Abstract
Objectives
We examined the in vitro cellular effects of the multi-targeted receptor tyrosine kinase inhibitors (TKIs) sunitinib and pazopanib on a series of human renal cell carcinoma (RCC) cell lines.
Methods
The human RCC cell lines 769-P, 786-O, HRC-24, HRC-31, HRC-45, HRC-78, RCC-26B, and SK-45 were treated with varying concentrations of sunitinib and pazopanib. Cellular proliferation and cellular death were assessed using the CellTiter-Blue Cell Viability Assay and the TUNEL assay, respectively. Effective doses (ED) for inhibition of cellular proliferation or induction of apoptosis were calculated for sunitinib and pazopanib in each RCC cell line.
Results
Both sunitinib and pazopanib exhibited anti-proliferative activity to varying degree against all human RCC cell lines; however, sunitinib’s effects were achieved at significantly lower concentrations. Moreover, sunitinib had a direct pro-apoptotic effect on all tested cell lines, while pazopanib failed to induce apoptosis in any of the examined human RCC cell lines even at maximal concentrations.
Conclusions
Although sunitinib and pazopanib are often used interchangeably in the clinical setting, our results suggest that in-vitro biological activity of the two agents differs. Sunitinib exhibits a cytotoxic effect on RCC cell lines, while pazopanib’s activity is solely cytostatic. These data may be clinically relevant given the current lack of comparative in-vivo studies between the two agents.
Keywords: advanced kidney cancer, tyrosine kinase inhibitors, pazopanib, sunitinib
INTRODUCTION
More than 13,000 patients in the United States some 24% of those diagnosed succumb annually to renal cell carcinoma (RCC), establishing RCC as the deadliest of all urologic malignancies.(1) Notwithstanding early detection, aggressive treatment strategies, and a greater understanding of RCC’s molecular pathogenesis, the mortality rates from renal malignancy are on the rise.(2, 3) Indeed, approximately one third of patients with RCC will receive the diagnosis, once their disease is already metastatic.(4) These patients generally face dismal prospects with a historical median survival of only 10 months.(5)
Because RCC is highly resistant to radiation therapy and cytotoxic chemotherapy, until recently the standard of care for patients with metastatic disease was surgical debulking combined with immunotherapy using interferon-alpha (INF-α) or interleukin-2 (IL-2).(6, 7) Due to limited efficacy and significant toxicity (8, 9), immunotherapy largely is being replaced by a new generation of targeted therapies that inhibit tumor angiogenesis. Agents such as sorafenib, sunitinib, temsirolimus, and bevacizumab target hypoxia response pathways, thereby hindering tumor growth. Since the introduction of these targeted therapies, median survival for patients with metastatic RCC (mRCC) has increased to nearly 24 months.(10, 11)
Sunitinib - the main line targeted therapy agent for patients with mRCC -- is an oral multi-targeted receptor tyrosine kinase inhibitor (TKI) with tumoricidal activity mediated mainly through inhibition of the platelet-derived growth factor receptor (PDGFR), the vascular endothelial growth factor receptor (VEGFR) 1 and 3, KIT, and FLT3. Sunitinib has proven to be more efficacious than interferon-alpha in a randomized phase III trial.(10) Pazopanib is another TKI with a comparable pharmacological profile as sunitinib. Similar to sunitinib, pazopanib also has demonstrated significantly improved progression-free survival and tumor response when compared to placebo (12). Nevertheless, currently, level I evidence for pazopanib’s superiority to immunotherapy or to another targeted therapy agent is lacking. Despite this lack of robust data, pazopanib is often prescribed as a first-line agent in clinical practice due to its perceived superior side-effect profile and ease of administration when compared with sunitinib.
To our knowledge, there have been no studies that have compared the direct in-vitro effect of sunitinib and pazopanib on RCC cell lines. As such, we investigated sunitinib’s and pazopanib’s in-vitro anti-tumor responses in a series of human RCC cell lines. Here, we demonstrate that while both agents exhibit anti-proliferative activity against all tested human RCC cell lines, only sunitinib has a direct pro-apoptotic effect on RCC cell lines.
METHODS
Cells and reagents
The 769-P, 786-O, HRC-24, HRC-31, HRC-45, HRC-78, and SK-45 human renal cell carcinoma cell lines were a kind gift of Dr. Joseph Testa (Fox Chase Cancer Center, Philadelphia, PA). The SK-26B cell line was obtained through the generosity of Dr. Finke (The Cleveland Clinic Foundation, Cleveland OH).(13) Cells were maintained in RPMI 1640 medium (Bio-Whittaker, Walkersville, MD) supplemented with 10% FBS (Hyclone, Logan, UT), penicillin (100 U/mL), streptomycin (100 μg/mL), sodium pyruvate (1 mM) and non-essential amino acids (0.1 mM).
