Abstract
Increasing evidence suggests that FOXO1 possesses a tumor suppressor function. Inactivation of FOXO1 has been documented in many types of human cancer, and restoring the activity of FOXO1 holds promise for cancer treatment. In this study, we identified a FOXO1-derived peptide termed FO1-6nls that inhibits cyclin-dependent kinases 1 and 2 (CDK1/2)-mediated phosphorylation of FOXO1 at the serine 249 residue in vitro and in vivo. Overexpression of FO1-6nls in prostate cancer (PCa) cells not only blocked CDK1-induced cytoplasmic localization of FOXO1 but also augmented FOXO1's transcriptional activity. This effect of FO1-6nls requires its binding to CDK1 and CDK2. Moreover, the ectopic expression of FO1-6nls inhibited the growth of PTEN-positive DU145 PCa cells. Importantly, the growth-inhibitory function of FO1-6nls is dependent on FOXO1. Finally, the ectopic expression of FO1-6nls overcame CDK1-mediated inhibition of FOXO1-induced apoptosis of PCa cells. These results indicate that the FOXO1-derived peptide FO1-6nls can restore FOXO1's tumor suppressor function by specifically opposing CDK1/2-mediated phosphorylation and inhibition of FOXO1 and hence may have a therapeutic potential for the treatment of PCa.
Introduction
FOXO transcription factors in humans, including FOXO1 (FKHR), FOXO3a (FKHRL1), FOXO4 (AFX), and FOXO6, play important roles in regulating many cancer-related functions, such as apoptosis, cell cycle arrest, DNA damage repair, and/or oxidative stress resistance, depending on the cellular context [1]. Activation of FOXOs induces transcription of the proapoptotic gene, FasL, by binding to the three consensus FOXO binding sites in the FasL promoter [2]. Indeed, forced expression of a constitutively active form of FOXO protein induces apoptosis in cerebellar granule neurons through a mechanism depending, at least in part, on FasL up-regulation [2]. Through an unbiased gene profiling study, tumor necrosis factor-related apoptosis inducing ligand (TRAIL), another death receptor ligand, was identified as a FOXO1-regulated gene in prostate cancer (PCa) cells [3]. In addition to the death receptor ligands, FOXO proteins have been shown to be involved in transactivation of the proapoptotic gene Bim [4–6]. In addition, members of the FOXO family regulate G1 cell cycle progression by upregulating the cyclin-dependent kinase inhibitor p27KIP1 and downregulating cyclin D1 and D2 [7–10]. FOXO transcription factors also play a role in the surveillance of DNA damage by transcriptionally regulating expression of the DNA damage response gene Gadd45 and scavenger genes, such as cytosolic catalase and superoxide dismutase [11–14]. These findings suggest that members of the FOXO family function as tumor suppressors. Support for this hypothesis also comes from data demonstrating that activation of FOXO1 induces apoptosis in PCa cells [3,8,15–17]. This observation further suggests that FOXO1 might be a potential target for PCa therapy.
The importance of FOXO proteins in human cancers is further revealed by the fact that their functions are often disrupted by oncogenic signaling pathways. The PTEN (also known as MMAC1/TEP1) tumor suppressor gene is frequently mutated in a variety of human cancers including PCa [18–20]. Biochemical and genetic studies demonstrate that PTEN acts as a tumor suppressor protein by primarily antagonizing the phosphoinositide 3-kinase (PI3K)/Akt pathway [21]. Activation of Akt due to loss of PTEN or treatment of cells with growth factors leads to phosphorylation and inhibition of FOXO proteins [2,22–24]. In addition, a recent study demonstrated that hemizygous and homozygous deletions in the FOXO1 gene locus are present in approximately 30% of PCa cell lines, xenografts, and a cohort of PCa specimens examined [25]. Thus, the function of FOXO1 is frequently abolished through various mechanisms in human PCas, suggesting that FOXO1 is a PCa-relevant tumor suppressor protein.
The tumor suppressor function of FOXO1 can also be inhibited by other protein kinase pathways [1]. CDK1 and CDK2, two cell cycle regulatory protein kinases that are important for cell cycle transitions from G1 to S and G2 to M, respectively, interact directly with and phosphorylate FOXO1 at the serine 249 (S249) residue in PCa cells [26,27]. This phosphorylation of FOXO1 attenuates the tumor suppressor function of FOXO1 and thereby favors PCa cell growth and survival. In this study, we identified a FOXO1-derived 70-amino acid peptide that antagonizes CDK1- and CDK2-mediated phosphorylation and inhibition of FOXO1. We further demonstrated that expression of this peptide not only restores the tumor suppressor function of FOXO1 but also inhibits growth and survival of PCa cells.
Materials and Methods
Plasmids, Small Interference RNA, and Chemicals
Plasmids for FLAG-tagged wild type (FOXO1-WT) and Akt phosphorylation-resistant mutant (FOXO1-A3) of FOXO1 and the luciferase reporter construct, 3xIRS, which contains three copies of the FOXO response element in the promoter of the IGFBP1 gene, were described previously [26]. The V5-tagged FO1-6nls (amino acids 211–280) that encompasses the intact nuclear localization signal (nls) was amplified by polymerase chain reaction using gene-specific primers (forward 5′-CACCATGAATTCAATTCGTCATAATCTGTCC-3′, reverse 5′-GCCAGACTGGAGAGATGCTTT-3′) and cloned in the pcDNA3.1D/V5-His vector (Invitrogen, Carlsbad, CA). Plasmids for active mutants of CDK1 (CDK1-AF) and CDK2 (CDK2-AF) and amino acid substitution mutant of FOXO1-S249A/S298A were described previously [26,27]. Various glutathione S-transferase (GST)-FOXO1 fusion constructs were generated with the backbone vector pGEX-4T-1 (GE Healthcare, Piscataway, NJ) as described [26]. The SMART pools of small interference RNAs (siRNAs) for human FOXO1 (5′-CCAGGCAUCUCAUAACAAA-3′; 5′-CCAGAUGCCUAUACAAACA-3′) and FOXO3a (5′-CGAAUCAGCUGACGACAGU-3′; 5′-GUACUCAACUAGUGCAAAC-3′) and nonspecific siRNA (5′-UAGCGACUAAACACAUCAA-3′) were purchased from Dharmacon (Lafayette, CO). The PI3K inhibitor LY294002 was purchased from Invitrogen. The working concentration of LY294002 was 20 µM.
