Abstract
Defects in apoptotic pathways can promote cancer cell survival and also confer resistance to antineoplastic drugs. One pathway being targeted for antineoplastic therapy is the anti-apoptotic B-cell lymphoma-2 (Bcl-2) family of proteins (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl1/A-1, and Bcl-B) that bind to and inactivate BH3-domain pro-apoptotic proteins. Signals transmitted by cellular damage (including antineoplastic drugs) or cytokine deprivation can initiate apoptosis via the intrinsic apoptotic pathway. It is controversial whether some BH3-domain proteins (Bim or tBid) directly activate multidomain pro-apoptotic proteins (e.g., Bax and Bak) or act via inhibition of those anti-apoptotic Bcl-2 proteins (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl1/A-1, and Bcl-B) that stabilize pro-apoptotic proteins. Overexpression of anti-apoptotic Bcl-2 family members has been associated with chemotherapy resistance in various human cancers, and preclinical studies have shown that agents targeting anti-apoptotic Bcl-2 family members have preclinical activity as single agents and in combination with other antineoplastic agents. Clinical trials of several investigational drugs targeting the Bcl-2 family (oblimersen sodium, AT-101, ABT-263, GX15-070) are ongoing. Here, we review the role of the Bcl-2 family in apoptotic pathways and those agents that are known and/or designed to inhibit the anti-apoptotic Bcl-2 family of proteins.
Apoptosis is one of the major mechanisms of cell death in response to cancer therapies (1). Alterations in susceptibility to apoptosis not only contribute to neoplastic development (2) but also can enhance resistance to conventional anticancer therapies, such as radiation and cytotoxic agents (3). One of the suggested mechanisms of resistance to cytotoxic antineoplastic drugs is the alteration in expression of B-cell lymphoma-2 (Bcl-2) family members. The Bcl-2 family of proteins consists of 25 pro- and anti-apoptotic members, which interact to maintain a balance between newly forming cells and old dying cells. When anti-apoptotic Bcl-2 family members are overexpressed, the ratio of pro- and anti-apoptotic Bcl-2 family members is disturbed and apoptotic cell death can be prevented. Targeting the anti-apoptotic Bcl-2 family of proteins can improve apoptosis and thus overcome drug resistance to cancer chemotherapy (4–6).
The key players that execute the apoptotic cascade are the initiator and the effector caspases, which are activated by cleavage early in apoptosis (3, 7). Two major pathways of apoptosis converge on the effector caspases, the intrinsic and extrinsic cell-death pathways. The intrinsic cell death pathway, also known as the mitochondrial apoptotic pathway, is activated by a wide range of signals, including radiation, cytotoxic drugs, cellular stress, and growth factor withdrawal, and involves the release of proteins (including cytochrome c) from the mitochondrial membrane space (8). Cytochrome c combines with an adaptor molecule, apoptosis protease-activating factor 1, and also with an inactive initiator caspase, procaspase-9, within a multiprotein complex called the apoptosome (9). This leads to the activation of caspase-9, which then triggers a cascade of caspase (caspase-3, caspase-6, caspase-7) activation, resulting in the morphologic and biochemical changes associated with apoptosis. By contrast, the extrinsic cell-death pathway can function independently of mitochondria and is activated by cell-surface death receptors, such as Fas and tumor necrosis factor–related apoptosis–inducing ligand (TRAIL) receptors, directly activating the caspase cascade via an “initiator” caspase (caspase-8) within a death-inducing signaling complex (10).
The intrinsic pathway (via mitochondria) plays a key role in regulating cell death in response to various stimuli (11). Mitochondrial outer membrane permeabilization is considered the “point of no return” for apoptotic cell death, triggering release into the cytoplasm of proteins that mediate cell death, such as cytochrome c (12). Outer membrane permeabilization is mediated by certain Bcl-2 family members that coordinately regulate apoptosis among a panoply of interacting pro- and anti-apoptotic proteins (13). Inner membrane permeabilization can be altered by the redox status of mitochondrial protein vicinal thiols (14) and through opening of the mitochondrial permeability transition pore (15). Although the mitochondrial permeability transition pore complex contains proteins on the outer and inner mitochondrial membranes, there is no clear involvement of inner membrane permeabilization in mitochondrial apoptosis and there is no compelling evidence that inner membrane permeabilization is necessary for outer membrane permeabilization (16).
The p53 tumor suppressor protein can induce the expression of numerous pro-apoptotic gene products that can initiate the extrinsic and intrinsic apoptotic pathways. Although the mechanisms through which p53 contributes to apoptosis remains to be clarified (and may act in a cell type–specific manner), current evidence points toward p53 functioning through transcriptional activation of pro-apoptotic target genes that contain p53-binding sites within their regulatory regions (17). Of the several apoptotic genes that p53 can directly target, NOXA and PUMA (pro-apoptotic Bcl-2 family) are induced by p53 in cell types that express pro-apoptotic Bcl-2 family members, although Noxa and Puma can also be induced by p53-independent mechanisms (18). In addition to Noxa and Puma, p53 causes transcriptional (17) and transcription-independent (13) activation of Bax, a pro-apoptotic member of the Bcl-2 family that is capable of directly triggering apoptosis.