Sunitinib and Pazopanib were obtained from LC Laboratories (Boston, MA). Stock solutions of both reagents were prepared in DMSO (Sigma, St. Louis, MO).
In vitro measurement of cell proliferation and apoptosis
Cell proliferation was determined by CellTiter-Blue assay (Promega, Madison, WI). Apoptosis was detected using the APO-BRDU kit (The Phoenix Flow Systems, Inc., San Diego, CA) followed by flow cytometry analysis.
Statistical Analysis
Data are shown as the mean of three assays run separately. Effective doses were calculated using the XL-Fit add in for the Microsoft Excel program (Microsoft Inc, Seattle, WA). Effective doses represent the calculated concentrations of sunitinib or pazopanib at which a certain percentage of cells were non-viable or apoptotic. For some effective doses, the concentration was so high that it was not calculable by the software.
RESULTS
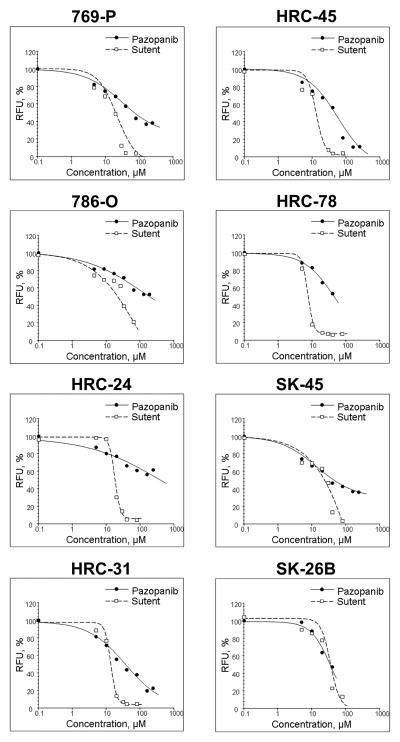
Effects of sunitinib and pazopanib on cellular proliferation were assessed using the CellTiter Blue assay. Both sunitinib and pazopanib inhibited cellular proliferation in all eight RCC cell lines, however, sunitinib demonstrated anti-proliferative effects at markedly lower concentrations than pazopanib (Fig. 1). The CellTiter Blue assay is based on the ability of living cells to convert a redox dye (resazurin) into a fluorescent end product (resorufin). As measured by the degree of fluourescence, sunitinib is able to completely suppress proliferation of all tested RCC cell lines, whereas pazopanib does not achieve the same degree of anti-proliferative activity. The divergence in the activity between sunitinib and pazopanib is further highlighted by the calculated range of effective doses (ED) for both compounds against the RCC cell lines displayed in Table 1. For example, at an ED 20 dose at which there is a reduction of cellular proliferation by 20 percent -- both compounds halt cellular growth at similar concentrations in all cell lines. When we used higher ED threasholds, pazopanib limitations at stopping cellular proliferation based on the CellTiter Blue Assay were apparent: at an ED50, pazopanib is only able to inhibit cellular proliferation at achievable concentrations in 5 of the 8 cell lines; and, at an ED70, pazopanib inhibits cellular viability only in the SK-26b cell line at its maximal concentration of ~60 μM. Conversely, sunitinib is able to halt proliferation in 90 percent of cells -- ED90 -- in five of the 8 cell lines tested.
Figure 1.
The effect of sunitinib and pazopanib on proliferation of human renal cell carcinoma cell lines. Cells were treated with indicated concentrations of sunitinib or pazopanib for 48 hours. Cellular proliferation was assessed using the CellTiter Blue assay.
Table 1. Inhibition of Cellular Proliferation.
