Abstract
Insects can learn, allowing them great flexibility for locating seasonal food sources and avoiding wily predators. Because insects are relatively simple and accessible to manipulation, they provide good experimental preparations for exploring mechanisms underlying sensory coding and memory. Here we review how the intertwining of memory with computation enables the coding, decoding, and storage of sensory experience at various stages of the insect olfactory system. Individual parts of this system are capable of multiplexing memories at different timescales, and conversely, memory on a given timescale can be distributed across different parts of the circuit. Our sampling of the olfactory system emphasizes the diversity of memories, and the importance of understanding these memories in the context of computations performed by different parts of a sensory system.
Introduction
How are sensory stimuli and their memories represented in the brain? Recent models suggest that traces of past input can endure in the transient dynamics of a network [1**], and that different network designs are capable of storing memories at different timescales [2]. We focus on the interplay of computation and memory in a neural system with relatively well understood network dynamics: insect olfaction.
The olfactory system of insects, like the vertebrate counterpart, is organized into successive stages (Figure 1). In the periphery (antennae, maxillary palps, and other body parts) odor molecules trigger responses in olfactory receptor neurons (ORNs). Axons of ORNs, sorted by receptor type, converge onto a set of neuropils known as glomeruli located in the antennal lobe (AL) where they activate specific sets of projection neurons (PN) and local neurons (LN). PNs transmit their responses to the Mushroom bodies (MBs) and the lateral horn (LH), regions suspected to mediate learned and innate behaviors, respectively [3]. Olfactory memories, broadly defined, are manifest at different timescales.
Figure-1.
Insect olfactory anatomy. (a) Olfactory receptor neurons (ORNs) on the antenna (and other body parts) send excitatory processes to the Antennal Lobe, where they synapse, within glomeruli, upon inhibitory (and some excitatory) local neurons (LNs) and excitatory (and, in some species, some inhibitory) projection neurons (PNs). Fast reciprocal connections between LNs and PNs generate synchronous oscillations. The receptor neurons, LNs, and PNs together generate the elaborate, temporally structured responses transmitted from the PNs to Kenyon cells (KCs) and the Lateral Horn Interneurons (LHIs). These interneurons send phase-locked feed-forward inhibition to KCs; this periodic inhibition prevents lengthy temporal integration and reinforces the ability of KCs to detect coincident spikes from the PN population. KCs provide phase-locked excitatory output to the β-lobe. The Giant GABAergic neuron (GGN) appears to broadly distribute inhibition. (b) Representative electrophysiological responses of olfactory circuit components to an odor stimulus. Traces show intracellular recordings, except the ORN, electroantennogram (EAG) and the Mushroom body local field potential (LFP, filtered, 15-30Hz). Diagram and recordings are based on the locust but reflect olfactory features of many insects.
1. Sub-second plasticity
In the AL, odors are represented by temporally structured patterns of spiking distributed across ensembles of PNs. This combinatorial spatiotemporal code provides a large high-dimensional space in which representations of huge numbers of odors can be well-separated from each other. This separation makes it easier to discriminate odors, and provides robustness against noise that can arise from odorant-receptor interactions and from subsequent processing steps [4].
State-based olfactory coding
An N-dimensional space with one bit of information along each dimension allows 2N distinct states (Figure 2a). Thus, the ensemble of about 800 PNs in the locust, conservatively allowed only two levels of activity per neuron, can represent 2800 (about 6 followed by 240 zeros!) distinct states. If each state represents an odor, more than enough are available for all the odors an animal could ever encounter, with wide separations between each.
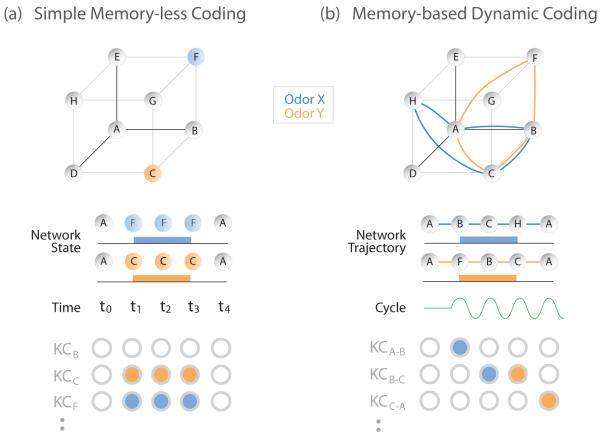
Figure-2.
Comparison of Memory-less and Memory-based coding schemes in a 3-dimensional space providing 8 states, A-H. (a) Simple (hypothetical) memory-less coding represents each odor as a specific state, independent of the stimulus history. Follower cells read out these instantaneous network states. (b) Dynamic coding represents each odor as trajectories through a state-space that reflect stimulus history. These trajectories – summarizing the temporal dynamics of the network – can be decoded piece-wise by downstream neurons, as Kenyon cells (KCs) decode the output from the Antennal Lobe, with each decoding-period defined by a cycle of network oscillation. KCs are shown responding to segments of trajectories through different regions of the state-space (marked in subscripts).
