Abstract
Aerobic fitness and adiposity are each independently associated with health outcomes among children, although the relationship between these two variables is unclear. Our objectives were to evaluate 1) the association of adiposity with aerobic fitness using objectively measured levels of percent body fat, compared to body mass index (BMI) as a percentile proxy for adiposity while controlling for genetic admixture, and 2) the congruence of BMI categories with high and low body fat categories of objectively measured percent body fat. Participants were 232 African-American, European-American, and Hispanic-American children aged 7-12 years (Tanner stage ≤ 3). Aerobic fitness was measured via a submaximal indirect calorimetry treadmill test (VO2-170), and physical activity levels with accelerometry. Genetic admixture estimates were obtained using 140 genetic ancestry informative markers to estimate European, African, and Amerindian admixture. Fat mass was determined using dual-energy x-ray absorptiometry. Children were classified into a low body fat group (< 25% in males, < 30% in females) or a high body fat group based on their percent body fat; children were also categorized according to BMI percentile. Children in the low body fat group had significantly higher aerobic fitness (P < 0.05) regardless of BMI percentile classification. Higher African genetic admixture was associated with lower aerobic fitness (P < 0.05), while physical activity was positively associated with fitness (P < 0.01). In conclusion, aerobic fitness levels differ by percent body fat and genetic admixture irrespective of BMI classification, and such differences should be taken into account when evaluating outcomes of health interventions.
Keywords: adiposity, body mass index, children, ethnic groups, physical fitness
Introduction
Aerobic fitness has been inversely associated with risk for several chronic diseases in both children and adolescents (1-3). Lower body fat levels are also indicative of decreased chronic disease risk, and an inverse association of fitness with body fat levels has been noted (4). Increased aerobic fitness may therefore be an important outcome of interventions designed to reduce the frequency of high body fat, insulin resistance, and elevated blood pressure among children. Studies that measure fitness levels among children often utilize gender- and age-adjusted body mass index (BMI; weight [kg]/height [m2]) percentiles to recruit participants classified as overweight/obese, in which a BMI ≥ 85th percentile is considered overweight, and BMI ≥ 95th percentile categorized as obese (5). However, a higher pediatric BMI is often caused by greater amounts of both lean and fat mass, and the actual contribution of excess adiposity to aerobic fitness may be masked if BMI cut points are used to estimate excess adiposity (6, 7). Recent studies have shown that not all children classified as overweight or obese present with excess body fat (8); however, no studies have evaluated whether this discrepancy could influence outcomes in aerobic fitness.
Recent investigations have also identified racial/ethnic differences in aerobic fitness, as well as in the relationship between BMI and body fat (4, 9-11). Racial/ethnic differences in fitness as measured by maximum oxygen consumption (VO2-max) among African-American (AA) and European-American (EA) children and adults are well documented, with a lower VO2-max in AA independent of body weight (9, 12). Other studies have indicated that Hispanic-American (HA) children may present with aerobic fitness levels similar to or lower than those of EA children (10, 11). Additionally, traditional cut points for overweight and obese classification may represent different levels of body fat at the same BMI or BMI percentile in AA and Hispanic-American (HA) compared to EA children (8, 13). For example, an AA child at the 85th percentile for BMI may have low body fat levels, while an HA child may present with excess body fat at a BMI < 85th percentile. These observed racial/ethnic differences in aerobic fitness and fat mass accrual may respond to cultural, behavioral and genetic factors. When exploring biological determinants of health, genetic admixture has been used to explain individual variability in metabolic outcomes (14-17). Many individuals in the United States exhibit mixed racial ancestry inherited from European, African, and Amerindian parental populations (17, 18). Use of genetic admixture in pediatric fitness studies could therefore allow for more accurate analyses of which factors are associated with higher aerobic fitness.
Our main objective was to evaluate the association of adiposity with aerobic fitness using objectively measured levels of percent body fat, compared to BMI as a percentile proxy for adiposity. Genetic admixture, pubertal status and moderate/vigorous physical activity were studied as covariates. Our secondary objective was to evaluate the congruence of the BMI overweight and normal weight categories with the high and low body fat categories of objectively measured percent body fat. We hypothesized that: 1) aerobic fitness levels (expressed as VO2-170/kg) would be lower among children with higher percent body fat independent of BMI classification, and 2) differences will be observed in the classification of children with excess adiposity when BMI percentile is used versus objectively measured percent body fat.