Bcl-2 Family of Proteins
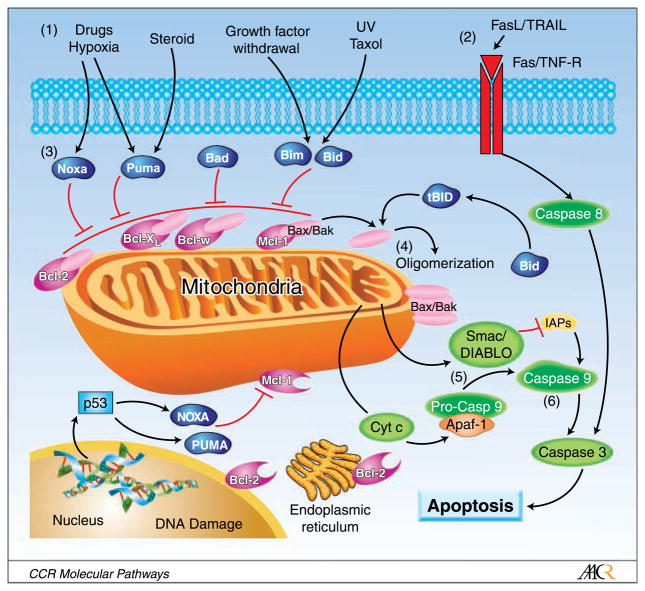
To date, 25 members of the Bcl-2 family of proteins have been identified (8). These proteins are localized to mitochondria, smooth endoplasmic reticulum, and perinuclear membranes in hematopoietic cells. Overexpression of several anti-apoptotic Bcl-2 family proteins has been reported in hematologic malignancies (19). Bcl-2 proteins are characterized by the presence of up to four relatively short sequence motifs, which are <20 amino acid residues in length, termed Bcl-2 homology domains (8). Bcl-2 family members can be divided into three subfamilies based on structural and functional features. The anti-apoptotic subfamily contains the Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl1/A-1, and Bcl-B proteins, which suppress apoptosis and contain all four Bcl-2 homology domains, designated Bcl-2 homology 1–4. Some pro-apoptotic proteins, such as Bax, Bak, and Bok, contain Bcl-2 homology 1–3 domains and are termed “multidomain proteins,” whereas other pro-apoptotic proteins, such as Bim, Bad, and Bid, contain only the BH3 domain and are termed “BH3-only” proteins (20). Figure 1 illustrates the role of the Bcl-2 family of proteins in apoptosis.
Fig. 1.
The apoptotic pathway to cell death from the perspective of the Bcl-2 family of proteins.1, The intrinsic pathway is initiated by various signals, principally extracellular stimuli. 2, The extrinsic pathway is activated by Fas ligand or TRAIL, subsequently activating caspase-8. Caspase-8 transforms Bid into truncated Bid. In addition, caspase-8 initiates a cascade of caspase activation. 3, BH3-only proteins (Bim, Bid, Bad, Noxa, Puma) engage with anti-apoptotic Bcl-2 family proteins to relieve their inhibition of Bax and Bak to activate them. 4, Next, Bax and Bak are oligomerized and activated, leading to mitochondrial outer membrane permeabilization. 5, Once mitochondrial membranes are permeabilized, cytochrome c and/or Smac/DIABLO is released into the cytoplasm, wherein they combine with an adaptor molecule, apoptosis protease-activating factor 1, and an inactive initiator caspase, procaspase-9, within a multiprotein complex called the apoptosome. Smac/DIABLO inhibits inhibitors of apoptosis proteins to activate caspase-9. 6, Caspase-9 activates caspase-3, which is the initiation step for the cascade of caspase activation. Intrinsic and extrinsic pathways converge on caspase-3. Bcl-2 family proteins are also found on the endoplasmic reticulum and the perinuclear membrane in hematopoietic cells, but they are predominantly localized to mitochondria.
How Bcl-2 family proteins interact to permeabilize the mitochondrial membrane remains controversial. There are two tentative models explaining how BH3-only proteins can interact with other Bcl-2 family proteins to induce apoptosis. The “direct activation model” suggested by Letai et al. (21) divides BH3-only domain proteins into groups, which are “sensitizers” and “activators.” The activator BH3-only molecules (Bim, Bid) directly bind and oligomerize Bax/Bak, leading to release of cytochrome c. A study supporting the latter theory showed that a Bid mutant that lacks the ability to interact with Bcl-2 (but maintains interaction with Bax) is still potently pro-apoptotic, suggesting that the interaction with Bax and/or Bak is important in the function of Bid (22). The sensitizers, such as Bad, Bik, and Noxa, cannot directly activate Bax/Bak, but they inhibit anti-apoptotic Bcl-2 proteins from engaging with activator proteins or Bax/Bak, thereby sequestering activators and Bax/Bak. The engagement of BH3-only proteins with anti-apoptotic Bcl-2 members is common between the two models.
The second model (indirect activation model) challenges the direct activation model with data showing that Bak did not bind to any of the BH3-only proteins and that Bax and Bak can mediate apoptosis without discernable association with the putative BH3-only activators (Bim, Bid), even in cells with no Bim or Bid and reduced Puma (23). Although further work is needed to fully elucidate the mechanisms regulating Bcl-2 family protein–induced apoptosis and to fully understand the biology of the Bcl-2 pathway, agents targeting the Bcl-2 family proteins are already entering oncology clinical trials.
