Abstract
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related deaths in America. Nearly two thirds of newly diagnosed CRC cases include lymph node (LN) involvement, and LN metastasis is one of the strongest negative prognostic factors for CRC. It is thought that CRC tumors contain a small population of drug-resistant CRC tumor-initiating cells (Co-TICs) that may be responsible for cancer recurrence. To evaluate the effects of the LN stromal cells on Co-TICs, we established a unique xenoplant model using CRC cells isolated by enzymatic digestion from consented patient specimens, HT-29 cells, HCA-7 cells, and LN stromal cell line HK cells. We found that HK cells and HK cell-conditioned media enhanced CRC tumor formation and tumor angiogenesis. Cells expressing CD133+ and the stromal cell-derived factor 1α (SDF-1α) receptor CXCR4 were enriched in chemotherapeutic-resistant CRC cells. CD133+CXCR4+ Co-TICs isolated from patient specimens are more tumorigenic than unsorted tumor cells. Furthermore, the inhibitors specific to HK cell-derived SDF-1α reduced tumor formation and tumor angiogenesis. Our results have demonstrated a role for Co-TICs in tumor growth and defined the influence of LN stromal cells on Co-TICs. We have identified a major Co-TIC/LN microenvironment-specific mechanism for CRC resistance to chemotherapeutic agents and established experimental platforms for both in vitro and in vivo testing, indicating that SDF-1α and its receptor, CXCR4, may be targets for clinical therapy.
Introduction
In the United States, colorectal cancer (CRC) is the third most common malignancy and the second most common cause of cancer-related mortality, with an estimated incidence of 143,000 cases and 51,000 deaths per year [1]. Despite the standard treatment of surgery and chemotherapy in advanced CRC, there is approximately 50% recurrence. Tumor-initiating cells (TICs) that are resistant to conventional chemotherapeutic and radiation treatments may be responsible for this tumor recurrence [2].
Current CRC therapies fail to effectively prevent extranodal CRC recurrence, even in cases of successful eradication of all visible tumors. These therapies inadequately treat the rare but highly significant population of CRC tumor-initiating cells (Co-TICs) [3] and ignore the tumor-nurturing role that the lymph node (LN) microenvironment plays.
LN metastasis is one of the strongest negative prognostic factors for CRC. Most CRC patients present with regional LN involvement, stage III disease, suggesting that the LN microenvironment plays a significant role promoting extranodal recurrence and further metastasis [4]. Follicular dendritic cells (FDCs), unique LN stromal cells that display both autocrine and paracrine properties, analogous to cancer-associated stromal fibroblasts, have been shown to nurture CRC cells through production of various cytokines and growth factors [5]. CRC cells interact with tumor-fostering stromal cells and the extracellular matrix in a protective fashion decreasing chemotherapy-induced apoptosis [6].
Various chemokines play important roles in TICs/stromal cell niche interaction. The stromal cell-derived factor 1α (SDF-1α) is a chemokine that regulates many essential biologic processes including revascularization, cellular adhesion, and tumorigenesis [7]. It has a negative effect in multiple cancers [8]. Previous studies have shown that SDF-1α and its membrane-bound receptor chemokine receptor 4 (CXCR4) are involved in tumor metastasis and extranodal recurrence in CRC [9–11] and that the metastatic activity of CD133+CXCR4+ TICs is increased in pancreatic cancer [12].
The accumulated evidence indicates that TICs are supported by microenvironmental factors produced by surrounding stromal cells. However, little is known about the role that LN stromal cells play in providing a supportive microenvironment to allow for Co-TICs survival, activation, and stimulation. Furthermore, it is unclear how this supportive LN microenvironment results in extranodal tumor recurrence despite appropriate oncologic treatment. We hypothesized that the LN stromal microenvironment, specifically FDC, primes Co-TICs activation through the SDF-1α/CXCR4 axis and plays a key role in supporting therapy-resistant Co-TICs that have metastasized to the LN. This microenvironment also facilitates cellular education, supporting and promoting extranodal metastasis. In this study, we evaluated the effect of LN stromal cells, specifically the effect of the SDF-1α/CXCR4 axis, on Co-TICs, using CRC patient specimens, HT-29, and HCA-7 cell lines in a unique CRC xenoplant model. By understanding the LN's stromal effect on Co-TICs, we hope to better predict tumor recurrence and develop targeted therapy for minimizing extranodal CRC recurrence.
Materials and Methods
Antibodies and Reagents
Antibodies (Abs) used were phycoerythrin (PE)-labeled mouse antihuman CD133 (clone 923C3; Miltenyi Biotec, Auburn, CA), CXCR4 (clone 12G5; R&D Systems, Inc, Minneapolis, MN), CD326 (EpCAM; Miltenyi Biotec), antimouse CD31 (PECAM-1, clone MEC13.3; BD Biosciences, San Jose, CA), and mouse isotype control IgG (clone MOPC-21; BD Biosciences); Allophycocyanin (APC)-conjugated CD133 (clone AC133; Miltenyi Biotec) and mouse isotype controls (clone MOPC-21 Inc. BD Biosciences); biotinylated goat antirabbit or antimouse IgG (R&D Systems); horseradish peroxidase-conjugated streptavidin ( Jackson Laboratory, Bar Harbor, ME); fluorescein isothiocyanate-conjugated goat antimouse IgG (BD Biosciences); rabbit anti-human CD133 (Abcam, Inc, Cambridge, MA); anti-cytokeratin 20 (Abcam, Inc); and anti-mucin 1 (Santa Cruz Biotechnology, Santa Cruz, CA). Reagents used were Sigma FAST 3,3-diaminobenzidine tablets (Sigma-Aldrich, St Louis, MO) and human SDF-1α-specific ELISA Kit (R&D Systems). The chemotherapeutic drugs used were 5-fluorouracil (5-FU; APP Pharmaceuticals LLC, Schaumburg, IL) and oxaliplatin (Hospira, Inc, Lake Forest, IL).
Cell Lines
An FDC cell line, HK, was maintained in RPMI medium supplemented with 10% fetal calf serum (FCS, Life Technologies, Grand Island, NY), 2 mMglutamine, 100 U/ml penicillin G, and 100 mg/ml streptomycin (Irvine Scientific, Santa Ana, CA) [13]. Colon cancer cell lines HT-29 and HCA-7 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). CRC-Pt1 is a colon cancer cell line derived from primary tumor in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice generated by coinjection of cancer cells isolated from colon cancer patient 1 and HK cells. All cell cultures were carried out in McCoy medium with supplements at 37°C in a 5% CO2 humidified incubator.