Methods and Procedures
Subjects
Two-hundred thirty-two children identified by parents/guardians as AA (n=75), EA (n=90), or HA (n=67) aged 7-12 years were recruited from the Birmingham, Alabama area to investigate genetic and environmental contributions to racial/ethnic differences in metabolic outcomes. The children were pubertal stage ≤ 3 as assessed by a pediatrician according to the criteria of Marshall and Tanner (19-21). One composite number is assigned for Tanner staging, representing the higher of the two values defined by breast/genitalia and public hair. Exclusion criteria included diagnoses of type1 or type 2 diabetes, any glucose or lipid disorders, or use of any medication known to affect body composition or metabolism. Before participating in the study, the nature, purpose, and potential risks were carefully explained to the parents and children. The children and parents provided informed consent and assent, respectively to the protocol, which was approved by the Institutional Review Board for human subjects at the University of Alabama at Birmingham (UAB).
Protocol
All measurements for this analysis were performed at the UAB Department of Nutrition Sciences (DNS) and General Clinical Research Center (GCRC) between 2004 and 2008. For this protocol, participants attended two visits. At the first, participants arrived at the DNS where a physician conducted a complete medical history and physical exam, during which pubertal status was assessed. Body composition, aerobic fitness measurements, and demographic information including socioeconomic status were also obtained. The children were then fitted with an accelerometer which they were asked to wear for seven days to objectively assess physical activity. During the second visit, blood samples for genetic admixture analysis were collected at the GCRC and accelerometers collected.
Body Composition
Body weight was measured to the nearest 0.1 kg in light clothing without shoes (Scale-tronix 6702W; Scale-tronix, Carol Stream, IL). Height was determined without shoes using a mechanical stadiometer. Body mass index (BMI; weight kg / height m2) was calculated from these values, and BMI percentile was determined using age- and sex-specific Center for Disease Control and Prevention growth charts (22).
Total fat mass and lean mass were evaluated via dual energy x-ray absorptiometry (DXA) with a GE Lunar Prodigy densitometer (Lunar Radiation Corp., Madison, WI). Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. DXA scans were analyzed with pediatric software enCORE 2002 version 6.10.029. Children were separated into two body fat groups according to the criteria established by Williams et al for a multiethnic population (23). All children meeting the criteria for normal body fat levels (boys with < 25% and girls with < 30% body fat) were placed into the low body fat group (n = 164), whereas children exceeding those parameters were placed into the high body fat group (n = 68).
Aerobic Fitness
VO2-170 was determined by indirect calorimetry on a treadmill. Children were given the opportunity to familiarize themselves with the treadmill prior to beginning the test. Participants then walked on a flat incline at two-and-a-half mph for four minutes. Speed was then increased to three mph for the remainder of the test, with the incline increasing 2% every two minutes until the child reached a heart rate greater than 170 beats per minute (bpm). Heart rate (HR) was monitored with the Polar Vantage XL HR monitor (Polar Beat, Port Washington, NY). Volumes of O2 and CO2 were measured continuously using open circuit spirometry until recording the VO2 level at a heart rate of 170 bpm using a Max-II metabolic testing system (PHYSIO-DYNE, Quogue, NY). Data were analyzed as described by Gutin et al. (24) and expressed as absolute VO2-170 (L·min-1) and VO2-170 adjusted for body weight (mL·kg-1·min-1). Additionally, it is possible that adjusting VO2-170 by body weight mathematically penalizes heavier individuals who may have more lean mass; hence we also present VO2-170 adjusted by total DXA lean mass (mL·kgLM-1·min-1).