Cell Culture, Transfection, and Luciferase Reporter Assay
The PCa cell lines LNCaP, DU145, and PC-3 were purchased from the American Type Culture Collection (Manassas, VA). The immortalized prostatic epithelial cell line BPH-1 was kindly provided by Dr S. W. Hayward (Vanderbilt University Medical Center). Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (Hyclone, South Logan, UT), 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were maintained at 37°C and 5% of CO2. Transfections were performed by electroporation using a Gene Pulse Electroporator (BTX, Holliston, MA). Approximately 50% to 90% transfection efficiencies were routinely achieved. For luciferase reporter assays, cells were harvested at 36 to 48 hours after transfection, and cell lysates were subjected to the measurement of activities of firefly and Renilla luciferases using a dual-luciferase kit (Promega, Madison, WI). Renilla luciferase activities in cells were used as an internal control. Both firefly and Renilla luciferase activities were measured using the Lumat LB 9507 luminometer (Berthold Technologies, Oak Ridge, TN).
Immunoprecipitation and Western Blot
Protein immunoprecipitations were carried out using an immunoprecipitation kit (Roche Applied Science, Indianapolis, IN). Western blot was performed as described [28]. Briefly, protein samples were prepared by lysing cells in modified radioimmune precipitation assay buffer (1x phosphate-buffered saline [PBS], 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail [Sigma-Aldrich, St Louis, MO]). Equal amounts of protein (50–100 µg) from cell lysates were denatured in the sample buffer, subjected to SDS-PAGE gels, and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The filters were immunoblotted with specific primary antibodies, horseradish peroxidase-conjugated secondary antibodies, and visualized by enhanced chemiluminescence (GE Healthcare). A polyclonal antibody against phosphorylated S249 of FOXO1 (S249-p) was raised by immunizing rabbits with the phosphorylated peptide NPEGGKSGKpSPRRRAAS and purified over a peptide-affinity column as described [26]. Other protein-specific antibodies were as follows: anti-FOXO1, anti-CDK1, anti-cyclin B1, and anti-poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology, Danvers, MA); anti-Erk2 mAb (Santa Cruz Biotechnology, Santa Cruz, CA); anti-V5 (Invitrogen); anti-FLAG (M2) (Sigma-Aldrich); anti-FOXO3a (Millipore, Billerica, MA); and anti-Bim (Chemicon, Billerica, MA).
Immunofluorescent Chemistry and Confocal Microscopy
Cells grown on coverslips (Eppendorf Scientific, Inc, Hauppauge, NY) were washed briefly in 1x PBS and fixed for 20 minutes in 2% paraformaldehyde (Thermo Fisher, Pittsburgh, PA) in 1x PBS. Cells were permeabilized by incubating with 0.2% Triton X-100 for 15 minutes. Cells were washed three times in 1x PBS and incubated in blocking buffer (5% goat serum in 1x PBS) for 1 hour at room temperature. Cells were incubated with a mouse anti-FLAG monoclonal antibody (1:1000) for 2 hours at room temperature. After washing with 1x PBS three times for 5 minutes each, cells were incubated for 1 hour at room temperature with the secondary Alexa Fluor 488-labeled goat antimouse IgG conjugate (Invitrogen) (1:5000) prepared in blocking buffer for 1 hour at room temperature. Coverslips were washed with three changes of 1x PBS for 5 minutes each and mounted in VECTASHIELD Mounting Medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Cells were analyzed with a laser-scanning microscope (FluoView 1000; Olympus, Center Valley, PA). An argon ion laser was used to excite Alexa Fluor 488, and a UV laser was used to excite 4′,6-diamidino-2-phenylindole.
Apoptosis and Cell Viability Assay
Apoptosis analysis was performed as described [27]. Briefly, cells were stained with a solution containing 0.1% Triton X-100, 20 µg/ml of propidium iodide, and 50 µg/ml of RNase A. Hypodiploid cells were determined by flow cytometry using a FACScan (Becton-Dickinson, Franklin Lakes, NJ). The percentage of sub-G1 cells was analyzed by BD CellQuest Pro software. Cell viability was assessed by means of trypan blue exclusion using Vi-Cell cell viability analyzer (Beckman Coulter, Brea, CA).
GST Fusion Protein Purification and GST Pull-down Assay
The plasmids expressing GST fusion genes were constructed and transformed into Escherichia coli BL21 star strains as described previously [26]. For protein purification, bacteria were grown to an optical density of 0.6 at 600 nm in Luria-Bertani medium, induced at 37°C with 0.1 mM of isopropyl-1-thio-D-galactopyranoside, and cultivated for 3 hours. Bacteria were then pelleted, resuspended in lysis/pull-down buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 1 mM phenylmethyl-sulfonyl-fluoride, 5 mM benzamidine, and bacterial protein inhibitors), and sonicated. Insoluble materials were removed by centrifugation. Glutathione S-Sepharose 4B beads (GE Healthcare) were added to the cleared lysate, incubated overnight at 4°C, and washed three times with the pull-down buffer. GST fusion proteins on beads were estimated by comparison to GST protein standard after SDS-PAGE and Coomassie blue staining. In vitro transcription and translation of CDK1 protein was performed according to the manufacturer's instruction (TnT Quick Coupled Transcription/Translation Systems; Promega). GST pull-down assays were carried out as described previously [26].
In Vitro Kinase Assay
Kinase assays were carried out in the presence of [γ-32P] ATP by using an in vitro kinase buffer system obtained from Cell Signaling Technology as described [26]. The reconstituted CDK1/cyclin B1 and CDK2/cyclin E complexes obtained from Cell Signaling Technology were incubated with various substrates at 30°C. Thirty minutes after reaction, proteins were subjected to SDS-PAGE and autoradiography.
