Abstract
Allogenic bone marrow transplantation is an effective therapy for hematological malignancies. However graft-verses-host disease (GVHD) is a major limiting factor for a successful patient outcome. GVHD is a result of alloimmune responses of donor T lymphocytes attacking the recipient’s cells and tissues. Chemokine receptor CCR5 plays a role in solid organ allograft rejection and mediates murine GVHD pathogenesis. Herein, we report that infiltrating lymphocytes in the skin of human acute GVHD samples are predominantly CCR5+ T cells. In addition we characterized the features of the CCR5 expression on alloreactive T lymphocytes. We found that the CCR5+ population exhibits the characteristics of the activated effector T cell phenotype. CCR5 expression is upregulated upon allogenic stimulation and CCR5+ cells are proliferating with co-expression of T cell activation markers. Furthermore, the activated T cells producing inflammatory cytokine TNFα, IL-2 or IFN-γ, are positive for CCR5. Thus, CCR5 is a marker for GVHD effector cells and that CCR5+ T cells are active participants in the pathogenesis of human acute GVHD.
INTRODUCTION
GVHD is the major cause of morbidity and mortality in allogeneic hematopoietic cell transplantation (HCT) recipients due to alloimmune responses (1). The disease is characterized primarily by targeted epithelial cell injury in skin, intestine and liver (2-4). The development of GVHD is thought to involve three phases: T cell activation, followed by proliferation and differentiation of allogeneic T cells into activated effector cells, and finally specific tissue damage (5-6). The main effector cells in GVHD are T lymphocytes, although their precise phenotype remains elusive. Current evidence suggests that GVHD represents a systemic Th1 type response: this characterization includes a CD4 response of Th1 type, resulting in CD8 cytotoxic T lymphocyte (CTL) generation and an inflammatory cytokine cascade (6).
Chemokines and their receptors play an important role in regulating leukocyte migration and activation (7). Recently, the functional state of T cells has been characterized by the chemokine receptor expression pattern (7-9). In particular, chemokine receptor CCR5 is a marker for effector T cells. CCR5 is a co-receptor for HIV entry and has been studied extensively (10). The expression of CCR5 is very low on naïve T cells, but is highly upregulated on both CD4+(Th1) T cells and activated antigen specific CD8+ T cells (11-12). The function of CCR5 and its ligands in GVHD has been primarily explored in murine models. It has been reported that CCR5+/CD8+ T cells mediate hepatic injury in mouse GVHD and blocking antibody to CCR5 reduces the damage (13). In addition, MIP-1α, one of the ligands for CCR5 has also been shown to mediate mouse GVHD (14-15). While two groups demonstrated that genetic deletion of CCR5 in the donor can reduce acute GVHD in mice (16-17); others reported that CCR5−/− or MIP-1α−/− donor T cells accelerate GVHD in liver and lung (17-18). These data suggested that the role of CCR5 in alloimmmune responses is complicated, and probably regulated in strain-, target organ- or pretransplant conditioning- dependant fashion in murine GVHD models (17). In addition to T cells, other cell types such as dendritic cells (DCs) and B cells are involved in the pathogenesis of GVHD (19-20). CCR5 has been shown to express on dendritic cells and specifically the dermal Langerhans cells (21-22). Langerhans cells represent the specialized DCs of the epidermis, and play an important role in skin GVHD (19). Clearly the role of CCR5 is intricate and complicated and the field is as of yet still unresolved regarding the role of CCR5 in human GVHD.
It has been shown that infiltration of CCR5+ T cells occurs in both acute and chronic phase of human renal allograft rejection (23). A large cohort of patients genetically lacking CCR5 (CCR5Δ32) demonstrated the functional importance of CCR5 in human renal allograft survival (24). Recently, we have identified donors genetically lacking CCR5 and correlated with the outcome of patients who received major histocompatibility complex (MHC)-matched unrelated HCT (25). We found a decreased incidence of GVHD in the absence of CCR5 on donor T cells. In this study, we report that lymphocyte infiltrates at the skin sites of human acute GVHD (aGVHD) are predominantly CCR5+ T cells, including both CD4+ and CD8+ subsets. Upon allogeneic stimulation, the majority of CCR5+ cells demonstrated characteristics of activated proliferating T cells with co-expression of activation marks and production of inflammatory cytokines.