Physical Activity
Children wore an MTI Actigraph accelerometer (GT1M, ActiGraph Health Services, Pensacola, FL) for seven days to objectively measure physical activity levels. Epoch length was set at one minute and results were expressed as counts per minute (counts/min-1). Children wore the monitor on an elastic belt at the waist over the right hip, with removal only occurring during activities such as sleeping, bathing and swimming. Actigraph monitors have been shown to exhibit a high degree of inter-instrument reliability (25). Daily counts per minute greater than 1952 counts/min-1 were summed and analyzed as the average time spent in moderate and vigorous physical activity (MVPA).
Genetic admixture
Genotyping of the ancestry informative markers (AIMs) for the measurement of genetic admixture was performed at Prevention Genetics (www.preventiongenetics.com) using the Chemicon Amplifuor SNPs Genotyping System coupled with ArrayTape technology (www.global-array.com). A panel of 140 AIMs was used to estimate the genetic admixture proportion of each subject. Molecular techniques for the allelic identification and methodology for genetic admixture application have been described elsewhere (15, 26) and information regarding marker sequences, experimental details, and parental population allele frequencies has been submitted to dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) under the handle PSU-ANTH (15). The ancestry estimates from AIMs was translated into estimates of African, European, and Native American admixture for each subject using maximum likelihood (ML) estimation based on the ML algorithm described by Hanis et al (14, 27). In brief, the ML method estimates the proportion of genetic ancestry for an individual, using a range of proportions from 0 to 1 and identifies the most probable value of admixture based on the observed genotypes.
Socioeconomic Status
Socioeconomic status (SES) has been shown to influence factors that impact aerobic fitness and was measured with the Hollingshead 4-factor index of social class, which combines the educational attainment and occupational prestige for the number of working parents in the child’s family (28). Scores ranged from 8 to 66, with the higher score indicating higher theoretical social status.
Statistical Analysis
Differences in descriptive statistics between low and high body fat groups were explored with independent samples t-tests. The percentage of children by body fat group within each BMI category (normal weight, overweight, obese) were also computed. Analysis of covariance (ANCOVA) was then used to evaluate the difference in aerobic fitness for low-versus high-body fat groups within the normal weight BMI category. Due to small sample size in the obese/high fat group, these participants were combined with the overweight/high fat group for ANCOVA analysis in an overweight/obese BMI category. Models evaluating differences in VO2-170 were controlled for lean mass, fat mass, pubertal status (Tanner stage), age, sex, MVPA, SES, and genetic admixture. Models evaluating differences in VO2-170/kg body weight and VO2-170/kg lean mass were controlled for pubertal status (Tanner stage), age, sex, MVPA, SES, and genetic admixture. The sum total of the three admixture estimates is equal to one; therefore, European admixture was used as the reference group and only African and American admixture were included in the models. Linear regression was then utilized to analyze the association of 1) lean mass, 2) fat mass, and 3) lean and fat mass, and genetic admixture with VO2-170 (L·min-1) in the total sample and within body fat groups after also controlling for the above covariates. Results reported here are for the models including both lean and fat mass. Orthogonal coding was utilized for pubertal status in all models, with contrasts chosen for vector analysis based on the hypothesis that children classified at Tanner stage 3 would be different from children classified at Tanner stages 1 and 2. Dummy coding was used for the covariate sex to test the association of female sex with aerobic fitness (0 = male, 1 = female). All analyses were performed using SAS statistical software version 9.2 (SAS Institute, Cary, NC, 2002) with a significance level established at P < 0.05.
Results
Table 1 presents the study population characteristics by body fat group. The high fat group presented with a greater percentage of Amerindian admixture and slightly higher pubertal stage (P < 0.05). Children in this group were also taller, had more fat mass and lean mass, and participated in less physical activity compared to the low fat group (all at P < 0.01). When analyzed by BMI percentile categories, approximately 12% of children classified as normal weight had high body fat (Figure 1). Conversely, approximately 49% of children classified as overweight and 9% classified as obese had low body fat levels.
Table 1.