Unlike most oncogenes that promote proliferation, Bcl-2 functions by preventing programmed cell death (24). As the anti-apoptotic Bcl-2 family proteins promote cancer cell survival by antagonizing apoptosis (25), they provide therapeutic targets, and inhibition of anti-apoptotic Bcl-2 family proteins is expected to predominantly induce apoptosis in cancer cells (26). Bcl-2 was identified because of a characteristic chromosomal translocation t(14;18) present in 85% of follicular lymphomas and 20% of diffuse B-cell lymphomas (27), which results in deregulated BCL-2 gene expression at the transcriptional level. The in vivo effects of Bcl-2 were initially investigated in BCL-2 transgenic mice in which Bcl-2 over-expression was targeted to B and T lymphocytes, which lead to follicular hyperplasia or T-cell lymphomas (28).
Constitutively high levels of Bcl-2 or Bcl-XL have been associated with a more aggressive malignant phenotype and/or drug resistance to various categories of chemotherapeutic agents in hematologic malignancies and solid tumors (5, 6, 19, 29). As an example, high Bcl-2 levels in primary prostate cancer were associated with high Gleason scores and a high rate of cancer recurrence after radical prostatectomy (6). Expression of BCL-XL in the NCI 60 cell line panel strongly correlated with resistance to most chemotherapy agents (30). Overexpression of Bcl-2 RNA and/or protein has been observed in acute myelogenous leukemia (AML) and in acute lymphoblastic leukemia (31, 32), and the Bax/Bcl-2 ratio inversely correlates with prognosis of AML and acute lymphoblastic leukemia (4, 33). Bcl-2 overexpression is commonly observed but is highly variable at diagnosis in acute lymphoblastic leukemia (31, 32, 34, 35). Despite observations that Bcl-2 modulation can increase the sensitivity to chemotherapeutic agents in vitro, the level of Bcl-2 expression has not been observed to impact event-free survival or aggressiveness of acute lymphoblastic leukemia (34, 35), possibly owing to the complexity of interactions among Bcl-2 family members. Thus, it has been speculated that the balance between anti- and pro-apoptotic Bcl-2 family members, rather than mere overexpression of Bcl-2, regulates the death of cancer cells (36).
Clinical and Translational Advances in Bcl-2 Inhibitors
Since the molecular cloning of Bcl-2 by Korsmeyer et al. (36), there has been tremendous progress in identifying Bcl-2 family members as targets for drug development. In the last 20 years, the anti-apoptotic properties of Bcl-2 were discovered, the overexpression of Bcl-2 conferring chemoresistance was reported, and the 3-dimentional protein structure of Bcl-XL was determined, which contributed to the development of protein inhibitors (37). The first agent targeting Bcl-2 that entered clinical trials is a Bcl-2 antisense (oblimersen sodium), which has shown chemosensitizing effects when combined with conventional chemotherapy drugs in chronic lymphocytic leukemia (CLL) patients, leading to improved survival (38). More recent advances include the discovery of small molecule inhibitors of the Bcl-2 family proteins. They are designed to bind the hydrophobic groove of anti-apoptotic Bcl-2 proteins in place of BH3-only proteins (i.e., BH3-mimetics). They can oligomerize Bax or Bak, which can subsequently depolarize mitochondrial membrane potential to release cytochrome c. Several agents targeting the Bcl-2 family of proteins have been developed, and three of these have entered clinical trials. Table 1 summarizes the various Bcl-2 inhibitors currently in preclinical and clinical development.
Table 1.
Agents targeting anti-apoptotic Bcl-2 family proteins
| Agents | Target proteins | Sponsor | Stage |
|---|---|---|---|
| Apogossypol | Bcl-2, Bcl-XL, Mcl-1 | Burnham (NCI) | Preclinical |
| HA-14 | Bcl-2 | Maybridge Chem | Preclinical |
| Antimycin A | Bcl-2, Bcl-XL | U of Washington | Preclinical |
| BH3Is | Bcl-XL | Harvard U | Preclinical |
| Oblimersen sodium | Bcl-2 | Genta | Phase III |
| Gossypol (AT-101) | Bcl-2, Bcl-XL, Bcl-w, Mcl-1 | Ascenta (NCI) | Phase I/II |
| ABT-737 (ABT-263) | Bcl-2, Bcl-XL, Bcl-w | Abbott | Phase I |
| GX15-070 | Bcl-2, Bcl-XL, Bcl-w, Mcl-1 | Gemin X | Phase I |
Abbreviations: BH3Is, BH3 inhibitors; NCI, National Cancer Institute; Maybridge Chem, Maybridge Chemical Co. Ltd.; U, University.