Results
Identification of a Peptide of FOXO1 That Inhibits CDK1/2 Phosphorylation of FOXO1 In Vitro
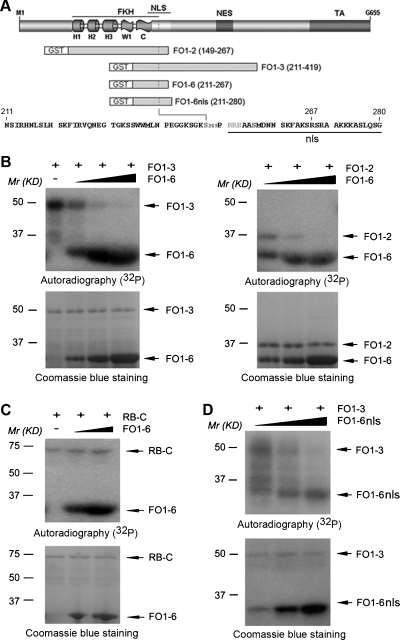
Previous studies show that CDK1 and CDK2 phosphorylate FOXO1 at S249 and that S249 phosphorylation diminishes FOXO1-mediated gene transactivation and inhibition of PCa cell growth and survival [26,27]. In the present study, we aimed to determine whether FOXO1-derived small peptides could function as a decoy that interferes with CDK1- and CDK2-mediated phosphorylation of FOXO1 at S249. Similar to the previous reports [26,27], two S249-containing fragments of FOXO1, FO1–3 (amino acids 211–419) and FO1–2 (amino acids 149–267) (Figure 1A), were phosphorylated by CDK2/cyclin E in vitro (Figure 1B). Importantly, CDK2-mediated phosphorylation of FO1–3 and FO1–2 was inhibited, in a dose-dependent manner, by the small peptide FO1–6 (amino acids 211–267), an S249-containing fragment of FOXO1 that is overlapped by FO1–3 and FO1–2 (Figure 1, A and B). As a control, addition of the same concentrations of GST proteins has no inhibitory effect on CDK2-mediated phosphorylation of FO1–2 and FO1–3 (data not shown). The COOH-terminus of the retinoblastoma (RB) protein (RB-C) is a well-known substrate of CDK2. However, CDK2-mediated phosphorylation of RB-C was not affected by FO1–6 (Figure 1C). These data suggest that the effect of FO1-6nls is FOXO1-specific. Similarly, CDK1-mediated phosphorylation of FO1–2 and FO1–3 was also inhibited by FO1–6 (data not shown). These results indicate that the FOXO1-derived small peptide FO1–6 can specifically inhibit CDK1/2-mediated phosphorylation of FOXO1 in vitro.
Figure 1.
FOXO1-derived peptides FO1–6 and FO1-6nls inhibit CDK2-mediated phosphorylation of FOXO1 in vitro. (A) A schematic diagram showing GST-FOXO1 recombinant proteins and the amino acid sequence of the small peptide FO1-6nls that contains the S249 phosphorylation site. FKH indicates forkhead domain; NLS/nls, nuclear localization signal; NES, nuclear export signal; TA, transactivation domain. (B) Reconstituted CDK2/cyclin E kinase assays using FO1–3 plus FO1–6 (left panels) or FO1–2 plus FO1–6 (right panels) as substrates. Mr(KD), relative molecular weight in kilodaltons. (C) Reconstituted CDK2/cyclin E kinase assays using the RB-C protein and FO1–6 as substrates. (D) Reconstituted CDK2/cyclin E kinase assays with substrates of FO1–3 and FO1-6nls. All the experiments were repeated at least two times.
FO1-6nls Inhibits FOXO1 Phosphorylation at S249 in PCa Cells
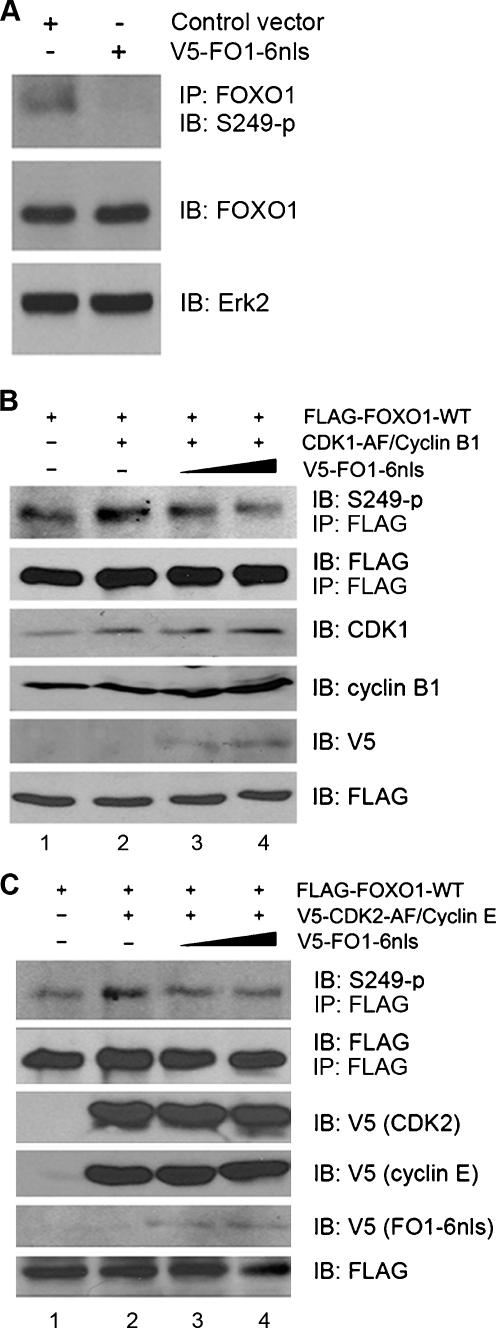
It has been shown recently that forced expression of CDK1 or CDK2 inhibits the transcriptional activity of the nuclear form of FOXO1, FOXO1-A3, in which three Akt phosphorylation residues are converted to alanine [26,27]. This finding suggests that CDK1/2 target FOXO1 in the nucleus. To examine the effect of the small peptide FO1–6 on S249 phosphorylation of FOXO1 in cells, we engineered a V5-tagged nucleus-targeting expression vector for this peptide by including 13 additional amino acids beyond the COOH-terminus of FO1–6, which constitute an essential portion of the functional nuclear localization signal (nls) in FOXO1 (Figure 1A) [29]. It was thus termed V5-FO1-6nls to reflect the nature of this construct that contains the intact nls. To determine the effect of FO1-6nls expression on S249 phosphorylation of endogenous FOXO1, the DU145 PCa cell line was used because it expresses high levels of endogenous FOXO1 [3]. As shown in Figure 2A, S249 phosphorylation of endogenous FOXO1 was completely abolished by the expression of FO1-6nls in these cells. In agreement with the previous reports [26,27], the ectopic expression of constitutively active CDK1 and CDK2 increased S249 phosphorylation of ectopically expressed FOXO1 in LNCaP PCa cells (Figure 2, B and C, lane 2 vs 1), in which expression of endogenous FOXO1 is relatively low [3]. Importantly, CDK1/2-induced increase in FOXO1 phosphorylation at S249 was inhibited by FO1-6nls in a dose-dependent manner (Figure 2, B and C, lane 3, 4 vs 2). These results are consistent with the finding from the in vitro kinase assay that in vitro phosphorylation of FO1–3 mediated by the constituted CDK2/cyclin E complex was inhibited by FO1-6nls (Figure 1D). These data indicate that FO1-6nls expression overcomes CDK1- and CDK2-mediated phosphorylation of FOXO1 at S249 in PCa cells.
