Abstract
Background
Fasting hyperglycemia has been associated with HIV protease inhibitor (PI) therapy.
Objective
To determine whether absolute insulin deficiency or insulin resistance with relative insulin deficiency and an elevated body mass index (BMI) contribute to HIV PI–associated diabetes.
Design
Cross-sectional evaluation.
Patients
8 healthy seronegative men, 10 nondiabetic HIV-positive patients naive to PI, 15 nondiabetic HIV-positive patients receiving PI (BMI = 26 kg/m2), 6 nondiabetic HIV-positive patients receiving PI (BMI = 31 kg/m2), and 8 HIV-positive patients with diabetes receiving PI (BMI = 34 kg/m2). All patients on PI received indinavir.
Measurements
Fasting concentrations of glucoregulatory hormones. Direct effects of indinavir (20 μM) on rat pancreatic [beta]-cell function in vitro.
Results
In hyperglycemic HIV-positive subjects, circulating concentrations of insulin, C-peptide, proinsulin, glucagon, and the proinsulin/insulin ratio were increased when compared with those of the other 4 groups (p < .05). Morning fasting serum cortisol concentrations were not different among the 5 groups. Glutamic acid decarboxylase (GAD) antibody titers were uncommon in all groups. High BMI was not always associated with diabetes. In vitro, indinavir did not inhibit proinsulin to insulin conversion or impair glucose-induced secretion of insulin and C-peptide from rat [beta]-cells.
Conclusions
The pathogenesis of HIV PI–associated diabetes involves peripheral insulin resistance with insulin deficiency relative to hyperglucagonemia and a high BMI. Pancreatic [beta]-cell function was not impaired by indinavir. HIV PI–associated diabetes mirrors that of non–insulin-dependent diabetes mellitus and impaired insulin action in the periphery.
Keywords: AIDS, Metabolic complications, Glucose metabolism, Pancreatic [beta]-cells, Insulin release
INTRODUCTION
HIV infection is characterized by active viral replication causing the immunodeficient state that typifies AIDS. The current goal of antiviral therapy is to maintain maximal suppression of viral replication using highly active antiretroviral therapy (HAART) (1–6). This usually involves a combination of reverse transcriptase inhibitors and an HIV-1 protease inhibitor (PI). HIV PIs have had a substantial impact on slowing HIV replication, reducing viral load, increasing CD4 lymphocyte numbers, reducing incidence of new opportunistic infections, reducing the length of hospitalization, and reducing AIDS-related mortality in the United States (1,3,4).
PIs have also been associated with undesirable metabolic complications (2,5–11). Hyperglycemia and diabetes have been observed in HIV-infected people taking any of the currently available PIs. Peripheral insulin resistance appears to contribute to this complication, but the mechanism is not completely understood (10,11). Peripheral insulin resistance and diabetes have been associated with the development of insulin secretory defects (12,13). This has not been evaluated in HIV PI–associated diabetes. One hypothesis suggests that HIV PIs can directly inhibit enzymes involved in [beta]-cell insulin processing and secretion (9,14,15). Improved understanding of the mechanism or mechanisms by which PIs mediate diabetes in HIV-infected people will assist in the development of treatment strategies.
Our objective was to evaluate potential mechanisms whereby PIs might cause diabetes. Type 1 diabetes (IDDM) is caused by autoimmune destruction of insulin-secreting pancreatic islet [beta]-cells and is associated with low levels of fasting plasma insulin and C-peptide and with serum antibodies against glutamic acid decarboxylase (GAD) (12,13). In contrast, Type 2 diabetes (NIDDM) occurs in the setting of insulin resistance and is associated with high fasting plasma insulin and C-peptide levels (12,13), an elevated proinsulin:insulin ratio (16–20), and the absence of GAD antibodies. All these parameters were determined in this study. In addition, we determined the circulating concentrations of the counterregulatory hormones cortisol and glucagon. Elevated concentrations of these hormones might increase hepatic glucose production, impair peripheral glucose uptake, or reduce insulin secretion by pancreatic [beta]-cells (12,13,21,22).
We determined whether indinavir directly inhibits proinsulin-to-insulin conversion or inhibits insulin and C-peptide secretion from rat pancreatic [beta]-cells in vitro. Pancreatic proteases (PC2, PC3) responsible for the proteolytic cleavage of proinsulin into insulin and C-peptide might be inhibited by HIV PIs (9,14,15). This would result in reduced insulin production with release of incompletely processed forms of proinsulin (20).
