Abstract
A water soluble zinc(II) phthalocyanine symmetrically appended with eight thioglucose units was synthesized from commercially available hexadecafluorophthalocyaninatozinc(II) by controlled nucleophilic substitution of the peripheral fluoro groups. The photophysical properties and cancer cell uptake studies of this nonhydrolyzable thioglycosylated phthalocyanine are reported. The new compound has amphiphilic character, is chemically stable, and can potentially be used as a photosensitizer in photodynamic therapy.
Keywords: Phthalocyanine, Photodynamic therapy, Glucose, Fluorescence, Quantum yield
Introduction
Free base phthalocyanines (Pcs) and their diamagnetic metallated complexes, e.g. Zn(II), Al(III), Ga(III), can be efficient photosensitizers for photodynamic therapeutics (PDT) because they can photosensitize the formation of singlet oxygen. Singlet oxygen is formed upon energy transfer from the triplet excited state of the dye to ground state triplet oxygen.1–4 Since red light penetrates deeper into tissues, Pcs can be more efficient PDT agents than the well studied porphyrins because the electronic bands of Pcs in the red spectral region are about two orders of magnitude stronger than the porphyrins. Pcs can be extraordinary stable.5,6 Zinc metallophthalocyanines are of interest because of their high triplet quantum yield and long triplet lifetimes.7 Long lived triplet states are advantageous since this increases the probability of a diffusional encounter between the excited triplet state of the photosensitizer and endogenous molecular oxygen. Significant research directed at improving the selectivity of the Pcs towards malignant tissues has resulted in limited success because of poor solubility in physiological fluids.8
In general, amphiphilic porphyrinoid photosensitizers are taken up by cells and tissues better than water soluble derivatives.9 Uptake and distribution are also a function of specific targeting motifs appended to the dye, and degree of aggregation.6, 9 Appending ionic groups on the Pc macrocycle such as sulfonic groups,10 quaternary amino or pyridinium groups11, 12 makes them water soluble but the purification of these compounds can be a problem. Neutral, polar Pcs can be prepared by attaching hydroxyl groups.13 Appending carbohydrates to porphyrinoids is of great interest because: (1) these impart amphipathic properties, and (2) many cancer cells over express carbohydrate receptors14 since they have a greater metabolic rate.15 The increased levels of glucose uptake and glycolysis may promote the uptake of glycosylated photosensitizers. There are examples of porphyrin-carbohydrate conjugates,16–19 and of carbohydrate substituted Pcs.20–24 The Pc compounds are generally prepared by cyclotetramerization of the glycosylated phthalonitrile, e.g. the work of Ziegler and coworkers, including thioglycosylated Pc.24–30
In addition to the inherently stronger red absorption bands, several important considerations distinguish the Pc from the porphyrins. Mono substitution of the four isoindoles results in a mixture of four isomers.31 The nature and number of functional groups appended to any position of the Pc macrocycle can have marked effect on the photophysical properties because they are attached directly to the chromophore. There are also strong solvent and aggregation effects on the electronic properties of Pcs.32 In contrast, the photophysical properties are minimally affected in most glycol-porphyrin conjugates, which have the sugar appended to the meso aryl moieties of tetraphenylporphyrin cores.33 The number and position of functional groups on porphyrinoids has been discussed in terms of PDT effects.16 O-glycoside attached sugars can be hydrolysed off of conjugates enzymatically, by lysosomal degradation,34, 35 and the decreased pH surrounding cancer cells and tissues due to the Warburg effect.36 Therefore, non-hydrolysable attachments may be more effective as therapeutics and for other applications.37, 38
Herein we report the facile, one pot, formation of a metallophthalocyanine appended with non-hydrolysable thioglucose units (Fig. 1) that takes advantage of the different reactivities of fluoro groups on the α and β positions. This work is the first example of base-promoted substitution of phthalocyanines with thioglucose that takes advantage of the excellent leaving group character of fluoride. This straightforward strategy allows commercially available zinc perfluorophthalocyanine, ZnPcF16, to serve as a core platform to rapidly make derivatives to assess the effectiveness of a broad array of biotargeting motifs for diverse applications.