Isolation of Single-Cell Suspension from Colon Cancer Specimens
Human CRC tissue specimens were obtained in accordance with the ethical standards of the institutional committee on human experimentation from consented patients undergoing a colon or rectal resection for colorectal adenocarcinoma. Histologic diagnoses were based on microscopic features of carcinoma cells determining the histologic type and grade. Colon cancer and normal colon tissues were collected in cold sterile McCoy medium containing penicillin G (500 U/ml) and streptomycin (500 mg/ml). Tissues were mechanically minced into small pieces. Enzymatic digestion was performed using collagenase IV (1.5 mg/ml), hyaluronidase (20 mg/ml), and deoxyribonuclease I (DNase I, 0.1 mg/ml; all from Sigma-Aldrich) in Hank's balanced salt solution for 2 to 3 hours at 37°C. The digests were used for phenotyping with Co-TIC markers, direct engraftment in immunodeficient mice, and purification of Co-TICs.
FACS Analysis
For cell surface Ag staining, enzyme-digested suspension colon cancer cells, control colon cells, or adhesion colon cancer cells detached by trypsin from monolayer cultures were incubated with the indicated Abs directly conjugated with PE or APC in the manufacturer's suggested concentration for 15 minutes at 4°C. Isotype-control Abs were used at the same concentration for background-level staining. After washing with phosphate-buffered saline containing 0.2% bovine serum albumin and 0.1% sodium azide, cells were acquired by a FACS Calibur using Cell Quest software (BD Biosciences) and were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR). Viable cells were gated by forward and side scatters.
Immunohistochemistry Using Frozen and Paraffin-Embedded Slides
For histologic evaluation, one portion of each colon cancer patient specimen and xenoplant tumor tissue were fixed in formalin and embedded in paraffin. Another portion was embedded in OCT compound (Tissue Tek, Sakura Finetek USA, Torrance, CO) and stored at -80°C. Tissue slides were subjected to hematoxylin and eosin staining as well as immunohistochemistry staining. For immunofluorescent staining on cryosections, PE-conjugated antimouse CD31 (1:50) was used to quantify angiogenesis in xenoplants. APC- or PE-conjugated antihuman CD133 (1:50) and PE- or peridinin chlorophyll protein (PerCP)-conjugated antihuman CXCR4 (1:50) Abs were used to identify Co-TICs. FITC-conjugated CD326 (1:50) was used as marker for colon cancer cells. The 4′,6-diamidino-2-phenylinodole (DAPI) was used for nuclei counterstaining (ProLong Gold antifade reagent with DAPI; Invitrogen, Eugene, OR).
Paraffin-embedded tissue sections (5 µm) were deparaffinized, rehydrated, and blocked for endogenous peroxidase activity. After high-temperature Ag retrieval in Trilogy unmasking solution (Cell Marque, Rocklin, CA), slides were biotin-blocked, serum-blocked, and immunostained using EnVision G/2 double-stain system, rabbit/mouse kit (DAB+/Permanent Red; Dako, Glostrup, Denmark) according to the manufacturer's instructions. Abs to CD133 and CXCR4 were applied at 1:50 for 1 hour at room temperature. Slides were counterstained with hematoxylin. All images were captured by a deconvoluting microscope using the SlideBook software (all from Intelligent Imaging Innovations, Inc, Denver, CO).
Isolation of Co-TICs Using Magnetic Cell Sorting and Flow Cytometry
The epithelial cell adhesion molecule (EpCAM or CD326) expression was used to ensure that the CD133+ cells corresponded to cancer cells of human epithelial origin. Magnetic cell separation was performed on cells obtained from enzymatic dissociation of colon cancer specimens and HT-29 cells using anti-CD326-APC followed by microbeads conjugated to anti-APC and then microbeads conjugated anti-CD133 (AC133) Ab after cleavage of the beads. The magnetic separation step was repeated twice, applying the eluted positive cells to a new magnetic column for positive selection. After magnetic sorting, cell viability was assessed using trypan blue exclusion. Quality of sorting was examined by flow cytometry with an Ab against CD133 (293C3-PE) on CD133+ cell population. To purify CD133+CXCR4+ cells from the patient's specimen, CD326-FITC-, CXCR4-PE-, and CD133-APC-positive cells were identified and electronically gated for cell sorting on a FACS Aria cell sorter using Becton Dickinson Diva software (BD Biosciences).
Preparation of GFP-Tagged CRCs
HT-29 or CRC-Pt1 cells were cultured in 500 µl of McCoy medium with 10% FCS without antibiotics in 12-well plates (5 x 105 cells/well). GFP-expressing lentivirus pLentiLox3.7 (200 µl), medium (300 µl), and 6 µg/ml polybrene were added to each well. After 12 hours of incubation, 2 ml of fresh medium was added. After expansion, GFP+ cells were enriched by FACS cell sorting twice.
In Vitro Colon Cancer Cell Proliferation Assay
GFP-tagged colon cancer cells (2 x 104 cells/well) were cultured in McCoy medium containing 10% fetal calf serum with HK cells (2 x 104 cells/well) that were seeded 1 day prior in 24-well plates. In some experiments, HK cell-conditioned McCoy medium was added to the cancer cells or HK cells were separated from the cancer cells by a transwell chamber with 0.4-µm pore filters (Corning Life Sciences, Lowell, MA). The chemotherapeutic drug 5-FU or oxaliplatin was added to the cell culture on day 2. GFP signals of viable cancer cells were measured by IVIS Lumina Imaging System; images were analyzed using Living Image Software (both from Caliper Life Sciences, Hopkinton, MA), and data were presented as efficiency x 10-3.
In some experiments, the cell proliferation reagent WST-1 was used according to the manufacturer's instructions to determine cell proliferation and viability (Roche Applied Science, Indianapolis, IN). Cells grown in a 96-well tissue culture plate were incubated with the ready-to-use WST-1 reagent for 1 hour to 3 hours. The formazan dye formed by metabolically active cells in the culture was quantified with an ELISA reader at OD650-450nm (Bio-Rad, Hercules, CA).
Colon Cancer Cell Sphere Formation by Extreme Limiting Dilution Analysis
Extreme limiting dilution analysis was performed as described by Hu and Smyth [14]. Briefly, serum-free stem cell medium (SCM) contains Dulbecco modified Eagle medium/F12 (GIBCO, Invitrogen Corporation, Carlsbad, CA) supplemented with 10 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor, 1 x B27 supplement (Sigma), 2 mM glutamine, 100 U/ml penicillin G, and 100mg/ml streptomycin. HK cell-conditioned medium was collected by culturing 0.5 million cells in 10 ml of SCM for 72 hours. HT-29 and HCA-7 single-cell suspension obtained from adherent cell cultures by trypsin were plated at concentrations of 10,000, 1000, 100, and 10 cells and 1 cell per well per 200 µl of SCM or 50% serum-free HK cell-conditioned medium in ultra-low-attachment 96-well plates and were incubated for 10 days (12–24 wells for each dilution). At the end of 10 days, the number of wells showing formation of colonospheres was counted using Leica Microscope (Leica Microsystems, Wetzlar, Germany). The frequency of sphere-forming cells in a particular cell type was determined using the extreme limiting dilution analysis Web tool at http://bioinf.wehi.edu.au/software/elda.
