suggesting that information on the rate of change will be a useful biomarker for tracking disease progression across the stages of the disease, during which good-quality MRI images can be obtained. They further suggest that if atrophy rates are used to monitor disease progression (or slowing of progression with treatment), different regions should be monitored at different disease stages [58,91], and that, when using measures of brain atrophy to differentiate AD from other dementing disorders [92–94], it may be crucial to take disease severity into account.
Figure 2. Annual atrophy rates as a function of degree of clinical impairment as assessed with baseline CDR-SB.
Mean atrophy rates are represented as a percent change in neocortical volume and mapped onto the lateral (left), ventral (middle) and medial (right) pial surface of the left hemisphere. These data demonstrate that atrophy rates are most prominent in medial and lateral temporal regions early in the course of disease, spreading to parietal and frontal regions as the level of impairment increases, with relative sparing of sensorimotor regions. The scale reflects annual percent change ranging from 0.5 (dark blue) to 3.0%. Note that the scale is optimized for showing disease-related change, rather than change in normal aging.
CDR-SB: Clinical Dementia Rating Sum of Boxes score.
Reprinted with permission from [58].
It is important to emphasize that although atrophy rates in MCI and mild AD significantly exceed rates observed in healthy older controls, subtle, but significant, changes in brain structure over time are detectable in healthy individuals [90,95], even over intervals as short as 6 months [84,96]. Using individually-specific regional measures of longitudinal change, atrophy rates in cognitively intact elders were found to vary across brain regions and to accelerate with increased age [95]. This acceleration was particularly apparent in regions most vulnerable to AD, and may be an early sign of impending cognitive impairment [95]. Consistent with this, a recent study demonstrated that 6-month atrophy rates in medial and lateral temporal areas in healthy elderly were predictive of 2-year memory decline [96]. Similarly, whole-brain and hippocampal atrophy rates increased 3 and 5 years, respectively, before the onset of AD in a small sample of pre-symptomatic autosomal dominant mutation carriers for AD [89]. The ability to differentiate mutation carriers from controls occurred 2 years earlier for atrophy rates than for cross-sectional comparisons of brain volumes [89], highlighting the sensitivity of longitudinal MRI measures for early detection of AD-related neurodegeneration.
In summary, cross-sectional and longitudinal studies convincingly demonstrate that quantitative MRI measures are sensitive to the neurodegeneration that occurs in prodromal and established AD, and suggest that structural loss can be detected prior to cognitive decline, providing firm support for the notion that structural MRI can be used to detect AD prior to the onset of dementia.
Structural MRI in relation to other biomarkers
Of the potential biomarkers currently under investigation for use in early detection and diagnosis of AD, structural MRI seems best suited for routine use as an adjunct to clinical diagnosis. It provides complementary information to that provided by the other biomarkers, and in isolation or in combination with other biomarkers, can provide valuable information to aid in AD diagnosis. The other promising bio-markers for early AD detection include PET measurements of glucose metabolism or cortical β-amyloid deposition, and CSF levels of β-amyloid and tau proteins.
FDG-PET
Neuron loss in AD, coupled with synaptic loss and dysfunction, results in reduced neuronal energy demand, evident as regions of hypometabolism on fluorodeoxyglucose (FDG)-PET images. AD is characterized by a distinct pattern of hypometabolism in posterior cingulate, precuneus and parietotemporal areas that correlates with clinical symptoms and predicts conversion to AD [36,37,97]. FDG-PET is currently used clinically to distinguish AD from frontotemporal dementia, and simple visual inspection of the images can reveal hypometabolism patterns characteristic of each disorder. As with MRI, FDG-PET appears to be sensitive to neuronal dysfunction prior to symptom onset. Significant hypometabolism in AD-related brain areas is observed in cognitively normal individuals at genetic risk for AD [98]. However, a recent study in which glucose metabolism in ADNI individuals was quantified in subject-specific regions of interest derived from coregistering an individual subject’s FDG-PET and MRI data, demonstrated that the largest structural effect sizes exceeded the largest metabolic effect sizes, not only in patients with mild AD, but also in those with MCI, including the subset of MCI patients with isolated memory impairment [99]. A recent longitudinal study of a small group of amnestic MCI subjects reported that hippocampal atrophy is an upstream event from posterior cingulate and medial orbital frontal hypometabolism [100]. These results contradict recent theoretical models of temporal ordering of relative biomarkers, which postulate that changes in FDG-PET metabolic measures precede changes in structural MRI measures and that FDG-PET measures begin to stabilize when measures of structural change begin to accelerate [35,91]. These findings imply that for early detection of AD in routine clinical practice, MRI may be just as sensitive, if not more so, than FDG-PET [99] and offers advantages of being free of ionizing radiation, less expensive, more widely available and more readily accepted for reimbursement by insurance companies.