Figure 1.
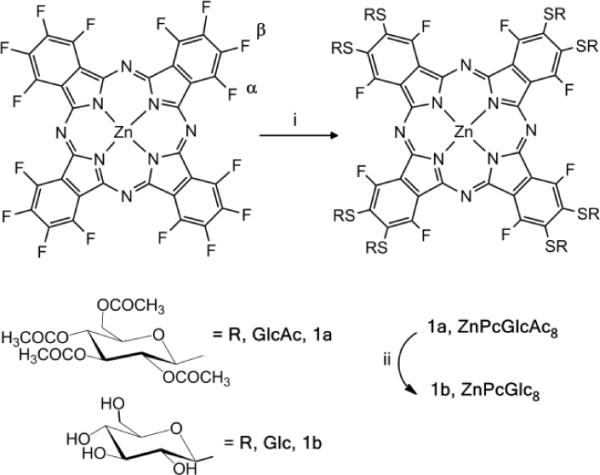
Synthesis of ZnPcGlc8. (i) 8.5 eq. GlcAc4SAc, K2CO3 in THF at 40–50 °C for three days; (ii) CH3ONa in CH2Cl2/CH3OH. The isolated yield of 1b = 72%. The α and β positions on the phthalocyanine are indicated on the starting ZnPcF16.
Though mixtures of compounds and/or isomers or atropisomers may have advantages in terms of PDT, because each may partition or localize in different parts of a tissue or cell resulting in oxidative damage at multiple sites, mixtures should be designed rather than accidental. For Pcs, derivatives with 16 or eight substituents can be pure compounds without isomers. The electronic absorption and luminescence properties of ZnPcF16 systematically changes as the fluoro groups are replaced with thioglucose.39–41 We focus on the octa-thioglycosylated compound since the remaining fluorine atoms can help protect the chromophore from oxidation. The main objective of this work was to synthesize ZnPcGlc8 in reasonable yields, study the photophysical properties, and examine cancer cell uptake.
Results and Discussion
Figure 1 shows the synthetic route for the octathioglycosylated substituted ZnPcGlc8. ZnPcF16 was treated under argon with 8.5 equivalents of thioglucose in presence of K2CO3 as a base and dry THF as the solvent at 40–50 °C. After 48 hours another four equivalents of thioglucose was added and the reaction continued for another 24 hours.42 The type of solvent and the base used for this reaction play an important role. Complex reaction mixtures and/or decomposition resulted when solvents such as diglyme or DMF were used with bases such as diethylamine, triethylamine, pyridine, Na/MeOH, or tetrabutylammonium hydroxide. The deprotection was done using a mixture of dichloromethane and methanol as solvent and sodium methoxide; using a slight excess relative to the equivalents of acetate moieties. Similar procedures using 4.5 equivalents of the thioglucose starting material yields the four isomers of the tetraglycosylated Pc wherein each isoindole has one thioglusoce unit. The water soluble Pc (1b) was purified by precipitation with water/methanol mixture. The reactivity of the ZnPcF16 progresses from a single substituent on one β position on each isoindole, to substitution of each β position, to substitution of the α positions. The diminished reactivity with succeeding additions is due to both thermodynamic effects from the exchange of a F atom for a S atom on the macrocycle, and kinetic effects from steric hindrance.