BCL-2 Antisense Effect on Cell Death
Oblimersen sodium (G3139, Genasense) is a phosporothioate BCL2 antisense oligodeoxynucleotide that targets BCL-2 mRNA. The cell death mechanisms of BCL-2 antisense can be classified in two categories exerting either an apoptotic or a nonapoptotic effect based on their involvement in cell death pathways. The apoptotic effect of G3139 antisense occurs via an increase in Bax and PARP, releasing cytochrome c to activate caspases and ultimately releasing Smac/DIABLO to antagonize inhibitors of apoptosis proteins or releasing apoptosis-inducing factors from mitochondria to induce DNA fragmentation (39). Inhibitors of apoptosis protein inhibition result in activation of caspase-3 and caspase-9, which initiates apoptosis, whereas apoptosis-inducing factor release induces necrosis of cells.
Autophagy is another cell death pathway that Bcl-2 interferes with via inhibition of Beclin-1, an autophagy-inducible gene in mammalian cells (40). Autophagy maintains cellular homeostasis by causing cell death or cell survival, depending on the nutritional status of cells (41). Autophagic (self-eating) cell death is considered an intracellular degradation of organelles by lysosomes for digestion or reuse of cellular components (41). Recent observations suggested that the BH3 domain of Beclin-1 interacts with Bcl-2 or Bcl-XL and that pharmacologic disruption of the interaction between Bcl-2 and Beclin-1 can stimulate autophagy (42). Bcl-2 down-regulation by BCL-2 antisense has been reported to induce autophagic cell death (a nonapoptotic cell death) in HL-60 cells, possibly via release of Beclin-1 (43). However, the contribution of apoptosis versus autophagy to cell death in cancer from various cytotoxic agents remains to be determined.
Substantial data are available on the nonantisense effects of oblimersen. CpG-motifs present in Bcl-2 antisense are recognized by toll-like receptor 9 expressed on subsets of B cells and lymphoid dendritic cells, which may result in production of polyclonal antibodies and promotion of dendritic-cell maturation, leading to secretion of multiple cytokines, including interleukin 6 and interferon-α (44). Immature plasmacytoid dendritic cells may be recruited to tumor sites from the bone marrow through a stromal-derived factor-1/CXCR4 interaction, wherein they are biochemically and functionally modulated into tumor-associated immature plasmacytoid dendritic cells that can weaken antitumor immunity by inhibiting dendritic-cell and T-cell activation (43, 45). Activation of immature plasmacytoid dendritic cells with Bcl-2 antisense may account for some of its activity due to stimulation of tumor antigen-presenting plasmacytoid dendritic cells to promote tumor immunity, a nonspecific effect (observed with other nucleic acids) that has been attributed to the two CpG motifs in the antisense molecule (43).
Oblimersen has been clinically tested in combination with other anticancer chemotherapeutic agents in CLL (46, 47), AML (48), multiple myeloma, small cell lung cancer (49), non-Hodgkin’s lymphoma, and melanoma (50). A summary of current phase II and III clinical trials of oblimersen is provided in Table 2. A randomized phase III study on dacarbazine +/− oblimersen in patients with advanced melanoma led to filing with the FDA of an unsuccessful new drug application (50). A randomized phase III trial comparing fludarabine/cyclophosphamide versus fludarabine/cyclophosphamide + oblimersen in relapse or refractory CLL was conducted seeking registration of oblimersen (47), but the new drug application was deemed nonapprovable by the Food and Drug Administration. However, because long-term follow-up data showed a significant increase in overall survival for patients who received oblimersen plus chemotherapy compared with patients treated with chemotherapy alone (38), an amended new drug application was submitted for oblimersen and is currently under review by the Food and Drug Administration.
Table 2.
Phase II and III clinical trials with oblimersen sodium
| Disease (group) | Phase | Regimen | n | Response/outcome | Survival benefit | Ref. |
|---|---|---|---|---|---|---|
| Melanoma | III | Dacarbazine ± oblimersen | 771 | 7.5% vs 13.5% overall | 9.7 vs 11.4 mos | 50 |
| CLL | III | FC ± oblimersen | 241 | 7% vs 17% CR/nPR | At 40 mos (P = 0.05) | 38, 47 |
| MM | III | Dexamethasone ± oblimersen | Ongoing | To be determined | ||
| NSCLC or SCLC | III | Docetaxel ± oblimersen | Ongoing | To be determined | ||
| AML (CALGB) | III | Ara-C/daunorubicin + HiDAC + oblimersen | 503 | No outcome benefit | No survival benefit | 48 |
| SCLC (CALGB) | II | Carboplatin/etoposide ± oblimersen | 56 | 61% vs 60% | No survival benefit | 49 |
| AML | II (nR) | Gemtuzumab ± oblimersen | 48 | 5 CR, 7 PR | nR | 87 |
| HRPCa (EORTC) | II (nR) | Docetaxel ± oblimersen | 28 | 4 of 12 (33%) | nR | 88 |
| Renal Ca | II (nR) | IFN-α + oblimersen | 23 | Objective response 1 PR | nR | 89 |
| MM | II (nR) | Dexamethasone/thalidomide + oblimersen | 33 | 2 CR, 4 near CR, 12 PR | nR | 90 |
| CLL | I/II (nR) | Oblimersen | 40 (26)* | 2 of 26 PR | nR | 46 |
| Hepatocellular | I/II (nR) | Doxorubicin + oblimersen | 27 (19)* | 6 of 19 SD | nR | 91 |
Abbreviations: MM, multiple myeloma; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; HRPCa, hormone-refractory prostate cancer; Renal Ca, renal cell cancer; HiDAC, high-dose cytosine arabinoside; CR, complete response; nPR, nodal partial response; PR, partial response; SD, stable disease; nR, not randomized; CALGB, Cancer and Leukemia Group B; EORTC, European Organization of Research and Treatment of Cancer; IFN, interferon; FC, fludarabine/cyclophosphamide.