Calculated concentrations of effective doses (ED) for sunitinib and pazopanib in inhibiting cellular proliferation against eight human renal cell carcinoma cell lines
| Cell line | Compound (μM) | ED20 | ED50 | ED70 | ED90 |
|---|---|---|---|---|---|
| 769-P | Sunitinib | 6.3 | 15.1 | 24.6 | 45.6 |
| Pazopanib | 6.8 | 60.3 | >100 | N/A | |
| 786-O | Sunitinib | 3.6 | 20.7 | 45.2 | 90.5 |
| Pazopanib | 7.9 | >100 | N/A | N/A | |
| HRC-24 | Sunitinib | 13.1 | 17.1 | 20.4 | 30.0 |
| Pazopanib | 11.2 | >100 | N/A | N/A | |
| HRC-31 | Sunitinib | 9.7 | 12.8 | 15.3 | 21.5 |
| Pazopanib | 4.8 | 32.4 | >100 | N/A | |
| HRC-45 | Sunitinib | 7.9 | 12.1 | 15.8 | 24.8 |
| Pazopanib | 9.1 | 36.7 | 79.8 | >100 | |
| HRC-78 | Sunitinib | 5.2 | 6.9 | 8.4 | 13.4 |
| Pazopanib | 10.3 | 44.0 | >100 | N/A | |
| SK-45 | Sunitinib | 4.5 | 21.4 | 39.6 | 64.6 |
| Pazopanib | 3.4 | 36.6 | >100 | N/A | |
| SK-26b | Sunitinib | 21.7 | 34.3 | 45.3 | 70.7 |
| Pazopanib | 13.4 | 34.6 | 61.6 | N/A |
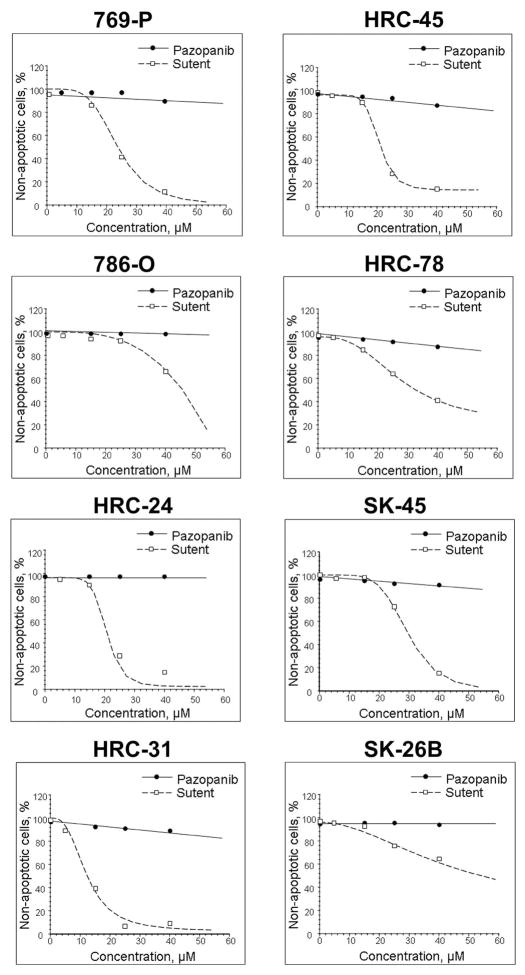
In addition to examining sunitinib’s and pazopanib’s effects on cytostasis, we examined cytotoxic effects of the two agents on RCC cell lines. We employed the TUNEL assay to test for presence of apoptosis following administration of the TKIs. In contrast to the cellular proliferation assay, only sunitinib exhibited a direct apoptotic effect on the human RCC cell lines tested. Pazopanib does not induce apoptosis in any of the tested cell lines. This difference in ability to induce programmed cellular death effects between the two compounds is illustrated in Figure 2. The percent viability for each cell line depicted on the Figure, independent of pazopanib’s concentration, remains essentially 100%. In marked distinction, sunitinib induces almost total apoptosis in five of the 8 cell lines: HRC-24, HRC-31, HRC-45, 769-P and SK-45. In a manner similar to the proliferative assay, we calculated the EDs of sunitinib and pazopanib at inducing programmed cellular death to underscore the differences in the effects of the two agents. As demonstrated in Table 2, pazopanib does not induce an ED20 in any of the cell lines at its maximal achievable concentration (approximately 60 μM). On the other hand, sunitinib has a calculated ED50, ED70, and ED90 for inducing apoptosis at a concentration between 20 and 40 μM in seven, six, and four cell lines, respectively. Although both pazopanib and sunitinib are able to halt in vitro cellular proliferation, only sunitinib is able to induce a direct apoptotic effect in human RCC cell lines. Thus, these results demonstrate that sunitinib is able to exhibit a cytotoxic effect on RCC cell lines in vitro, while pazopanib exerts only a cytostatic effect.
Figure 2.
The effect of sunitinib and pazopanib on apoptosis in human renal cell carcinoma cell lines. Cells treated with indicated concentrations of sunitinib or pazopanib for 24 hours. Apoptosis was measured by the TUNEL assay followed by flow cytometry analysis.
Table 2.