The compounds were characterized by 1H, 13C, 19F NMR, UV-visible spectra and MALDI-TOF. In the 1H NMR of compound 1a, the acetyl peaks appear at 1.90–2.34 ppm, and resonances of the other protons of the carbohydrate units appear as two multiplets at 3.7–4.2 ppm and 5.2–5.5 ppm. The 1H NMR of compound 1b in DMSO-d6 is well resolved and confirms the deprotection of the carbohydrate units. The resonances of the anomeric protons appear as doublets between 5.2–5.6 ppm. The 13C NMR displays the typical chemical shifts for Pcs but multiple resonances are observed near 125–132 ppm for the α position carbons that remain coupled to the F atoms. The 19F NMR of the compound shows a singlet around −109 ppm and disappearance of the peak at around −85 ppm indicating that the β-F atoms are substituted by the thioglucose (Supplementary Materials).
The UV-visible spectra of compound 1b in different solvents are shown in Fig. 2. Notably, the spectrum in DMSO shows defined peaks and no apparent aggregation, but the marked decrease and broadening of the Q-band at 727 nm in both less polar solvents and phosphate buffered saline (PBS) indicates significant aggregation of this compound.25 Similarly, there is a gradual decrease and broadening of this Q band with increasing amounts of water in the DMSO solution. The nearly 60 nm blue shift of both the Q bands and somewhat smaller blue shift in the B bands in the optical spectra in these solvents indicates the ZnPcGlc8 aggregates are generally in a cofacial arrangement (H-aggregates).22,43 This aggregation behavior has been observed with other glycosylated Pcs.1,13
Figure 2.
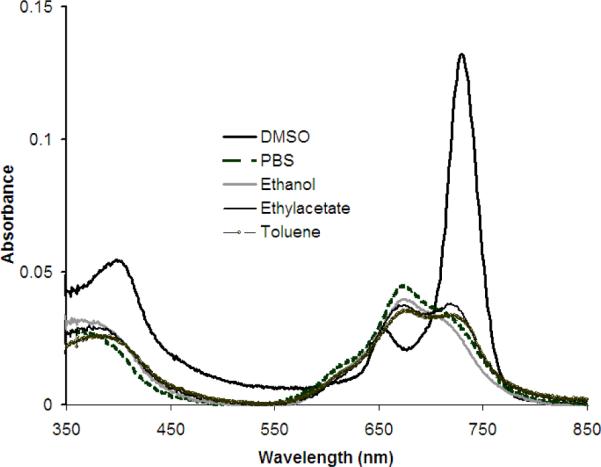
UV-visible spectra of 2 μM ZnPcGlc8 in different solvents, from a 1 mM ZnPcGlc8 in DMSO stock solution, indicating aggregation in most solvents.
The detailed photophysical data of ZnPcGlc8 was measured in dry DMSO, PBS, and 1:1 mixture solvent of DMSO:H2O and is summarized in Table 1. The UV-visible spectra of ZnPcGlc8 are significantly different than the starting ZnPcF16 compound. The Q-band at 727 nm of ZnPcGlc8 is red shifted by ~55 nm compared to the 672 nm Q-band for ZnPcF16 and other ZnPc compounds (Supplementary materials). The systematic red-shift with successive exchange of the F for the S attached to the macrocycle arises from the decrease in the optical band gap as reported earlier.41
Table 1.
Photophysical properties ZnPcGlc8 measured in air
| Compound | Solvent | Absorption λ (log ε) | Emission λ | ΦF air | ΦF N2 | τf, ns air (under N2) | Φ Δ |
|---|---|---|---|---|---|---|---|
| ZnPc | DMSO | 360, 605, 641, 672(4.72) | 679, 747 | 0.20 | 3.1 (3.3) | 0.67 ref. 46 | |
| ZnPcF16 | DMSO | 356, 636, 672 (4.38) | 686 | 0.01 | 0.013 | 2.7 (2.75) | 0.13 (acetone) ref. 47 |
| ZnPcGlc8 | DMSO | 397, 650, 727 (4.64) | 740 | 0.06 | 0.064 | 2.1 (2.2) | 0.41 D2O Tris buffer |
| PBS | 358, 671, 711 (4.25) | 727 | 0.0021 | 0.0026 | 17% T1=0.3 (0.33) | 0.41 DMSO | |
| 83% T2=2.1 (2.24) | |||||||
| DMSO:H2O (1:1) | 376, 672, 719 (4.36) | 738 | -- | -- | -- |
The fluorescence quantum yield, ΦF, was measured by exciting the molecule at 647 nm where all compounds have an absorbance 0.027. Both quantum yield and singlet state life time was measured in air and under N2 by purging N2 gas through the solution for 10 min. Time correlated single photon counting measurements of the singlet state life time used a 401 nm laser to excite the molecule and emission decay was recorded at 740 nm, and band pass = 3 nm. The instrument response time is ~200 ps. The 1O2 quantum yield, ΦΔ, was measured as described.