Number of patients whose responses were assessed is marked in parenthesis.
Small Molecule Inhibitors of the Bcl-2 Family of Proteins
Molecules affecting gene or protein expression
Several classes of drugs have been found to regulate gene expression of anti-apoptotic Bcl-2 members. Sodium butyrate is a histone deacetylase inhibitor that decreases Bcl-XL protein levels by down-regulating its RNA expression in mesothelioma cell lines (51). Another histone deacetylase inhibitor, depsipeptide, decreases the expression of Bcl-2, Bcl-XL, and Mcl-1 in multiple myeloma cells (52). Fenretinide, a synthetic cytotoxic retinoid, down-regulates Bcl-2, Bcl-XL, and Mcl-1 in leukemia cells (53, 54). Flavopiridol, a cyclin-dependent kinase inhibitor, reduces Mcl-1 levels in lung cancer cells (55). These agents may achieve cytotoxicity for cancer cells in part by affecting Bcl-2 family members. Alternatively, the observed effects on Bcl-2 proteins could be secondary to other cytotoxic actions of the drugs. Although the specificity for the anti-apoptotic Bcl-2 family members for drugs such as histone deacetylase inhibitors, fenretinide, or flavopiridol is lower than for agents that are designed to directly target anti-apoptotic Bcl-2 family members, the ability of such agents to modulate drug resistance mechanisms via the Bcl-2 family proteins provides opportunities for potentially synergistic drug combination studies.
Molecules acting on proteins
Recently, small molecules have been developed that directly interact with anti-apoptotic Bcl-2 proteins. These agents mimic the action of BH3 proteins and interact with anti-apoptotic Bcl-2 proteins at their BH3-binding groove.
Gossypol is the first compound that showed inhibition of Bcl-2, Bcl-XL, and Mcl-1. Gossypol is a potentially toxic phenolic pigment found in the seed, stem, and root of the cotton plant, and was initially identified as an antifertility agent in China during the 1950s (56). Natural gossypol is a racemic mixture, and levo gossypol (AT-101, Ascenta) phase II clinical trials are ongoing in CLL (in combination with rituximab) and in hormone refractory prostate cancer (in combination with docetaxel). AT-101 exhibits submicromolar binding affinity for Bcl-2 and Mcl-1. Gastrointestinal toxicity was dose limiting in a phase I/II clinical trial in prostate cancer patients (57). A gossypol analog, apogossypol (Burnham Institute), is in preclinical development. Apogossypol seems to better target Bcl-2 and Mcl-1 and may decrease the systemic toxicities observed with gossypol.
ABT-737 (A-779024, Abbott Laboratories) is a small molecule that targets anti-apoptotic Bcl-2 family proteins (Bcl-2, Bcl-XL, and Bcl-w), thereby sequestering pro-apoptotic BH3 domain proteins, promoting Bax and Bak oligomerization and ultimately programmed cell death of malignant cells (58). ABT-737 was developed using nuclear magnetic resonance–based screening and structure-based design to target the Bcl-2 family of proteins, with linking of two different molecules of modest affinity into a single molecule of high affinity. ABT-737 binds the anti-apoptotic Bcl-2 family of proteins with an affinity two or three orders of magnitude more potent than previously reported compounds (Ki ≤ 1 nmol/L for Bcl-2, Bcl-XL, and Bcl-w; Ki ≅ 0.46 nmol/L for Bcl-B, Mcl-1, Bfl1/A-1).
ABT-737 markedly increased the response to radiation as well as multiple chemotherapy agents in vitro and showed good activity as a single agent in two small cell lung cancer xenograft models (58). Other studies have shown preclinical activity of ABT-737 as a single agent or in combination with various cytotoxic agents against AML (59, 60), multiple myeloma (61), lymphoma (62), CLL (63), acute lymphoblastic leukemia (64), and small cell lung cancer (65, 66). Consistent with the low affinity of ABT-737 for Mcl-1, multiple reports have suggested that high basal levels of Mcl-1 expression are associated with resistance to ABT-737 (60, 62, 63, 67). Combining ABT-737 with a cyclin-dependent kinase inhibitor (flavopiridol), arsenic trioxide, or fenretinide have achieved synergy via inactivation of Mcl-1 by the second agent (54, 68), paving the way for future combination chemotherapy strategies targeting the Bcl-2 family of proteins.
ABT-263, an oral version of ABT-737, shares similar biological properties with ABT-737. ABT-263 is active as a single agent in small cell lung cancer xenografts, and it enhanced the activity of other chemotherapy agents in preclinical studies of B-cell lymphoma and multiple myeloma (69). Although dose-dependent transient thrombocytopenia (possibly due to Bcl-XL inhibition) was observed (69), ABT-263 is non-myelosuppressive and has shown activity as a single agent against refractory or relapsed lymphoid malignancies with minimal systemic toxicities (70, 71). ABT-263 is also under clinical investigation in adults with chronic myelogenous leukemia and small cell lung cancer (72).