Figure 2.
FO1-6nls inhibits phosphorylation of FOXO1 at S249 in PCa cells. (A) DU145 cells grown in the exponential phase were harvested and lysed for immunoprecipitation with anti-FOXO1 antibodies and Western blot analysis with antibodies as indicated. Immunoblot analysis of Erk2 was used as a loading control. (B, C) LNCaP cells were transfected with FLAG-tagged FOXO1 plus cyclin B1 and CDK1-AF (B) or cyclin E and CDK2-AF (C). At 48 hours after transfection, cells were lysed and subjected to immunoprecipitation with anti-FLAG (M2) antibodies. Immunoprecipitates were analyzed by Western blot using antibodies as indicated. Similar results were obtained from at least two independent experiments.
FO1-6nls Overcomes CDK-Mediated Inhibition of FOXO1's Transcriptional Activity and Nuclear Exclusion of FOXO1
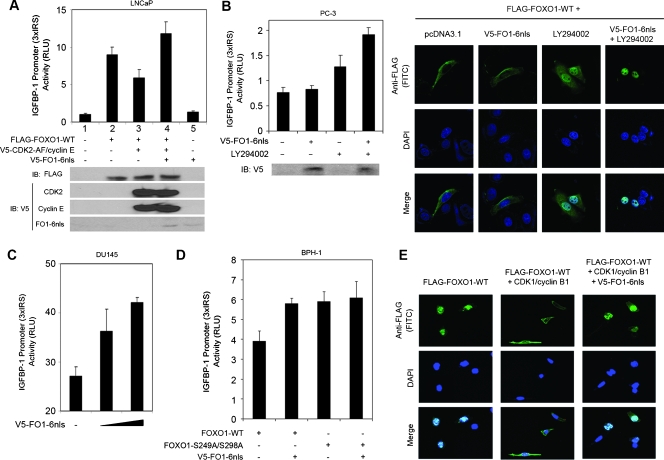
It has been reported previously that CDK1- and CDK2-mediated phosphorylation of FOXO1 at S249 inhibits FOXO1's transcriptional activity [26,27]. Next, we sought to determine whether expression of FO1-6nls affects CDK1/2 inhibition of FOXO1 activity. As expected, forced expression of constitutively active CDK2 (CDK2-AF) and cyclin E inhibited the transcriptional activity of FOXO1 in LNCaP cells as demonstrated by luciferase reporter gene assays (Figure 3A). However, this effect of CDK2/cyclin E was completely reversed by the expression of FO1-6nls (Figure 3A). It is worth noting that transfection of FO1-6nls alone resulted in a limited increase in the transcriptional activity of endogenous FOXO1 (Figure 3A, lane 5 vs 1). This is presumably because of the low level of endogenous FOXO1 protein [30] and the inhibition of FOXO1 caused by Akt-mediated phosphorylation in this PTEN-mutated PCa cell line [18]. A similar result was observed in PC-3 cells (Figure 3B, left), another PCa cell line that expresses a low level of endogenous FOXO1 [3] and lacks PTEN [18]. In line with this notion, pretreatment of PC-3 cells with the PI3K inhibitor LY294002 increased the transcriptional activity of FOXO1 proteins, and this effect was further enhanced in cells transfected with FO1-6nls (Figure 3B, left). Similar to a previous report [8], wild-type FOXO1 proteins were primarily localized in the cytoplasm of PTEN-null PC-3 cells cultured in media containing growth factors (Figure 3B, right, column 1). In agreement of the gene reporter results (Figure 3B, left), transfection of FO1-6nls had a minimal effect on nuclear localization of FOXO1 under these conditions (Figure 3B, right, column 2). As expected, most FOXO1 proteins were localized in the nucleus of PC-3 after LY294002 treatment (Figure 3B, right, column 3). Importantly, FOXO1 proteins were primarily localized in the nucleus of PC-3 cells treated with FO1-6nls and LY294002 (Figure 3B, right, column 4). Thus, similar to the results obtained from the gene reporter assays (Figure 3B, left), the effect of FO1-6nls on the cellular localization of FOXO1 proteins is markedly affected by the activation of PI3K/Akt. Consistent with these observations, the ectopic expression of FO1-6nls alone was sufficient to enhance the transcriptional activity of endogenous FOXO proteins in a dose-dependent manner in PTEN-positive DU145 cells (Figure 3C). These data indicate that the expression of FO1-6nls increases FOXO1 transcriptional activity, and this effect can be attenuated by loss of PTEN and/or activation of Akt.
Figure 3.