The organization and size of organic nanoparticles (aggregates) depends on a variety of factors including concentration, solvent, mixing, and the architecture of the component molecules.44 In PBS, dynamic light scattering (DLS) measurements shows two different sized aggregate populations with diameters of 40±6 nm and 265±25 nm after shaking in 0.1 mM 1b. After sonication of this solution for about 15 minutes, the organic nanoparticles reorganize to yield only particles that were 65±8 nm in diameter. The nanoparticle size and organization affects the photophysical properties and cell uptake.
An intense fluorescence emission for ZnPcGlc8 was observed at 740 nm in dry DMSO with a 13 nm Stokes shift, which is consistent with other ZnPc derivatives.45–49 Because of self-quenching in aggregates, the fluorescence is very weak in PBS, ethanol and a 2% solution of DMSO in water used for the cell studies. The fluorescence decreases as water is added to a DMSO solution such that it is almost completely quenched in 1:1 DMSO: water and corresponds to the aggregation observed in the UV-visible spectra. Similar fluorescence quenching due to aggregation was observed in toluene, ethanol, and ethyl acetate. The solvent dependence of the photophysics of 1b impacts the observation of this compound in breast cancer cells by fluorescence microscopy (see below).
The fluorescence quantum yield, ΦF was calculated to be 0.06 (Table 1). The low ΦF value of the 1b compared to ZnPcF16 or ZnPc is due to both electronic and heavy atom effects arising from replacing the F with S on the chromophore. The appended sugars may also effect internal conversion. The fluorescence lifetime for the chromophore in DMSO was measured to be 2.1 ns. These are consistent with other ZnPc derivatives.45–48 Photobleaching of the chromophore was measured by exposing a 5 μM solution of compound in DMSO to sunlight with a power of about 60–95 W/m2. The photodegradation was measured by taking UV-visible and emission spectra after different time intervals. About 26 % of 1b decomposed after 5 minutes, and after 2 hours almost complete photodegradation was observed. The photo-decomposition product(s) of the chromophore were not explored.
Direct observation of 1O2 luminescence kinetics at 1270 nm after irradiation of ZnPcGlc8 at 532 nm in pH 7.4 D2O Tris buffer was done as previously described.49,50 The quantum yield of 1O2 production (ΦΔ) was determined according to previously reported method52 in air-saturated pH 7.4 D2O Tris buffer on a relative basis by steady-state photolysis. 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (TCPP) in pH 7.4 D2O Tris buffer and zinc(II)Pc (ZnPc) in DMSO were used as references for excitation at 532 nm and 700 nm, respectively. ΦΔ for TCPP in weakly alkaline solution is 0.53 and for ZnPc in DMSO is 0.67 (Supplementary materials). A phosphine, [2-(dicyclohexylphosphino)ethyl]trimethylammonium chloride] in water, and 9,10-dimethylanthracene (DMA) in DMSO were used as 1O2 traps. Measurement of phosphine oxidation by 31P NMR, and DMA oxidation by 1H NMR allows the yield of 1O2 to be calculated (Table 1). Since ΦΔ values are 0.42 ± 0.01 and 0.41 ± 0.01 with 532 nm and 700 nm excitation, respectively, sensitization comes from a common excited state.
