Abstract
The normal expression of myocardial mitochondrial enzymes is essential to maintain the cardiac energy reserve and facilitate responses to stress, but the molecular mechanisms to maintain myocardial mitochondrial enzyme expression have been elusive. Here we report that congestive heart failure is associated with a significant decrease of myocardial Estrogen-Related Receptor alpha (ERRα), but not PPAR gamma coactivator-1 alpha (PGC1α), in human heart failure samples. In addition, chronic pressure overload in mice caused a decrease of ERRα expression that was significantly correlated to the degree of LV dysfunction, pulmonary congestion and decreases of a group of myocardial energy metabolism related genes. We found that the metabolic sensor AMP activated protein kinase (AMPK) regulates ERRα expression in vivo and in vitro. AMPKα2 KO decreased myocardial ERRα (both mRNA and protein) and its downstream targets under basal conditions, with no change in myocardial PGC1α expression. Using cultured rat neonatal cardiac myocytes, we found that overexpression of constitutively active AMPKα significantly induced ERRα mRNA, protein and promoter activity. Conversely, selective gene silencing of AMPKα2 repressed ERRα and its target gene levels, indicating that AMPKα2 is involved in the regulation of ERRα expression. In addition, over-expression of ERRα in AMPKα2 KO neonatal cardiac myocytes partially rescued the repressed expression of some energy metabolism related genes. These data support an important role for AMPKα2 in regulating the expression of myocardial ERRα and its downstream mitochondrial enzymes.
Keywords: AMP activated protein kinase, estrogen-related receptor alpha, energy metabolism, gene regulation, heart failure
Introduction
The normal expression of myocardial mitochondrial enzymes is essential to maintain cardiac energy reserve and facilitate compensatory responses to stress. During the past few years, significant progress has been made in understanding the basic molecular mechanism of mitochondrial biogenesis. Using genetically modified mice and cells, the biological significance of a group transcriptional factors and co-activators that modulate mitochondrial function has been revealed. Estrogen related receptor alpha (ERRα) is one of these transcriptional factors that plays an important role in regulating energy homeostasis and mitochondrial biogenesis 1, 2. ERRα is expressed at high levels in tissue that is utilizing diet-derived lipid as fuel 3. It has been demonstrated that ERRα induces PDK2 and PDK4 expression in hepatoma cells 4, 5 and skeletal muscle 6, indicating that ERRα may promote fatty acid oxidation and repress glycolysis. Using ChIP-on-chip, Dufour et al demonstrated that in adult mouse hearts, ERRα and ERRγ direct the activity of a set of genes that are implicated in intracellular fuel sensing, uptake of energy substrate, fatty acid oxidation and TCA cycle activity, transport of ATP across the mitochondrial membranes, and Ca2+ handling 7. This suggests that ERRα is involved in a broad range of cardiac functions related to myocardial energetics. Consistent with these observations, Kelly and associates demonstrated that ERRα deficiency caused decreased expression of several ERRα target genes involved in myocardial substrate utilization and energy production 8. This abnormal gene expression profile is responsible for the reduced high-energy phosphate reserve and lower ATP synthesis rate in response to an increased work load in ERRα null mice 8. ERRα expression can be induced in human skeletal muscle by exercise 9, in brown fat and skeletal muscle of mice by cold exposure 10, and in mouse liver by fasting 11. However, little is known about regulation of ERRα expression in the heart, and whether ERRα expression changes during the development of heart failure.
The metabolic sensor AMP activated protein kinase (AMPK) integrates signals regarding energy expenditure and energy production to regulate cellular metabolic processes. In response to metabolic stress, AMPK is rapidly activated to preserve energy homeostasis. During myocardial ischemia, AMPK activation increases glucose uptake, glycolysis and fatty acid oxidation 12. In mice subjected to left coronary artery occlusion/reperfusion, activation of AMPK by metformin significantly increased survival and improved ventricular function 13. Conversely, adult male mice over-expressing a dominant negative AMPKα2 subunit exhibited greater injury after ischemia/reperfusion 14, 15. Both AMPKα1 and AMPKα2 activities were elevated in rat hearts subjected to pressure overload, implying that AMPK is involved in the response to chronic hemodynamic overload 16, 17. Furthermore, our previous study using AMPKα2 deficient mice demonstrated that AMPK exerts cardioprotective effects against systolic pressure overload induced left ventricular hypertrophy and dysfunction partially by repressing mTOR signaling 16. The inhibition of mTOR signaling by AMPK may help to decrease energy consumption associated with mTOR dependent increases in translation. However, whether AMPK is also involved in regulation of myocardial energy generation is not clear.
It has been reported that peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α) is the key regulator that controls cardiac energy metabolism 18, 19. However, we found that the protein level of PGC-1α was unchanged in the failing human heart, while the protein content of ERRα was significantly decreased. Furthermore, the decrease of ERRα in response to chronic pressure overload in mouse hearts was significantly correlated with the decrease of LV ejection fraction, increase of pulmonary congestion, and dysregulation of the expression of several energy metabolism related genes. Using AMPKα2 knockout mice, as well as by manipulating AMPKα2 expression in isolated neonatal cardiomyocytes, we demonstrated that AMPKα2 plays an important role in regulating ERRα promoter activity, mRNA and protein level, and ERRα downstream metabolic gene expression. These data indicate that AMPKα2 not only plays an important role in regulating the myocardial mTOR signaling pathway, but that it also maintains cardiac energy levels and cardiac function through regulation of ERRα expression.
Material and Methods
Please see http://hyper.ahajournals.org for expanded “material and methods”.