FO1-6nls augments FOXO1's transcriptional activity and antagonizes CDK1-induced nuclear exclusion of FOXO1. (A) The antagonistic effect of FO1-6nls on CDK2-mediated inhibition of FOXO1's transcriptional activity. LNCaP cells were transfected with plasmids as indicated. At 36 hours after transfection, luciferase activities were measured and analyzed as described in the Materials and Methods section. Error bars, SD of three independent experiments. (B) Effect of FO1-6nls on the transcriptional activity (left) and cellular localization (right) of FOXO proteins in PTEN-null PC-3 cells. Left, PC-3 cells were transfected with FO1-6nls and/or pretreated with the PI3K inhibitor LY294002, and luciferase activities were measured as described in A. Right, PC-3 cells were transfected with plasmids as indicated and plated in serum-containing media. At 24 hours after transfection, cells were treated with LY294002 (20 µM) or vehicle for 12 hours. Cells were then subjected to immunofluorescent chemistry with antibodies indicated. (C) Effect of FO1-6nls on the transcriptional activity of endogenous FOXO1 proteins in PTEN-positive DU145 cells. Cells were transfected with the reporter gene 3xIRS-Luc and different amount of FO1-6nls. At 36 hours after transfection, luciferase activities were measured and analyzed as described in A. Error bars, SD of three independent experiments. (D) BPH-1 cells were transfected with plasmids as indicated and subjected to luciferase activity measurement as in A. (E) DU145 cells transfected with plasmids as indicated and plated in serum-free media. At 48 hours after transfection, cells were subjected to immunofluorescent chemistry with antibodies indicated.
Next, we sought to determine whether FO1-6nls-induced activation of FOXO1 is mediated by its inhibition of CDK phosphorylation of FOXO1. To this end, BPH-1 cells were used because, in this cell line, CDKs are highly active owing to the inactivation of RB and p53 mediated by SV40 large T antigen [31]. Cotransfection of FO1-6nls with FOXO1 increased the transcriptional activity of wild-type FOXO1 in BPH-1 cells (Figure 3D). The expression of FO1-6nls failed to increase the transcription activity of a mutant of FOXO1 that is resistant to CDK-mediated phosphorylation and inhibition of FOXO1 [26,27]. As expected, this mutant exhibited a higher basal transcriptional activity than the wild-type FOXO1 (Figure 3D). These results suggest that FO1-6nls acts as a “decoy” molecule that activates FOXO1's transcriptional activity by antagonizing CDK1/2-mediated phosphorylation of FOXO1.
Because CDK2 inhibits FOXO1 by promoting FOXO1 phosphorylation and nuclear exclusion in PTEN-positive DU145 PCa cells [26], we sought to determine whether the expression of CDK1 also affects cellular localization of the FOXO1 protein and whether this process could be blocked by the expression of FO1-6nls. In accordance with the previous report [26], ectopically expressed wild-type FOXO1 proteins were mainly localized in the nucleus of DU145 cells when they were cultured in growth factor-depleted conditions (Figure 3E). Similar to the effect of CDK2 [26], overexpression of constitutively active CDK1 (CDK1-AF) and cyclin B1 resulted in the nuclear exclusion of FOXO1 in DU145 cells (Figure 3E). Importantly, cotransfection of FO1-6nls blocked CDK1-induced nuclear exclusion of wild-type FOXO1 (Figure 3E). Thus, FO1-6nls prohibits nuclear exportation of FOXO1 induced by CDK1 in DU145 PCa cells. This result is consistent with the FO1-6nls-enhanced transcriptional activity of FOXO1 in this cell line.
FO1-6nls Binds to CDK1 and CDK2 Proteins
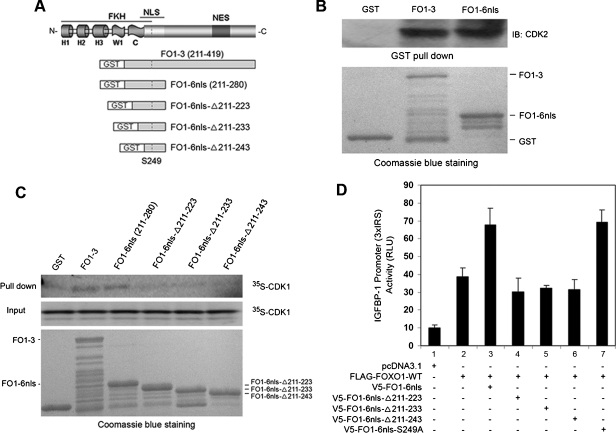
To further explore the molecular mechanism underlying the antagonistic effect of FO1-6nls on CDK1/2-mediated phosphorylation of FOXO1, we sought to determine whether FO1-6nls binds to CDK1 and CDK2. Toward this end, bacterially expressed GST recombinant FO1-6nls and FO1–3 fusion proteins (Figure 4A) were purified, and GST pull-down assays were performed by incubating the fusion proteins with whole-cell lysate of BPH-1 prostatic cells. Consistent with previous reports [26,27], FO1–3 bound to CDK2 (Figure 4B). FO1-6nls also bound toCDK2 in a manner similar to FO1–3 (Figure 4B). No binding of CDK2 to GST proteins was detected in these experiments (Figure 4B), suggesting that the binding of FO1-6nls to CDK2 is specific. In vitro protein binding assays demonstrated that FO1-6nls binds to the CDK1 protein produced from in vitro transcription and translation (Figure 4C), suggesting that FO1-6nls binds directly to CDK1. Different from FO1-6nls, however, three NH2-terminal truncated mutants of FO1-6nls, including FO1-6nls-Δ211–223, FO1-6nls-Δ211–233 and FO1-6nls-Δ211–243, failed to bind to CDK1 (Figure 4C). This result suggests that the 13 amino acids (211–223) in the NH2-terminus of FO1-6nls are important for the binding of FO1-6nls to CDK1.
Figure 4.