CSF biomarkers & amyloid imaging
Elevated levels of CSF tau and phosphorylated tau proteins, as well as decreased levels of amyloid-β42, are reliably associated with AD [44,101–103] and predictive of clinical decline in MCI [44,102]. Elevated tau levels are believed to be a nonspecific reflection of neuronal degeneration, whereas phosphorylated tau is thought to reflect NFT formation [104]. Decreased levels of amyloid-β42, which are inversely correlated with postmortem measures of β-amyloid accumulation [105,106], are thought to reflect sequestering of β-amyloid in amyloid plaques in the brain, and thus serve as an indirect biomarker for brain amyloid pathology.
Brain amyloid pathology can also be directly measured in vivo through the use of PET tracers that bind to amyloid deposits in the brain [33,107]. PET imaging measures of β-amyloid binding are highly directly correlated with postmortem β-amyloid pathology [107], and highly inversely correlated with CSF amyloid-β42 levels [108]. Like CSF amyloid-β42 measures, PET measures of β-amyloid binding are very sensitive to AD and predictive of progression to AD in individuals with MCI and in healthy controls [109].
Using either CSF or PET imaging methods, estimates of β-amyloid pathology, indistinguishable from those of AD patients, have been observed in approximately 30% of healthy individuals, consistent with the frequency of findings of AD neuropathology in nondemented individuals at autopsy [106,110,111]. Current theories suggest that levels of β-amyloid pathology rise slowly, many years before clinical symptoms of AD emerge, and that by the time cognitive impairment and neurodegeneration are detected, β-amyloid levels have stabilized [35,91,112]. However, there is evidence to suggest that when individually-specific measures of brain structural changes are used, regional atrophy can be detected in cognitively normal individuals with abnormal levels of amyloid biomarkers [57,113].
Amyloid biomarkers are very sensitive in discriminating AD and MCI patients from healthy controls, and at discriminating individuals with MCI who will progress to AD from those who remain stable [110]. However, the specificity of amyloid pathology is uncertain, as it is not accompanied by dementia in approximately 30% of cases and may not inevitably lead to dementia in these individuals. Current research thus suggests that negative amyloid findings provide strong evidence for the absence of AD, whereas positive findings, in the absence of other biomarker evidence, do not necessarily imply that an individual will develop dementia.
Studies that have directly compared the predictive ability of amyloid biomarkers with structural measures have found that structural measures are better at predicting risk of decline in MCI over the near term, but that amyloid biomarkers provide important complementary information [79,112,114,115]. Taken together, these findings suggest that positive evidence of amyloid pathology in the presence of phenotypic AD brain atrophy would strongly suggest that an individual is in a prodromal stage of AD [112].
Translation to clinical practice
MRI is commonly used in current clinical practice for the evaluation of cognitive impairment. However, the translation of research results on the sensitivity of structural MRI to prodromal AD into clinical practice has been slow. As recently reviewed in detail [116], several barriers have prevented routine adoption of structural MRIs in the clinical assessment of suspected AD. These include a lack of standardized image acquisition parameters, spatial distortions and motion artifact in MRI data, lack of automated methods for individual-specific quantification of affected brain regions, lack of normative data and difficulties integrating quantitative MRI measures into the clinical workflow. Many of these barriers are being overcome with the aid of large-scale, multisite clinical trials, such as ADNI, and other large-scale efforts aimed at improving automated MRI processing, such as the Morphometry Biomedical Informatics Research Network (mBIRN) [117,118].
A critical first step in ADNI was the development of standardized image acquisition protocols that would be robust to intersite variation and would maximize the contrast between gray and white matter in the brain – a vital consideration for the use of automated image analysis algorithms [119]. Clinical MRI scans, currently used to rule out other causes of impairment in suspected AD cases, are suboptimal for use in quantitative structural analysis, due to their relative lack of sensitivity to gray–white contrast, and to their susceptibility to artifacts, such as spatial distortions related to gradient field nonlinearities, field inhomogeneities, and intensity nonuniformities due to B1 motion-induced artifacts caused by patient movement. These artifacts do not pose a significant problem for visual detection of gross brain abnormalities, but can cause large errors in automated quantification algorithms.