Descriptive characteristics by body fat group
| Total Sample (n=232) | Low Body Fat (n=164) | High Body Fat (n=68) | |
|---|---|---|---|
| Female Sex (n,%) | 108 (46.6) | 77 (46.7) | 31 (46.3) |
| Race(AA/EA/HA) | 75/90/67 | 58/73/33 | 17/17/34b |
| Tanner stage | 1.5 ± 0.7 | 1.4 ± 0.7 | 1.7 ± 0.7a |
| Age | 9.6 ± 1.5 | 9.5 ± 1.6 | 9.9 ± 1.4 |
| Height (cm) | 139.5 ± 10.1 | 138.4 ± 10.2 | 142.2 ± 9.7 b |
| Weight (kg) | 36.7 ± 9.0 | 33.4 ± 6.4 | 44.7 ± 9.7 b |
| BMI (kg/m2) | 18.6 ± 2.9 | 17.3 ± 1.6 | 21.9 ± 2.8 b |
| BMI percentile | 66.7 ± 25.4 | 57.8 ± 24.7 | 88.6 ± 8.2 b |
| Lean mass (kg) | 25.8 ± 5.1 | 25.3 ± 4.9 | 27.2 ± 5.3 b |
| Fat mass (kg) | 8.8 ± 5.4 | 6.1 ± 2.3 | 15.3 ± 5.1 b |
| Body fat (%) | 23.2 ± 9.0 | 18.5 ± 5.6 | 34.2 ± 5.5 b |
| MVPA (min/day) | 59.4 ± 34.1 | 66.2 ± 36.3 | 44.2 ± 23.2 b |
| SES | 38.8 ± 14.8 | 41.6 ± 14.0 | 33.2 ± 14.7 b |
| Genetic Admixture: | |||
| European | 53.9 ± 37.3 | 55.9 ± 39.0 | 48.7 ± 32.9 |
| African | 27.4 ± 36.8 | 31.2 ± 38.7 | 19.3 ± 30.5a |
| Amerindian | 18.7 ± 25.3 | 13.0 ± 20.7 | 31.9 ± 30.1 b |
| VO2-170 (L·min-1) | 1.08 ± 0.30 | 1.04 ± 0.29 | 1.17 ± 0.32 b |
| VO2-170 (mL·kg-1·min-1) | 29.68 ± 6.04 | 31.13 ± 5.86 | 26.33 ± 5.13 b |
| VO2-170 (mL·kgLM-1·min-1) | 41.63 ± 7.29 | 41.14 ± 7.07 | 42.89 ± 7.82 |
Values are mean ± Std or n, %.
= P < 0.05;
= P < 0.01 for significant body fat group difference.
BMI = Body Mass Index; MVPA = Moderate/Vigorous Physical Activity; SES = Socioeconomic Status
Figure 1.
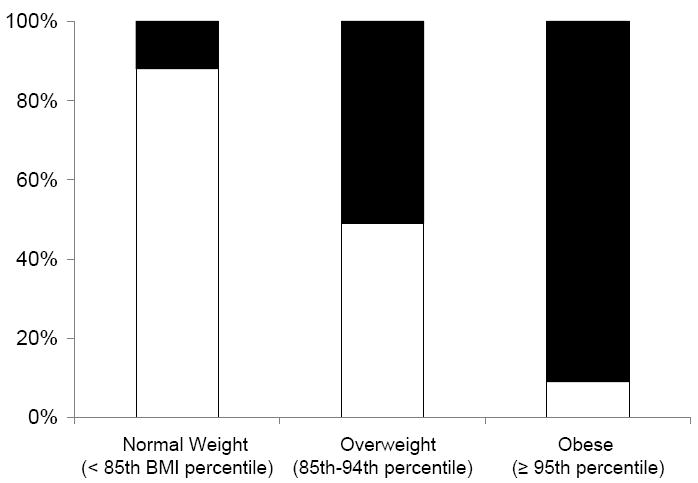
Percentage of children classified at low-fat and high-fat by BMI percentile category
White bars = low fat group; black bars = high fat group
Results of ANCOVA models by BMI category and body fat group are presented in Table 2. Participants at normal weight but with high body fat presented with higher unadjusted VO2-170 (L·min-1; P < 0.05) but lower VO2-170/kg total weight than those with low body fat. There was no difference between the two groups in VO2-170/ mL·kgLM-1·min-1. Participants classified as overweight/obese but with low body fat had a higher VO2-170/kg compared to those with high body fat. There was no difference by body fat group in VO2-170/ mL·kgLM-1·min-1among children classified as overweight/obese. However, when only participants classified as overweight were analyzed, there was a trend for greater VO2-170/ mL·kgLM-1·min-1 in those classified as overweight but with low body fat (P = 0.075).