The 13 amino acids in the NH2-terminus of FO1-6nls are important for the binding of FO1-6nls to CDK1 and FO1-6nls-induced activation of FOXO1. (A) Schematic diagram depicting GST-FOXO1 recombinant peptides. (B) GST pull-down assay with GST-FOXO1 fusion proteins FO1–3 and FO1-6nls using BPH-1 prostatic cell lysate. (C) Binding assays with CDK1 proteins produced by in vitro transcription and translation and GST-FOXO1 recombinant peptides shown in A. Top, Autoradiography of 35S-labeled CDK1 protein bound to GST-FOXO1 recombinant peptides. Middle, Input of 35S-labeled CDK1 protein used in the in vitro protein binding assays. Bottom, GST and GST-FOXO1 recombinant peptides for in vitro protein binding assays indicated by Coomassie blue staining. (D) DU145 cells were transfected with plasmids as indicated. At 36 hours after transfection, cells were harvested and lysed for luciferase activity measurement. Error bars, SD of three independent experiments.
Thirteen Amino Acids in the NH2-Terminus of FO1-6nls Are Important for the Antagonistic Effect of FO1-6nls on CDK-Mediated Inhibition of FOXO1
Next, we sought to determine whether the 13-amino acid motif is important for FO1-6nls-mediated activation of FOXO1. Unlike FO1-6nls, expression of all three truncation mutants of FO1-6nls, including FO1-6nls-Δ211–223, FO1-6nls-Δ211–233, and FO1-6nls-Δ211–243, failed to increase FOXO1 transcriptional activity in DU145 cells (Figure 4D). Because all these truncation mutants contain the CDK phosphorylation receptor site S249 (Figure 4A), these results suggest that the presence of the CDK phosphorylation residue in FO1-6nls may not be important for its decoy function. Indeed, this notion was further supported by the result that the expression of FO1-6nls-S249A, a substitution mutant of FO1-6nls in which the S249 residue was converted into alanine, increased the transcriptional activity of FOXO1 as effectively as the unmutated FO1-6nls did (Figure 4D, column 7 vs 3). These observations suggest that the binding of FO1-6nls to CDK1 and CDK2, but not the phosphorylation acceptor residue S249 in FO1-6nls, is important for its antagonistic effect on CDK1- and CDK2-mediated inhibition of FOXO1.
FO1-6nls-Mediated Inhibition of PCa Cell Growth Is Dependent on FOXO1
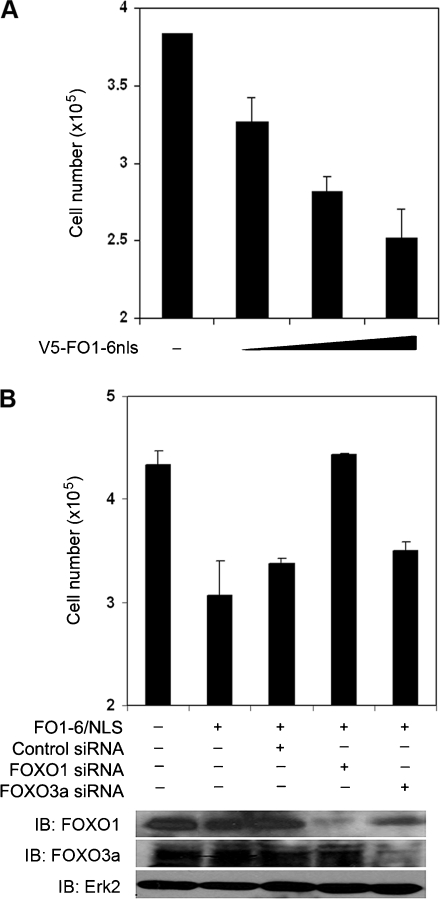
As shown in Figure 3C, the expression of FO1-6nls alone enhanced transcriptional activity of endogenous FOXO proteins in PTEN-positive DU145 cells. This observation prompted us to evaluate the antitumor effect of FO1-6nls in this cell line without exogenous expression of FOXO1. As demonstrated in Figure 5A, expression of FO1-6nls inhibited growth of DU145 cells in a dose-dependent manner. To test the possibility that expression of FO1-6nls may affect the growth of PCa cells by acting on other FOXO proteins, we individually knocked down FOXO1 and FOXO3a, two members of the FOXO family that are predominantly expressed in PCa cell lines [3,26]. As expected, treatment of cells with a nonspecific control siRNA had little or no effect on FO1-6nls-mediated inhibition of cell growth (Figure 5B). Silencing of FOXO1, but not FOXO3a by a pool of gene-specific siRNAs, completely abolished FO1-6nls-mediated inhibition of DU145 cell growth, suggesting that the inhibitory effect of FO1-6nls on the growth of DU145 PCa cells is mediated by FOXO1. This observation is consistent with the previous finding that the consensus CDK phosphorylation residue is present in FOXO1 (S249) but not in FOXO3a and FOXO4 proteins and that forced expression of CDK2/cyclin E inhibits FOXO1's but not other FOXO protein's transcriptional activity [26].
Figure 5.
FO1-6nls expression inhibits PCa cell growth in a FOXO1-dependent manner. (A) DU145 cells were transfected with different amounts of FO1-6nls plasmids. At 48 hours after transfection, cells were harvested, and viable cells were counted as described in the Materials and Methods section. (B) DU145 cells were transfected with plasmids and control or gene-specific siRNAs as indicated. At 48 hours after transfection, cells were harvested and subjected to cell viability measurement or Western blot analysis. Data are shown as mean ± SD of three independent experiments.