Animals
Adult (12–15 weeks) male AMPKα2 deficient mice (background of C57B6J) 20 and their wild type littermates were used in this study. All animal studies were performed according to a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee.
Results
Human heart failure samples show decreased LV ERRα expression but unchanged PGC-1α expression
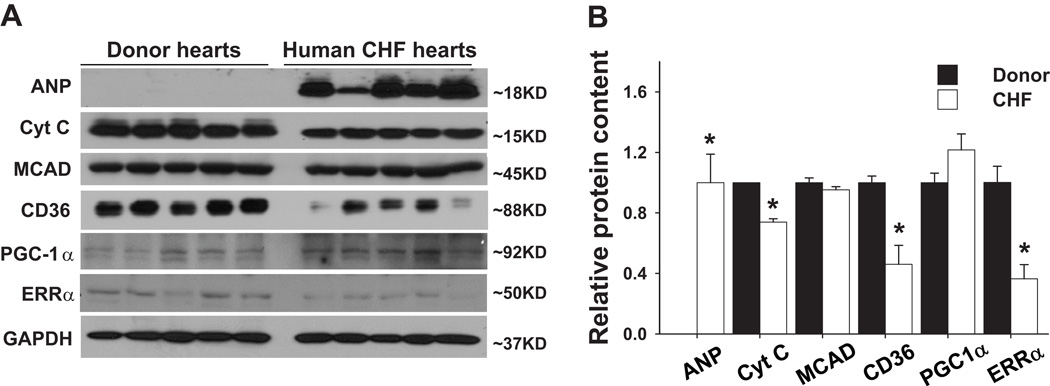
We studied the expressions of LV ERRα and PGC-1α in 5 patients with CHF and 5 normal donor hearts (Table S1). ERRα expression was significantly decreased by ~ 40% in the CHF hearts as compared with the donor hearts (Figure 1). Interestingly, the protein level of PGC-1α, a well defined transcriptional coactivator regulating energy metabolism and mitochondrial biogenesis, was not decreased. Myocardial ANP protein, a marker associated with ventricular hypertrophy and heart failure, was significantly increased in the CHF hearts. In addition, the myocardial mitochondrial respiratory chain components cytochrome C and fatty acid transporter CD36 were significantly decreased in the CHF samples (Figure 1).
Figure 1.
Expression of ERRα and energy metabolism related enzymes in failing human hearts. Western blots of human heart tissue samples (A). Myocardial ERRα was significantly decreased in the failing heart, which this was associated with decreases in some mitochondrial enzymes (B). * p< 0.05 as compared with normal donor hearts.
LV dysfunction and decreased energy metabolism related gene expression are correlated with decreased myocardial ERRα expression
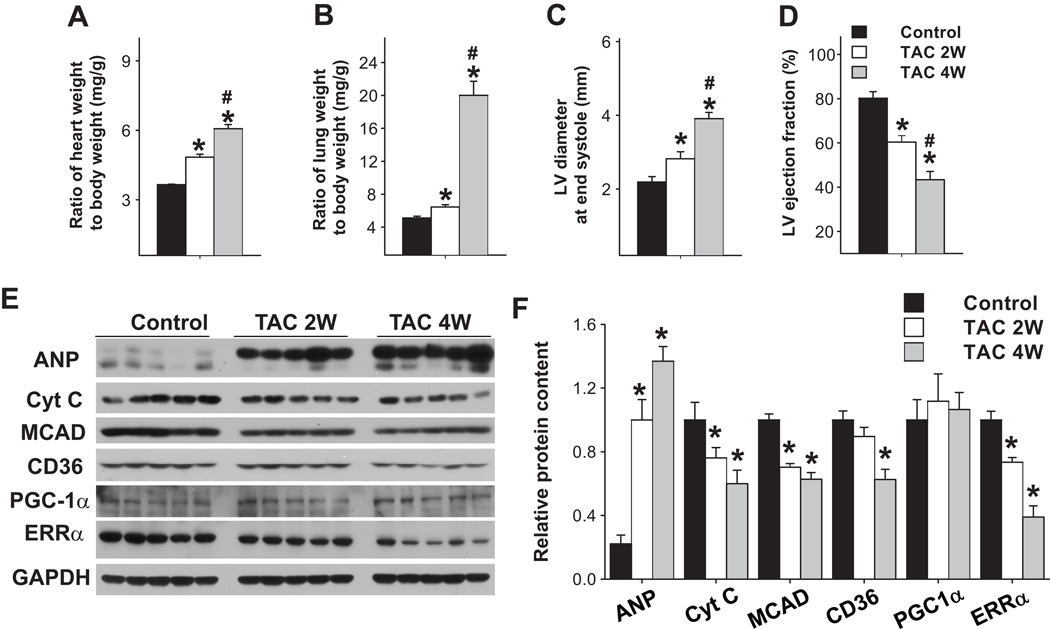
As ERRα expression is decreased in human failing heart samples, we speculated that there might be a correlation between the repressed ERRα expression and the down-regulation of fatty acid metabolism related genes as well as LV dysfunction. To test this hypothesis, wild type mice were subjected to severe pressure overload using the TAC procedure and LV samples from these mice were collected 2 weeks after TAC (when mice had moderate LV dysfunction) or 4 weeks after TAC (when mice had severe LV dysfunction). TAC caused a progressive increase of the ratio of ventricular weight to body weight (Figure 2A), and the ratio of lung weight to body weight (Figure 2B), a reliable marker of LV dysfunction 21–23. TAC also caused increased LV end systolic diameter (Figure 2C), and a decrease of LV ejection fraction (Figure 2D). At 2 weeks after TAC, electron microscopy revealed no apparent mitochondrial damage. At 4 weeks after TAC, electron microscopy revealed swollen and vacuolated cristae in some cardiac myocyte mitochondria, with some mitochondria completely disrupted (Figure S1).