Another pan-Bcl-2 inhibitor, GX15-070 (obatoclax, Gemin X), is being tested in a phase I clinical trial for CLL. The agent has been granted orphan drug status by the U.S. Food and Drug Administration (FDA) for the treatment of CLL. It is a small molecule indole bipyrrole compound that inhibits most anti-apoptotic Bcl-2 family of proteins. Compared with ABT-737, GX15-070 has a relatively low affinity for Bcl-2, Bcl-XL, Bcl-w, and Mcl-1 (Ki = 220 nmol/L for Bcl-2 and ~0.5 μmol/L for Bcl-XL, Bcl-w, and Mcl-1; ref. 73). However, interaction between Mcl-1 and Bak was inhibited by GX015-070 relative to vehicle controls with an apparent IC50 of about 1.5 μmol/L (73). A more recent report suggested that the cell death induced by GX15-070 and gossypol is in part independent of Bax and Bak, by showing that the compounds exhibited a similar cell killing spectrum in wild-type and Bcl-X–, Mcl-1–, and Bax/Bak–deficient mouse embryonic fibroblasts (72). Preclinical experiments showed that GX15-070 has single agent activity and also enhanced the in vitro cytotoxicity of bortezomib against human multiple myeloma (74) and mantle cell lymphoma cell lines (75). A phase I study of GX15-070 has established a dose for GX15-070 in combination with docetaxel (in non–small cell lung cancer patients) that did not aggravate neutropenia of docetaxel (76).
HA14-1 (in preclinical development) is a small-molecule inhibitor of the anti-apoptotic Bcl-2 family of proteins that was identified by structure-based screening (77). HA14-1 induced apoptosis in various cancer cells, including leukemia, lymphoma, glioblastoma, neuroblastoma, and colon cancer (78, 79). It enhanced the cytotoxicity of doxorubicin, TRAIL ligand, dexamethasone, bortezomib, and flavopiridol, and caused a growth delay in glioblastoma xenograft s.c. model (78). However, the binding affinity of HA14-1 to Bcl-2 (IC50, ~9 μmol/L) is relatively high compared with other inhibitors of anti-apoptotic Bcl-2 proteins (77). A recent study suggested that HA14-1 decomposes rapidly in solution, but a chemical modification has improved its stability (80).
Other small molecules of anti-apoptotic Bcl-2 proteins that are in preclinical development include BH3 inhibitor-1 and -2 and antimycin A. Antimycin A generates reactive oxygen species and inhibits mitochondrial electron transport, which results in mitochondrial membrane depolarization, Bcl-2 down-regulation, and Bax up-regulation (81). However, a limitation of using antimycin A in vivo is that it requires a high concentration to inhibit the growth of cancer cells (82). BH3 inhibitors are small molecule cell-permeable inhibitors of BH3 domain–mediated dimerization, which interfere with cell-intrinsic signaling pathways. The BH3 inhibitors (BH3I) are reported to sensitize TRAIL-induced apoptosis in leukemia (83) and prostate cancer cells (84) and to enhance radiation sensitivity in non–small cell lung cancer (85). The BH3ls also require high concentrations (~20 μmol/L) to exhibit sensitizing activity in cancer cells (86), which may limit their clinical application.
Therapeutic Implications
Many agents have been identified or designed to target the Bcl-2 family at the mRNA or protein level. Pharmacologic and cellular aspects of agents targeting the Bcl-2 family should be considered when exploring their potential application as chemotherapy. The binding affinity for inhibiting the anti-apoptotic Bcl-2 family members, a large and redundant family of proteins, should optimally be in the clinically achievable concentrations for each agent. Agents with high specificity provide ready opportunities for cancer cell drug resistance, whereas broader acting agents may introduce unexpected responses to the agents and/or increase systemic toxicities. There have been six anti-apoptotic Bcl-2 family members identified and several of these seem to contribute to drug resistance in cancer cells (8), suggesting that inhibition of multiple Bcl-2 family members will be necessary to achieve optimal therapeutic effect. One approach to enhancing the therapeutic effect of the inhibitors of anti-apoptotic Bcl-2 family members would be to use a combination of drugs that target different Bcl-2 family members.