Tumorigenicity
Male nude and NOD/SCID mice, 6 to 8 weeks old, were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD) and were acclimated for 1 week. All animal studies were conducted under approved guidelines of the Animal Care and Use Committee of the Ochsner Clinic Foundation. Varying doses of HT-29, HCA-7, CRC-Pt1, or purified CD133+ CRC cells were mixed with HK cells (1 x 106) in 0.1 ml of McCoy medium and then injected subcutaneously with a 25-gauge needle into the posterior flank of the mice. In some experiments, CRC cells were pre-incubated with 50 µM AMD3100 (Sigma) for 30 minutes at 37°C. Tumorigenesis was assessed by measuring solid tumors in three dimensions with calipers twice a week. Tumors were removed when they became 1 cm3.
Statistical Analysis
Data presented are representatives of at least three similar independent experiments. To assess statistical significance of differences, an unpaired t test (Prism Version 4.03; GraphPad Software, Inc, La Jolla, CA) was used. P < .05 was considered significant (*), and P < .001 was considered very significant (***). Linear regression method was used to analyze the correlation between GFP signal and WST-1 assay for viable cell quantification. The R2 value was used to assess significance.
Results
FDC/HK Cells Promote Colon Cancer Cell Tumor Formation in Immunodeficient Mice
LN involvement positively correlates to poor prognosis of CRC. It was reported that the addition of stromal cells to a small number of HT-29 cells, which, in themselves, are unable to induce tumors, allowed the development and growth of tumors in nude mice [15]. To evaluate the role of LN stromal cells in tumor growth and to quantitatively evaluate the presence of TICs, we established an in vivo CRC tumor model using HT-29 cells and FDC cell line HK cells. In this model, varying doses of HT-29 cells were coinoculated subcutaneously with or without HK cells into nude mice for tumor formation. As shown in Figure 1A and Table 1, the addition of HK cells increased tumor formation especially in lower doses of HT-29 cell.
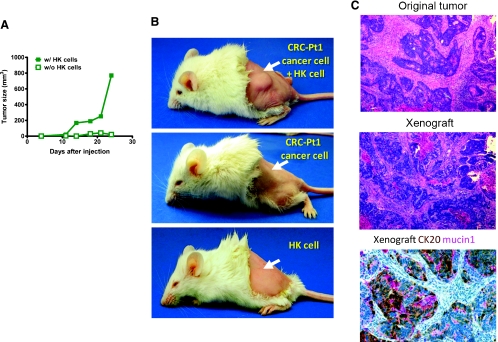
Figure 1.
FDC/HK cells support HT-29 cell and CRC patient cancer cell tumor formation in immunodeficient mice. (A) HT-29 cell tumor formation in nude mice. HT-29 cells (2 x 105) were subcutaneously injected with or without HK cells (1 x 106) into the posterior flank of nude mice. Graph shows tumor size measured with a caliper in three dimensions at the indicated days after cell injection. (B) Single-suspension cancer cells from patient 1 (2 x 105) were subcutaneously injected with or without HK cells (1 x 106) into the left posterior flank of NOD/SCID mice (arrows). Images show patient's colon cancer cells form tumor in the presence of HK cells (top), whereas no tumor was formed without HK cells (middle) or with HK cells alone (bottom). (C) Hematoxylin and eosin staining of paraffin-embedded original tumor and tumor from xenograft showing similarity in cancer morphology, and cytokeratin 20 (CK20) and Mucin 1 staining positive in the xenograft tumor. Images were captured by a deconvoluting microscope using SlideBook software. Original magnification, x400.
Table 1.
CRC Cells Are Tumorigenic in Immunodeficient Mice.
| Cancer Cells Injected | Tumor Formation/Injection | ||||
| 1 x 106 | 1 x 105 | 1 x 104 | 1 x 103 | 1 x 102 | |
| HT-29 | 4/4 | 3/5 | 0/4 | 0/2 | |
| HT-29 + HK | 4/4 | 6/6 | 5/6 | 1/3 | |
| HT-29 CD133+ | 0/6 | 0/2 | 0/2 | ||
| HT-29 CD133+ + HK | 4/6 | 5/6 | 1/3 | ||
| Pt cancer | 2/20 | 0/14 | 0/2 | ||
| Pt cancer + HK | 6/20 | 6/14 | 1/2 | ||
| Pt cancer CD133+ | 0/2 | 0/6 | |||
| Pt cancer CD133 ++ HK | 2/2 | 1/6 | |||
| Pt cancer CD133+CXCR4+ + HK | 2/2 | ||||
To examine whether CRC cells from patients are tumorigenic in this model, enzyme-digested single-cell suspensions of human cancer cells were inoculated into NOD/SCID mice. As indicated in Table 1 and Figure 1B, 13 of 36 injections of suspension cancer cells (0.01–1 x 106 cells/site) formed tumors in the presence of HK cells, while only 2 tumors were formed in 36 cancer cell injections without HK cells. Injection of HK cells alone did not form tumors. The xenograft resembles the original tumors in morphology as well as cytokeratin 20 and Mucin 1 expression (Figure 1C). These data suggest that HK cells play a key role in cancer cell survival, tumor initiation, and growth in vivo and that HT-29 cells and patients' specimens contain TICs.
Soluble Factors Produced by FDC/HK Promote Colon Cancer Cell Proliferation, Sphere Formation, and Tumor Formation
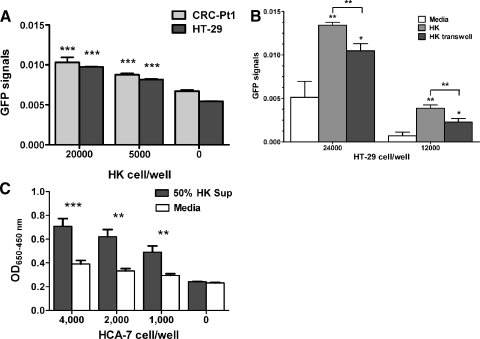
To evaluate the FDCs'/HK cells' effects on CRC cells, we then established an in vitro CRC proliferation assay. First, we confirmed that the GFP signal from viable cells correlated with the WST-1 assay (Figure W1). To monitor cancer cell proliferation in CRC cell and stromal cell cocultures, HT-29 cells and tumor cells isolated from patient 1 (CRC-Pt1) were tagged with GFP and then cultured with and without HK cells. Results show that HK cells promoted CRC cell proliferation in a dose-dependent manner as measured by IVIS Lumina Imaging System by quantifying the GFP signals (Figure 2A). When HK cells were separated from HT-29 cells by transwells (Figure 2B) or when HK cell-conditioned medium was added to the HT-29 or HCA-7 cell cultures (Figures 2C and 4, B and C), a significant supporting effect was observed, indicating that soluble factors secreted from stromal cell HK, rather than cell-cell contact, are involved.