Figure 2.
LV function and expression of myocardial ERRα and energy metabolism related enzymes in mice after TAC. Wild type mice were subjected to TAC for 2 or 4 weeks, which caused gradual deterioration of LV function (A–D) and reduction of myocardial ERRα and some mitochondrial enzymes (E,F). * p<0.05 as compared with control mice; # p<0.05 as compared with mice after 2 weeks of TAC.
Hearts subjected to TAC demonstrated a gradual increase of ANP, as well as decreases of ERRα and energy metabolism related genes including cytochrome C, CD36 and medium chain aceyl-CoA dehydrogenase (MCAD) (Figure 2E,F). As in the human failing heart, myocardial PGC-1α expression was unchanged in the mice in response to pressure overload for 4 weeks of TAC. Thus, the decrease of LV ERRα was correlated with the decreased expression of energy metabolism related genes and impaired cardiac function. These data suggest that the decreased expression of ERRα, but not PGC-1α, may be a marker of LV dysfunction. As previous studies have demonstrated that ERRα gene deletion attenuates the expression of metabolism related genes such as MCAD and cytochorome C, and exacerbates TAC- induced LV hypertrophy and dysfunction 8, it is plausible that the decreased LV ERRα would contribute to the development of LV dysfunction in the pressure overloaded heart.
AMPKα2 deficiency attenuated expression of myocardial ERRα protein and energy metabolism related genes
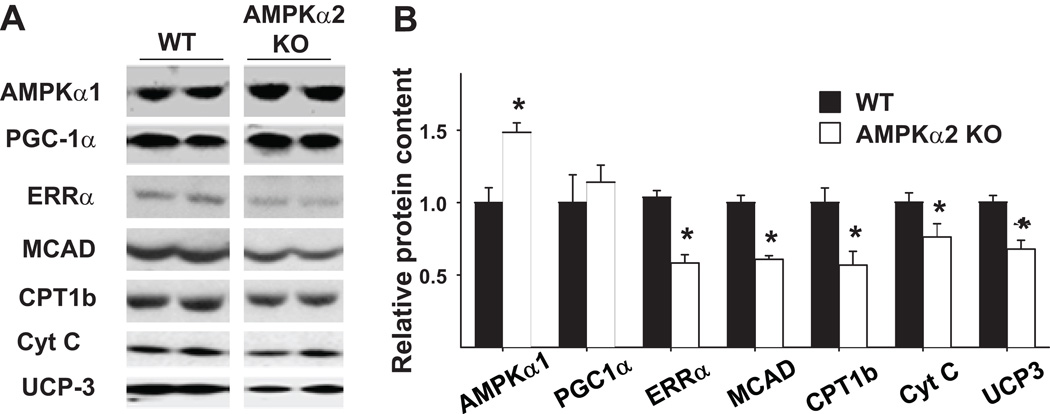
Our previous study using AMPKα2 null mice demonstrated that AMPK suppresses the energy consuming process of protein synthesis through mTOR signaling and attenuates cardiac hypertrophy induced by pressure overload 16. Furthermore, we found that in the AMPKα2 null heart, the protein levels of several energy metabolism related genes, including MCAD, carnitine palmitoyl transferase 1 muscle isoform (CPT1b), cytochrome C and uncoupling protein 3 (UCP3), were significantly decreased as compared with their wild type littermates (Figure 3). In addition, the ERRα protein level was significantly reduced in AMPKα2 deficient hearts, while the protein level of PGC1α was similar between the wild type and AMPKα2 knockouts (Figure 3). Thus, depletion of AMPKα2 attenuated the expression of mitochondrial enzymes involved in energy production. Similar to our observations in the human CHF samples and in the mouse hearts with pressure overload induced hypertrophy, the repression of energy metabolism related genes in AMPKα2 deficient heart was associated with decreased expression of ERRα, but not PGC1α.
Figure 3.
Protein levels of myocardial ERRα and energy metabolism related enzymes in wild type and AMPKα2 deficient mice. Protein levels were determined by Western blots (A). Protein levels of ERRα and some energy metabolism related enzymes were significantly decreased in the AMPKα2 deficient mice (B). * p<0.05 as compared with wild type mice.