Automated methods for spatial distortion correction and image intensity normalization that can be applied to post-acquisition data have been developed [118,120]. Techniques that can correct for motion artifact during image acquisition, with minor impact on acquisition time, are under development [121] and have shown very promising results in motion-prone populations [122]. High-throughput, automated methods have been developed for efficient segmentation and quantification of various brain structures in individual patients [52–54,123–126]. These methods, when applied to data acquired with ADNI-compatible protocols and that have undergone corrections for spatial distortion and intensity inhomogeneities, provide sensitive, reliable measures of global and regional volumes [50,126]. However, they do require qualitative visual review by a trained technician or imaging expert to detect cases where significant image artifacts or gross brain abnormalities result in segmentation errors.
Initial clinical experience
As was recently described [116,126], US FDA-approved image analysis software (NeuroQuant; CorTechs, Inc., La Jolla, CA, USA) currently exists that provides fully automated volumes of several brain structures, including cerebral white and gray matter, hippocampus, amygdala, basal ganglia nuclei and ventricular volumes from ADNI-compatible, 3D T1-weighted sagittal MRI scans. These measures have been validated against manual segmentation and shown to be sensitive to neurodegeneration associated with AD [50,126]. NeuroQuant software compares an individual patient’s regional brain volumes with those of a normative database, correcting for sex, head size and age. Visual quality review of the results by an imaging expert remains important, and the imaging expert, as well as the referring physician, have the ability to scroll through the segmented MRI images, in all three dimensions, to determine accuracy of the segmentation. NeuroQuant is currently in use in a number of clinics, including the University of California, San Diego (UCSD; CA, USA). During the past 2 years of clinical use of volumetry, Medicare reimbursement for the additional post-processing has been consistent. As advised by the local office for Center for Medicare and Medicaid Services, records are kept to document that the referring physicians have specifically requested quantitative segmental volume reporting and assessment (CPT code 76377) for the patient.
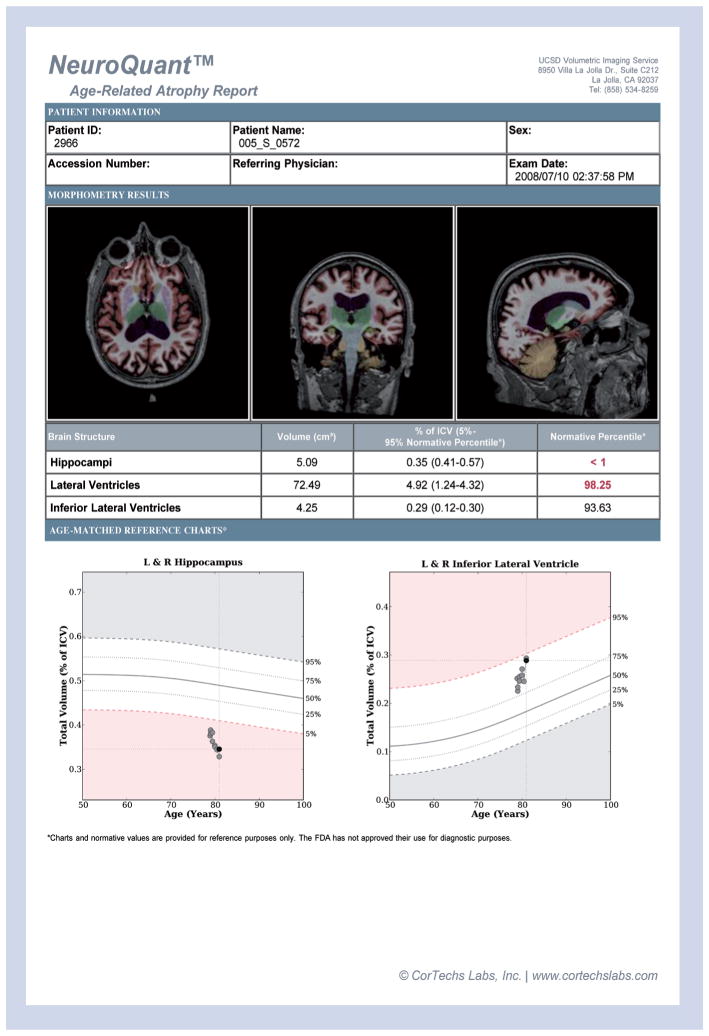
An example of a clinical report generated by this method is shown in Figure 3. The report provides axial, coronal and sagittal views of a patient’s MRI, with color-labeled structures to provide information on image and segmentation quality. Raw volumes and volumes expressed as a percentage of intracranial volume are provided, along with the age-specific normative range. Referring physicians have reported that the information contained in the reports have provided valuable complementary information to the history, neurological and neuropsychological findings of their patients, often providing a biological foundation for their clinical impressions [116].
Figure 3. NeuroQuant report from a mild cognitive impairment patient scanned longitudinally for 2 years as a volunteer in the Alzheimer’s Disease Neuroimaging Initiative study.