Figure 2.
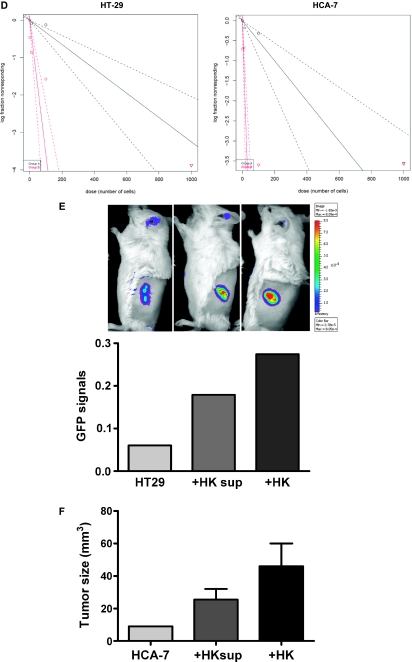
FDC/HK cells support CRC cell proliferation, sphere formation, and tumor formation. (A–C) CRC cell proliferation. GFP-tagged HT-29 (3000 cells/well) and CRC-Pt1 (1000 cells/well) cells were cocultured with indicated numbers of HK cells in 96 well plates for 72 hours (A). GFP-tagged HT-29 cells were cultured alone, with HK cells, or with HK cells separated by a transwell in 24-well plates for 48 hours (B). GFP signals were evaluated by IVIS Lumina Imaging System (A and B), and WST-1 assay for HCA-7 cells was measured by OD650−450 nm after 72 hours of incubation with or without 50% HK cell-conditioned medium (C). (D) A log-fraction plot of the limiting dilution model fitted to the data in Table W1. The slope of the line is the sphere formation cell fraction for HT-29 and HCA-7 cells. The dotted lines give the 95% confidence interval. Black line for SCM and red lines for 50% HK cell-conditioned medium. (E and F) HK cells and HK cell-conditioned medium support HT-29 and HCA-7 cell tumor formation in NOD/SCID mice. CRC tumor formation was indicated by GFP signal imaging (for GFP-HT-29 cells), and tumor size was measured by a caliper (for HCA-7 cells) in the absence or the presence of HK cell or HK cell-conditioned media.
Figure 4.
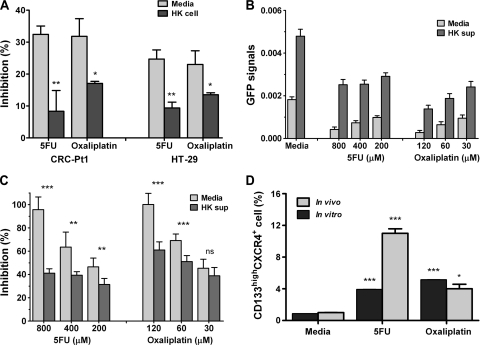
FDC/HK cells support chemoresistant colon cancer cells in vitro and in vivo. (A) Suboptimal doses of 5-FU (400 µM) and oxaliplatin (60 µM) were added to the coculture of GFP-tagged CRC cells with HK cells in 96-well plates for 72 hours. Percentage inhibition of cell growth by GFP signal levels was calculated against that of untreated cells. (B–C) GFP-tagged HT-29 cells were cultured with or without 50% HK cell-conditioned media and then treated with 5-FU and oxaliplatin for 72 hours. GFP signals (B) and percentage inhibition of viable cells by GFP signal levels (C) are shown. (D) CD133+CXCR4+ cells are enriched in chemoresistant HT-29 cells. HT-29 cells after 72 hours of chemotherapy in A were collected. Tumor-bearing mice were treated with control and chemotherapeutic drugs for 2 weeks as described in Results. Enzyme-digested single-cell suspension tumor cells were collected. Both cells were stained with CD133 and CXCR4 and analysis by FACS similar to Figure 3C. Data are presented as mean ± SD. Data show percentage of double-positive cells.
The ability of CRC cells to form spheres in serum-free culture conditions is not only an indication of cell self-renewal but also a characteristic of Co-TICs [16,17]. When HT-29 and HCA-7 cells were tested, the addition of HK cell-conditioned media increased HT-29 and HCA-7 cell sphere formation significantly (Figure 2D and Table W1). One of 308 HT-29 cells and 1 of 200 HCA-7 cells formed spheres in 10 days when cells were seeded in serum-free SCM. When a 50% serum-free HK cell-conditioned medium was added to the cultures, 1 of 29 HT-29 cells and 1 of 8 HCA-7 cells formed spheres. This is consistent with the idea that TICs are only a small population of cancer cells. Supporting effects of HK cell-conditioned medium are also confirmed by in vivo tumor formation assay. In NOD/SCID mice, the addition of HK cells significantly enhanced HT-29 and HCA-7 cell tumor formation, whereas the presence of HK cell-conditioned media partially supported HT-29 and HCA-7 cell tumor formation (Figure 2, E and F). These data suggest that soluble factors produced by HK cells are sufficient to support sphere-forming Co-TICs in HT-29 and HCA-7 cells.
Identification of Co-TICs in CRC Cells
It has been documented that Co-TICs express a variety of surface markers; the most promising marker seems to be CD133 [18]. Although CD133 expression is a prognostic marker for colon cancer, it may lack a functional role in tumor with initiation [19], and CD133+ CRC cells represent a heterogeneous population of cells. Because soluble factors are involved in supporting Co-TICs, chemokine SDF-1α is one of the soluble factors known to be produced by FDCs in LNs, and TICs express an additional marker CXCR4, the membrane-bound receptor for SDF-1α, that has increased metastatic activity in pancreatic cancer [12], we examined CXCR4 as a Co-TIC marker in addition to CD133. When the two markers CD133 and CXCR4 were used in CRC specimens, a distinct population of cancer cells was detected in paraffin-embedded tissue as well as in fresh frozen tumor specimens, indicating that CRC tumors contain a small fraction of CD133+ CXCR4+ Co-TIC (Figures 3, A and B, and W2A). To identify and quantify the presence of Co-TIC, colon cancer cell lines HT-29 and HCA-7 and enzyme-digested single-cell suspension tumor cells from 21 CRC patients were examined using FACS analysis with Abs specific to CD133 and CXCR4. Figure 3C shows that HT-29, HCA-7 cells, and patient tumors cells all contain small populations of CD133+CXCR4+ cells. Although consistently present (17/18 patients tested), the percentage of Co-TIC in patient specimens varies (from 0.05 to 7.39; Table 2). This double-positive population is not seen in normal control colon cell suspensions (Figure 3C). CXCR4 is known to be expressed on a variety of cell subtypes. In Figure W2A, a triple-Ab staining shows that the CD133 and CXCR4 double-positive cells are also CD326-positive.