Finally, many PIs are also esterase inhibitors. HIV PIs might reduce insulin secretion by inhibiting an esterase that participates in the [beta]-cell insulin secretory process. For example, the bromoenol lactone suicide substrate (BEL) (23) is a PI (24) that inhibits the esterase group VI phospholipase A2 (iPLA2) (25,27). BEL suppresses glucose-induced insulin secretion and the glucose-induced rise in [beta]-cell cytosolic [Ca2+] that triggers insulin secretion from the [beta]-cell (23,25–27). We determined whether rat [beta]-cell iPLA2 activity and glucose-induced insulin and C-peptide secretion in vitro are impaired by indinavir.
METHODS
Human Studies
These studies were approved by the Human Studies Review Committee at Washington University Medical School and all participants signed an informed consent document.
We enrolled 47 men into this study (Table 1). Eight normal, healthy HIV-seronegative men served as controls. We assessed 10 nondiabetic HIV-infected study subjects receiving antiretroviral therapy that did not include an HIV PI (i.e., PI-naive), 15 nondiabetic HIV-infected study subjects receiving combined antiretroviral therapy including indinavir, 8 HIV-infected study subjects with newly diagnosed diabetes (fasting glucose, >7 mM) who were receiving combined antiretroviral therapy including indinavir, and 6 nondiabetic HIV-infected study subjects receiving indinavir who were matched to the body mass index (BMI; kg/m2) of the group with diabetes. A family history of diabetes (parents or siblings) was reported by 2 of 8 study subjects with diabetes, 4 of 8 reported no known family history, and 2 of 8 could not accurately report on family history. The nondiabetic, high-BMI group was enrolled as a comparison group to control for the high BMI of the study subjects with diabetes. The HIV PI was indinavir (800 mg three times per day).
TABLE 1.
Descriptive characteristics of study subjects
| Group | n | Age (y) |
Height (cm) |
Weight (kg) |
BMI (kg/m2) |
|---|---|---|---|---|---|
| HIV-seronegative | 8 | 40 ± 2 | 178 ± 3 | 89 ± 6 | 28 ± 1 |
| HIV-positive, no PI, normoglycemic | 10 | 41 ± 3 | 176 ± 2 | 83 ± 4 | 27 ± 1 |
| HIV-positive, ART + PI, normoglycemic | 15 | 38 ± 2 | 174 ± 2 | 79 ± 2 | 26 ± 1 |
| HIV-positive, ART + PI, normoglycemic, BMI > 28 | 6 | 40 ± 3 | 180 ± 2 | 100 ± 4b | 31 ± 1b |
| HIV-positive, ART + PI, diabetic, BMI > 28 | 8 | 43 ± 2 | 178 ± 3 | 108 ± 13b | 34 ± 3b |
P < .01 versus groups withoutb. Groups with BMI > 28 were not significantly different from each other with respect to age, height, weight, or BMI.
BMI, body mass index (kg/m2); ART, antiretroviral therapy; PI, protease inhibitor (i.e., indinavir).
Subjects were recruited from the Washington University Medical School AIDS Clinical Trials Unit (ACTU). From 10:00 PM on the night prior to blood sampling until between 9:00 and 10:00 AM, when baseline blood samples were collected, the study subjects discontinued food and fluid intake (except water). Blood was collected from an antecubital vein into evacuated tubes containing the appropriate preservatives. Serum or plasma were isolated, transferred to cryotubes, and frozen (0°C) until analysis. Plasma glucose concentration was determined on an automated glucose analyzer (Beckman Instruments, Fullerton, CA, USA). Plasma proinsulin, insulin, C-peptide, and glucagon concentrations were determined using human specific radioimmunoassays (RIA, Linco Laboratories, Inc., St. Charles, MO, USA). Serum cortisol levels and GAD antibody titers were determined by Specialty Laboratories (Santa Monica, CA, USA). GAD antibody titers >2.5 U/ml were considered positive. Specificity of the GAD antibody assay was 90%. Interassay and intraassay variability for RIA’s were: insulin (3.9%, 3.2%, coefficient of variation [CV]), C-peptide (4.7%, 4.6% CV), proinsulin (6.6%, 3.8% CV), and cortisol (8.4%, 6.0% CV). Cross-reactivity of the human specific proinsulin antibody with insulin was <0.2%, and not detectable with C-peptide (Linco Laboratories).