To date, one Bcl-2 antisense and three small molecule Bcl-2 protein inhibitors are being tested in clinical trials. Preclinical studies seem promising, especially in combination with additional chemotherapy agents. Ongoing and planned phase II clinical trials to define the activity of single agents and drug combinations will determine the direction of future clinical development of the Bcl-2 inhibitors.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Reed JC. Mitochondria and apoptosis [review] [59 refs] Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML) Blood. 2003;101:2125–31. doi: 10.1182/blood-2002-06-1714. [DOI] [PubMed] [Google Scholar]
- 5.Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903–10. [PubMed] [Google Scholar]
- 6.Yoshino T, Shiina H, Urakami S, et al. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–24. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 7.Spierings D, McStay G, Saleh M, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310:66–7. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies [review] [119 refs] Blood. 2005;106:408–18. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 9.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 10.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 11.Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000;150:1027–36. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 14.Fagian MM, Pereirada-Silva L, Martins IS, Vercesi AE. Membrane protein thiol cross-linking associated with the permeabilization of the inner mitochondrial membrane by Ca2+ plus prooxidants. J Biol Chem. 1990;265:19955–60. [PubMed] [Google Scholar]
- 15.Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–9. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 17.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 18.Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008;15:1019–29. doi: 10.1038/cdd.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–30. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114:441–9. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–69. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 23.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 24.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–2. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 25.Lauria F, Raspadori D, Rondelli D, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia. 1997;11:2075–8. doi: 10.1038/sj.leu.2400854. [DOI] [PubMed] [Google Scholar]
- 26.Reed JC. Fenretinide: the death of a tumor cell. J Natl Cancer Inst. 1999;91:1099–100. doi: 10.1093/jnci/91.13.1099. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell TJ, Deane N, Platt FM, et al. bcl-2-Immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 29.Perego P, Righetti S, Supino R, et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis. 1997;2:540–8. doi: 10.1023/a:1026442716000. [DOI] [PubMed] [Google Scholar]
- 30.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–10. [PubMed] [Google Scholar]
- 31.Campana D, Coustan-Smith E, Manabe A, et al. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993;81:1025–31. [PubMed] [Google Scholar]
- 32.Campos L, Sabido O, Sebban C, et al. Expression of BCL-2 proto-oncogene in adult acute lymphoblastic leukemia. Leukemia. 1996;10:434–8. [PubMed] [Google Scholar]
- 33.Osorio LM, De Santiago A, guilar-Santelises Ha M, Mellstedt K, Jondal M. CD6 ligation modulates the Bcl-2/Bax ratio and protects chronic lymphocytic leukemia B cells from apoptosis induced by anti-IgM. Blood. 1997;89:2833–41. [PubMed] [Google Scholar]
- 34.Gala JL, Vermylen C, Cornu G, et al. High expression of bcl-2 is the rule in acute lymphoblastic leukemia, except in Burkitt subtype at presentation, and is not correlated with the prognosis. Ann Hematol. 1994;69:17–24. doi: 10.1007/BF01757343. [DOI] [PubMed] [Google Scholar]
- 35.Coustan-Smith E, Kitanaka A, Pui CH, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87:1140–6. [PubMed] [Google Scholar]
- 36.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–32. [PubMed] [Google Scholar]
- 37.Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–41. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 38.Rai KR, Moore J, Wu J, Novick SC, O’Brien SM. Effect of the addition of oblimersen (Bcl-2 antisense) to fludarabine/cyclophosphamide for relapsed/refractory chronic lymphocytic leukemia (CLL) on survival in patients who achieve CR/nPR: Five-year follow-up from a randomized phase III study [abstract] J Clin Oncol. 2008;26:7008. [Google Scholar]
- 39.Emi M, Kim R, Tanabe K, Uchida Y, Toge T. Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res. 2005;7:R940–52. doi: 10.1186/bcr1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiuri MC, LeToumelin G, Criollo A, et al. Functional and physical interaction between Bcl-XL and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim R, Emi M, Matsuura K, Tanabe K. Antisense and nonantisense effects of antisense Bcl-2 on multiple roles of Bcl-2 as a chemosensitizer in cancer therapy. Cancer Gene Ther. 2007;14:1–11. doi: 10.1038/sj.cgt.7700986. [DOI] [PubMed] [Google Scholar]
- 44.Jahrsdorfer B, Jox R, Muhlenhoff L, et al. Modulation of malignant B cell activation and apoptosis by bcl-2 antisense ODN and immunostimulatory CpGODN. J Leukoc Biol. 2002;72:83–92. [PubMed] [Google Scholar]
- 45.Zou W, Machelon V, Coulomb-L’ Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–20. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 48.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy {+/−} oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old [abstract] J Clin Oncol. 2007;25:7012. [Google Scholar]
- 49.Rudin CM, Salgia R, Wang X, et al. Randomized phase II study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–6. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group [see comment] J Clin Oncol. 2006;24:4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 51.Cao XX, Mohuiddin I, Ece F, McConkey DJ, Smythe WR. Histone deacetylase inhibitor downregulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am J Respir Cell Mol Biol. 2001;25:562–8. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 52.Khan SB, Maududi T, Barton K, Ayers J, Alkan S. Analysis of histone deacetylase inhibitor, depsipeptide ( FR901228), effect on multiple myeloma. Br J Haematol. 2004;125:156–61. doi: 10.1111/j.1365-2141.2004.04882.x. [DOI] [PubMed] [Google Scholar]