Table 2.
Adjusted means ± SE of aerobic fitness by BMI percentile category and body fat group
| Normal Weight | Overweight/Obese | |||
|---|---|---|---|---|
| Low Fat | High Fat | Low Fat | High Fat | |
| n | 134 | 18 | 30 | 50 |
| VO2-170 (L·min-1) | 1.04 ± 0.02 | 1.11 ± 0.05a | 1.17 ± 0.06 | 1.18 ± 0.05 |
| VO2-170 (mL·kg-1·min-1) | 31.44 ± 0.51 | 28.72 ± 1.12b | 31.97 ± 1.02 | 25.61 ± 0.82a |
| VO2-170 (mL·kgLM-1·min-1) | 41.41 ± 0.60 | 43.94 ± 1.74 | 45.00 ± 1.45 | 42.48 ± 1.35 |
Means for VO2-170 (L·min-1) adjusted for lean mass, fat mass, sex, pubertal status, age, moderate/vigorous physical activity, socioeconomic status, African and Amerindian admixture. Means for VO2-170 (mL·kg-1·min-1) and VO2-170 (mL·kgLM-1·min-1) adjusted for sex, pubertal status, age, moderate/vigorous physical activity, socioeconomic status, African and Amerindian admixture.
SE = standard error; BMI = body mass index; P values are for comparisons of Low Fat versus High Fat groups within each BMI percentile category
= P < 0.05;
= P < 0.10 trend for significance
Among the total sample, linear regression analysis indicated an inverse association between VO2-170 (L·min-1) and African Admixture (P < 0.01), while increased lean mass (P < 0.01) and MVPA were associated with higher fitness (P = 0.01). When analyzed by body fat group, African admixture, lean mass and MVPA remained associated with VO2-170 (L·min-1) in participants with low body fat (P < 0.05). However, only African admixture (P = 0.05) and lean mass (P < 0.01) were associated with VO2-170 (L·min-1) in the high fat group (P < 0.05). Results were consistent when aerobic fitness was analyzed as VO2-170/kg of total body weight and lean mass. Total fat mass and female sex were significantly, negatively associated with aerobic fitness only when MVPA was not included in the model (data not shown). Since MVPA was associated with aerobic fitness in the low fat but not the high fat group, a fat mass by MVPA interaction was tested for in the total sample. However, no significant interaction was identified.
Discussion
This investigation evaluated the association of adiposity with aerobic fitness and the extent to which genetic admixture may play a role in this relationship in a multi-ethnic pediatric population. Lower body fat was associated with greater aerobic fitness irrespective of BMI classification, while African genetic admixture was associated with lower aerobic fitness. This suggests that levels of body fat, rather than BMI category, should be considered when designing interventions that include aerobic fitness as an outcome.
Several studies in children and adults have suggested that BMI may be an imprecise measure of body fat, which is of concern when evaluating the effect of adiposity on aerobic fitness (8, 13). Such interventions may improve a participant’s body composition even though no significant change is weight is noted. Hence, the use of BMI alone as an index of adiposity might lead to incorrect conclusions about the outcome of the intervention. Romero-Corral and colleagues found that BMI was a poor indicator of percent body fat among men and women (29). Similarly, Flegal et al noted that less than half of children and adolescents who were classified as overweight actually had high levels of body fat (8). This is consistent with our finding that half of the children categorized as overweight in this study actually had low body fat. Interestingly, when aerobic fitness in these two groups was compared, those with low body fat had higher fitness levels than children with high body fat. This suggests that when interventions are designed to improve body fat and aerobic fitness levels in children considered overweight/obese, estimation of body fat percent may be a more accurate indicator than BMI category to determine which children might benefit most from the intervention.