In Vitro Studies
We evaluated the ability of the HIV PI indinavir to inhibit proinsulin-to-insulin conversion in rat INS-1 cells; an insulinoma cell line derived from rat [beta]-cells (28). We also determined whether indinavir inhibits the glucose-induced secretion of insulin or C-peptide from isolated rat pancreatic islets (23,25–27). These experiments were approved by the Animal Welfare Committee at Washington University Medical Center.
Materials
Indinavir was obtained from Merck Research Laboratories (Rahway, NJ, USA). Immediately prior to use, it was dissolved (2 mM) in distilled deionized water. This solution was then diluted into the incubation medium to achieve a final concentration of 20 μM. The [14C]labeled phospholipase substrate ([14C]-PAPE; 1-hexa-decanoyl-2-[1-14C]eicosa-5′,8′,11′,14′-tetra-enyl-sn-glycero-3-phosphoethanolamine) was obtained from American Radiochemical Corporation (St. Louis, MO, USA). Male Sprague-Dawley rats (180–220 g) were obtained from Sasco (O’Fallon, St. Louis, MO, USA); ampicillin, kanamycin, and carbachol from Sigma Chemical (St. Louis, MO, USA). D-Glucose was obtained from the National Bureau of Standards (Washington, D.C., USA). The suicide substrate (BEL) [(E)-6-(bromomethylene)tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one] was obtained from Cayman Chemical (Ann Arbor, MI, USA). Tissue culture medium CMRL-1066, penicillin, streptomycin, and Hanks’ balanced salt solution (HBSS) were obtained from Gibco (Grand Island, NY, USA). The chemical composition of the islet incubation media has been described elsewhere (23,25–28).
Pancreatic Islet Isolation, Culture, and Secretory Experiments
Pancreatic islets were isolated aseptically from male Sprague-Dawley rats, as described previously (23,26,27). In experiments with indinavir, the islets were incubated with 20 μM of indinavir for 48 hours before the secretion experiment, and 20 μM of indinavir was added to the incubation medium during the incubation with secretagogues. Control islets were incubated for the same period in a medium that did not contain indinavir. For the secretion experiments, the islets were incubated for 30 minutes at 37°C under an atmosphere of 95% air/5% CO2. The incubation medium contained 3, 5, 8, 17, or 22 mM D-glucose without or with 0.50 mM carbachol (CCh) and/or 20 μM indinavir. Islets were collected by centrifugation (14,000 rpm for 30 seconds), the medium was removed, and assayed for rat insulin and C-peptide by RIA (RIA Core Facility, Washington University Diabetes Research and Training Center).
Group VI phospholipase A2 activity (iPLA2) was determined by the ability of rat islet homogenates to convert the substrate [14C]-PAPE to [14C]-arachidonic acid in Ca2+-free media(23,25–27). Isolated rat pancreatic islets were homogenized at 4°C in 50 mM 2-[N-morpholino]ethanesulfonic acid, 250 mM sucrose, at pH 7.2. In some cases, the assay solution was supplemented with 1 mM adenosine triphosphate (ATP) or 20 μM indinavir. Hydrolysis of [14C]-arachidonic acid from the radiolabeled phospholipid substrate was determined as previously described (23,25–27) after TLC isolation and liquid scintillation spectrometry.
Ability of the pancreatic convertase enzymes to convert proinsulin to insulin was determined in rat INS-1 (transformed pancreatic [beta]) cells (28–30). Cells were cultured in the presence or absence of 20 μM of indinavir for 4 days followed by a 15 minute pulse-label with [3H]leucine and a 90-minute chase incubation in the continued presence of the drug. Cellular proteins were extracted in 1M acetic acid and 0.1% bovine serum albumin (BSA). Insulin, proinsulin, and intermediate conversion products were separated by high performance liquid chromatography (HPLC) (28–30). The total radioactivity in all insulin-related products was determined using liquid scintillation counting and the extent of proinsulin conversion to mature insulin expressed as a percentage (of insulin + proinsulin + conversion intermediates) (20,28).
Statistics
All sera and plasma determinations were compared among the 5 groups of study subjects using a one-way analysis of variance. [14C]-arachidonic acid release, insulin and C-peptide secretion, and proinsulin-to-insulin conversion in isolated islets incubated in the presence and absence of BEL or indinavir were compared using a one-way analysis of variance. When a significant difference was identified (p < .05), a Student-Newman-Keuls post-hoc test was used to identify which groups differed. Mean ± standard error of the mean (SEM) are reported.