MATERIALS AND METHODS
Immunohistochemistry
Skin and lip biopsies were retrieved from the GVHD tissue repository at Fred Hutchinson Cancer Center from patients transplanted between 1988 and 2000 (25-26). Formalin-fixed paraffin-embedded sections of skin and lip were examined by hematoxylin and eosin stain, and CD3, CD4, CD8, CCR5, CD1a and CD20 immunostains performed using avidin-biotin-conjugated reagents. The co-expression of CCR5 in combination with CD4 or CD8 was performed using DAKO Envision Double System (DAKO System) that can detect two antigens from the same species within one specimen. The skin biopsies were double stained with CCR5 antibody and either CD4 or CD8 antibodies (Texas-Red). The same slides were photographed by light and fluorescence microscopy for Texas-Red.
Dendritic Cell Culture
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat preparations derived from whole blood of healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX). The buffy coat was diluted with PBS at 1:2 ratio and centrifuged over Histopaque-1077 (Sigma Diagnostics) for 30 minutes. The PBMC interface was collected and analyzed for purity by flow cytometry and frozen for future use. Dendritic cells were generated from CD14 positive selected monocytes using magnetic beads (Miltenyi Biotec). The CD14 positive fraction was cultured in RPMI plus 50ng/ml GM-CSF and 10ng/ml IL4. On day 6, 1/100 dilution of maturation cytokine factor 100X PITIP (1 ug/ml Poly (cytidylic-inosinic) acid, 1ug/ml IL-1β, 1ug/ml TNFα, 1.5 ug/ml IL-6, 100 ug/ml Prostaglandin E2) was added to the differentiation culture as described (27). DCs were collected on day 9 of culture.
Mixed Lymphocyte Reaction
Frozen PBMCs from healthy volunteer donors were thawed and cultured in RPMI plus 10% FBS and 1% streptomycin/penicillin. After 24 hours the cells were counted and resuspended to 1×106 cells/ml and labeled either responder or stimulator. This day is labeled Day 0 and cells were assayed on day indicated in figure legends. The responders were mixed in a 1:1 ratio with irradiated stimulator (allogeneic) or irradiated responders (autologous) mixed lymphocyte reaction (MLR) respectively. Where indicated the MLR was carried out using DCs, allogeneic or autologous DC MLR was performed using a DC:PBMC ratio of 1:100. Cells were collected and stained on Days 3, 6, 9 and/or day 12 as indicated in figure legends.
Intracellular Cytokine Assay
The intracellular cytokine staining was carried out as previously described (28). In brief, the MLR cultures were treated on the indicated day with Brefeldin A (final concentration of 10 ug/ml) for 5 hours at 37°C. Cells were then collected, washed and stained for surface expression of CD4, CD8, and CCR5. Cells were washed and permeabilized using BD FACS Permeabilizing Solution 2 (BD Biosciences) for 10 minutes with occasional mild vortex. Cells were washed and stained for intracellular cytokines using fluorescent-tagged antibodies against TNFα, IL-2 and IFN-γ (BD Biosciences).
RESULTS
Infiltrating T cell lymphocytes in the skin of human aGVHD are predominantly CCR5+ cells
Based on our recent report, patients who received bone marrow from CCR5Δ32 homozygous donors had lower risk of skin GVHD than the control group, with an odds ratio of 0.19 and 95% confidence interval of 0.05-0.80 and the difference is statistically significant (p=0.02) (25). This implicated CCR5 expression might mediate the invasion of lymphocytes into the skin, liver and gut, which is the characteristic feature of GVHD (6). We therefore examined the degree of expression of CCR5 in the lymphocytes in the skin of human aGVHD. We screened our GVHD tissue repository of allograft recipients, to select ten samples from skin and lip that showed histological features of aGVHD (epithelial apoptosis associated with lymphocyte infiltration) and had the most abundant number of lymphocytes. Samples were stained with CCR5, CD3, CD4 and CD8 antibodies.