Induction of Apoptosis. Calculated concentrations of effective doses (ED) for sunitinib and pazopanib in inducing apoptosis against eight human renal cell carcinoma cell lines
| Cell line | Compound (μM) | ED20 | ED50 | ED70 | ED90 |
|---|---|---|---|---|---|
| 769-P | Sunitinib | 17.4 | 23.7 | 28.7 | >40 |
| Pazopanib | >100 | N/A | N/A | N/A | |
| 786-O | Sunitinib | 34.5 | 45.9 | 50.9 | 55.1 |
| Pazopanib | >100 | N/A | N/A | N/A | |
| HRC-24 | Sunitinib | 18.0 | 21.0 | 22.4 | 25.5 |
| Pazopanib | >100 | N/A | N/A | N/A | |
| HRC-31 | Sunitinib | 7.7 | 12.4 | 16.7 | 27.3 |
| Pazopanib | >100 | N/A | N/A | N/A | |
| HRC-45 | Sunitinib | 17.4 | 21.4 | 24.8 | 32.8 |
| Pazopanib | 76.1 | N/A | N/A | N/A | |
| HRC-78 | Sunitinib | 17.5 | 32.7 | 48.9 | 88.9 |
| Pazopanib | 80.6 | N/A | N/A | N/A | |
| SK-45 | Sunitinib | 23.2 | 29.6 | 34.3 | 43.4 |
| Pazopanib | >100 | N/A | N/A | N/A | |
| SK-26b | Sunitinib | 22.4 | 54.5 | 99.3 | >100 |
| Pazopanib | N/A | N/A | N/A | N/A |
DISCUSSION
Our work demonstrates that although both pazopanib and sunitinib are able to suppress proliferation of human RCC cell lines in vitro, only sunitinib completely halts proliferation of malignant cells. Moreover, using the TUNEL assay, we show that sunitinib is able to induce a direct pro-apoptotic effect on RCC cells. In contrast, pazopanib fails to induce apoptosis in all tested RCC lines. Our data appear to demonstrate significant biological differences between the two agents in cell culture. These results raise the possibility that pazopanib’s in vivo activity may also significantly differ from sunitinib’s. As such, these findings may have clinical implications.
The targeted therapy era was ushered in with clinical data showing sunitinib’s improved efficacy over interferon-alpha in patients with metastatic RCC. Two clinical trials demonstrated that sunitinib was able to achieve a 40–43% partial response rate as well as halt disease progression for at least 3 months in 22–27% patients who were immunotherapy failures. In these studies, the median progression-free survival was 8.3 and 8.7 months, respectively.(14, 15) Furthermore, in a multicenter, randomized phase 3 trial comparing sunitinib to interferon-alpha in previously untreated patients with metastatic RCC, patients treated with sunitinib experienced an objective response rate of 31% compared to 6% for the interferon-alpha arm.(10) Based on these trials, sunitinib received approval by the Food and Drug Administration for patients with metastatic RCC in 2006, and sunitinib still remains the first-line treatment for patients with metastatic RCC(11) despite its significant toxicities, which include hypertension, hand-foot skin reaction, mucositis, and myelosuppression.(16)
Since these initial landmark trials with sunitinib, other TKIs, monoclonal antibodies against vascular endothelial growth factors (VEGF), and inhibitors of mammalian target of rapamycin (mTOR) have been introduced as targeted agents to treat mRCC. Most of these agents, including temsirolimus, everolimus, and bevacizumab, have shown improved efficacy when compared to interferon-alpha or other existing targeted therapies. Nevertheless, the role for each of these therapies in the complex therapeutic landscape of advanced RCC is yet to be delineated.(16)
Pazopanib is a novel multitargeted angiogenesis inhibitor that has shown efficacy as a single agent in patients with metastatic RCC in phase I/II trials.(17–19) A recent randomized, double-blind, placebo-controlled phase III trial comparing pazopanib versus placebo in treatment naïve and cytokine-pretreated patients with locally advanced or metastatic RCC demonstrated an objective response rate of 30% with a median PFS of 9.2 months. (12) Compared to historical data from trials with sunitinib, pazopanib demonstrated an improved side effect profile. Specifically, a lower incidence of grades 3 or 4 myelosuppression as well as decreased incidence of hand-foot syndrome and stomatitis/mucositis were noted. (20) Currently data regarding direct comparisons between pazopanib and immunotherapy or other targeted agents is absent. Indeed, despite promising initial data, some experts have expressed concern in drawing conclusions regarding pazopanib’s superiority over sunitinib. Specifically, equivalent therapeutic efficacy and superior side-effect profile are largely inferred and head to head prospective comparisons are lacking. (21) Nevertheless, in clinical practice, sunitinib and pazopanib are starting to be used interchangeably, and, in some instances, pazopanib is chosen as a first line agent due to its apparent lower toxicity.(20)