RESULTS
Human Studies
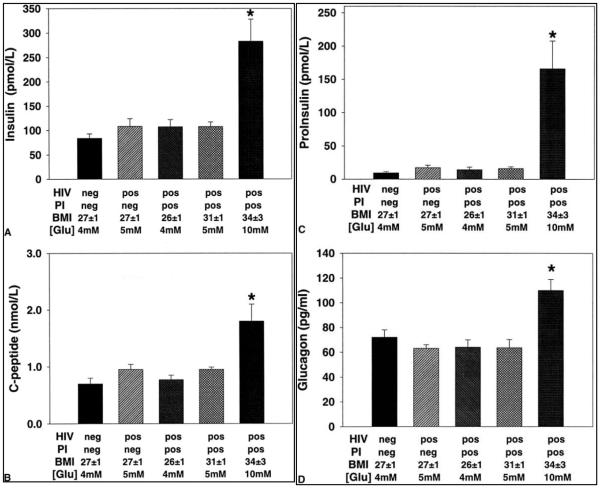
None of the study subjects in the hyperglycemic HIV PI group had diagnosed diabetes prior to receiving PI therapy. In the hyperglycemic HIV-infected group, circulating concentrations of insulin, C-peptide, proinsulin, and glucagon were increased when compared to the other 4 groups (p < .05; Fig. 1). The proinsulin:insulin ratio was also greater in the hyperglycemic HIV-infected group (p < .05; Table 2). Despite elevated insulin concentrations, blood glucose concentrations were elevated in the HIV PI group selected on the basis of their fasting hyperglycemia. The BMI of the HIV PI group with diabetes (34 ± 3 kg/m2) was greater than the other groups. The high BMI may have predisposed to the development of diabetes and account for greater glucoregulatory hormone concentrations. To control for the effects of a high BMI on glycemia, we enrolled an additional group of HIV-infected study subjects with a similarly high BMI who were receiving indinavir therapy but did not have diabetes (Table 1). In the 6 non-diabetic, PI-treated subjects with a BMI ranging from 28 to 34 kg/m2, fasting insulin, C-peptide, proinsulin, glucagon, and the proinsulin/insulin ratio were identical to the other non-diabetic groups. Values for all these parameters were significantly lower in that group than in the group with a high BMI who were hyperglycemic and treated with indinavir.
FIG. 1.
Morning fasting blood glucoregulatory hormone concentrations in each of the 5 groups (p < .05 versus other 4 groups). [Glu], blood glucose concentration in mM; PI, protease inhibitor; BMI, body mass index.
TABLE 2.
Morning, fasting blood glucose, proinsulin/insulin ratio, cortisol concentrations, and islet cell antibody titers in the five groups of study subjects
| Fasting measure | HIV-negative | HIV-positive, no PI |
HIV-positive, ART + PI |
HIV-positive, ART + PI, BMI > 28 |
HIV-positive, ART + PI, BMI > 28, diabetes |
|---|---|---|---|---|---|
| Glucose (mM) | 4.4 ± 0.2 | 5.2 ± 0.4 | 4.4 ± 0.2 | 5.1 ± 0.2 | 10 ± 1.3 |
| ProIns/Ins | 0.11 ± 0.02 | 0.17 ± 0.04 | 0.13 ± 0.02 | 0.16 ± 0.03 | 0.54 ± 0.10b |
| Cortisol (μg/dl) | 13 ± 2 | 16 ± 2 | 14 ± 2 | 15 ± 2 | 15 ± 1 |
| GAD antibody titer | all negative | all negative | 1 positive | all negative | 1 positive |
p < .05 versus all other groups.
PI, protease inhibitors; ART, antiretroviral therapy; BMI, body mass index; GAD, glutamic acid decarboxylase
Morning fasting serum cortisol concentrations did not differ among the 5 groups (Table 2). GAD antibody titers were detected in 1 hyperglycemic study subject and 1 normoglycemic HIV-infected study subject receiving indinavir (Table 2). The normoglycemic seronegative and normoglycemic HIV-infected groups did not differ with respect to any of the measured variables (Fig. 1; Table 2).