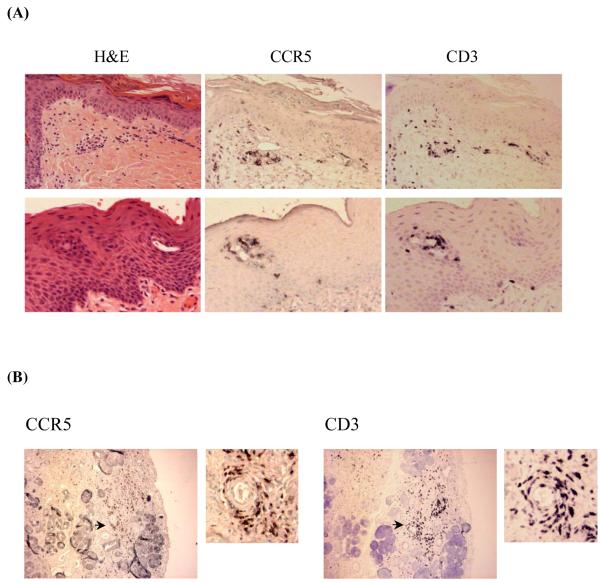
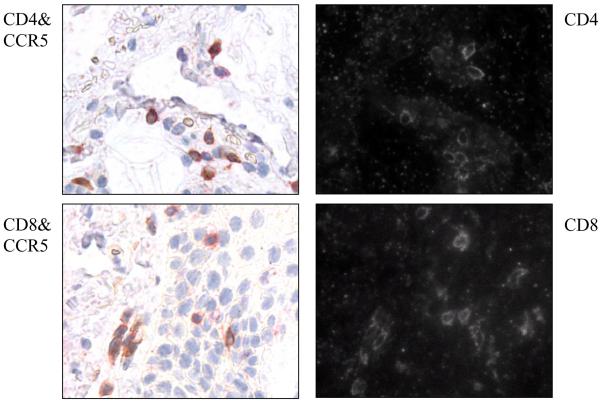
As shown in Figure 1A, a representative example of GVHD skin (top panel) and lip (lower panel), lymphocytes were found near the dermal and epidermal junctions, and were concentrated in the basal layer. Adjacent sections that were stained with CCR5 antibody or CD3 antibody showed a similar pattern. Figure 1B is a representative example of the lip biopsy of a GVHD patient: the salivary gland was damaged by lymphocytes which resulted in the dry mouth symptoms often seen in GVHD patients. The pattern of CCR5 antibody staining in these lip biopsies is similar to that of CD3 staining, suggesting that the gland-infiltrating lymphocytes were CCR5+ T cells. To determine whether the CCR5+ T cells were CD4+ versus CD8+, we examined the co-expression of CCR5 in combination with CD4 or CD8, using the DAKO Envision Double System that can detect two antigens from the same species within one specimen. As shown in Figure 2, the majority of infiltrating cells were double stained with either CD4 or CD8 and CCR5. Thus, CCR5 expression is present on the majority of both CD4+ and CD8+ T cells involved in skin GVHD.
Figure 1. Infiltrating T cell lymphocytes in human aGVHD tissues are predominantly CCR5+.
(A) Skin and lip biopsies of GVHD patients. Three adjacent sections of skin (top panel) and lip (lower panel) biopsies of a representative GVHD patient were stained with H&E, CCR5 antibody or CD3 antibody. CCR5+ and CD3+ lymphocyte infiltrates were found in both the dermal and epidermal layers. (B) Lip biopsy of a representative GVHD patient with damaged salivary gland. Right and left panels are adjacent sections stained with CCR5 or CD3 antibodies. In each panel, the right side is an enlarged picture of the area with the arrow. CCR5+ and CD3+ lymphocyte infiltrates were found in the damaged area of salivary gland.