Our initial studies with sunitinib and pazopanib demonstrate that the agents behave differently in cell culture. Sunitinib appears to be superior to pazopanib in inhibiting growth of all RCC cell lines tested. Furthermore, sunitinib, but not pazopanib, induces apoptosis of all RCC cell lines used for the current study. Both pazopanib and sunitinib inhibit multiple tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR). VEGFR and PDGFR are both expressed on endothelial cells and perivascular cells (pericytes), playing a critical roles in angiogenesis. In vivo, the ability of multi-targeted TKIs to inhibit angiogenesis and thereby affect tumor responses has been well-established. The direct in vivo effect of multi targeted TKI on tumor cells is ill-defined.(22) As such, the importance of our finding for in vivo activity of sunitinib and pazopanib is unclear. Furthermore, the relevance of the concentrations employed in our studies to in vivo mechanisms of the agents also needs to be further investigated, however, regardless of the concentrations, the cellular effects were durable across all cell lines. Despite these limitations, our data, to our knowledge, are the first to directly compare activity of pazopanib and sunitinib in vitro and to demonstrate that the two agents have profoundly different activity against RCC. The strength of these findings is underscored by their universal nature, as results for pazopanib and sunitinib were consistent across all 8 RCC cell lines tested.
CONCLUSIONS
In an in vitro model, sunitinib exhibits a cytotoxic effect on human RCC cell lines while pazopanib only exhibits a cytostatic effect. These divergent effects were consistent across all eight cell lines tested. These findings cast doubt on the equivalency of pazopanib to sunitinib in clinical application.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 CA108890 and CA134463, CCSG Pilot Project Award (VMK); American Institute for Cancer Research Grant (RGU); Department of Defense, Physician Research Training Award (AK).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Aben KK, Luth TK, Janssen-Heijnen ML, Mulders PF, Kiemeney LA, van Spronsen DJ. No improvement in renal cell carcinoma survival: a population-based study in the Netherlands. Eur J Cancer. 2008;44(12):1701–9. doi: 10.1016/j.ejca.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23(3):202–12. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163(2):408–17. [PubMed] [Google Scholar]
- 7.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 8.Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;(1):CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Dutcher J, Atkins MB, Margolin K, Weiss G, Clark J, Sosman J, Logan T, Aronson F, Mier J. Kidney cancer: the Cytokine Working Group experience (1986–2001): part II. Management of IL-2 toxicity and studies with other cytokines. Med Oncol. 2001;18(3):209–19. doi: 10.1385/MO:18:3:209. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Haas NB, Uzzo RG. Targeted therapies for kidney cancer in urologic practice. Urol Oncol. 2007;25(5):420–32. doi: 10.1016/j.urolonc.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 13.Kolenko V, Uzzo RG, Bukowski R, Bander NH, Novick AC, Hsi ED, Finke JH. Dead or dying: necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Cancer Res. 1999;59(12):2838–42. [PubMed] [Google Scholar]
- 14.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27(19):3225–34. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, Dowlati A, Savage S, Fernando N, Lasalvia S, Whitehead B, Suttle B, Collins D, Ho P, Pandite L. Safety, tolerability and pharmacokinetics of oral administration of GW786034 in patients with solid tumors (abstract) J Clin Oncol. 2005;23(16S):3012. [Google Scholar]
- 18.Hutson T, Davis I, Machiels J, DeSouza PL, Baker K, Bordogna W, Westlund R, Crofts T, Pandite L, Figlin RA. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J Clin Oncol. 2008;26(15S):5046. [Google Scholar]
- 19.Hutson T, Davis I, Machiels J, deSouza PL, Baker K, Pandite L, McCann L, Hodge JP, Lin Y, Figlin RA. Predictive and prognostic factors in phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor (abstract) Annals of Oncology. 2008;19(Supplement 8):viii187–viii207. [Google Scholar]
- 20.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, Epstein RJ, Baker KL, McCann L, Crofts T, Pandite L, Figlin RA. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28(3):475–80. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 21.Jeldres C, Sun M, Perrotte P, Karakiewicz PI. Pazopanib trial data cannot support first-line use. Nat Rev Urol. 2010;7(6):307–8. doi: 10.1038/nrurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Ding Y, Li Y, Luo WM, Zhang ZF, Snider J, Vandenbeldt K, Qian CN, Teh BT. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70(3):1053–62. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]