Ten scans (two from each timepoint) were analyzed using NeuroQuant. From top to bottom, the report consists of demographic information; a row of axial, coronal and sagittal MR images with a color overlay of the segmentation results; a table reporting the volume, the volume as a percent of intracranial volume and the volume’s percentile score based on the normative database for bilateral hippocampus, lateral ventricle and temporal horn of the lateral ventricle; and normative graphs plotting the patient’s hippocampus volume and temporal horn volume, corrected for intracranial volume, relative to the expected range (in white) across the age range included in the normative database. For this patient, the baseline hippocampal volume of 5.09cc –corrected for age, sex and intracranial volume – was more than two standard deviations below that expected for their age, and continued to atrophy at a rate faster than would be expected in normal aging. The baseline temporal horn volume of 4.25cc was at the 77th percentile of that expected for age, and continued to enlarge at a rate faster than would be expected in normal aging. The patient progressed to Alzheimer’s disease at year 2.
Quantitative structural MRI in AD clinical trials
In addition to its potential in clinical practice, quantitative structural MRI also has an important role to play in AD clinical trials. As an enrichment strategy, structural MRI offers the ability to selectively enroll individuals with the highest likelihood of experiencing significant decline over a 1–2 year period. Constraining enrollment to amnestic MCI individuals with a regional atrophy pattern characteristic of AD on a baseline screening MRI, as shown in Figure 1, would enable reductions in sample sizes by 40–60%, considerably reducing the expense of a trial [46]. As an outcome variable, structural MRI measures would also enable further reductions in sample size due to their lower inter-individual variability and higher sensitivity to change in early disease stages than standard outcome measures, such as the Alzheimer’s Disease Assessment Scale – Cognitive subscale (ADAS-Cog) [46,87,127–129]. Importantly, unlike standard clinical and functional outcome measures, which cannot distinguish symptomatic benefits from disease-altering effects, structural MRI measures can provide evidence of slowing of the progressive neurodegeneration associated with AD.
Structural MRI outcome measures are now being included in a number of clinical trials. Although no trials have yet demonstrated disease-modifying effects in planned primary analyses, secondary analyses of two amyloid agents have shown promising effects. In the Phase III trial of tramiprosate, an amyloid-β antagonist, secondary analyses revealed that tramiprosate treatment was associated with significantly less hippocampal volume loss and less decline on the ADAS-Cog (although effects on the latter were marginal) relative to placebo [130]. Similarly, secondary analyses in the Phase II trial of bapineuzumab, a passive amyloid-β immunotherapy, showed that with treatment, MCI individuals without the APOE ε4 genetic risk factor experienced a reduced rate of whole-brain atrophy and reduced decline on functional and cognitive measures, relative to the placebo group. Individuals with the genetic risk factor did not show any beneficial effect of therapy [131].
However, the interrupted trial of the active amyloid-β immunotherapy, AN1792, produced unexpected MRI results. Antibody responders showed increased ventricular expansion and whole-brain volume loss, but with less cognitive decline, than the placebo group [132]. Hippocampal loss did not significantly differ between groups. The basis for the contradictory MRI and cognitive results is not understood. The authors noted that increased neuronal degeneration in the responder group was unlikely, due to the beneficial effect of treatment on cognitive function. The observed decrease in CSF tau levels with treatment also argues against greater neurodegeneration in the treated group. The authors speculated that increased CSF outflow resistance, mediated by the mobilization of amyloid, could have resulted in ventricular enlargement and reduced brain volumes. Although not available in the study, information on subregional atrophy rates across the cortex may have been able to aid in the interpretation of the nonspecific effects and shed more light on the effects of treatment on AD-related neurodegeneration.
Expert commentary
Qualitative structural neuroimaging, available for more than two decades, has been revolutionary in identifying gross structural lesions, but has failed thus far in identifying and differentiating between neurodegenerative illnesses. Recent advances in quantitative structural neuroimaging, however, offer hope that this scenario is about to change. As discussed above, US FDA-approved image analysis methods, operating on MRI data collected using ADNI-compatible image acquisition protocols can currently provide computer-assisted detection of objective signs of neurodegeneration consistent with AD. This, coupled with clinical impression, can be a tremendous tool for assisting diagnoses in clinical settings where a myriad of causes may underlie a patient’s subjective or objective cognitive impairment.