The mechanism of cancer cell uptake of nonhydrolysable thioglycosylated porphyrinoids is not well understood. Our hypothesis is that the glucose receptors on the cell surface bind the chromophore, thereby increasing its concentration around the cell, but these are unable to transport the large conjugate inside the cell. The amphipathic properties of the molecule allow diffusion across the cell membrane. Additionally, nano-aggregates less than about 50 nm can be endocytosed into the cell.52 Fluorescence microscopy indicates that ZnPcGlc8 is taken up by MDA-MB-231 breast cancer cells mostly as poorly fluorescent nano-aggregates (Fig. 3) because initially small, diffuse spots are just visible. Four days after fixing, the cell morphology remains, but the nanoparticles of ZnPcGlc8 have disaggregated; therefore the fluorescence significantly increases. This indicated uptake of the dyes into these cells.
Figure 3.
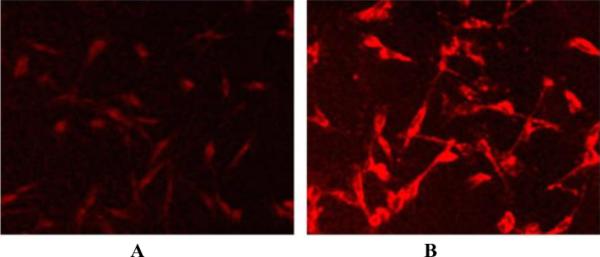
MDA-MB-231 cells were incubated with 50 nM ZnPcGlc8 for 24 h, rinsed three times with PBS buffer to remove unbound dye and fixed with 4% paraformaldehyde solution. Fluorescence images were captured by exciting at 540–580 nm, magnification 20× under identical conditions. (A) Just after preparation of the fixed cells slide, and (B) 4 day after later. The contrast of each was enhanced by 40% for publication.
Non-hydrolysable thioglycosylated Pc from the commercially available ZnPcF16 core platform can be made in high yields. The amphiphilic character of 1b, the cancer cell targeting carbohydrate units, and the photonic properties enable potential therapeutic applications. Other treatment modalities, e.g. photothermal with the Ni(II) complex,53 may also be enabled by this molecular architecture. The chromophore was found to aggregate in water resulting in quenching of the fluorescent signal, but uptake and disaggregation in breast cancer cells indicates this may be a viable strategy for new PDT agents.
Supplementary Material
Acknowledgements
A. Aggarwal and S. Singh contributed equally to this work. Support from the National Science Foundation (NSF) CHE-0847997 to C.M.D, and NSF-PREM (DMR-0611539) to R.G.; the National Institutes of Health (NIH) NIH-RCMI (G12-RR-013459) at Jackson State; collaboration fostered by NIH-RTRN (U54RR022762). Hunter College Chemistry infrastructure is supported by the NSF, NIH, including the RCMI program (G12-RR-03037), and the City University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zorlu Y, Ermeydan MA, Dumoulin F, Ahsen V, Savoie H, Boyle RW. Photochem. Photobiol. Sci. 2009;8:312–318. doi: 10.1039/b817348f. [DOI] [PubMed] [Google Scholar]
- 2.Josefsen LB, Boyle RW. Metal-Based Drugs. 2008;2008:276109. doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Zhang S, Han G. Molecules. 2009;14:3688–3693. doi: 10.3390/molecules14093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hillegersberg R, Kort WJ, Wilson JHP. Drugs. 1994;48:510–527. doi: 10.2165/00003495-199448040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bonnett R, Martínez G. Tetrahedron. 2001;57:9513–9547. [Google Scholar]