Figure 3.
Detecting Co-TICs in CRC. (A) Paraffin-embedded tumor tissue sections from CRC patients 1 and 4 were double stained for CD133 (brown) and CXCR4 (pink) and control Ab staining. Arrows show double-positive cells. Hematoxylin was used as a counterstain. Original magnification, x400. (B) Frozen tumor tissue sections from CRC patients 5 and 8 were double stained for CD133 (red) and CXCR4 (green, arrows). DAPI was used as a nuclear counterstain. Original magnification, x400. (C) Double staining of CD133 and CXCR4 on patient's normal colon cells or CRC patient cells (left panels) and HT-29 cells or HCA-7 cells (right panels). Numbers indicate percentage of cells in quadrants. Isotype control mouse IgGs (mIgG) were used for background-level staining.
Table 2.
Selected CRC (Adenocarcinoma) Patient Biopsy Characteristics.
| Specimens | Age (year) | Sex | Stage | Differentiation | Tumor Location | Markers | ||
| Positive (%) in Cancer Cells | ||||||||
| CD133 | CXCR4 | CD133/CXCR4 | ||||||
| Pt1 | 61 | F | III | M | Left | 0.56 | ND | ND |
| Pt2 | 44 | M | II | M | Sigmoid | 0.21 | ND | ND |
| Pt3 | 66 | F | II | M | Transverse | 0.48 | ND | ND |
| Pt5 | 85 | M | II | P | Cecum | 1.71 | 5.11 | 1.72 |
| Pt6 | 55 | M | II | M | Transverse | 3.62 | 5.6 | 1.02 |
| Pt7 | 63 | M | II | M | Right | 4.59 | 20.32 | 4.84 |
| Pt8 | 71 | F | III | P | Hepatic flexure | 2.24 | 18.44 | 1.64 |
| Pt12 | 67 | M | II | M | Sigmoid | 0.92 | 0.94 | 0 |
| Pt16 | 75 | M | III | M | Rectum | 0.73 | 0.48 | 0.27 |
| Pt20 | 39 | F | III | M | Cecum | 0.98 | 1.28 | 0.08 |
| Pt24 | 77 | M | III | M | Rectosigmoid | 0.78 | 1.47 | 0.7 |
| Pt25 | 80 | F | II | P | Right | 0.19 | 4.66 | 0.16 |
| Pt26 | 55 | M | III | P | Rectosigmoid | 0.33 | 3.41 | 0.25 |
| Pt27 | 44 | F | III | M | Right | 30.09 | 12.49 | 7.39 |
| Pt28 | 79 | M | III | M | Rectosigmoid | 1.3 | 4.62 | 0.38 |
| Pt30 | 65 | M | II | M | Transverse | 0.2 | 2.6 | 0.05 |
| Pt32 | 86 | F | I | P | Right | 0.5 | 40.5 | 0.5 |
| Pt34 | 47 | F | II | M | Sigmoid | 27.8 | 4.2 | 1.67 |
| Pt35 | 60 | M | II | M | Rectum | 0.86 | 3.22 | 0.34 |
| Pt36 | 51 | F | II | M | Sigmoid | 43.32 | 14.42 | 6.22 |
| Pt38 | 80 | F | III | W | Right | 0.89 | 3.26 | 0.25 |
Differentiation: M indicates moderately; ND, not tested; P, poor; W, well.
CD133+ CRC Cells Are More Tumorigenic than Unseparated Cells
CD133 has been documented as a TIC marker in HT-29 cells [20]. To enrich CD133 highly expressed CRC cells, HT-29 and CRC patient's cells were magnetically labeled with anti-CD133 and separated by MACS column. As indicated by flow cytometry analysis for CD133 staining, magnetic cell sorting significantly enriched CD133high HT-29 cells (purity was enriched from 57% to 82% and mean fluorescence intensity for CD133 increased from 29 to 252) and CRC-Pt7 cells (from 7% to 71% and mean fluorescence intensity increased from 6 to 46; Figure W3).
In a limiting dilution assay [18], using different doses of unsorted HT-29 cells or MACS column-purified CD133high HT-29 cells (1 x 102 to 1 x 106) for tumor formation in nude mice with or without HK cells (1 x 106), we found, in the presence of HK cells, that CD133high HT-29 cells formed tumors with lower doses of cells compared with unsorted cells (Table 1). In fact, we were able to form tumors in 10 of 15 nude mice with as little as 100 CD133high HT-29 cells in the presence of HK cells (Table 1). At these low doses of HT-29 cells, no tumor was formed without HK cells. These data further demonstrate the CD133high cell population-enriched Co-TIC and the importance of HK cells in supporting tumor formation. When MACS column-purified CD133+ cancer cells and FACS sorting-enriched CD133+CXCR4+ cancer cells from CRC patients were used for tumor formation in NOD/SCID mice, in the presence of HK cells, 10,000 CD133+ cancer cells and 100 CD133+CXCR4+ cancer cells formed tumors. There was no tumor formation in the absence of HK cells (Figure 1B and Table 1). These data suggest that CD133+CXCR4+ CRC contain enriched Co-TICs.
FDC/HK Cells Protect Chemoresistant Co-TICs In Vitro and In Vivo
5-FU and oxaliplatin are standard chemotherapeutic agents used in the treatment of LN-positive CRC, but the development of chemoresistance and extranodal recurrences does occur [21]. Because Co-TICs are thought to be resistant to chemotherapy, we hypothesized that Co-TICs can be enriched by exposing CRC cells to chemotherapy and that HK cells will protect these chemoresistant cancer cells. Indeed, GFP-tagged HT-29 and CRC-Pt1 cell growth was inhibited by 5-FU or oxaliplatin treatment, and HK cells prevented this inhibition (Figure 4). After 72 hours of incubation in the presence of HK cells, CRC cell growth inhibition by suboptimal doses of chemotherapeutic agents was significantly reduced in both HT-29 cells and CRC-Pt1 cells (Figures 4A and W4). When HK cell-conditioned medium was used to substitute HK cells in the HT-29 cell culture, the inhibitory effects of the chemotherapeutic drugs were partially blocked (Figure 4, B and C). The surviving HT-29 cells from chemotherapy were enriched with CD133highCXCR4+ double-positive cell populations compared with media-treated control cells (Figure 4D).