In cases where the clinical impression points to neurodegenerative illness, quantitative neuroimaging can provide objective and direct supportive evidence of such neurodegeneration, allowing the physician to more confidently provide forewarning to the patient and family, with stronger justification for pursuing a more aggressive course of intervention. Currently, this might include earlier social, financial and safety planning, or recommendation for enrollment in clinical trials with a higher risk:benefit ratio, given the increased likelihood of poor outcome if no treatment is pursued.
In other cases, the clinical impression may be an absence of neurodegenerative illness, which is not uncommon when treating educated, knowledgeable and, often, very worried elderly individuals. Quantitative neuroimaging can be quite helpful in these cases as well, providing evidence of preserved brain structure consistent with the lack of clinical evidence of objective decline. This reassurance may itself be therapeutic in individuals for whom extreme anxiety and fear of developing AD contributes to their perceived symptoms. Although one can never ‘rule out’ prodromal disease, current evidence would suggest reduced immediate risk in such cases, warranting an approach of watchful waiting.
Finally, in cases where the clinical impression is consistent with early AD, but structural imaging results show minimal neurodegeneration in memory structures, the physician may be appropriately motivated to perform a more thorough search for less common or reversible causes of cognitive impairment, such as medication side effects or depression-related ‘pseudodementia’. In the rushed world of clinical practice, an elderly patient with memory impairment might currently be given only a brief evaluation before being prescribed an acetylcholinesterase inhibitor. Whereas inappropriate acetlycholinesterase treatment can be problematic, inappropriate treatment with future aggressive disease-modifying agents could be devastating. Current evidence suggests that such treatments may be accompanied by risk of significant side effects, such as brain edema and possible hemorrhage [133]. Their administration will only be warranted when it is clear that the potential benefit of treatment justifies the risk. The ability to make this determination will necessitate greater confidence in the diagnosis of early AD.
In preparation for such a time when patient safety will depend on accurate diagnosis of early AD, it is important to gain more clinical experience with the available biomarkers. While the validation of accuracy in large, public datasets of carefully screened subjects, such as ADNI, have been invaluable, more data must be collected in actual clinical settings, where improvement of diagnostic accuracy can be documented in relatively unselected clinical populations. Thus, a concerted effort to fund and support clinical research on the most promising biomarkers in actual clinic settings will be critical for completing translation of the enormous strides made in research into the clinical realm. Only in this way will the payoff from the large prior investment in this research be delivered directly to patients in the clinic.
Five-year view
Late stage clinical trials are evaluating the efficacy of medications that reduce the production of amyloid oligomers by inhibiting enzymatic cleavage of the amyloid precursor protein [134]. The targeted enzyme, γ-secretase, also has a role in the notch pathway, important for a number of normal biological functions [135]. Inhibition of the notch pathway was probably the cause of increased skin cancer in the treatment arm of the semagacestat Phase III trial and may have caused the decline in cognitive and functional abilities among treated patients that led to the trial’s recent stoppage. This has not discouraged other trials of γ-secretase inhibitors from moving forward, but has raised attention to the importance of minimizing notch pathway inhibition in attempts to target the amyloid cleaving function of γ-secretase.
Another therapeutic approach that is in late-stage clinical trials is through passive immunization against the amyloid protein. This approach also has risks that are perhaps associated with nonselective removal of both vascular- and plaque-based amyloid. The removal of vascular amyloid may predispose patients to vasogenic brain edema and hemorrhage [133]. A higher degree of risk might be accepted in the treatment of such a devastating disease, but results of the clinical trials to date highlight the importance of improving confidence in early diagnosis before exposing patients to the risks associated with potential disease-modifying medications.
Given the possible near-term availability of AD medications targeting amyloid, it will soon be important to identify not only whether an individual suffers from progressive neurodegeneration, but whether or not that degeneration is mediated by increased amyloid deposition. For this decision, the combination of an amyloid biomarker, either reduced CSF levels of amyloid-β42 or elevated binding of amyloid-sensitive PET tracers in the brain, will complement structural imaging evidence of brain atrophy. Biomarker evidence of elevated amyloid, in the absence of ongoing neuro-degeneration, would likely be insufficient to warrant aggressive treatment that may be associated with risk of serious side effects, since such individuals may remain cognitively stable throughout the rest of their lives. An analogy from the cardiovascular realm seems appropriate: treating elevated cholesterol, a risk-factor for heart disease, would be appropriate only if the medicine is associated with minimal side effects, whereas treating angina, a sign of ongoing heart damage, warrants more aggressive, immediate therapy and even angiocatheterization. Much like elevated cholesterol, elevated amyloid is not necessarily associated with imminent decline, whereas, like angina or active myocardial infarction, ongoing neurodegeneration will likely warrant an aggressive approach, such as infusion of anti-amyloid therapy, once available. For other neurodegenerative illnesses, where complementary biomarkers may be lacking, there may still be hope for prodromal differential diagnosis using quantitative structural MRI. Although current automated and US FDA-approved techniques for quantitative structural neuroimaging have yet to be evaluated for assisting with differential diagnosis, strong evidence exists for selective vulnerability of cortical networks in various neurodegenerative diseases. Therefore, a more specific diagnosis is likely to be obtained through quantitative examination of the integrity of these networks. However, to inform differential diagnosis in clinical practice, the technique must be sensitive enough not only to identify the regional atrophy pattern in individual subjects, but to differentiate the early stage AD pattern from atrophy patterns associated with early stages of other neurodegenerative diseases. Furthermore, the technique has to be easily integrated into the workflow of clinical practice and be robust enough to be applied to a wide range of patients with variable compliance, with an acceptable, very low, failure rate. Understandably, the field might be more than 5 years from developing such a robust procedure for differential diagnosis based on structural imaging, but progress will be made in this direction.