Expression of FO1-6nls Antagonizes CDK1-Mediated Inhibition of FOXO1-Induced Apoptosis of PCa Cells
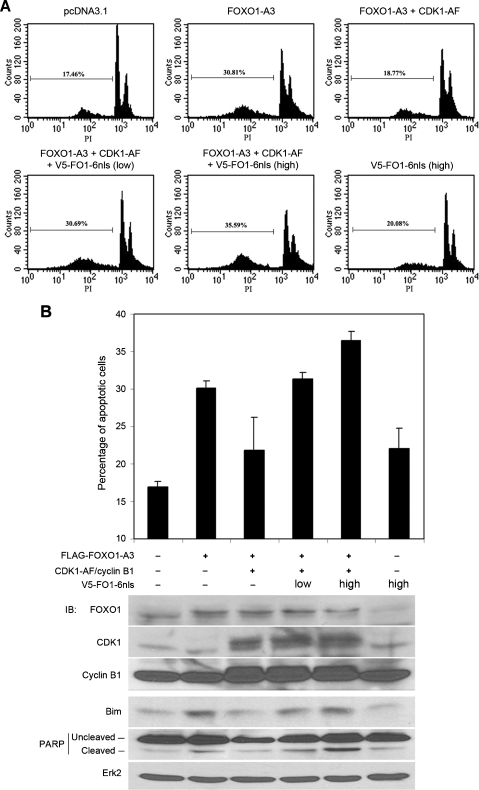
Previous studies demonstrated that the ectopic expression of FOXO1-A3, a constitutively active mutant of FOXO1, induces apoptosis in PTEN-mutated LNCaP PCa cells [8,17]. Moreover, the ectopic expression of CDK1 inhibits FOXO1-A3-induced apoptosis, and this effect is mediated by CDK1 phosphorylation of FOXO1 at S249 [27]. We examined whether FO1-6nls expression affects CDK-mediated inhibition of FOXO1-induced apoptosis of PCa cells. As expected, the expression of FOXO1-A3 in LNCaP cells resulted in an increase in apoptosis in comparison to the expression of the empty vector (Figure 6A). This effect was completely inhibited by cotransfection with the constitutively active CDK1 (CDK1-AF). However, CDK1-mediated inhibition of FOXO1-induced apoptosis was reversed by FO1-6nls in a dose-dependent manner (Figure 6A). Transfection of FO1-6nls alone resulted in a slight increase in apoptosis relative to the transfection of the empty vector (Figure 6A), which is consistent with the very limited increase in the transcriptional activity of endogenous FOXO1 in this PTEN-mutated cell line when transfected with FO1-6nls alone (Figure 3A, column 5 vs lane 1). Background apoptosis was observed when cells were transfected with the empty vector. This is presumably because of the cell debris generated by the transfection method of electroporation and the weak-adhesive nature of LNCaP cells as reported previously [27]. The quantitative results of these experiments are shown in Figure 6B (upper panel). In line with these results, the ectopic expression of FOXO1-A3 induced expression of Bim (Figure 6B), a proapoptosis gene transcriptionally activated by FOXO1 [6]. This effect was inhibited by the expression of CDK1-AF (Figure 6B). However, CDK1-mediated inhibition of Bim expression was abolished in FO1-6nls-transfected cells (Figure 6B). A similar result was observed for the cleavage of PARP, an apoptotic marker (Figure 6B). These data suggest that FO1-6nls can antagonize CDK-mediated inhibition of FOXO1, thereby restoring the apoptotic function of FOXO1 in PCa cells.
Figure 6.
FO1-6nls expression overcomes CDK1-mediated inhibition of FOXO1-induced apoptosis in LNCaP PCa cells. (A) Representative images of flow cytometry. LNCaP cells were transfected with plasmids as indicated. At 48 hours after transfection, cells were harvested, fixed, and stained with propidium iodide (PI), and FACS analysis was performed. The percentage of cells at sub-G1 (apoptotic cells) is indicated in each experiment. (B) Quantification results from three independent experiments. The expression of FOXO1, CDK1, cyclin B1, and the proapoptotic protein Bim and the cleavage of PARP protein were examined by Western blots. These experiments were repeated at least twice.
Discussion
Findings from human and mouse studies suggest that FOXO transcription factors function as tumor suppressors by promoting cell cycle arrest and apoptosis [26,32]. The relevance of these proteins to human cancers is further evident in the fact that their tumor suppressor functions are often disrupted by many oncogenic pathways [1]. Thus, specifically restoring the tumor suppressor function of FOXO1 in cancerous cells would be of great interest for the treatment of human cancers. In the present study we demonstrated that FO1-6nls, a 70-amino acid peptide of FOXO1 that contains the CDK phosphorylation site S249, binds directly to CDK1 and CDK2 and blocks CDK1/2-mediated phosphorylation of FOXO1 both in vitro and in vivo. Consistent with the previous findings that S249 phosphorylation is important for CDK1/2-induced nuclear exclusion and inhibition of FOXO1 [26,27], the expression of FO1-6nls impedes CDK-induced nuclear exclusion of FOXO1, augments FOXO1's transcriptional activity and inhibits PCa cell growth and survival. Therefore, we have identified a novel, biologically active polypeptide of FOXO1 that enables to restore FOXO1's transcriptional and antitumor activities by specifically overcoming CDK1- and CDK2-mediated phosphorylation and inhibition of FOXO1.
The finding that the decoy function of FO1-6nls was not affected by the S249A mutation in this peptide suggests that the antagonistic effect of FO1-6nls on CDK phosphorylation of FOXO1 is independent of the phosphorylation acceptor site S249. Mechanistically, we further showed that FO1-6nls binds directly to CDK1 and CDK2 and that deletion of 13 amino acids at the N-terminus of FO1-6nls abolishes the interaction between FO1-6nls and CDK1 and CDK2. This deletion also completely abrogates the ability of FO1-6nls in activation of FOXO1. On the basis of these observations, we envision a working model wherein the competitive binding of FO1-6nls to CDK1 and CDK2 disrupts the binding of FOXO1 by CDK1 and CDK2, thereby abolishing CDK1- and CDK2-mediated phosphorylation and nuclear exclusion of FOXO1 and inhibition of FOXO1's transcriptional activity. The finding that the biologic activity of FO1-6nls is mediated by its specific disruption of FOXO1-CDK interaction provides a plausible explanation for the observation that the effect of FO1-6nls on cell growth is dependent on FOXO1, but not FOXO3a, although FOXO3a is also dominantly expressed in PCa cells. Importantly, this result is consistent with the previous finding that no functional CDK phosphorylation sites were identified in other FOXO proteins including FOXO3a and FOXO4 [26].