- 53.Delia D, Aiello A, Formelli F, et al. Regulation of apoptosis induced by the retinoid N-(4-hydroxy-phenyl) retinamide and effect of deregulated bcl-2. Blood. 1995;85:359–67. [PubMed] [Google Scholar]
- 54.Kang MH, Wan Z, Kang Y, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–95. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- 55.Rosato RR, Almenara JA, Kolla SS, et al. Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- 56.Jones L. Gossypol and some other terpenoids, flavonoids, and phenols that affect quality of cottonseed protein. J Am Oil Chemists Soc. 1979;56:727–30. [Google Scholar]
- 57.MacVicar GR, Kuzel TM, Curti BD, et al. An open-label, multicenter, phase I/II study of AT-101 in combination with docetaxel (D) and prednisone (P) in men with hormone refractory prostate cancer (HRPC) [abstract] J Clin Oncol. 2008;26:16048. [Google Scholar]
- 58.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 59.Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–86. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 60.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2006;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 62.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang MH, Kang YH, Szymanska B, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110:2057–66. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 65.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 66.Shoemaker AR, Oleksijew A, Bauch J, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res. 2006;66:8731–9. doi: 10.1158/0008-5472.CAN-06-0367. [DOI] [PubMed] [Google Scholar]
- 67.Lin X, Morgan-Lappe S, Huang X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X(L) inhibitorABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 68.Morales MC, Perez-Yarza G, Nieto-Rementeria N, et al. Intracellular glutathione levels determine cell sensitivity to apoptosis induced by the antineoplasic agent N-(4-hydroxyphenyl) retinamide. Anticancer Res. 2005;25:1945–51. [PubMed] [Google Scholar]
- 69.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 70.Wilson WH, Tulpule A, Levine AM, et al. A phase1/2a study evaluating the safety, pharmacokinetics, and efficacy of ABT-263 in subjects with refractory or relapsed lymphoid malignancies [abstract] Blood. 2008;110:1371. [Google Scholar]
- 71.Wilson WH, Czuczman MS, LaCasce AS, et al. A phase1 study evaluating the safety, pharmacokinetics, and efficacy of ABT-263 in subjects with refractory or relapsed lymphoid malignancies [abstract] J Clin Oncol. 2008;26S:8511. [Google Scholar]
- 72.Roberts A, Gandhi L, O’Connor OA, et al. Reduction in platelet counts as a mechanistic biomarker and guide for adaptive dose-escalation in phase I studies of the Bcl-2 family inhibitorABT-263 [abstract] J Clin Oncol. 2008;26:3542. [Google Scholar]
- 73.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–9. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 76.Haura EB, Williams CA, Chiappori AA, et al. A phase I trial of the small molecule pan-bcl-2 inhibitor obatoclax (GX15-070) in combination with docetaxel in patients with relapsed non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2008;26:19035. [Google Scholar]
- 77.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–9. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manero F, Gautier F, Gallenne T, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 79.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284–92. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 80.Tian D, Das SG, Doshi JM, Peng J, Lin J, Xing C. sHA 14-1, a stable and ROS-free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer Lett. 2008;259:198–208. doi: 10.1016/j.canlet.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campo ML, Kinnally KW, Tedeschi H. The effect of antimycin A on mouse liver inner mitochondrial membrane channel activity. J Biol Chem. 1992;267:8123–7. [PubMed] [Google Scholar]
- 82.Park WH, Han YW, Kim SH, Kim SZ. An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J Cell Biochem. 2007;102:98–109. doi: 10.1002/jcb.21280. [DOI] [PubMed] [Google Scholar]
- 83.Hao JH, Yu M, Liu FT, Newland AC, Jia L. Bcl-2 inhibitors sensitize tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by uncoupling of mitochondrial respiration in human leukemic CEM cells. Cancer Res. 2004;64:3607–16. doi: 10.1158/0008-5472.CAN-03-3648. [DOI] [PubMed] [Google Scholar]
- 84.Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells toApo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis. 2005;10:1411–8. doi: 10.1007/s10495-005-2490-y. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz PS, Manion MK, Emerson CB, et al. 2-Methoxy antimycin reveals a unique mechanism for Bcl-xL inhibition. Mol Cancer Ther. 2007;6:2073–80. doi: 10.1158/1535-7163.MCT-06-0767. [DOI] [PubMed] [Google Scholar]
- 86.Degterev A, Lugovskoy A, Cardone M, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–82. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 87.Moore J, Seiter K, Kolitz J, et al. A phase II study of Bcl-2 antisense (oblimersen sodium) combined with gemtuzumab ozogamicin in older patients with acute myeloid leukemia in first relapse. Leuk Res. 2006;30:777–83. doi: 10.1016/j.leukres.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 88.Tolcher AW, Chi K, Kuhn J, et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:3854–61. doi: 10.1158/1078-0432.CCR-04-2145. [DOI] [PubMed] [Google Scholar]
- 89.Margolin K, Synold TW, Lara P, et al. Oblimersen and á-interferon in metastatic renal cancer: a phase II study of the California Cancer Consortium. J Cancer Res Clin Oncol. 2007;133:705–11. doi: 10.1007/s00432-007-0200-6. [DOI] [PubMed] [Google Scholar]
- 90.Badros AZ, Goloubeva O, Rapoport AP, et al. Phase II study of G3139, a Bcl-2 antisense oligonucleotide, in combination with dexamethasone and thalidomide in relapsed multiple myeloma patients. J Clin Oncol. 2005;23:4089–99. doi: 10.1200/JCO.2005.14.381. [DOI] [PubMed] [Google Scholar]
- 91.Knox J, Chen X, Feld R, et al. A phase I-II study of oblimersen sodium (G3139, Genasense) in combination with doxorubicin in advanced hepatocellular carcinoma (NCI # 5798) Invest New Drugs. 2008;26:193–4. doi: 10.1007/s10637-007-9104-1. [DOI] [PubMed] [Google Scholar]