Progress will also be made within the next 5 years to permit enhanced detection of progressive change. As reviewed above, measures of longitudinal change over time provide improved predictive prognosis in MCI. Risk stratification can occur at the initial visit, based on comparison to age-matched normative data, and later, as the patient is followed, the initial assessment can be adjusted based on rates of subregional change. Increased rates of change in AD-related regions may increase confidence in a diagnosis of early AD, and allow staging of the disease. Conversely, if atrophy rates are similar to those of age-matched controls, or increased atrophy rates are observed in unexpected areas, the initial diagnosis may be revised, prompting consideration of other causes of impairment. The ability to examine an overlay map of change projected on the three-dimensional cortical reconstruction might allow identification of the cortical pattern of neurodegeneration that would aid in differential diagnosis.
The future for use of quantitative structural MRI in clinical practice for early detection and monitoring of AD progression is highly promising. Future efforts aimed at more fully translating research results into clinical practice will result in improved diagnostic ability, allowing for more reliable identification of patients in very early stages of AD. This will provide physicians with better information on which to base risk/benefit decisions when deciding how aggressively to treat an individual patient, once disease-modifying treatments become available.
Key issues.
There is strong interest in using biological markers to identify Alzheimer’s disease (AD) before the onset of dementia, since disease-modifying treatments, once they become available, are more likely to be successful when given early.
Quantitative structural MRI measures are a promising potential biomarker to aid in early detection of AD since they are highly sensitive to the neurodegeneration that occurs in mild and prodromal AD.
AD is associated with a characteristic pattern of subregional atrophy, and the degree to which this atrophy pattern is present in individuals with mild cognitive impairment is predictive of conversion to AD.
Atrophy rates in regions vulnerable to AD may be able to identify individuals at risk of developing AD even prior to the onset of cognitive impairment.
Barriers to routine clinical use of quantitative structural MRIs are being overcome through the aid of large-scale clinical trials, such as the Alzheimer’s Disease Neuroimaging Initiative.
Initial use of US FDA-approved methods for obtaining objective evidence of atrophy in brain structures vulnerable to AD clinical setting has shown that these measures provide important complementary data to the history, neurological and neuropsychological findings.
Since new disease-modifying therapies may be associated with significant side effects, quantitative structural MRI measures, which can predict risk of imminent decline, will be critical for determining whether potential benefits of preventing or delaying decline outweigh risk of significant side effects, such as brain edema or hemorrhage.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Linda K McEvoy is supported by NIA K01AG029218; James B Brewer is supported by NINDS K02 NS067427. Linda K McEvoy’s spouse is President of CortTechs, Inc. James B Brewer is an investigator for, and receives research funds from, Janssen Alzheimer Immunotherapy; he also receives research funds from GE medical foundation and has served on an advisory board for Elan Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.The Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafii MS, Aisen PS. Recent developments in Alzheimer’s disease therapeutics. BMC Med. 2009;7:7. doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 2007;69(16):1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 6.Aisen PS. Commentary on “a roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” Facilitating Alzheimer’s disease drug development in the United States. Alzheimers Dement. 2009;5(2):125–127. doi: 10.1016/j.jalz.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis KA, Rowe CC, Villemagne VL, et al. Addressing population aging and Alzheimer’s disease through the Australian imaging biomarkers and lifestyle study: collaboration with the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2010;6(3):291–296. doi: 10.1016/j.jalz.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Frisoni GB. Alzheimer’s disease neuroimaging initiative in Europe. Alzheimers Dement. 2010;6(3):280–285. doi: 10.1016/j.jalz.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsubo T. Japanese Alzheimer’s Disease Neuroimaging Initiative: present status and future. Alzheimers Dement. 2010;6(3):297–299. doi: 10.1016/j.jalz.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Weiner MW, Aisen PS, Jack CR, Jr, et al. The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6(3):202–211. e207. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aisen PS, Petersen RC, Donohue MC, et al. Clinical core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement. 2010;6(3):239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2010;6(3):212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagust WJ, Bandy D, Chen K, et al. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6(3):221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 17.Grill JD, Cummings JL. Current therapeutic targets for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2010;10(5):711–728. doi: 10.1586/ern.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braak H, Braak E. Staging of Alzheimer-related cortical destruction. Int Psychogeriatr. 1997;9(Suppl 1):257–261. discussion 269–272. [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 20.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HG, Zhu X, Castellani RJ, Nunomura A, Perry G, Smith MA. Amyloid-β in Alzheimer disease: the null versus the alternate hypotheses. J Pharmacol Exp Ther. 2007;321(3):823–829. doi: 10.1124/jpet.106.114009. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12(1):38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellani RJ, Lee HG, Siedlak SL, et al. Reexamining Alzheimer’s disease: evidence for a protective role for amyloid-β protein precursor and amyloid-β. J Alzheimers Dis. 2009;18(2):447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl ) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, Picard G, Sarazin M. Early detection of Alzheimer’s disease: new diagnostic criteria. Dialogues Clin Neurosci. 2009;11(2):135–139. doi: 10.31887/DCNS.2009.11.2/bdubois. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69(19):1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson MW, McEvoy LK, Dale A, Fennema-Notestine C. Cognitive phenotypes, brain morphometry and the detection of cognitive decline in preclinical AD. Behav Neurol. 2009;21(1):29–37. doi: 10.3233/BEN-2009-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesisin vivo. Behav Neurol. 2009;21(1):117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fennema-Notestine C, McEvoy LK, Hagler DJ, Jr, Jacobson MW, Dale AM. The Alzheimer’s Disease Neuroimaging I. Structural neuroimaging in the detection and prognosis of pre-clinical and early AD. Behav Neurol. 2009;21(1):3–12. doi: 10.3233/BEN-2009-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 37.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80(Spec No 2):S160–S167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrillo MC, Blackwell A, Hampel H, et al. Early risk assessment for Alzheimer’s disease. Alzheimers Dement. 2009;5(2):182–196. doi: 10.1016/j.jalz.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 43.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 44.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 45.Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81(5):541–546. doi: 10.1136/jnnp.2008.171066. [DOI] [PubMed] [Google Scholar]
- 46.McEvoy LK, Edland SD, Holland D, et al. Neuroimaging enrichment strategy for secondary prevention trials in Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(3):269–277. doi: 10.1097/WAD.0b013e3181d1b814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, et al. Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum Brain Mapp. 2009;30(10):3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morra JH, Tu Z, Apostolova LG, et al. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp. 2009;30(9):2766–2788. doi: 10.1002/hbm.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walhovd KB, Fjell AM, Dale AM, et al. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging. 2010;31(7):1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacevic S, Rafii MS, Brewer JB. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2009;23(2):139–145. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2(2):79–88. doi: 10.1016/s1474-4422(03)00304-1. [DOI] [PubMed] [Google Scholar]
- 52.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 54.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Van Der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 56.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39(3):1186–1197. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald CR, McEvoy LK, Gharapetian L, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73(6):457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan Y, Batmanghelich N, Clark CM, Davatzikos C. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39(4):1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McEvoy LK, Fennema-Notestine C, Roddey JC, et al. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251(1):195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73(4):287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vemuri P, Whitwell JL, Kantarci K, et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008;42(2):559–567. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain. 2007;130(Pt 4):1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22(6):474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(Pt 3):708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62(9):1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 69.Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129(Pt 11):2885–2893. doi: 10.1093/brain/awl256. [DOI] [PubMed] [Google Scholar]
- 70.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70(7):512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20(1):1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 72.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 73.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 75.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31(7):1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 77.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desikan RS, Cabral HJ, Fischl B, et al. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of Alzheimer disease. AJNR Am J Neuroradiol. 2009;30(3):532–538. doi: 10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73(4):294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer’s disease via pattern classification of MRI. Neurobiol Aging. 2008;29(4):514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McEvoy LK, Holland D, Hagler DJ, Jr, Fennema-Notestine C, Dale AM. Structural neuroimaging for individual patient risk assessment in mild cognitive impairment. Alzheimers Dement. 2010;6(4 Suppl):S300–S301. [Google Scholar]