We also noted an association of African genetic admixture with lower aerobic fitness among the total sample and when analyzed within body fat groups. Previous studies that have used the racial/ethnic group classifications of African-American and European-American have reported lower fitness in African-American children and adults (10, 30). Roy et al attributed ethnic differences in VO2-max between AA and EA women to reduced ability of the cardiovascular system to deliver oxygen to muscle and reduced mitochondrial function as measured by prolonged adenosine diphosphate (ADP) time constants (measure of post-exercise recovery time) (12). That work suggests contributions to fitness aberrations by both lower oxygen carrying capacity and muscle oxidative aerobic capacity, and complements other findings of a lower proportion of type I oxidative skeletal muscle fibers among AA women (12, 31). Hence, it is likely that variations in fitness within our sample are at least partially attributable to inherent differences in muscle physiology that could result from genetic background.
Although we controlled for both pubertal status and African admixture, it is also plausible that the higher pubertal status noted in AA (Tanner stage 1.7 ± 0.8) versus EA and HA (both 1.4 ± 0.66) could have been associated with the lower fitness noted in individuals with higher African admixture. Higher pubertal status is associated with lower aerobic fitness and our AA children tended to have a higher pubertal status at a given age compared to other racial/ethnic groups. Additionally, Lohman et al. reported a fat mass by racial/ethnic group (African-American versus other) interaction effect on aerobic fitness in a multiethnic sample of eighth-grade girls (4). When we included race/ethnic group as a covariate in our model in place of genetic admixture, we also noted a fat mass by racial/ethnic group interaction (data not shown). However, this interaction was not present when genetic admixture was included in the models. Race/ethnicity represents a social (rather than biological) construct that is typically based on unique cultural and behavioral practices that could affect metabolic pathways and disease risk independent of genetic admixture. Unmeasured environmental factors associated with racial/ethnic group, rather than African racial ancestry itself, could account for the interaction with fat mass seen with racial/ethnic group but not with genetic admixture estimates.
We did not detect an association of Amerindian admixture with aerobic fitness, though participants in the high body fat group had higher estimates of Amerindian ancestry. Among our participants, Amerindian ancestry was highest in those self-classified as Hispanic-American. Current literature regarding fitness levels in HA compared to EA and AA youth is equivocal. Shaibi and colleagues evaluated fitness differences by VO2-peak in 73 early pubertal and pubertal normal- and overweight boys and girls. Both AA and HA groups had significantly lower fitness than EA children and adolescents (10). Conversely, Treuth et al observed no differences in fitness between EA and HA girls (11). In our investigation, HA participants presented with aerobic fitness levels similar to EA children. Additional investigations are warranted to determine how Amerindian admixture and Hispanic-American culture may be associated with aerobic fitness levels.
We also reported that total body fat and sex were only associated with aerobic fitness when physical activity (MVPA) was not included in statistical models. Participants with high body fat were significantly less physically active, suggesting that physical activity may mediate the relationship between adiposity and fitness. Several studies have investigated the association of physical activity and aerobic fitness during weight loss or longitudinal follow-up studies; however, further research is needed to evaluate whether increased physical activity significantly improves pediatric aerobic fitness without changes in body fat (4, 32-34). Additionally, investigations among adults have reported higher aerobic fitness in men compared to women (35). Among children, aerobic fitness is also higher in boys compared to girls (36); however, during early puberty this difference is less likely due to sex hormone differences compared to physical activity levels. Other studies are in agreement that increased physical activity is associated with higher fitness levels (4, 34), and may have a stronger influence on aerobic fitness than sex prior to puberty.
Our study benefited from measurement of racial genetic ancestry in a multiethnic population with both boys and girls, and an increased precision of body composition measures using DXA. This study was limited by the use of a sample of HA children predominately of Central- and South American origin with higher levels of Amerindian ancestry. Hence, these results may not be applicable to HA children with origins outside those regions. Additionally, the cut-off values used to determine low and high fat for this study were those computed to detect cardiovascular disease risk by Williams et al. from skinfold measures. It is possible that these values may differ for other chronic disease risk factors, and that the values may slightly differ when using DXA versus anthropometric methodology. We also determined aerobic fitness levels from a submaximal measure (VO2-170), which may have lower validity than VO2-peak or VO2-max values. However, studies that have previously utilized VO2-170 as a measure of pediatric physical fitness report similar outcomes to those that measure VO2-peak or VO2-max.