- 6.Macdonald IJ, Dougherty TJ. J. Porph. Phthal. 2001;5:105–129. [Google Scholar]
- 7.van Lier JE, Spikes JD. In: Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use (CIBA Foundation Symposium 146) Block G, Harnett S, editors. Vol. 17. Wiley; Chichester: 1989. pp. 17–39. [Google Scholar]
- 8.Allen CM, Sharman WM, Van Lier JE. J. Porph. Phthal. 2001;5:161–169. [Google Scholar]
- 9.Ali H, van Lier JE. Chem. Rev. 1999;99:2379–2450. doi: 10.1021/cr980439y. [DOI] [PubMed] [Google Scholar]
- 10.Arslan S, Yilmaz I. Polyhedron. 2007;26:2387–2394. [Google Scholar]
- 11.Dinçer HA, Koca A, Gül A, Koçak MB. Dyes and Pigments. 2008;76:825–831. [Google Scholar]
- 12.Sesalan BS, Koca A, Gül A. Dyes and Pigments. 2008;79:259–264. [Google Scholar]
- 13.Boyle RW, Leznoff CC, van Lier JE. Br. J. Cancer. 1993;67:1177–1181. doi: 10.1038/bjc.1993.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Airley RE, Mobasheri A. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 15.(a) Warburg O. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]; (b) Toschi A, Lee E, Thompson S, Gadir N, Yellen P, Drain CM, Ohh M, Foster DA. Cancer Lett. 2010;299:72–79. doi: 10.1016/j.canlet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Chen X, Hui L, Foster DA, Drain CM. Biochem. 2004;43:10918–10929. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pasetto P, Chen X, Drain CM, Franck RW. Chem. Commun. 2001:81–82. [Google Scholar]; (c) Thompson S, Chen X, Hui L, Toschi A, Foster DA, Drain CM. Photochem. Photobio. Sci. 2008;7:1415–1421. doi: 10.1039/b806536e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S, Aggarwal A, Thompson S, Tomé JPC, Zhu X, Samaroo D, Vinodu M, Gao R, Drain CM. Bioconjugate Chem. 2010;21:2136–2146. doi: 10.1021/bc100356z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Pandey SK, Graham A, Dobhal MP, Mehta R, Chen Y, Gryshuk A, Rittenhouse-Olson K, Oseroff A, Pandey RK. J. Org. Chem. 2004;69:158–172. doi: 10.1021/jo030280b. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto K, Miyata T, Aoyama Y. J. Am. Chem. Soc. 2000;122:3558–3559. [Google Scholar]
- 20.Álvarez-Micó X, Calvete MJF, Hanack M, Ziegler T. Synthesis. 2007;2007:2186–2192. [Google Scholar]
- 21.(a) Choi C-F, Huang J-D, Lo P-C, Fong W-P, Ng DKP. Org. Biomol. Chem. 2008;6:2173–2181. doi: 10.1039/b802212g. [DOI] [PubMed] [Google Scholar]; (b) Liu J-Y, Lo P-C, Fong W-P, Ng DKP. Org. Biomol. Chem. 2009;7:1583–1591. doi: 10.1039/b822128f. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Mico X, Calvete MJF, Hanack M, Ziegler T. Tetrahedron Lett. 2006;47:3283–3286. [Google Scholar]
- 23.Ribeiro AO, Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Iamamoto Y, Torres T. Tetrahedron Lett. 2006;47:9177–9180. [Google Scholar]
- 24.Maillard P, Gaspard S, Guerquin-Kern JL, Momenteau M. J. Am. Chem. Soc. 1989;111:9125–9127. [Google Scholar]
- 25.Lyubimtsev A, Iqbal Z, Crucius G, Syrbu S, Taraymovich ES, Ziegler T, Hanack M. J. Porph. Phthal. 2011;15:39–46. [Google Scholar]
- 26.Iqbal Z, Hanack M, Ziegler T. Tetrahedron Lett. 2009;50:873–875. [Google Scholar]