AMPKα2 regulates ERRα gene expression in rat neonatal cardiac myocytes
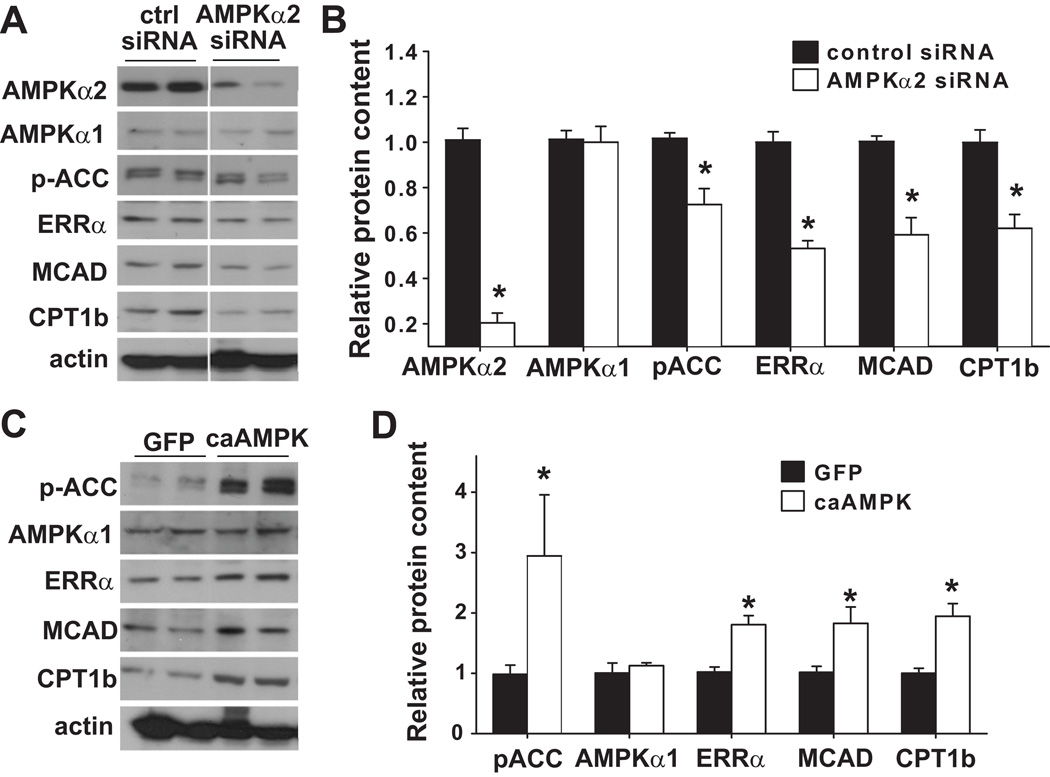
To be sure that the down regulation of ERRα in the AMPKα2 knockout mouse hearts was not due to neurohormonal alterations in the intact animal, we examined the regulation of ERRα by AMPK using rat neonatal cardiac myocytes. First, we inhibited AMPKα2 expression in the cardiac myocytes using specific siRNA. The expression of AMPKα2 was decreased by 80%; the protein level of AMPKα1 was not significantly changed (Figure 4A,B). The phosphorylation of acetyl-CoA carboxylase (ACC), a substrate of AMPK, was significantly decreased, suggesting that AMPKα2 is the dominant isoform in cardiac myocytes. Furthermore, decreasing the expression of AMPKα2 significantly decreased the protein levels of ERRα and its targets, MCAD and CPT1b (Figure 4A,B), suggesting that AMPKα2 is required for maintaining normal expression levels of ERRα and its target genes.
Figure 4.
Alterations of ERRα, MCAD and CPT1b in rat neonatal cardiac myocytes in response to selective gene silencing of AMPKα2 or overexpression of constitutively active AMPKα. siRNA knockdown of AMPKα2 repressed the expression of ERRα, MCAD and CPT1 (A), while constitutively active AMPK increased expression of these proteins (C). * p<0.05 as compared with control siRNA transfected cells (B); or cells infected with adenovirus over expressing GFP (D).
Consistent with the above observations, in rat neonatal cardiac myocytes, activation of AMPK with AICAR caused an induction of ERRα protein 6 hours after AICAR treatment (please see supplementary Figure S2). Furthermore, overexpression of constitutively active AMPKα (caAMPK, gift from Dr. Ming-Hui Zou) in rat neonatal cardiac myocytes caused significant increases of ERRα, MCAD and CPT1b (Figure 4C,D). Taken together, these data suggest that AMPKα2 can regulate the expression of ERRα and its target genes.
AMPKα2 regulates myocardial ERRα at the transcriptional level both in vivo and in vitro
To understand the mechanism of ERRα gene regulation by AMPKα2, RT and real-time PCR was performed to determine the mRNA contents of ERRα and its target genes in the AMPKα2 deficient mice and their wild type littermates. AMPKα2 gene deletion significantly decreased the mRNA levels of ERRα, very long chain acetyl CoA dehydrogenase (VLCAD), MCAD, CPT1b, cytochrome C oxidase I (COX1) and cytochrome C oxidase III (COX3) (Figure S3A). Interestingly, the mRNA level of PGC1α was significantly increased in the AMPKα2 deficient hearts. This result suggested that PGC1α was not directly involved in the down regulation of the mitochondrial enzymes caused by AMPKα2 depletion.
In rat neonatal cardiac myocytes, selective gene silencing of AMPKα2 by specific siRNA caused significant reductions in the mRNA levels of ERRα and VLCAD (Figure S3B). There was a trend toward down regulation of CPT1b, but this was not significant. Thus, both the in vivo and in vitro data suggest that AMPKα2 can regulate ERRα expression at the transcriptional level.