In conclusion, children with higher percent body fat had lower aerobic fitness compared to children in the same BMI category that had a lower percent body fat. Greater African genetic admixture and less physical activity were also associated with lower fitness levels. Our findings suggest that percent body fat, rather than BMI percentile, is associated with aerobic fitness levels, and use of BMI percentile alone may bias the findings of interventions that recruit children classified as overweight by BMI percentile alone. Further investigations are required to determine if these differences also impact the change over time in aerobic fitness levels during longitudinal health interventions.
Table 3.
Regression models predicting aerobic fitness (VO2-170; L·min-1)
| Total Sample | Low Body Fat | High Body Fat | ||||
|---|---|---|---|---|---|---|
| Betaa (β) | P | beta (β) | P | beta (β) | P | |
| Lean mass | 0.78 (0.04) | <0.01 | 0.76 (0.95) | <0.01 | 0.78 (0.05) | <0.01 |
| Fat mass | -0.10 (-0.01) | 0.10 | <0.01 (<0.01) | 0.99 | -0.22 (-0.01) | 0.13 |
| Sex (female) | -0.05 (-25.63) | 0.36 | -0.14 (-0.04) | 0.23 | 0.02 (10.29) | 0.89 |
| Pubertal Status | -0.10 (-83.75) | 0.06 | -0.02 (-0.01) | 0.76 | -0.18 (-141.00) | 0.13 |
| Age | -0.03 (-6.16) | 0.63 | -0.07 (-0.09) | 0.43 | 0.06 (14.87) | 0.70 |
| African Admixture | -0.27 (-218.91) | <0.01 | -0.29 (-0.03) | <0.01 | -0.22 (-220.39) | 0.05 |
| Amerindian Admixture | 0.10 (114.69) | 0.10 | 0.09 (0.17) | 0.20 | 0.16 (168.46) | 0.23 |
| MVPA | 0.13 (1.07) | 0.01 | 0.14 (0.05) | 0.02 | 0.05 (0.69) | 0.60 |
| Socioeconomic status | -0.04 (-0.82) | 0.46 | -0.14 (-0.08) | 0.03 | 0.08 (1.78) | 0.48 |
MVPA = moderate/vigorous physical activity (min/d);
Beta (ß) = standardized beta (ß estimate)
Acknowledgments
We thank Alexandra Luzuriaga McPherson, Robert Petri, and David Bryan for their assistance with data collection. The authors would also like to acknowledge the reviewers for their thoughtful assessments that greatly improved the manuscript. This work was funded by the National Institutes of Health grants R01-DK067426, P30-DK56336, P60-DK079626, M01-RR-00032, and the National Cancer Institute Cancer Prevention and Control Training Program CA47888.
Footnotes
Conflict of Interest Disclosures: none
Reference List
- 1.Gutin B, Yin Z, Humphries MC, Hoffman WH, Gower B, Barbeau P. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr. 2004;145(6):737–43. doi: 10.1016/j.jpeds.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Ortega FB, Tresaco B, Ruiz JR, et al. Cardiorespiratory fitness and sedentary activities are associated with adiposity in adolescents. Obesity (Silver Spring) 2007;15(6):1589–99. doi: 10.1038/oby.2007.188. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Arslanian SA. Cardiorespiratory fitness and abdominal adiposity in youth. Eur J Clin Nutr. 2007;61(4):561–5. doi: 10.1038/sj.ejcn.1602541. [DOI] [PubMed] [Google Scholar]
- 4.Lohman TG, Ring K, Pfeiffer K, et al. Relationships among fitness, body composition, and physical activity. Med Sci Sports Exerc. 2008;40(6):1163–70. doi: 10.1249/MSS.0b013e318165c86b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. About BMI for children and teens. 2003 [Google Scholar]
- 6.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107(2):344–50. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 7.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child. 2001;85(1):67–72. doi: 10.1136/adc.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Ogden CL, Yanovski JA, et al. High adiposity and high body mass index-forage in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91(4):1020–6. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trowbridge CA, Gower BA, Nagy TR, Hunter GR, Treuth MS, Goran MI. Maximal aerobic capacity in African-American and Caucasian prepubertal children. Am J Physiol. 1997;273(4 Pt 1):E809–E814. doi: 10.1152/ajpendo.1997.273.4.E809. [DOI] [PubMed] [Google Scholar]