- 27.Álvarez-Micó X, Calvete MJF, Hanack M, Ziegler T. Carbohydrate Res. 2007;342:440–447. doi: 10.1016/j.carres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Iqbala Z, Lyubimtseva A, Hanack M, Ziegler T. J. Porph. Phthal. 2010;14:494–498. [Google Scholar]
- 29.Soares ARM, Tomé JPC, Neves MGPMS, Tomé AC, Cavaleiro JAS, Torres T. Carbohydrate Res. 2009;344:507–510. doi: 10.1016/j.carres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Taquet J.-p., Frochot C, Manneville V, Barberi-Heyob M. Curr. Med. Chem. 2007;14:1673–1687. doi: 10.2174/092986707780830970. [DOI] [PubMed] [Google Scholar]
- 31.(a) Samaroo D, Vinodu M, Chen X, Drain CM. J. Combi. Chem. 2007;9:998–1011. doi: 10.1021/cc070067j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Drain CM, Singh S. Combinatorial Chemistry of Porphyrins. In: Kadish K, G. R, Smith K, editors. The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Vol. 2. World Scientific Publishers; Singapore: 2010. pp. 485–540. [Google Scholar]
- 32.Goslinski T, Osmalek T, Konopka K, Wierzchowski M, Fita P, Mielcarek J. Polyhedron. 2011;30:1538–1546. [Google Scholar]
- 33.Vedachalam S, Choi B-H, Pasunooti KK, Ching KM, Lee K, Yoon HS, Liu X-W. Med. Chem. Commun. 2011;2:371–377. [Google Scholar]
- 34.Laville I, Pigaglio S, Blais JC, Loock B, Maillard P, Grierson DS, Blais J. Bioorg. Med. Chem. 2004;12:3673–3682. doi: 10.1016/j.bmc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X, Pandey RK. Anti-Cancer Agents Med. Chem. 2008;8:241–268. doi: 10.2174/187152008783961897. [DOI] [PubMed] [Google Scholar]
- 36.Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornia M, Menozzi M, Ragg E, Mazzini S, Scarafoni A, Zanardi F, Casiraghi G. Tetrahedron. 2000;56:3977–3983. [Google Scholar]
- 38.Ahmed S, Davoust E, Savoie H, Boa AN, Boyle RW. Tetrahedron Lett. 2004;45:6045–6047. [Google Scholar]
- 39.(a) Leznoff CC, Hiebert A, Ok S. J. Porph. Phthal. 2007;11:537–546. [Google Scholar]; (b) Leznoff CC, Sosa-Sanchez JL. Chem. Commun. 2004:338–339. doi: 10.1039/b313253f. [DOI] [PubMed] [Google Scholar]
- 40.(a) Nemykin VN, Lukyanets EA. Key Role of peripheral substitution in the chemistry of phthalocyanines. In: Kadish K, G. R, Smith K, editors. The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Vol. 3. World Scientific Publishers; Singapore: 2010. pp. 1–323. [Google Scholar]; (b) Lukyanets EA, Nemykin VN. J. Porph. Phthal. 2010;14:1–40. [Google Scholar]
- 41.Varotto A, Nam C-Y, Radivojevic I, Tomé JPC, Cavaleiro JAS, Black CT, Drain CM. J. Am. Chem. Soc. 2010;132:2552–2554. doi: 10.1021/ja907851x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Synthesis of the protected thioglycosylated phthalocyanine, ZnPcGlcAc8 (1a). To a solution of 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (60 mg, 139.04 μmol, 8 equiv) in THF (2 mL) was added K2CO3 (25 mg), the mixture was stirred for 10 min and then zinc perfluorophthalocyanine, ZnPcF16 (15 mg, 17.38 μmol) was added. The reaction mixture was stirred at 40–50°C for 48 h and then additional 2 equivalents of 2,3,4,6-tetra-O-acetyl-glucosylthioacetate and K2CO3 dissolved in THF was added and the reaction mixture was stirred at 40–50°C for additional 24 h with total reaction time of 3 days. The reaction mixture was then precipitated with water and the solid was filtered through a short column of Celite and washed with water. The crude mixture was recovered in CH2Cl2 and purified by flash chromatography (silica gel) using a mixture of toluene/acetone as a gradient. Compound 1a was obtained after crystallization in CH2Cl2/hexanes, as a green powder. Peaks in the spectrum are broader due to aggregation. 