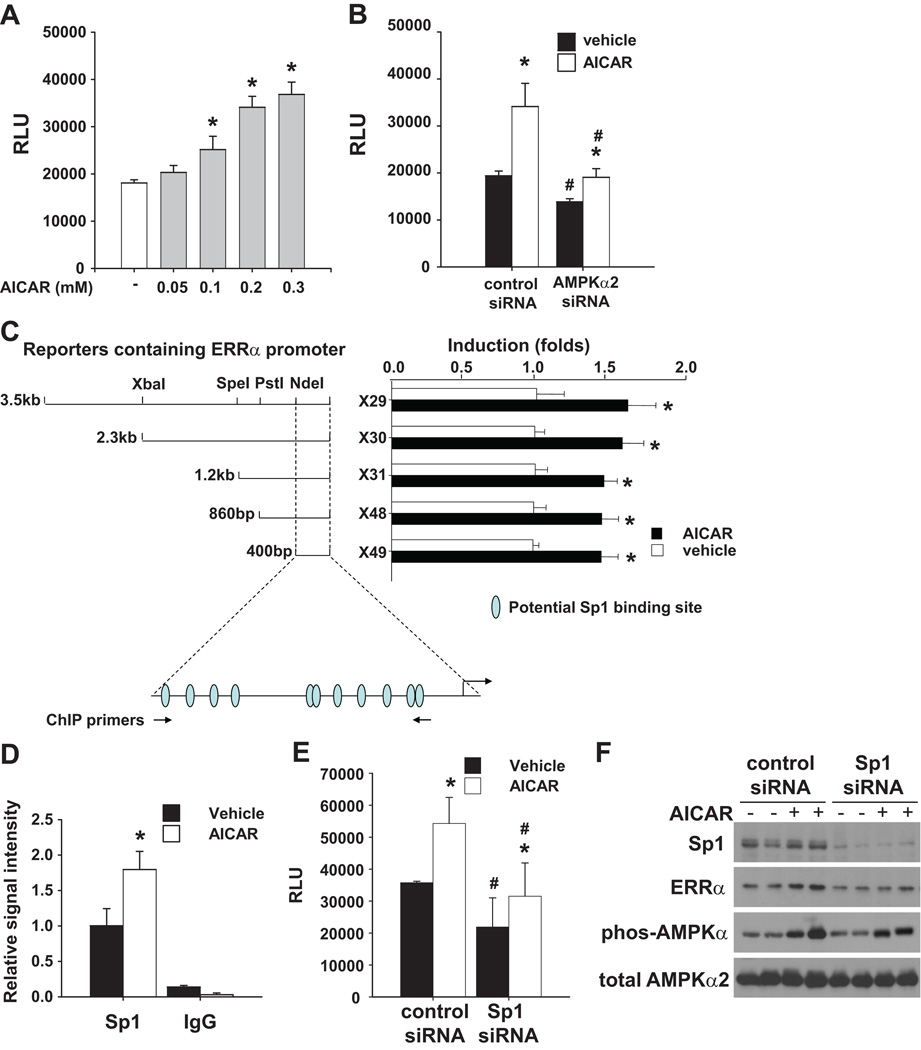
To test if AMPKα2 can regulate ERRα promoter activity, a reporter construct driven by 3.5kb of the ERRα promoter was transfected into rat neonatal cardiac myocytes and the cells were treated with AICAR for 24 hours. As shown in Figure 5A, treatment with AICAR dose dependently increased reporter gene activity. Conversely, ablation of AMPKα2 expression by specific siRNA caused a significant decrease of the reporter gene activity in vehicle treated cells, and also repressed its activation by AICAR (Figure 5B). These results indicate that the induction of ERRα promoter activity by AICAR is AMPKα2 dependent and suggest that AMPK regulates ERRα expression at the transcriptional level. The induction of reporter activity by AICAR was slightly decreased but still observed when the reporter was driven by only ~400bp of the ERRα promoter (Figure 5C). This 400bp of the mouse ERRα promoter contains multiple Sp1 sites, which are conserved in humans (Figure 5C) 24. ChIP assay using rat neonatal cardiac myocytes demonstrated that Sp1 was recruited to this region, and that treatment with AICAR enhanced the interaction between Sp1 and the ERRα promoter (Figure 5D). Indeed, knocking down the expression of Sp1 in rat neonatal cardiac myocytes repressed basal ERRα promoter activity and its induction by AICAR (Figure 5E), which resulted in decreased ERRα expression at the protein level (Figure 5F). Thus, Sp1 is involved in AMPK regulation of ERRα.
Figure 5.
AMPK regulates ERRα promoter activity. ERRα promoter activity was measured in rat neonatal cardiac myocytes transfected with ERR-promoter-luciferase constructs. ERRα promoter activity was increased after treatment with AICAR in a dose dependent manner (A). Inhibition of AMPKα2 expression by specific siRNA repressed ERRα promoter activity and its activation by AICAR (B). The reporter activity of serial deletions of ERRα promoter and their responses to AICAR induction (C). ChIP assay demonstrated that AICAR enhanced Sp1 binding to ERRα promoter in cardiac myocytes (D). Gene silencing of Sp1 by specific siRNA repressed ERRα promoter activity (E) and ERRα expression (F) at basal conditions, and attenuated their induction in response to AICAR. * p<0.05 as compared with vehicle treated cells; # p<0.05 as compared with non-specific siRNA transfected cells.