This phenomenon was confirmed with in vivo tumor formation experiments. HT-29 cells were coinjected with HK cells in nude mice for tumor formation. Three weeks after the injection, tumor-bearing mice were randomly assigned to three groups: one was injected with phosphate-buffered saline (intravenously) as medium control, the second was injected with 5-FU (50 mg/kg, intravenously, three times per week), and the third group was injected with oxaliplatin (10 mg/kg, intraperitoneally, once a week). Tumor cells were collected for Co-TIC marker analysis by FACS 2 weeks after the treatments. Figure 4D shows CD133highCXCR4+ Co-TICs were enriched in residual tumor cells after chemotherapy treatments, suggesting that the presence of FDCs/HK cells promotes drug-resistant Co-TICs' survival and proliferation.
FDCs/HK Cells Support Co-TICs by Providing SDF-1α and Enhancing Tumor Angiogenesis
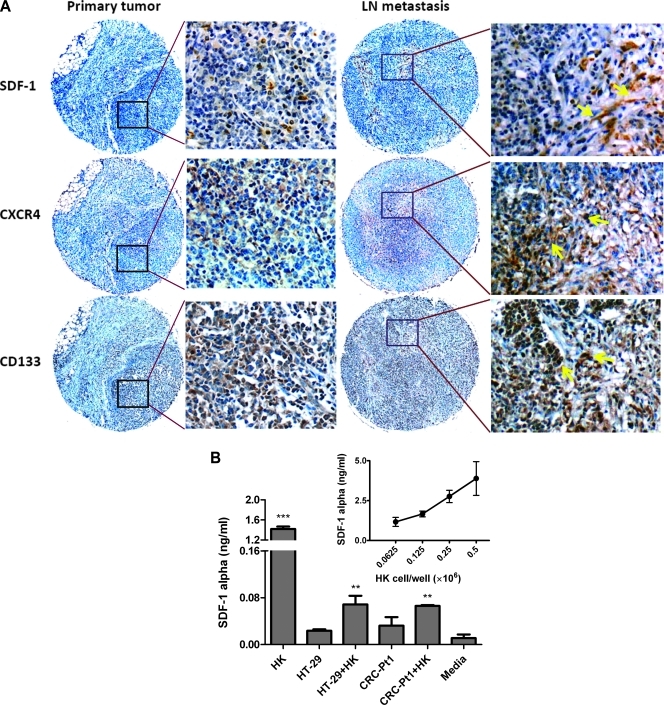
There are two receptors for SDF-1: CXCR4 and CXCR7 [22]. Studies have shown that SDF-1 and CXCR4 are involved in the metastatic process of CRC [22]. We first investigated the SDF-1α expression pattern in CRC LN metastasis. When a CRC specimen and its LN metastatic tumor from the same patient were stained with SDF-1α and CXCR4 (Figure 5A), spindle-like stromal cells in the LN but not CRC cells showed SDF-1α expression. CD133 and CXCR4 expressions were also increased in CRC metastatic LN compared with original tumor. Thus, it is possible that soluble SDF-1α produced by LN stromal cells supports CD133+CXCR4+ Co-TIC, which metastasized to the LN.
Figure 5.
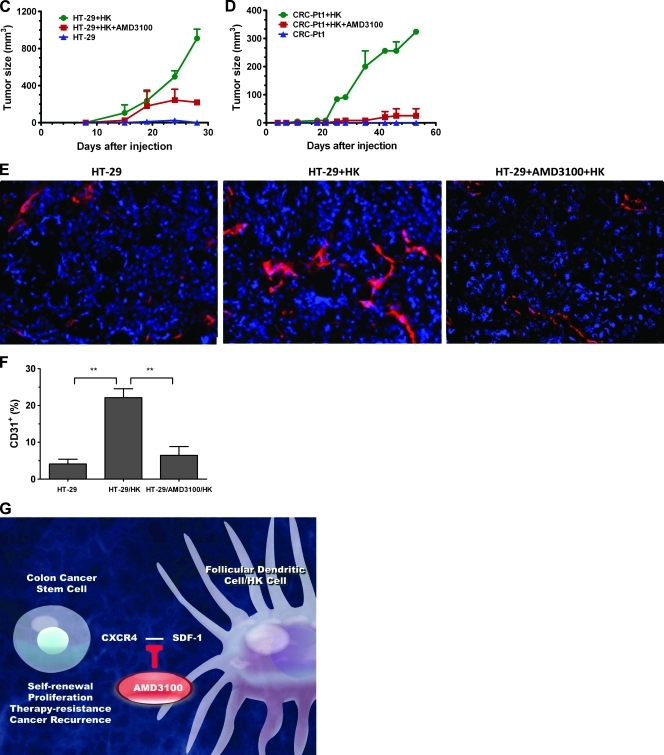
FDC/HK cells support Co-TIC by providing SDF-1α and enhance host angiogenesis. (A) Immunohistochemistry staining of colon cancer original tumor, LN metastasis paraffin slides for SDF-1α, CXCR4, and CD133 expression. Arrows show Ab staining-positive cells. (B) SDF-1α protein levels in 72 hours of culture supernatants of HK cells and/or colon cancer cells were measured by specific ELISA (R&D Systems). Insert shows SDF-1α levels in HK cell dose titration. (C and D) HK cells promote HT-29 (C) and CRC-Pt1 (D) cells to form tumors in nude and NOD/SCID mice, respectively. HT-29 or CRC-Pt1 cells (1 x 105) with or without HK cells (1 x 106) were subcutaneously injected into the posterior flank of immunodeficient mice. In some experiments, cancer cells were pretreated with AMD3100. Tumor sizes (mm3) were measured at the indicated time points. (E) Immunohistochemistry staining of frozen slides from tumors formed by HT-29 cells with or without HK cells using antimouse CD31 Abs (red). DAPI is used for nuclear staining (blue). Images were captured by a deconvoluting microscope using SlideBook software. Original magnification, x400. (F) Quantitative analysis of CD31-positive staining (% area) in tumors formed with HT-29 cells. The analysis was done with PhotoShop 7.0 software. (G) Cartoon showing in vitro and in vivo Co-TIC/stromal cell coculture models.