Figure 2. Infiltrating CCR5+ T cell lymphocytes in the skin of aGVHD are both CD4+ and CD8+ T cells.
The skin biopsies of a representative GVHD patient were double stained with CCR5 antibody and either CD4 antibody (top panel) or CD8 antibody (bottom panel). On the right side, CCR5+ cells are brown, and both CD4+ and CD8+ cells are Texas-Red. The same slides were photographed by light (right panel) and fluorescence microscopy (left panel) for Texas-Red.
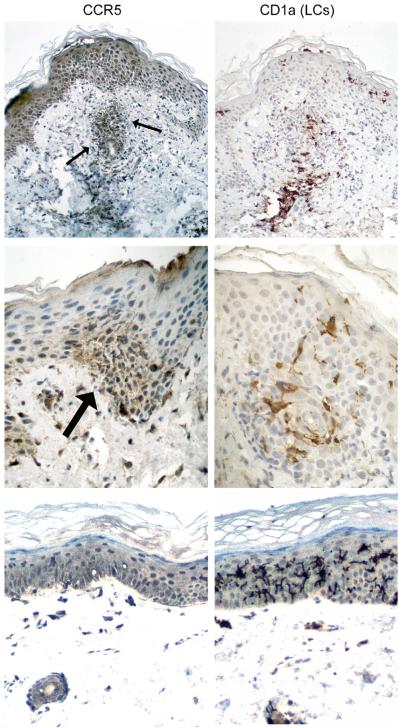
In addition, we examined all ten skin and lip biopsies for Langerhans DC marker CD1a and B-cell marker CD20. No CD20+ B cells were noted (data not shown) and CD1a+ epidermal Langerhans cells were abundant in five samples. The samples with heavy Langerhans cell infiltrates were retrieved for CCR5 staining. Representative sections are shown in Figure 3. In the top panel, the skin biopsy with extensive epidermal damage has CCR5 expression on the vast majority of T cell infiltrates in the superficial dermis (arrows) and epidermis, with increased CD1a+ Langerhans DCs at sites of lymphocytic infiltration. The foci of epidermal damage has high-level CCR5 expression on T cells but weak to minimal CCR5 staining on Langerhans DCs (middle panel). In the bottom panel, lip biopsy from a case with minimal lymphoid infiltrate or epithelial damage shows no detectable CCR5 expression on CD1a+ Langerhans DCs. Thus, strong CCR5 expression is present on T cells but not dendritic cells in the skin of aGVHD.
Figure 3. Strong CCR5 expression on T cells but not dendritic cells in aGVHD tissues.
Immunohistochemistry was performed on paraffin sections for CCR5 (left panel) and Langerhans DC marker CD1a (right panel). Top panel: skin biopsy with CCR5+ T cells in the superficial dermis (arrows) and epidermis in addition to CD1a+ Langerhans DCs at sites of lymphocytic infiltration. Middle panel: foci of epidermal damage with high-level CCR5 expression in T cells (arrow) but weak CCR5 staining in CD1a+ Langerhans DCs. Bottom panel: lip biopsy from a case with minimal epithelial damage and no detectable CCR5 expression in CD1a+ Langerhans DCs.