Overexpression of ERRα or constitutively active AMPK partially rescued energy metabolism related gene expression in AMPKα2 deficient mice
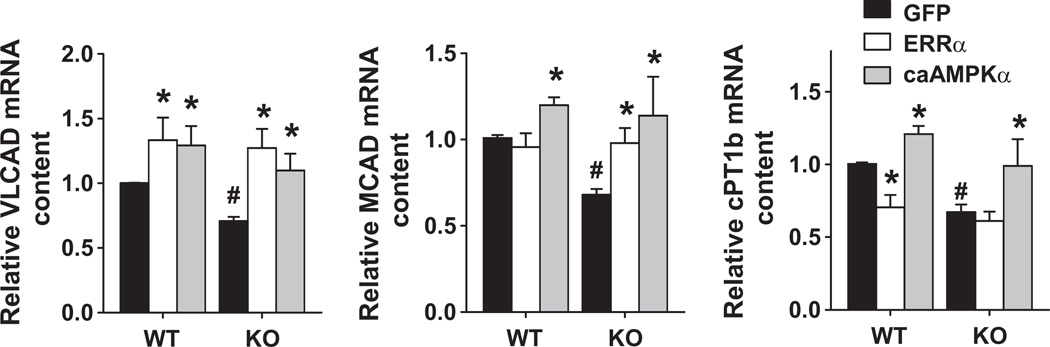
AMPKα2 deficiency resulted in a significant decrease of myocardial ERRα at both mRNA and protein levels. Furthermore, the phenotype and gene expression profile of AMPKα2 KO mice are similar to those of the ERRα KO mice. Therefore, down regulation of ERRα might contribute to the abnormal myocardial energy metabolism related genes in the AMPKα2 KO mice. To test this hypothesis, neonatal cardiac myocytes were isolated from wild type and AMPKα2 deficient mice and infected with adenovirus over-expressing either caAMPK or ERRα. Overexpression of caAMPK significantly increased the mRNA levels of the ERRα target genes, VLCAD, MCAD, and CPT1b in both wild type and AMPKα2 knockout cells (Figure 6). In the wild type cells where ERRα levels were normal, increasing ERRα expression caused an increase of VLCAD mRNA, but had no effect on the expression of MCAD and even repressed the expression of CPT1b. Thus, other factors, such as the levels of co-regulators, may influence the function of ERRα. In the AMPKα2 deficient cells, where ERRα expression is reduced, overexpression of ERRα increased the mRNA levels of VLCAD and MCAD. These data indicate that overexpression of ERRα was able to partially rescue the repressed expression of the energy metabolism related enzymes in the AMPKα2 deficient cardiac myocytes.
Figure 6.
mRNA levels of MCAD, VLCAD and CPT1b in wild type and AMPKα2 deficient mouse neonatal cardiac myocytes after overexpression of constitutively active AMPKα2 or ERRα. Wild type and AMPKα2 deficient neonatal cardiac myocytes were infected with adenovirus expressing constitutively active AMPKα2 or ERRα. mRNA levels of MCAD, VLCAD and CPT1b were determined by RT and qPCR. Over-expression of constitutively active AMPKα2 or ERRα partially rescued expression of MCAD, VLCAD and CPT1b in AMPKα2 deficient neonatal cardiac myocytes. * p<0.05 as compared with cells infected with GFP adenovirus. # p<0.05 as compared with wild type cells.
Previous studies have shown that activation of AMPK by metformin protects cardiac myocytes from oxidative stress induced cell death 25. Indeed, when we treated rat neonatal cardiac myocytes with H2O2, AMPKα2 specific gene silencing caused a decrease of cell viability and an increase of cleaved caspase 3 and TUNEL positive cells (please see Figure S4). Furthermore, the increased vulnerability to oxidative stress damage caused by AMPKα2 knockdown was partially rescued by over expression of ERRα (please see Figure S4). The finding that AMPK/ERRα is involved in protection of cardiac myocytes against oxidative stress is in agreement with the report that ERRα plays a critical role in the expression of antioxidant genes in embryonic fibroblasts 26.
Discussion
Dysregulation of energy metabolism related genes is an important aspect in the development of pathologic ventricular hypertrophy and heart failure. In human heart failure samples, we found that ERRα protein levels were decreased, and this was associated with down regulation of several enzymes that are involved in energy production, while the protein level of PGC-1α was not changed. Similarly, we observed significant down regulation of ERRα and several energy metabolism related genes in mouse ventricular tissue after 4 weeks of TAC, with no alterations of PGC1α at the protein level. These findings support the concept that the expression of ERRα is of vital importance in preserving energy production and cardiac function under stress conditions 27.
Detailed studies by Dr. Teng’s group identified a multi-hormone response element and multiple Sp1 sites in the ERRα promoter, and demonstrated that the transcription of ERRα can be modulated by ERRα, ERRγ, estrogen receptor and Sp124, 28, 29. In the current study, we demonstrated for the first time that AMPKα2 contributes to regulation of myocardial ERRα expression. Several lines of evidence suggested that AMPKα2 is one of the regulators of myocardial ERRα expression. First, selective gene silencing of AMPKα2 in rat neonatal cardiac myocytes repressed ERRα expression. Similarly, under basal conditions, depletion of AMPKα2 in the knockout mice resulted in decreased ERRα at both mRNA and protein levels. Thus, AMPKα2 can regulate ERRα expression under physiological conditions. Second, activation of AMPK with AICAR or overexpression of constitutively active AMPK in rat neonatal cardiac myocytes induced ERRα promoter activity and expression. This induction was blunted by knocking down the expression of AMPKα2. We further demonstrated that Sp1 is involved in the regulation of ERRα by AMPK. In addition to regulating ERRα expression, AMPK may also influence ERRα function by adjusting its subcellular distribution 30.