To investigate the role of SDF-1α in our CRC tumor model, we examined the expression of SDF-1α in HK cells. Figure 5B (insert) shows accumulative SDF-1α production in the culture supernatant after 72 hours of incubating with titrated doses of HK cells. Minimal expression of SDF-1α was detected in either HT-29 or CRC-Pt1 cell cultures. The protein level of SDF-1α was depleted when HK cells and CRC cells were cocultured, indicating CRC cells' consumption of this chemokine (Figure 5B). To determine the role of SDF-1α produced by HK cells in tumor formation, we used AMD3100, a CXCR4-specific small-molecule inhibitor that blocks SDF-1α/CXCR4 interaction. Pretreatment of CRC cells with AMD3100 partially inhibits the HK cell-assisted tumor formation in both HT-29 and CRC-Pt1 cells (Figure 5, C and D).
SDF-1 is also known to support tumor angiogenesis [23]. Tumor vasculature was detected in tumors formed by HT-29 cells and HK cells. An Ab against the mouse endothelial cell marker CD31 was used to reveal host angiogenesis. Comparing CD31-positive staining between tumors formed by HT-29 cells alone and by HT-29 plus HK cells, the presence of HK cells significantly enhanced host vasculature development (CD31-positive; Figure 5, E and F, red staining). This effect was inhibited by AMD3100 treatment of CRC cells before cell injection, further supporting our hypothesis that stromal HK cells may assist CRC cells' tumor formation through SDF-1α and CXCR4 paracrine signaling.
Discussion
The current study indicates that stromal FDCs enhance Co-TIC tumor formation in the LN microenvironment (Figure 5G). We have established in vitro and in vivo experimental methods using CRC patient specimens, HT-29, and HCA-7 cells with FDC (in the form of the human FDC cell line HK) [24]. HK cells express smooth muscle antigen, von Willebrand factor, and vimentin, but not CD31, indicating that it is a stromal cell (Figure W5, A and B). Although derived from tonsilar cells, the HK cell line functionally resembles primary FDCs in supporting germinal center B cells and lymphomas [25,26]. Using co-culture and coimplantation tumor xenograft models, we demonstrated that FDCs/HK cells significantly promote the survival and growth of CRC cells, preferentially CD133+CXCR4+ Co-TICs. FDCs play a central role in promoting tumor growth and tumor angiogenesis by recruiting CD31+ endothelial progenitor cells. These effects are mediated in part by a soluble factor SDF-1α because the CXCR4 antagonist AMD3100 partially inhibited FDC-enhanced tumor formation in immunodeficient mice. This function of stromal FDC is similar to that of carcinoma-associated fibroblasts as seen in breast cancer [27]. These in vitro and in vivo models are valuable in further elucidating the effects of LN stromal microenvironment on Co-TICs.
Co-TICs have been found to express a variety of surface markers, including CD133 [3]. CD133 is a transmembrane glycoprotein molecule with a molecular weight of 120 kDa on chromosome 4p15.32. CD133+ cancer cells were shown to have the ability to self-renew, retain tumorigenicity, and regenerate a tumor after treatment [18]. Li et al. [28] have shown that, in stage IIIB colon cancer, recurrence correlated with the percentage of CD133+ cells present in the original tumor. Whereas CD133+ cells have been shown to meet all the characteristics of TIC, its specificity as a true Co-TIC marker has been questioned [29]. Other markers such as aldehyde dehydrogenase 1, epithelial specific antigen (Ep-CAM or CD326), CD44, and CD166 have also been used to identify Co-TICs. More recently, Wnt signaling activity combined with cellular interaction with the microenvironment has been deemed as a marker for Co-TIC [30]. In our experiments, we primarily used CD133 as Co-TIC marker to enrich TICs from HT-29 cells and CRC patient cells. These cells were able to initiate tumors in immunodeficient mice in the presence of HK cells and HK cell-conditioned media. Although the ideal Co-TIC marker is yet to be determined, we were able to show that a small proportion of Co-TICs display CD133+CXCR4+. Cell sorting with an additional marker increased the target cancer cell's tumor-initiating ability by 100-fold (Table 1), agreeing with the observations of Dalerba et al. [31]. In addition, double-positive cells were enriched in chemotherapy-resistant cell population. Our data indicate that CD133 and CXCR4 may be better markers for drug-resistant Co-TICs.
The 5-year survival rate of the American Joint Committee on Cancer stage I CRC is 90% and decreases to 75% and 50% for stage II and III patients, respectively. Tumor invasion and regional LN metastases are important factors for determining CRC prognosis. Prognostic markers are urgently needed for CRC, especially for stage II patients. Recently, several studies have focused on such prognostic markers. Saiki et al. [32], using reverse transcription-polymerase chain reaction analysis, revealed that the expression of stem cell-related genes, including LIN28 and SOX2, correlated with LN metastasis. Although the CRC tumor tissues that were examined had been collected by laser microdissection, these results may indicate that the Co-TIC population is increased in metastatic sites. When putative TIC markers were tested in CRC tumor buds, ABCG5 and EpCAM were significantly associated with a poorer prognosis [33]. However, most published studies have been conducted with bulk tumor samples without differentiating the response between Co-TIC and non-TIC. Because Co-TICs are such a small population, precise analysis of prognostic markers within Co-TICs may yield more accurate prognostic significance.
SDF-1α is one of many soluble microenvironmental factors produced by FDCs in a paracrine fashion [34]. The SDF-1α/CXCR4 axis is associated with in vitro CRC migration, lymphatic and distant dissemination, disease recurrence, and decreased survival rate [9,11,35]. In our experiments, drug-resistant CRC cells showed an increased expression of CD133 and CXCR4. Our observation is in agreement with those of Dessein et al. [36]; they recently demonstrated that induction of CXCR4 acts as a major mechanism underlying invasion in drug-resistant colon carcinoma HT-29 cells. This phenomenon is not limited to CRC because CD133+CXCR4+ migrating TICs are shown to play a crucial role in tumor initiation, growth, and metastasis in human pancreatic, prostate, and breast cancers [12,37].
Although it has been widely accepted that only rare TIC populations within human leukemia can seed leukemia in mice [38], this assertion about TICs is still controversial for solid tumors. In solid tumors, most in vivo studies generate human tumor xenografts from immunodeficient mice, a foreign microenvironment for human cancers. It was shown that as few as 100 purified TICs could initiate tumors in immunodeficient mice [39]. It is unclear whether this phenomenon represents a selection of surviving cells in a foreign environment or whether it is due to the presence of TIC because, in syngeneic animals, a dominant cell population rather than rare TICs seems to sustain many tumors. In our in vivo model, human stromal HK cells were coinoculated with CRC patient cells, recreating a humanized microenvironment similar to a LN, a common destination for CRC metastasis. A similar method was also reported in breast cancer studies [40], where an orthotopic xenograft model was established in which both the stromal and epithelial components of the reconstructed mammary gland were of human origin, providing the proper environment for the development of human mammary epithelium. Our data show that coinjection of HK cells is essential for CD133+ Co-TIC to form tumor in immunodeficient mice and that HK cells selectively support Co-TICs' survival from chemotherapeutic drugs. Indeed, cells positive for ABCG2, a multidrug pump, were also positive for CD133 and CXCR4 (Figure W2B).