CCR5 is expressed on activated T cells and upregulated on proliferating T lymphocytes following allogeneic activation
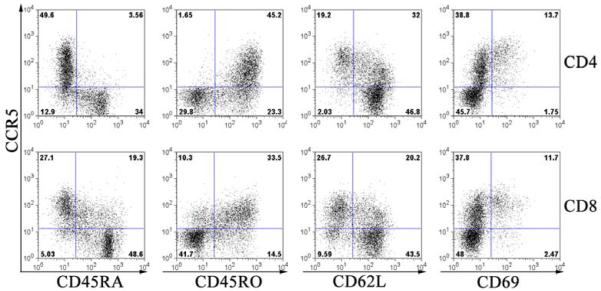
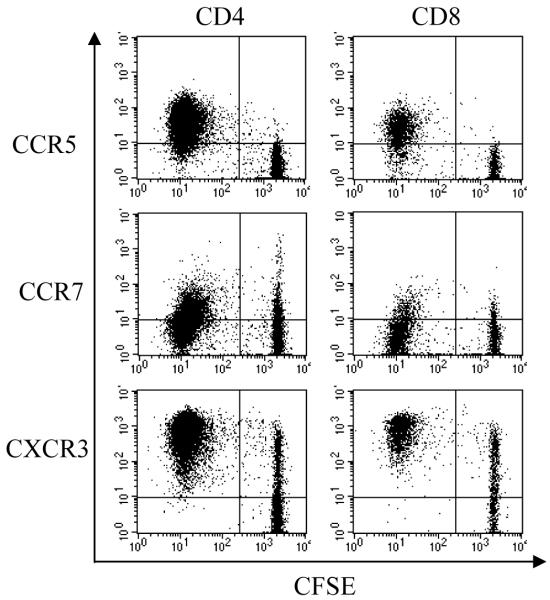
In order to dissect the human alloimmune responses mediated by CCR5+ T cells, we used MLR with allogeneic DCs mixed with responder PBMCs for lymphocyte activation and proliferation. We found that the expression level of CCR5 on both the CD4+ and CD8+ T cells increased from day 3 and peaked at day 6-9 after allogeneic MLR (data not shown). We further examined the characteristics of the CCR5+ T cells after allogeneic MLR. As shown in Figure 4, the majority of CCR5+ were CD45RA−/CD45RO+ with decreased expression of CD62L and CD69, representing the activated T cell phenotype. Furthermore, we examined whether the CCR5+ population was actively proliferating. CFSE (Carboxy Fluorescein Succinimidyl Ester)-labeled responder cells were plated with stimulator cells and cell proliferation was measured by CFSE intensity with FACS. As shown in Figure 5, the proliferating CD4+ and CD8+ T cells with low CFSE staining were positive for CCR5. Thus, the expression of CCR5 is greatly restricted to the proliferating T lymphocytes. In contrast, both CCR7 and CXCR3 do not appear to have the same restriction to proliferating cells as CCR5.
Figure 4. CCR5 is co-expressed with T cell activation markers.
Mixed lymphocyte culture (MLR) were used to activate PBMC from normal donors with allogenic dendritic cells (DC) mixed with responder PBMCs (DC-MLR) at a DC:PBMC ratio of 1:100. The cells were collected 7 days after stimulation and stained with antibodies CD4, CD8, CCR5 and a panel of activation markers including CD45RA, CD45RO, CD62L and CD69. The representative dot plots of CD4 (upper panel) and CD8 (lower panel) T cells demonstrated the co-expression of CCR5 and activation marks. Data were representative of experiments from three independent samples. Cells were collected on a FACscaliber Flow Cytometer and data was analyzed by Cell Quest software (Becton Dickinson).
Figure 5. CCR5 is up-regulated on proliferating T cell lymphocytes following allogeneic activation.
Responder PBMCs were incubated in CFSE for 10 minutes and then washed with PBS for co-culture with allogenic DCs. Cells were collected 9 days after stimulation and stained with CD4 and CD8 antibodies. The gated lymphoblast population of CD4+ or CD8+ T cells were analyzed separately for CCR5, CCR7 and CXCR3 expression. Data shown here are representative of three independent samples.