In addition to ERRα, several energy metabolism related genes including CPT1b, MCAD, VLCAD, CD36, FATP1 and UCP3 were also increased in response to activation of AMPK in neonatal cardiac myocytes or depletion of AMPKα2 in KO mice. This suggested that AMPKα2 plays an important role in regulating myocardial energy metabolism related gene expression. Interestingly, a similar set of genes was down regulated in the AMPKα2 deficient hearts as those observed in the global ERRα deficient hearts 7, 8. Furthermore, the changes in expression of these energy metabolism related genes in the AMPKα2 knockout mice or rat neonatal cardiac myocytes was associated with changes in the expression of ERRα. Most importantly, in the AMPKα2 deficient neonatal cardiac myocytes, overexpression of either ERRα or caAMPKα rescued the repressed expression of VLCAD and MCAD. These results imply that AMPKα2 regulates energy metabolism related genes at least partially through ERRα. CPT1b has been reported as a target gene for both ERRα and ERRγ 7, 31 and a potential ERR responsive element was identified in its promoter 1. ERRα has been shown to regulate the expression of CPT1b in mouse embryonic fibroblasts 26, 32 and brown adipose tissue 33. However, the expression of CPT1b was not changed in ERRα deficient hearts, but was decreased in the ERRγ knockout hearts. Therefore, in the heart, ERRγ, but not ERRα, appears to play the dominant role in regulation of CPT1b expression. In the current study, we found that depletion of AMPKα2 in rat neonatal cardiac myocytes or in the heart tissue of AMPKα2 knockout mice repressed CPT1b expression, while activation of AMPK induced CPT1b in rat neonatal cardiac myocytes. However, in the AMPKα2 deficient neonatal cardiac myocytes, overexpression of ERRα did not rescue CPT1b expression, indicating that the decreased expression of ERRα in these cells is not directly responsible for the down regulation of CPT1b. These findings imply that the reduced ERRα expression in the AMPKα2 KO mice contributed to the dysregulation of only some of the energy metabolism related genes. Changes of other transcription factors, such as ERRγ, might also contribute to the phenotypes observed in AMPKα2 KO mice. Intriguingly, in the wild type cells, overexpression of ERRα led to different responses in the expression of its potential targets. The function of ERRα is modulated by its co-regulators, such as coactivators PGC1α/β and corepressor RIP140. Thus, in some cases, ERRα overexpression caused significant increases of its responsive genes 32, 34, but in several other studies overexpression of ERRα alone failed to induce some of those target genes 35–37. These results suggest that the ratio of ERRα and its coactivators may be critical for its target gene activation, and that this effect seems to be gene specific. On the other hand, overexpressed ERRα may induce expression of corepressors, such as RIP140 38and SHP 39, which forms a negative feedback loop and represses gene expression 40. Furthermore, ERRα may compete for coactivators with other transcription factors, which in turn, could result in gene repression 11.
Similar to our observations in AMPKα2 KO mice, several recent studies have demonstrated that moderate decreases of the myocardial energy metabolism related genes caused no apparent LV dysfunction in mice with disruption of ERRα 8, PGC-1α 18, 41, PGC-1β 42 mice or cardiac KATP channel activity 21 under unstressed conditions. Nevertheless, disruption of AMPKα2 16, ERRα 8, PGC-1α 43 or cardiac KATP channel activity 21 all significantly exacerbated TAC-induced LV hypertrophy and heart failure. Thus, a moderate decrease of energy metabolism related genes which has no effect on LV function under unstressed conditions may compromise the myocardial energy reserve and impair the cardiac adaptation to hemodynamic stresses such as chronic systolic pressure overload.
Clinical Perspectives.
Myocardial mitochondrial enzymes are essential to maintain the cardiac energy reserve and to facilitate adaptive responses to stress, but the underlying molecular mechanisms for maintaining myocardial mitochondrial enzyme expression have been elusive. We demonstrated that the expression level of ERRα, but not PGC-1α, correlates with the down regulation of cardiac energy producing enzymes in myocardium from both patients and mice with heart failure. These results imply that PGC-1α independent pathway(s) can regulate myocardial energetics which might involve ERRα. We further demonstrated that AMPKα2 (but not AMPKα1) regulates ERRα expression in cardiac myocytes. Our data support an important role for AMPKα2 in regulating the expression of myocardial ERRα and its downstream mitochondrial enzymes. These results suggest that treatments that increase AMPK activity may have potential therapeutic value in heart failure.
Supplementary Material
Acknowledgements
We thank Dr. Janice Huss for the adenoviruses expressing ERRα.
Sources of Funding
This study was supported by U.S. Public Health Service Grants HL20598, HL021872, R21HL098669, R21HL098719 and R21HL102597 from the National Heart, Lung and Blood Institute; Research Grants 0330136N, 09SDG2170072 and 0160275Z from the American Heart Association; and Beijing Natural Science Foundation of China (No. 5042006 and 7072012). Drs Hu and Zhang are recipients of Scientist Development Awards from the American Heart Association National Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Giguere V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 2.Villena JA, Kralli A. ERR[alpha]: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical Profiling of Nuclear Receptor Expression Reveals a Hierarchical Transcriptional Network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related Receptors Stimulate Pyruvate Dehydrogenase Kinase Isoform 4 Gene Expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 5.Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol. 2010;315:159–167. doi: 10.1016/j.mce.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makoto A, Kiyoto M. Identification of ERRa; as a specific partner of PGC-1a; for the activation of PDK4 gene expression in muscle. FEBS J. 2006;273:1669–1680. doi: 10.1111/j.1742-4658.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- 7.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguère V. Genome-wide Orchestration of Cardiac Functions by the Orphan Nuclear Receptors ERRa and g. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Huss JM, Imahashi K-i, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguère V, Murphy E, Kelly DP. The Nuclear Receptor ERR[alpha] Is Required for the Bioenergetic and Functional Adaptation to Cardiac Pressure Overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener J-L, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRa expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The Transcriptional Coactivator PGC-1 Regulates the Expression and Activity of the Orphan Nuclear Receptor Estrogen-Related Receptor alpha (ERRalpha) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 11.Ichida M, Nemoto S, Finkel T. Identification of a Specific Molecular Repressor of the Peroxisome Proliferator-activated Receptor g Coactivator-1a (PGC-1a) J Biol Chem. 2002;277:50991–50995. doi: 10.1074/jbc.M210262200. [DOI] [PubMed] [Google Scholar]