In Vitro Studies
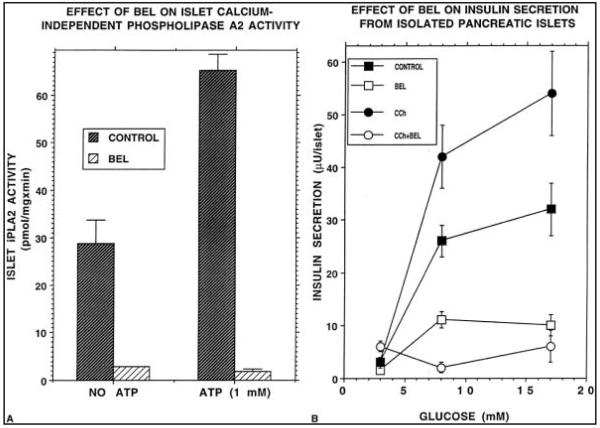
Figure 2A illustrates that islet iPLA2 activity is stimulated by ATP and inhibited by 20 μM BEL. Figure 2B illustrates that treatment of isolated rat pancreatic islets with BEL suppresses insulin secretion induced by D-glucose at concentrations of 8 or 17 mM and prevents amplification of glucose-induced insulin secretion by CCh. These observations are consistent with previous reports on effects of BEL on islet iPLA2 activity and insulin secretion (23–25).
FIG. 2.
(A) The protease inhibitor bromoenol lactone (BEL; 20 μM) inhibits iPLA2 activity in basal and ATP-stimulated rat islets. (B) BEL, 20 μM, suppressed D-glucose-induced insulin secretion from rat islets. D-Glucose concentrations were 8 or 17 mM. BEL also prevented the amplification of D-glucose–induced insulin secretion by a muscarinic agonist of insulin secretion. (CCh, carbachol.).
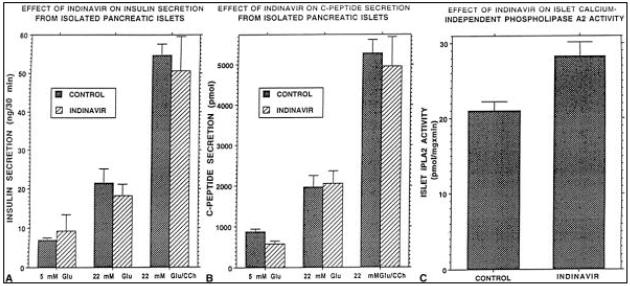
Similar inhibitory effects were not observed with indinavir (Fig. 3). When administered to humans in therapeutic doses, peak indinavir concentrations achieved in plasma are 12.4 ± 4 μM and trough concentrations are 0.25 ± 0.18 μM (31). A concentration of 20 μM indinavir was therefore selected as the highest likely to be encountered in the usual clinical usage of the agent. Isolated rat pancreatic islets incubated without indinavir (i.e., control islets) secreted greater amounts of insulin and C-peptide (compared with basal [5 mM] glucose] when incubated with 22 mM glucose alone or with 22 mM glucose plus CCh. Indinavir-treated islets secreted amounts of insulin and C-peptide that were not significantly different from amounts secreted by control islets under identical incubation conditions (Fig. 3A, B). Islet iPLA2 activity was also not suppressed relative to control islet homogenates when incubated with 20 μM indinavir for 48 hours (Fig. 3C).
FIG. 3.
Rat islets were incubated for 48 hours in the presence and absence of indinavir (20 μM) at basal (5 mM) and hyperglycemic (22 mM) concentrations of glucose. (A, B) Islets incubated in the absence of indinavir (dark bars) and when carbachol was added to the incubation media. Islets treated with indinavir (light bars) secreted insulin and C-peptide in amounts that were not significantly different from amounts secreted by control islets when glucose concentrations were 5 and 22 mM. (C) Rat islet iPLA2 activity was not suppressed in [beta]-cells treated with indinavir when compared with iPLA2 activity in control [beta]-cells incubated in the absence of indinavir.
The pulse-chase [3H]-leucine experiments indicated that insulin production from proinsulin in INS-1 cells was not reduced following incubation with 20 μM of indinavir for 4 days. The percentage of 3H-insulin formed was not reduced when 20 μM of indinavir was present during the incubation period and pulse-chase experiment (indinavir, 76.3% ± 0.6%; control, 74.1% ± 0.9%). Indinavir did not appear to be toxic for the rat INS-1 cells because the total [3H]-leucine incorporation into the cells was not reduced by indinavir. Indinavir did not reduce the total insulin content of the INS-1 cells.