- 82.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72(11):999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2005;65(1):119–124. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- 85.Ridha BH, Anderson VM, Barnes J, et al. Volumetric MRI and cognitive measures in Alzheimer disease: comparison of markers of progression. J Neurol. 2008;255(4):567–574. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- 86.Barnes J, Bartlett JW, van de Pol LA, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging. 2009;30(11):1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holland D, Brewer JB, Hagler DJ, Fenema-Notestine C, Dale AM. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(49):20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald CR, Gharapetian L, McEvoy LK, et al. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiol Aging. 2010 doi: 10.1016/j. neurobiolaging.2010.03.015. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006;5(10):828–834. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 90.Jack CR, Jr, Weigand SD, Shiung MM, et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70(19 Pt 2):1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scahill RI, Fox NC. Longitudinal imaging in dementia. Br J Radiol. 2007;80(Spec No 2):S92–S98. doi: 10.1259/bjr/78981552. [DOI] [PubMed] [Google Scholar]
- 93.Barnes J, Godbolt AK, Frost C, et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol Aging. 2007;28(1):20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 94.Krueger CE, Dean DL, Rosen HJ, et al. Longitudinal rates of lobar atrophy in frontotemporal dementia, semantic dementia, and Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2010;24(1):43–48. doi: 10.1097/WAD.0b013e3181a6f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy EA, Holland D, Donohue M, et al. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. Neuroimage. 2010;53(4):1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Langbaum JB, Chen K, Caselli RJ, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67(4):462–468. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karow DS, McEvoy LK, Fennema-Notestine C, et al. Relative capability of MR Imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology. 2010;256(3):932–942. doi: 10.1148/radiol.10091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villain N, Fouquet M, Baron JC, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010 doi: 10.1093/brain/awq203. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4(1):38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 102.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18(2):413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 103.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 105.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 106.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 107.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1(8–9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Meyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 112.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. Brain atrophy in healthy aging is related to CSF levels of Aβ1–42. Cereb Cortex. 2010;20(9):2069–2079. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci. 2010;30(6):2088–2101. doi: 10.1523/JNEUROSCI.3785-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Desikan RS, Cabral HJ, Settecase F, et al. Automated MRI measures predict progression to Alzheimer’s disease. Neurobiol Aging. 2010;31(8):1364–1374. doi: 10.1016/j.neurobiolaging.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brewer JB. Fully-automated volumetric MRI with normative ranges: translation to clinical practice. Behav Neurol. 2009;21(1):21–28. doi: 10.3233/BEN-2009-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 118.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 119.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 121.White NW, Roddey C, Shankaranaraynan A, et al. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 2010;63(1):91–105. doi: 10.1002/mrm.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown TT, Kuperman JM, Erhart M, et al. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage. 2010;53(1):139–145. doi: 10.1016/j.neuroimage.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cognitive Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 124.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 125.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 126.Brewer JB, Magda S, Airriess C, Smith ME. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol. 2009;30(3):578–580. doi: 10.3174/ajnr.A1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schott JM, Frost C, Whitwell JL, et al. Combining short interval MRI in Alzheimer’s disease: Implications for therapeutic trials. J Neurol. 2006;253(9):1147–1153. doi: 10.1007/s00415-006-0173-4. [DOI] [PubMed] [Google Scholar]
- 128.Saumier D, Aisen PS, Gauthier S, et al. Lessons learned in the use of volumetric MRI in therapeutic trials in Alzheimer’s disease: the ALZHEMED (Tramiprosate) experience. J Nutr Health Aging. 2009;13(4):370–372. doi: 10.1007/s12603-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 129.Hua X, Lee S, Yanovsky I, et al. Optimizing power to track brain degeneration in Alzheimer’s disease and mild cognitive impairment with tensor-based morphometry: an ADNI study of 515 subjects. Neuroimage. 2009;48(4):668–681. doi: 10.1016/j.neuroimage.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gauthier S, Aisen PS, Ferris SH, et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13(6):550–557. doi: 10.1007/s12603-009-0106-x. [DOI] [PubMed] [Google Scholar]
- 131.Salloway S, Sperling R, Gilman S, et al. A Phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fox NC, Black RS, Gilman S, et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 133.Black RS, Sperling RA, Safirstein B, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Panza F, Frisardi V, Imbimbo BP, et al. γ-secretase inhibitors for the treatment of Alzheimer’s disease: the current state. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755–5949. 2010.00164.x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li H, Wolfe MS, Selkoe DJ. Toward structural elucidation of the γ-secretase complex. Structure. 2009;17(3):326–334. doi: 10.1016/j.str.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Alzheimer’s Association: Recommendations to Update Diagnostic Criteria. www.alz.org/research/diagnostic_criteria.