It is well established that cyclin-CDK complexes, such as cyclin A-CDK2 and cyclin E-CDK2, target their inhibitors, activators, and other cell cycle regulators (e.g. p21WAF1, E2F1, p107, and p130) through the binding of the cyclin subunits to a cyclin-binding sequence (also known as a Cy or RXL motif) present in their substrates [33,34]. A number of primary sequence elements, which loosely resembles the RXL motif, are also identified in a region (amino acids 792–928) of RB protein. Conversion of one of these elements to three alanine residues diminishes cyclin A-CDK2-mediated phosphorylation of RB [33]. It is worth noting that there is no RXL motif present in FO1-6nls (Figure 1A). Moreover, unlike RXL-containing substrates such as p21WAF1 and RB, which bind directly to the cyclin subunit, rather than the CDK catalytic subunit of the CDK complex, FOXO1 directly binds to CDK1 and CDK2 [26,27]. Thus, CDK may inhibit the function of FOXO1 and RB through distinctive mechanisms.
Deregulation of the cell cycle is one of the hallmarks of cancer cells. Most importantly, expression and/or activity of CDK1 and CDK2 often increase in human PCas. The expression of p27KIP1, a well-characterized inhibitor of CDK2, frequently decreases in advanced PCa [35]. Decreased levels of this protein are correlated with poor clinical outcome [35,36]. These clinical findings suggest that increased activity of CDK2 owing to loss of p27KIP1 is important for PCa progression. Because p27KIP1 can also bind to and inhibit CDK1 [37,38], loss of p27KIP1 may lead to increased activity of CDK1 in PCa. Moreover, several groups have shown increased expression of the CDK1 protein in human PCas relative to normal prostatic tissues [39–41]. Furthermore, Cdc25C, the upstream activator of CDK1, not only is upregulated in PCa but also is present exclusively in its active form [42]. Loss-of-function mutations of the Chk2 gene, an upstream negative regulator of CDK1 and CDK2, have been detected in PCa specimens [43,44]. These observations in clinic specimens support the view that aberrant activation of CDK1 and CDK2 may lead to the inhibition of the tumor suppressor function of FOXO1, thereby promoting PCa survival and/or resistance to therapy. The prevalence of CDK1/2-mediated FOXO1 inhibition in human PCas and the effectiveness of FO1-6nls in reversing this process highlight a therapeutic potential of FO1-6nls for the treatment of PCa.
In summary, we have identified a bioactive, FOXO1-derived peptide FO1-6nls that blocks S249 phosphorylation of FOXO1 mediated by CDK1 and CDK2. In addition, expression of FO1-6nls overcomes CDK1-induced nuclear exclusion and inhibition of FOXO1. Consistently, treatment of PTEN-positive PCa cells with FO1-6nls alone induces the expression of FOXO1 target genes, such as Bim, and promotes apoptosis and inhibits cell growth. Thus, FO1-6nls may be harnessed for the development of novel therapeutics for the treatment of human cancers such as PCa.
Acknowledgments
The authors thank D. J. Tindall for reagents and S. W. Hayward for BPH-1 cells.
Abbreviations
- CDK
cyclin-dependent kinase
- PCa
prostate cancer
- nls
nuclear localization signal
- GST
glutathione S-transferase
Footnotes
References
- 1.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 2.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 3.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 4.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 5.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 6.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–544. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N. FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem. 2002;277:26729–26732. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- 13.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 14.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Muddiman DC, Tindall DJ. Androgens negatively regulate forkhead transcription factor FKHR (FOXO1) through a proteolytic mechanism in prostate cancer cells. J Biol Chem. 2004;279:13866–13877. doi: 10.1074/jbc.M314143200. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitinmediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 19.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 20.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 21.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5- trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 22.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 23.Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 25.Dong XY, Chen C, Sun X, Guo P, Vessella RL, Wang RX, Chung LW, Zhou W, Dong JT. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27:4733–4744. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ. PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem. 2001;276:38830–38836. doi: 10.1074/jbc.M103632200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 31.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 32.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza N, Fong S, Marsters J, Koeppen H, Schwall R, Wickramasinghe D. Selective cyclin-dependent kinase 2/cyclin A antagonists that differ from ATP site inhibitors block tumor growth. Cancer Res. 2003;63:1020–1024. [PubMed] [Google Scholar]
- 35.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, et al. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- 36.Freedland SJ, deGregorio F, Sacoolidge JC, Elshimali YI, Csathy GS, Dorey F, Reiter RE, Aronson WJ. Preoperative p27 status is an independent predictor of prostate specific antigen failure following radical prostatectomy. J Urol. 2003;169:1325–1330. doi: 10.1097/01.ju.0000054004.08958.f3. [DOI] [PubMed] [Google Scholar]
- 37.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 39.Mashal RD, Lester S, Corless C, Richie JP, Chandra R, Propert KJ, Dutta A. Expression of cell cycle-regulated proteins in prostate cancer. Cancer Res. 1996;56:4159–4163. [PubMed] [Google Scholar]
- 40.Kallakury BV, Sheehan CE, Ambros RA, Fisher HA, Kaufman RP, Jr, Ross JS. The prognostic significance of p34cdc2 and cyclin D1 protein expression in prostate adenocarcinoma. Cancer. 1997;80:753–763. doi: 10.1002/(sici)1097-0142(19970815)80:4<753::aid-cncr15>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Kallakury BV, Sheehan CE, Ambros RA, Fisher HA, Kaufman RP, Jr, Muraca PJ, Ross JS. Correlation of p34cdc2 cyclin-dependent kinase overexpression, CD44s downregulation, and HER-2/neu oncogene amplification with recurrence in prostatic adenocarcinomas. J Clin Oncol. 1998;16:1302–1309. doi: 10.1200/JCO.1998.16.4.1302. [DOI] [PubMed] [Google Scholar]
- 42.Ozen M, Ittmann M. Increased expression and activity of Cdc25C phosphatase and an alternatively spliced variant in prostate cancer. Clin Cancer Res. 2005;11:4701–4706. doi: 10.1158/1078-0432.CCR-04-2551. [DOI] [PubMed] [Google Scholar]
- 43.Dong X, Wang L, Taniguchi K, Wang X, Cunningham JM, Donnell SK, Qian C, Marks AF, Slager SL, Peterson BJ, et al. Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet. 2003;72:270–280. doi: 10.1086/346094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Dong X, Liu W, Chen J. Characterization of CHEK2 mutations in prostate cancer. Hum Mutat. 2006;27:742–747. doi: 10.1002/humu.20321. [DOI] [PubMed] [Google Scholar]