DISCUSSION
Fasting plasma concentrations of insulin, C-peptide, and proinsulin were higher in the patients with HIV PI–associated diabetes than in any of the 4 normoglycemic comparison groups. These findings indicate that insulin secretion was increased in the study subjects with diabetes. Absolute insulin deficiency was not the cause of HIV PI–associated diabetes in these study subjects. This conclusion is consistent with the absence of an inhibitory effect of indinavir on proinsulin-to-insulin conversion by rat INS-1 cells and the lack of inhibition on glucose-induced insulin and C-peptide secretion from rat islets in vitro. The scarcity of GAD antibody positivity (1 of 6 people with diabetes) is not consistent with autoimmune [beta]-cell destruction common in newly diagnosed IDDM.
These findings are consistent with previous reports of peripheral insulin resistance and NIDDM in HIV PI–treated patients (10,11). Although sensitivity to insulin’s action at the same plasma glucose concentration was not assessed directly with euglycemic, hyperinsulinemic clamp studies, the finding of fasting hyperglycemia in conjunction with increased proinsulin, insulin, and C-peptide levels implies resistance to the glucose-lowering effects of insulin. This is consistent with previous findings that elevated fasting proinsulin and insulin concentrations and an elevated proinsulin/insulin ratio are inversely correlated with an index of insulin sensitivity (SI) determined in insulin-resistant study subjects (16–20). Insulin resistance has been associated with either an increased or unchanged proinsulin:insulin ratio (16–20,33,34). In the present study, the circulating proinsulin/insulin ratio was greater in the study subjects with diabetes than in the other groups, which is consistent with the impaired glucose tolerance and peripheral insulin resistance associated with NIDDM (10,11,16,18–20). It may also suggest that hyperglycemia placed an increased demand on [beta]-cell secretory function, which may have resulted in a disproportionate release of proinsulin or incompletely processed proinsulin into the circulation (14,20).
Despite increased fasting insulin concentrations in the HIV PI group with diabetes, hyperglycemia occurred. The hyperglucagonemia observed in the PI-treated study subjects with diabetes suggests that insulin deficiency relative to the plasma glucagon concentration may play a role in the pathogenesis of hyperglycemia. The mechanism by which indinavir might increase glucagon release, reduce its clearance, or increase tissue sensitivity to its glucose mobilizing effects is unclear.
Plasma cortisol concentrations were not elevated in the patients with HIV PI–associated diabetes. Cortisol excess can cause insulin resistance and hyperglycemia, at least in susceptible individuals, but this does not appear to mediate HIV PI–associated diabetes (7).
The in vitro studies strongly suggest that indinavir did not directly impair [beta]-cell function. Indinavir did not interfere with the proteolytic processing events involved in the production of mature, bioactive insulin in rat INS-1 cells. Indinavir did not inhibit glucose-induced secretion of insulin and C-peptide from isolated rat [beta]-cells. Further, indinavir did not inhibit islet iPLA2 enzymatic activity and therefore, does not mimic the effects of the protease/esterase inhibitor BEL on this enzyme (23,25). Collectively, these findings indicate that HIV PI–associated diabetes is induced at the level of insulin action. This implies a direct effect on skeletal muscle, the major site of insulin-stimulated glucose disposal.
In this study, all participants treated with HIV PI therapy received indinavir. At the time of enrollment, the ACTU at Washington University Medical School was conducting several trials of indinavir therapy in combination with and without other antiviral medications. This facilitated our evaluation of potential mechanisms by which this PI might induce diabetes. Our findings should not be interpreted to indicate that diabetes only occurs in indinavir-treated patients. In fact, diabetes has been associated with several currently available PIs (8,9,34–36).
Although not clear, the prevalence of PI-associated diabetes appears to be greater than that in the non-infected population. Of the 8 patients with diabetes who were studied, 4 reported no family history of diabetes. Given the small number of study subjects, we cannot rule out the possibility that PI therapy unmasked an underlying genetic susceptibility to diabetes that was not evident in HIV-infected study subjects prior to the availability of PIs when HIV viral load was higher, malabsorption or malnutrition were common, and concurrent opportunistic infections were frequent. It seems likely that PI therapy is a causative factor because the initiation of HIV PI therapy and the development of diabetes and dyslipidemias appear to be temporally related (5,8–11,34). Also, switching patients from PI-containing to non–PI-containing regimens substituted with nevirapine appears to reduce hyperglycemia and hyperlipidemia in most patients (35,36).