CCR5+ T cells produce cytokine TNFα, IL-2 and IFN-γ
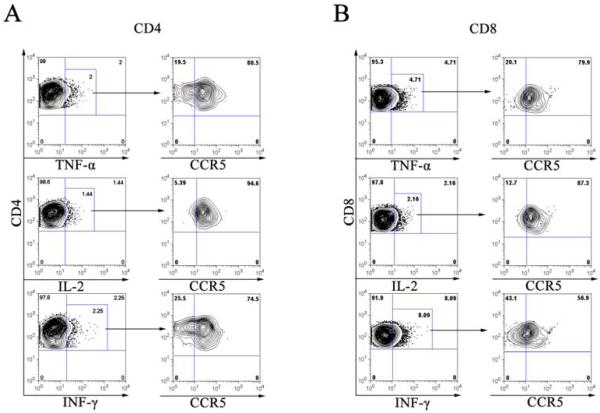
The mechanism of GVHD has been described as a “cytokine storm” due to the central role of cytokines in mediating tissue injury and inflammation characteristic of GVHD pathology (6). Therefore, we assessed whether cells producing the cytokine TNFα, IL-2 and IFN-γ were positive for CCR5. Cells were treated with brefeldin A seven days post MLR and were stained for CD4, CD8, and CCR5. The expression of cytokines was measured by intracellular staining with antibodies against TNFα, IL-2 or IFN-γ. As shown in Figure 6 (top panel), the CD4+ (A) or CD8+ (B) T cells producing intracellular TNFα were gated and analyzed for CCR5 expression. The majority of TNFα producing cells were positive for CCR5 with about 80.5% for CD4+ and 79.9% for CD8+ T cells respectively. We found a similar pattern of CCR5 expression in IL-2 producing cells (Figure 6, middle panel). The majority of IFN-γ producing cells (74.5%) were positive for CCR5 in the CD4+ T cell population, whereas only about 56.9% were positive for CCR5 in the CD8+ T cell population (Figure 6, bottom panel). In conclusion, CD4+ T cells that produce cytokine TNFα, IL-2 or IFN-γ, were expressing CCR5. However, CCR5 expression was only detected on TNFα or IL-2 producing CD8+ T cells and about half of IFN-γ producing CD8+ T cells were negative for CCR5 expression.
Figure 6. CCR5+ T lymphocytes and intracellular cytokine production of TNFα, IL-2 and IFN-γ.
Allogenic dendritic cells mixed with responder PBMCs (DC-MLR) at a DC:PBMC ratio of 1:100. The MLR cultures were treated with brefeldin-A on day 7 for 5 hours. The cells were harvested and stained with CD4, CD8, CCR5 and intracellular cytokines TNFα (top panel), IL-2 (middle panel) and IFN-γ (bottom panel). The left panel of A (CD4) and B (CD8) was the representative dot-plots of intracellular cytokine production and the gated cells were positive for the cytokine using autologous MLR sample as the reference. The right panel of A and B was the CCR5 expression of the gated cells from the respective upper panel and the quadrant gates were decided based on isotype control. Results were representative of four independent samples.
DISCUSSION
In the current study, we investigated the role of chemokine receptor CCR5 in skin GVHD and allo-immune responses. We demonstrated that CCR5 expression is present on both CD4+ and CD8+ T cell infiltrates in the skin biopsies of human aGVHD. CCR5 is upregulated upon allostimualtion and expressed on activated T cells. In addition, the expression of CCR5 is restricted to the proliferating T lymphocytes. Furthermore, we found that activated T cells producing cytokine TNFα, IL-2 or IFN-γ are positive for CCR5. Together, our data indicated that CCR5 expression on T cells mediates alloimmune responses in GVHD.
CCR5 is considered one of the “inflammatory” chemokine receptors regulated by proinflammatory stimuli to orchestrate immune responses, in comparison to the “homeostatic “ group which are important in immune surveillance such as CCR7. Inflammatory chemokine receptors are upregulated during tissue damage or inflammation, and involved in T cell polarization (29-31). Gene expression profiles have shown that CCR5 was upregulated during aGVHD in human (32-33). Our data further demonstrated that CCR5 expression is on activated and proliferating T cells upon allogeneic stimulation. In contrast, CCR7 was found within both proliferating and non-proliferating T cells, and there was no significant increase in the expression of CCR7. Interestingly, CXCR3, another inflammatory chemokine receptor, does not show the same restriction to proliferating cells as CCR5. Although almost all proliferating T cells were positive for CXCR3, there were high percentage of non-proliferating T cells expressing this chemokine receptor. Thus, the data suggests a critical role of CCR5 expression in alloimmune responses and GVHD.