19F NMR (CDCl3): δ −109. 1H NMR (CDCl3): δ 3.7–5.5 (m, 56H, Glc-H), 1.90–2.36 (m, 96H, acetyl-H). 13C NMR (CDCl3): δ 20.6 (CH3CO2), 61.85, 68.32, 71.10, 73.84, 75.70, 85.15 (Glc), 127.28–127.94, 151.42, 151.53, 154.13, 154.22, 156.24, 156.28, 156.31, 169.35 (Pc-C), 169.90, 170.04, 170.71(CH3CO2). MALDI Calcd for C144H152F8N8O72S8Zn M+: 3620.67 found 3620.33.Synthesis of thioglycosylated phthalocyanine, ZnPcGlc8 (1b). Phthalocyanine 1a (20 mg, 8.8 μmol) was dissolved in methanol/CH2Cl2 (3:1, 2 mL) and treated with sodium methoxide (0.5 M solution in methanol, 0.4 mL). The reaction mixture was stirred at room temperature for 2 h and then neutralized by an aqueous citric acid solution. The mixture is filtered through Waters Sep-Pak C18 35 cm3 reverse-phase prep column and washed with methanol. The deprotected phthalocyanine 1b was eluted with water/methanol mixture. Phthalocyanine 1b was obtained after crystallization in water/methanol as a green powder. mp> 250°C. 19F NMR (DMSO-d6): δ −108.3. 1H NMR (DMSO-d6): δ 3.18–5.59 (m, 56H, Glc-H) 13C NMR (DMSO-d6): δ 61.35, 70.48, 75.23, 78.57, 81.60, 86.48 (Glc), 125.56, 128.67, 151.55, 153.28, 155.32 (Pc-C). MALDI Calcd for C80H88F8N8O40S8Zn (M+H)+: 2276.49 found 2276.44.
- 43.Tau P, Ogunsipe AO, Maree S, Maree MD, Nyokomg T. J Porph. Phthal. 2003;7:439–446. [Google Scholar]
- 44.(a) Drain CM, Smeureanu G, Patel S, Gong X, Garno J, Arijeloye J. New J. Chem. 2006;30:1834–1843. [Google Scholar]; (b) Gong XC, Milic T, Xu C, Batteas JD, Drain CM. J. Am. Chem. Soc. 2002;124:14290–14291. doi: 10.1021/ja027405z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogunsipe A, Chen J-Y, Nyokong T. New. J. Chem. 2004;28:822–827. [Google Scholar]
- 46.Atilla D, Durmus M, Gürek AG, Ahsena V, Nyokong T. Dalton Trans. 2007:1235–1243. doi: 10.1039/b617773e. [DOI] [PubMed] [Google Scholar]
- 47.Beveridge AC, Bench BA, Gorun SM, Diebold GJ. J. Phys. Chem. A. 2003;107:5138–5143. [Google Scholar]
- 48.Nyokong T. Coord. Chem. Rev. 2007;251:1707–1722. [Google Scholar]
- 49.Li W, Gandra N, Ellis E, Cartney S, Gao R. ACS Appl. Mater. Interfaces. 2009;1:1778–1784. doi: 10.1021/am9003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Gandra N, Courtney SN, Gao R. ChemPhysChem. 2009;10:1789–1793. doi: 10.1002/cphc.200900155. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Zhu X, Smith J, Haygood MT, Gao R. J. Phys. Chem. B. 2011;115:1889–1894. doi: 10.1021/jp109590t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M. Trends in Biotechnology. 2008;26:612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Busetti A, Soncin M, Reddi E, Rodgers MAJ, Kenney ME, Jori G. J. Photochem. Photobiol. B: Biol. 1999;53:103–109. doi: 10.1016/s1011-1344(99)00132-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