On the basis of the glucoregulatory factors determined here, the pathogenesis of HIV PI–associated diabetes is similar to that of NIDDM. The importance of this complication and its long-term implications require further study. In this regard, our findings suggest that HIV-infected people with a high BMI may be predisposed to the development of diabetes when treated with HIV PIs. We found that some HIV-infected study subjects with a high BMI did not develop diabetes when treated with indinavir. This indicates that a high BMI (i.e., obesity) alone is not sufficient to induce development of diabetes when PI therapy is initiated.
In summary, diabetes in HIV PI–treated subjects was associated with peripheral insulin resistance and hyperglucagonemia. Indinavir did not reduce proteolytic processing of proinsulin to insulin or glucose-induced insulin secretion from [beta]-cells. The mechanisms by which peripheral insulin action is impaired in some individuals treated with HIV PIs remain to be identified.
Acknowledgments
Indinavir for in vitro experiments was obtained from Merck Research Laboratories. This work was supported by National Institutes of Health grants DK49393, AI25903, RR00036, and DK20579; The Campbell Foundation; and Swiss National Research Fund grant 31-50811-97.
REFERENCES
- 1.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society—USA panel. JAMA. 1997;277:1962–9. Serial Solutions [Context Link] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services (HHS) the Henry J. Kaiser Family Foundation Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. MMWR Morb Mortal Wkly Rep. 1998;47:4382. Serial Solutions [Context Link] [PubMed] [Google Scholar]
- 3.HaMmer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 5.Flexner C. HIV-Protease inhibitors. N Engl J Med. 1998;338:1281–92. doi: 10.1056/NEJM199804303381808. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 6.Shader RI, Greenblatt DJ. Protease inhibitors and drug interactions—an alert. J. Clin Psychopharmacol. 1996;16:343–4. doi: 10.1097/00004714-199610000-00001. Ovid Full Text Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–70. doi: 10.1016/S0140-6736(97)11443-X. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 8.Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 9.Eastone JA, Decker CF. New-onset diabetes mellitus associated with use of protease inhibitor [Letter] Ann Intern Med. 1997;127:948. doi: 10.7326/0003-4819-127-10-199711150-00017. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 10.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [Context Link] [DOI] [PubMed] [Google Scholar]
- 11.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–73. doi: 10.1097/00002030-199815000-00001. Ovid Full Text Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 12.Fajans SS. Definition and classification of diabetes including maturity onset diabetes of the young. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus: a fundamental and clinical text. Lippincott-Raven; Philadelphia: 1996. pp. 249–60. [Context Link] [Google Scholar]
- 13.Weir GC, Bonner-Weir S. Insulin secretion in non-insulin-dependent diabetes mellitus. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus: a fundamental and clinical text. Lippincott-Raven; Philadelphia: 1996. pp. 503–8. [Context Link] [Google Scholar]
- 14.O’Rahilly S, Gray H, Humphreys PJ, Krook A, Polonsky KS, White A, et al. Brief Report: impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N Engl J Med. 1995;333:1386–90. doi: 10.1056/NEJM199511233332104. [Context Link] [DOI] [PubMed] [Google Scholar]
- 15.Azaryan AV, Krieger TJ, Hook VYH. Purification and characteristics of the candidate prohormone processing proteases PC2 and PC1/3 from bovine adrenal medulla chromaffin granules. J Biol Chem. 1995;270:8201–8. doi: 10.1074/jbc.270.14.8201. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 16.Mykkänen L, Haffner SM, Hales CN, Rönneman T, Laakso M. The relation of proinsulin, insulin, and proinsulin-to-insulin ratio to insulin sensitivity and acute insulin response in normoglycemic subjects. Diabetes. 1997;46:1990–5. doi: 10.2337/diab.46.12.1990. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 17.Phillips DIW, Clark PM, Hales CN, Osmund C. Understanding oral glucose tolerance:comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–92. doi: 10.1111/j.1464-5491.1994.tb00273.x. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 18.Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D., Jr Disproportionate elevation of immunoreactive proinsulin in Type-2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30:698–702. doi: 10.1007/BF00296991. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Mykkänen L, Valdez RA, et al. Disproportionately increased proinsulin levels are associated with the insulin resistance syndrome. J Clin Endocrinol Metab. 1994;79:1806–10. doi: 10.1210/jcem.79.6.7989488. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes. 1997;46:1725–32. doi: 10.2337/diab.46.11.1725. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 21.Weinstein SP, Paquin T, Pritsker A, Haber RS. Glucocorticoid-induced insulin resistance: dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin-and non-insulin-related stimuli. Diabetes. 1995;44:441–5. doi: 10.2337/diab.44.4.441. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 22.Delaunay F, Khan A, Cintra A, et al. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–8. doi: 10.1172/JCI119743. Serial Solutions [Context Link] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J Biol Chem. 1997;272:11118–27. Serial Solutions [Context Link] [PubMed] [Google Scholar]
- 24.Daniels SB, Katzenellenbogen JA. HaloEnol lactones. Studies on the mechanism of inactivation of alpha-chymotrypsin. Biochemistry. 1986;25:1436–44. doi: 10.1021/bi00354a037. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 25.Balsinde J, Dennis EA. Function and inhibition of intracellular calcium-independent phospholipase A2. J Biol Chem. 1997;272:16069–72. doi: 10.1074/jbc.272.26.16069. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 26.Ramandham S, Gross RW, Han X, Turk J. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta cell cytosolic calcium concentration. Biochemistry. 1993;32:337–46. doi: 10.1021/bi00052a042. [Context Link] [DOI] [PubMed] [Google Scholar]
- 27.Gross R, Ramanadham S, Kruszka K, Han X, Turk J. Rat and human pancreatic islet cells contain a calcium-independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by ATP and specifically localized to islet beta-cells. Biochemistry. 1993;32:327–36. doi: 10.1021/bi00052a041. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 28.Irminger JC, Meyer K, Halban P. Proinsulin processing in the rat insulinoma cell line INS after overexpression of the endoproteases PC2 or PC3 by recombinant adenovirus. Biochem J. 1996;320:11–15. doi: 10.1042/bj3200011. Serial Solutions [Context Link] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neerman-Arbez M, Sizonenko SV, Halban PA. Slow cleavage at the proinsulin B-chain/connecting peptide junction associated with low levels of endoprotease PC1/3 in transformed beta cells. J Biol Chem. 1993;268:16098–16100. Serial Solutions [Context Link] [PubMed] [Google Scholar]
- 30.Neerman-Arbez M, Cirulli V, Halban PA. Levels of the conversion endoproteases PC1 (PC3) and PC2 distinguish between insulin-producing pancreatic islet beta cells and non-beta cells. Biochem J. 1994;300:57–61. doi: 10.1042/bj3000057. Serial Solutions [Context Link] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arky R, editor. Crixivan. Physicians’ desk reference. 52nd edition. Medical Economics; Montvale, NJ: 1998. pp. 1625–32. [Context Link] [Google Scholar]
- 32.Kahn SE, McCulloch DK, Schwartz MW, Palmer JP, Porte D., Jr Effect of insulin resistance and hyperglycemia on proinsulin release in a primate model of diabetes mellitus. J Clin Endocrinol Metab. 1992;74:192–7. doi: 10.1210/jcem.74.1.1727820. Serial Solutions. [DOI] [PubMed] [Google Scholar]
- 33.Wang P-W, Abbasi F, Carantoni M, Chen Y-DI, Azhar S, Reaven GM. Insulin resistance does not change the ratio of proinsulin to insulin in normal volunteers. J Clin Endocrinol Metab. 1997;82:3221–4. doi: 10.1210/jcem.82.10.4053. Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 34.Gharakhanian S, Salhi Y, Nguyen TH, et al. Frequency of lipodystrophy and factors associated with glucose/lipid abnormalities in a cohort of 650 patients treated by protease inhibitors [abstract 642]. VI Conference on Retroviruses and Opportunistic Infections; Chicago. 1999. [Context Link] [Google Scholar]
- 35.Martinez E, Conget I, Lozano L, Casamitjana R, Gatell JM. Reversion of metabolic abnormalities after switching from HIV-1 protease inhibitors to nevirapine. AIDS. 1999;13:805–10. doi: 10.1097/00002030-199905070-00009. Ovid Full Text Serial Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- 36.Carr A, Thorisdottir A, Samaras K, Kaufmann GR, Chisholm DJ, Cooper DA. Reversibility of protease inhibitor (PI) lipodystrophy syndrome on stopping PIs or switching to nelfinavir [abstract 668]. VI Conference on Retroviruses and Opportunistic Infections; Chicago. 1999. [Context Link] [Google Scholar]