We and others have demonstrated that the protective effect of the CCR5 deletion mutation in GVHD (25, 34). In a small cohort study, we found that in the absence of CCR5 on donor cells, there is a decreased incidence of GVHD and increased relapse rate in recipients received HLA-matched unrelated marrow donors (25). In particular, there is a significant reduction of skin GVHD. We further examined the skin and lip biopsies from aGVHD patients for CCR5 expression in situ, and found the majority of infiltrating T cells were positive for CCR5. Altogether, these data confirms that CCR5 expression plays an important role in mediating human GVHD.
CCR5 is highly upregulated on both CD4+(Th1) T cells and activated antigen specific CD8+ T cells (11-12). Using intracellular cytokine assay, we found that activated CD4+ T cells that produce inflammatory cytokine TNFα, IL-2 or IFN-γ, were positive for CCR5. In addition, the TNFα and IL-2 producing CD8+ T cells also expressed CCR5. This observation supported the notion that CCR5 is a marker for effector T cells that actively participate in the “cytokine storm” of GVHD (6).
Chemokines and their receptors are important in many human diseases, including HIV, autoimmune disease, inflammatory disease and organ graft rejection (35). Currently small molecule antagonists and humanized monoclonal antibodies targeting chemokine receptors are developed by industry and tested in preclinical models as well as in phase I trials (36-39). Our data demonstrated that CCR5+ T cells are important in mediating GVHD in human. The results will provide rationale to develop novel treatment for GVHD.
Acknowledgments
This work was supported by grants from the Wendy Will Case Cancer Fund (to Q.M.), and CA18029, CA15704 and CA78902 from the National Institutes of Health (to R.F.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Sale GE. The Pathology of Organ Transplantation. Butterworth Publishers, Inc.; London, Stoneham: 1990. pp. 229–260. [Google Scholar]
- 3.Ferrara JLM, Antin JH. The Pathophysiology of graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Blackwell Scientific Publications; Boston: 1994. pp. 305–315. [Google Scholar]
- 4.Sale GE. Does graft-versus-host disease attack epithelial stem cells? Mol Med Today. 1996;2:114–119. doi: 10.1016/1357-4310(96)88721-1. [DOI] [PubMed] [Google Scholar]
- 5.Fowler DH, Gress RE. Graft-versus-host disease as a Th1-type process: regulation by donor cells of Th2 cytokine phenotype. In: Ferrara JLM, Deeg HJ, Burakoff SJ, editors. Graft-vs.-Host Disease. Marcel Dekker Inc.; New York: 1997. pp. 479–500. [Google Scholar]
- 6.Ferrara JL. Pathogenesis of acute graft-versus-host disease: cytokines and cellular effectors. J Hematother Stem Cell Res. 2000;9:299–306. doi: 10.1089/15258160050079407. [DOI] [PubMed] [Google Scholar]
- 7.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 8.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 9.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 10.Garzino-Demo A, DeVico AL, Conant KE, Gallo RC. The role of chemokines in human immunodeficiency virus infection. Immunol Rev. 2000;177:79–87. doi: 10.1034/j.1600-065x.2000.17711.x. [DOI] [PubMed] [Google Scholar]
- 11.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8+ T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–5550. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 12.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol. 2002;168:2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 13.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Related Active participation of CCR5+CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serody JS, Cook DN, Kirby SL, Reap E, Shea TC, Frelinger JA. Murine T lymphocytes incapable of producing macrophage inhibitory protein-1 are impaired in causing graft-versus-host disease across a class I but not class II major histocompatibility complex barrier. Blood. 1999;93:43–50. [PubMed] [Google Scholar]
- 15.Serody JS, Burkett SE, Panoskaltsis-Mortari A, Ng-Cashin J, McMahon E, Matsushima GK, Lira SA, Cook DN, Blazar BR. T-lymphocyte production of macrophage inflammatory protein-1alpha is critical to the recruitment of CD8+ T cells to the liver, lung, and spleen during graft-versus-host disease. Blood. 2000;96:2973–2980. [PubMed] [Google Scholar]