The existence of Co-TICs may be the underlying reason for chemotherapeutic drug resistance. Indeed, when treated with standard chemotherapeutic agents for CRC, both in vitro and in vivo chemotherapeutic treatment-resistant HT-29 cells were enriched with CD133+CXCR4+ Co-TIC. Our data are consistent with the findings by Dylla et al. [41] and Todaro et al. [42] in that Co-TICs were enriched in colon tumors after treatment with chemotherapeutic drugs. These cells remained capable of rapidly regenerating phenotypical similar tumors. In our models, both HT-29 and CRC patient cells, Co-TICs were enriched by chemotherapy in the presence of HK cells. This enrichment suggests that targeting Co-TICs alone may not suffice to minimize recurrence and that future treatments must target LN microenvironmental support as well.
The intriguing result from our stromal cell or stromal cell-conditioned media/colon cancer cell coculture and coimplantation studies is that LN stomal cells provide SDF-1α to assist CXCR4-expressing CD133+ Co-TICs in xenoplant tumor formation of both CRC patient tumor cells and HT-29 cells. It is likely that drug-resistant CD133+CXCR4+ Co-TICs are regulated in a paracrine manner by SDF-1α derived from HK cells (Figure 5G). AMD3100 partially blocked the stromal/colon cancer cell tumor formation, indicating that SDF-1α is crucial to CD133+CXCR4+ Co-TICs. This finding is in agreement with a recent report that Co-TIC marker Wnt-signaling activity was regulated by the microenvironmental myofibroblast-secreted factors [30], implicating the microenvironment as a dominant factor in Co-TICs. FDCs may promote Co-TIC tumor growth in two ways: 1) by supporting Co-TIC survival and activation for tumor initiation by providing soluble factors, such as SDF-1α; and 2) by enhancing the host's response to increase tumor angiogenesis.
In addition to providing evidence in support of the TIC hypothesis, this study provides multiple lines of evidence that Co-TICs serve a crucial role in CRC tumor growth and chemotherapy drug resistance. Even more importantly, it determines the influence of LN stromal cells on Co-TICs' survival, chemoresistance, and stimulation. We have identified a major Co-TIC/LN microenvironment-specific mechanism of chemotherapeutic resistance and established both in vitro and in vivo platforms for testing novel agents against both Co-TIC and LN microenvironmental factors. Our data indicate that SDF-1α from LN stromal cells and its receptor CXCR4 are possible targets for clinical therapy (Figure 5G). Closer scrutiny of TIC and its supporting LN microenvironmental factors will lead to a better understanding of mechanisms of extranodal CRC recurrence and metastasis and, ultimately, to novel targeted therapies.
Supplementary Material
Acknowledgments
The authors thank Yong Sung Choi and David Beck for scientific discussion, Rosemary Velasquez for pathology expertise, Rachel Regn and Yang D. Zhao for excellent technical assistance, and Heidi Davis for editorial assistance.
Footnotes
This article refers to supplementary materials, which are designated by Table W1 and Figures W1 to W5 and are available online at www.neoplasia.com.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 4.SEER Cancer Statistics Review. [April 18, 2011]. Available at: http://seer.cancer.gov/statfacts/html/colorect.html.
- 5.Nakagawa H, Liyanarachchi S, Davuluri RV, Auer H, Martin EW, Jr, de la Chapelle A, Frankel WL. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene. 2004;23:7366–7377. doi: 10.1038/sj.onc.1208013. [DOI] [PubMed] [Google Scholar]
- 6.Kouniavsky G, Khaikin M, Zvibel I, Zippel D, Brill S, Halpern Z, Papa M. Stromal extracellular matrix reduces chemotherapy-induced apoptosis in colon cancer cell lines. Clin Exp Metastasis. 2002;19:55–60. doi: 10.1023/a:1013880326925. [DOI] [PubMed] [Google Scholar]
- 7.Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, Shenoy AK, Brank A, Russell KJ, Otero J, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114:4310–4319. doi: 10.1182/blood-2009-03-211342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 10.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 11.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 12.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;155:1101–1109. [PubMed] [Google Scholar]
- 14.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Picard O, Rolland Y, Poupon MF. Fibroblast-dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res. 1986;46:3290–3294. [PubMed] [Google Scholar]
- 16.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 17.Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 19.Horst D, Scheel SK, Liebmann S, Neumann J, Maatz S, Kirchner T, Jung A. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009;219:427–434. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 20.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 21.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, II, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–2961. [PubMed] [Google Scholar]
- 25.Li L, Choi YS. Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells. Semin Immunol. 2002;14:259–266. doi: 10.1016/s1044-5323(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Yoon SO, Fu DD, Zhang X, Choi YS. Novel follicular dendritic cell molecule, 8D6, collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line, L3055. Blood. 2004;104:815–821. doi: 10.1182/blood-2004-01-0292. [DOI] [PubMed] [Google Scholar]
- 27.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Li CY, Li BX, Liang Y, Peng RQ, Ding Y, Xu DZ, Zhang X, Pan ZZ, Wan DS, Zeng YX, et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med. 2009;7:56. doi: 10.1186/1479-5876-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 31.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saiki Y, Ishimaru S, Mimori K, Takatsuno Y, Nagahara M, Ishii H, Yamada K, Mori M. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann Surg Oncol. 2009;16:2638–2644. doi: 10.1245/s10434-009-0567-5. [DOI] [PubMed] [Google Scholar]
- 33.Hostettler L, Zlobec I, Terracciano L, Lugli A. ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J Gastroenterol. 2010;16:732–739. doi: 10.3748/wjg.v16.i6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husson H, Carideo EG, Cardoso AA, Lugli SM, Neuberg D, Munoz O, de Leval L, Schultze J, Freedman AS. MCP-1 modulates chemotaxis by follicular lymphoma cells. Br J Haematol. 2001;115:554–562. doi: 10.1046/j.1365-2141.2001.03145.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 36.Dessein AF, Stechly L, Jonckheere N, Dumont P, Monte D, Leteurtre E, Truant S, Pruvot FR, Figeac M, Hebbar M, et al. Autocrine induction of invasive and metastatic phenotypes by the MIF-CXCR4 axis in drug-resistant human colon cancer cells. Cancer Res. 2010;70:4644–4654. doi: 10.1158/0008-5472.CAN-09-3828. [DOI] [PubMed] [Google Scholar]
- 37.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 38.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 39.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 40.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.