- 12.Dyck JRB, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Yong Ji S, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-Activated Protein Kinase by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose Metabolism and Energy Homeostasis in Mouse Hearts Overexpressing Dominant Negative a2 Subunit of AMP-activated Protein Kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP Activated Protein Kinase-{alpha}2 Deficiency Exacerbates Pressure-Overload-Induced Left Ventricular Hypertrophy and Dysfunction in Mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased Adenosine Monophosphate-Activated Protein Kinase Activity in Rat Hearts With Pressure-Overload Hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 18.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu P-H, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1a controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Sano MD, Schneider M. Energizer: PGC-1a keeps the heart going. Cell Metab. 2005;1:216–218. doi: 10.1016/j.cmet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JFP, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha 2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of Sarcolemmal ATP-Sensitive Potassium Channel Activity Impairs the Cardiac Response to Systolic Overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 Receptor Deficiency Exerts Unanticipated Protective Effects on the Pressure-Overloaded Left Ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, Remedios Cd, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative Stress Regulates Left Ventricular PDE5 Expression in the Failing Heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen Stimulates Estrogen-Related Receptor {alpha} Gene Expression through Conserved Hormone Response Elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin Prevents Progression of Heart Failure in Dogs: Role of AMP-Activated Protein Kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 26.Rangwala SM, Li X, Lindsley L, Wang X, Shaughnessy S, Daniels TG, Szustakowski J, Nirmala NR, Wu Z, Stevenson SC. Estrogen-related receptor [alpha] is essential for the expression of antioxidant protection genes and mitochondrial function. Biochem Biophys Res Commun. 2007;357:231–236. doi: 10.1016/j.bbrc.2007.03.126. [DOI] [PubMed] [Google Scholar]
- 27.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA Replication Impairs Mitochondrial Biogenesis In Human Failing Hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Zhang Z, Teng CT. Estrogen-related receptor-{gamma} and peroxisome proliferator-activated receptor-{gamma} coactivator-1{alpha} regulate estrogen-related receptor-{alpha} gene expression via a conserved multi-hormone response element. J Mol Endocrinol. 2005;34:473–487. doi: 10.1677/jme.1.01586. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Teng CT. Interplay between estrogen-related receptor alpha (ERR[alpha]) and gamma (ERR[gamma]) on the regulation of ERR[alpha] gene expression. Mol Cell Endocrinol. 2007;264:128–141. doi: 10.1016/j.mce.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun P, Sehouli J, Denkert C, Mustea A, Könsgen D, Koch I, Wei L, Lichtenegger W. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med. 2005;83:457–467. doi: 10.1007/s00109-005-0639-3. [DOI] [PubMed] [Google Scholar]
- 31.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker Johan W, Giles W, Naviaux RK, Giguère V, Evans RM. ERR[gamma] Directs and Maintains the Transition to Oxidative Metabolism in the Postnatal Heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-Related Receptor {alpha} Directs Peroxisome Proliferator-Activated Receptor {alpha} Signaling in the Transcriptional Control of Energy Metabolism in Cardiac and Skeletal Muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villena JA, Hock MB, Chang WY, Barcas JE, Giguère V, Kralli A. Orphan nuclear receptor estrogen-related receptor a is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A. 2007;104:1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajalin A-M, Pollock H, Aarnisalo P. ERR[alpha] regulates osteoblastic and adipogenic differentiation of mouse bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2010;396:477–482. doi: 10.1016/j.bbrc.2010.04.120. [DOI] [PubMed] [Google Scholar]
- 35.Araki M, Motojima K. Identification of ERRα as a specific partner of PGC-1α for the activation of PDK4 gene expression in muscle. FEBS J. 2006;273:1669–1680. doi: 10.1111/j.1742-4658.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- 36.Mirebeau-Prunier D, Le Pennec S, Jacques C, Gueguen N, Poirier J, Malthiery Y, Savagner F. Estrogen-related receptor α and PGC-1-related coactivator constitute a novel complex mediating the biogenesis of functional mitochondria. FEBS J. 2010;277:713–725. doi: 10.1111/j.1742-4658.2009.07516.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L-L, Liu Y, Cui A-F, Shao D, Liang J-C, Liu X-J, Chen Y, Gupta N, Fang F-D, Chang Y-S. PGC-1a coactivates estrogen-related receptor-a to induce the expression of glucokinase. Am J Physiol Endocrinol Metab. 2010;298:E1210–E1218. doi: 10.1152/ajpendo.00633.2009. [DOI] [PubMed] [Google Scholar]
- 38.Nichol D, Christian M, Steel JH, White R, Parker MG. RIP140 Expression Is Stimulated by Estrogen-related Receptor a during Adipogenesis. J Biol Chem. 2006;281:32140–32147. doi: 10.1074/jbc.M604803200. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal S, Kim J-Y, Kim H-J, Takeda J, Lee Y-K, Moore DD, Choi H-S. Differential Regulation of the Orphan Nuclear ReceptorSmall Heterodimer Partner (SHP) Gene Promoter by Orphan Nuclear Receptor ERR Isoforms. J Biol Chem. 2002;277:1739–1748. doi: 10.1074/jbc.M106140200. [DOI] [PubMed] [Google Scholar]
- 40.Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen ZO, Holloszy J, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1a Deficiency Causes Multi-System Energy Metabolic Derangements: Muscle Dysfunction, Abnormal Weight Control and Hepatic Steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Strömstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1b Results in Defective Mitochondrial Activity, Thermogenesis, Hepatic Function, and Cardiac Performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-g coactivator 1a. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.