- 16.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 17.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 18.Panoskaltsis-Mortari A, Hermanson JR, Taras E, Wangensteen OD, Serody JS, Blazar BR. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood. 2003;101:3714–3721. doi: 10.1182/blood-2002-08-2465. [DOI] [PubMed] [Google Scholar]
- 19.Merad M, Hoffmann P, Ranheim E, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;5:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B Cells in the pathogenesis of graft-versus-host disease. Blood. 2009 Sep 11; doi: 10.1182/blood-2008-10-161638. 2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Gros E, Bussmann C, Bieber T, Förster I, Novak N. Expression of chemokines and chemokine receptors in lesional and nonlesional upper skin of patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:753–760. doi: 10.1016/j.jaci.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Santegoets SJ, Gibbs S, Kroeze K, van de Ven R, Scheper RJ, Borrebaeck CA, de Gruijl TD, Lindstedt M. Transcriptional profiling of human skin-resident Langerhans cells and CD1a+ dermal dendritic cells: differential activation states suggest distinct functions. J Leukoc Biol. 2008;84:143–151. doi: 10.1189/jlb.1107750. [DOI] [PubMed] [Google Scholar]
- 23.Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, Cartron JP, Colin Y, Schlondorff D, Alpers CE. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am J Kidney Dis. 2001;37:518–531. [PubMed] [Google Scholar]
- 24.Fischereder M, Luckow B, Hocher B, Wuthrich RP, Rothenpieler U, Schneeberger H, Panzer U, Stahl RA, Hauser IA, Budde K, Neumayer H, Kramer BK, Land W, Schlondorff D. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 25.Ma Q, Gooley TA, Storb RF. CCR5 expression on cells from HLA-matched unrelated marrow donors and graft-versus-host disease. Biol Blood Marrow Transplant. 2009 doi: 10.1016/j.bbmt.2009.05.017. DOI: 10.1016/j.bbmt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, Petersdorf EW, Radich J, Sanders JE, Storb RF, Sullivan KM, Anasetti C. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 27.Xing D, Decker WK, Li S, Robinson SN, Yang H, Segal H, O’Connor S, Yao X, Komanduri KV, McMannis JD, Jones RB, de Lima M, Champlin RE, Shpall EJ. AML-loaded DC generate Th1-type cellular immune responses in vitro. Cytotherapy. 2006;8:95–104. doi: 10.1080/14653240600620093. [DOI] [PubMed] [Google Scholar]
- 28.Martins SL, St John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ, Komanduri KV. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–3436. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 29.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv Immunol. 2001;78:233–266. doi: 10.1016/s0065-2776(01)78005-4. [DOI] [PubMed] [Google Scholar]
- 32.Jaksch M, Remberger M, Mattsson J. Increased gene expression of chemokine receptors is correlated with acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:280–287. doi: 10.1016/j.bbmt.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Sato N, Takahashi S, Tojo A. Gene-expression profiles of peripheral blood mononuclear cell subpopulations in acute graft-vs-host disease following cord blood transplantation. Exp Hematol. 2008;36:1760–1770. doi: 10.1016/j.exphem.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Bogunia-Kubik K, Duda D, Suchnicki K, Lange A. CCR5 deletion mutation and its association with the risk of developing acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91:1628–1634. [PubMed] [Google Scholar]
- 35.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 36.Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Clercq E. New anti-HIV agents and targets. Medical Research Reviews. 2002;22:531–565. doi: 10.1002/med.10021. [DOI] [PubMed] [Google Scholar]
- 38.Horuk R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods. 2003;29:369–375. doi: 10.1016/s1046-2023(02)00361-4. [DOI] [PubMed] [Google Scholar]
- 39.Dhami H, Fritz CE, Gankin B, Pak SH, Yi W, Seya MJ, Raffa RB, Nagar S. The chemokine system and CCR5 antagonists: potential in HIV treatment and other novel therapies. J Clin Pharm Ther. 2009;34:147–160. doi: 10.1111/j.1365-2710.2008.00978.x. [DOI] [PubMed] [Google Scholar]