- 10.Shaibi GQ, Ball GD, Goran MI. Aerobic fitness among Caucasian, African-American, and Latino youth. Ethn Dis. 2006;16(1):120–5. [PubMed] [Google Scholar]
- 11.Treuth MS, Butte NF, Puyau M, Adolph A. Relations of parental obesity status to physical activity and fitness of prepubertal girls. Pediatrics. 2000;106(4):E49. doi: 10.1542/peds.106.4.e49. [DOI] [PubMed] [Google Scholar]
- 12.Roy JL, Hunter GR, Fernandez JR, et al. Cardiovascular factors explain genetic background differences in VO2max. Am J Hum Biol. 2006;18(4):454–60. doi: 10.1002/ajhb.20509. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol. 2010;115(5):982–8. doi: 10.1097/AOG.0b013e3181da9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeigue PM. Mapping genes that underlie ethnic differences in disease risk: methods for detecting linkage in admixed populations, by conditioning on parental admixture. Am J Hum Genet. 1998;63(1):241–51. doi: 10.1086/301908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet. 2004;36(11 Suppl):S54–S60. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- 16.Shriver MD, Smith MW, Jin L, et al. Ethnic-affiliation estimation by use of population-specific DNA markers. Am J Hum Genet. 1997;60(4):957–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Bonilla C, Shriver MD, Parra EJ, Jones A, Fernandez JR. Ancestral proportions and their association with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Hum Genet. 2004;115(1):57–68. doi: 10.1007/s00439-004-1125-7. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez JR, Allison DB. Understanding racial differences in obesity and metabolic syndrome traits. Nutr Rev. 2003;61(9):316–9. doi: 10.1301/nr.2003.sept.316-319. [DOI] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 23.Williams DP, Going SB, Lohman TG, et al. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health. 1992;82(3):358–63. doi: 10.2105/ajph.82.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutin B, Barbeau P, Owens S, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75(5):818–26. doi: 10.1093/ajcn/75.5.818. [DOI] [PubMed] [Google Scholar]
- 25.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008:1–9. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 26.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63(6):1839–51. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986;70(4):433–41. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead AB. Unpublished Doctoral Dissertation. New Haven, Connecticut: Yale University; 1975. Four factor index of social status; pp. 1–24. [Google Scholar]
- 29.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32(6):959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne NM, Weinsier RL, Hunter GR, et al. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: comparison of responses in white and black women. Am J Clin Nutr. 2003;77(6):1368–73. doi: 10.1093/ajcn/77.6.1368. [DOI] [PubMed] [Google Scholar]
- 31.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282(6):E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 32.Byrd-Williams CE, Shaibi GQ, Sun P, et al. Cardiorespiratory fitness predicts changes in adiposity in overweight Hispanic boys. Obesity (Silver Spring) 2008;16(5):1072–7. doi: 10.1038/oby.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson MS, Figueroa-Colon R, Herd SL, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106(4):E50. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 34.Barbeau P, Johnson MH, Howe CA, et al. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity (Silver Spring) 2007;15(8):2077–85. doi: 10.1038/oby.2007.247. [DOI] [PubMed] [Google Scholar]
- 35.Uth N. Gender difference in the proportionality factor between the mass specific VO2max and the ratio between HR(max) and HR(rest) Int J Sports Med. 2005;26(9):763–7. doi: 10.1055/s-2005-837443. [DOI] [PubMed] [Google Scholar]
- 36.Rowlands AV, Eston RG, Ingledew DK. Relationship between activity levels, aerobic fitness, and body fat in 8- to 10-yr-old children. J Appl Physiol. 1999;86(4):1428–35. doi: 10.1152/jappl.1999.86.4.1428. [DOI] [PubMed] [Google Scholar]