But odor stimuli are actually represented by specific successions of these states (Figure 2b). That’s because switching an odor on or off elicits from PNs not binary state changes like step increases or decreases in firing, but rather elaborate, time-varying spiking patterns. These patterns are generated as odorants traverse enzyme-filled fluids surrounding ORNs to activate complex, adapting transduction machinery, resulting in sequences of spiking consisting of periods of excitation and inhibition. These relatively simple peripheral patterns of activity then drive the central circuitry of the AL, where inhibitory LNs greatly expand the temporal complexity of firing patterns in PNs, which endure for tens to thousands of milliseconds [5*]. Superimposed onto these slowly-varying patterns are fast, ~20Hz oscillations that cause the AL to present their follower neurons with a succession of discrete “snapshots” of activity, each corresponding to one oscillatory cycle of ~50ms [6**,7,8].
Considered from the perspective of follower neurons – the Kenyon cells (KCs) in the MBs – these patterns form a trajectory through the N-dimensional space, with a pathway and velocity determined by the identity and concentration of the odor stimulus [9]. Ongoing sensory input interacts with these memory-like state sequences to determine new states to be read out downstream (Figure 2b). Recordings from KCs reveal spiking patterns whose timing and specificity are consistent with a process for decoding the trajectories one snapshot at a time; periodic inhibition prevents the KCs from integrating their input across longer stretches of time. Further, the responses of KCs reveal information content inconsistent with decoding instantaneous sensory input [9,10,11]. Besides keeping stimulus history, a dynamic code offers several advantages: (1) Transient dynamics are highly resistant to noise [12]; (2) trajectories are organized so different concentrations of an odor closely co-vary, but representations for different odors are more distinct, so same circuitry can instantiate a hierarchical classification of odor identity and concentration [9]; (3) dynamic representations enable a time-varying sliding scale of coding from odor categories to odor identity [13].
Olfactory coding of overlapping stimuli
That brief odor stimuli can elicit neural responses enduring well after the input [14] poses a possible conundrum: rapid, plume-like sequences of odor pulses elicit firing patterns from individual PNs that change unpredictably as lengthy responses to successive stimuli collide [10]. However, this confound could be resolved by analyzing the time-varying responses of the population of PNs in brief snapshots defined by oscillatory cycles. The analysis, designed to reflect the way KCs receive input from the AL, showed that variance introduced by changes in the timing of the stimulus was much less than variance introduced by changes in the odorant or its concentration [10]. Overlapping presentations of different odors could also be resolved similarly [11]. Thus, temporal coding by ensembles of neurons allows robust and invariant olfactory coding in the face of temporally-varying input [15].
2. Seconds to minutes
Spontaneous correlations and perireceptor memory
Measurements of spontaneous activity reveal that spikes occurring in different AL neurons become more correlated even many minutes after the conclusion of an odor presentation [16]. A potential source this activity is the persistent, seconds-long firing of some ORNs (Joseph et al, in preparation), with correlations arising from patterns of connectivity between ORNs and AL neurons [17]. Odor molecules must traverse aqueous sensillar lymph to reach the ORNs [18,19]. Persistence of these odor molecules in the lymph beyond the duration of the stimulus can be regarded as a perireceptor memory, and, it may partly explain the sustained organization of persistent, apparently spontaneous activity observed downstream.
Receptor adaptation
Sensory adaptation, a reduction in response to a sensory stimulus upon prolonged or rapid, repeated exposures, occurs in ORNs [20]. By reducing responses to sustained stimuli, adaptation emphasizes changes, allowing extraction of temporal features such as odor onset and offset, potentially helping animals orient toward odor sources [20]. In insects, adaptation may endure for seconds or minutes [21], constituting a form of fast peripheral memory. Interestingly, olfactory responses reduced through adaptation (fewer spikes in responsive ORNs) differ from responses to reduced concentrations of odors (fewer types of ORNs are recruited)[6**].
Odor exposure creates lasting effects on oscillatory synchronization
Odors also induce changes in AL circuitry that may last for several minutes beyond the odor exposure [22*]. Over the course of multiple odor presentations, total spikes elicited in PNs decrease while the precision of oscillatory synchrony in firing among PNs increases [23]. This increase in synchrony endures for 5-10min and is consequential because downstream KCs act as coincidence detectors [24]. This “fast learning” occurs entirely within the AL, independently of, and on a different timescale from adaptation in ORNs, and is odor-specific [23].
How is this form of memory stored, and how is it useful? Animals repeatedly sample odors through sniffing or antennal-flicking behaviors, and often encounter repeated presentations of odors through plumes. In a computational model, activity-dependent facilitation of the inhibitory synapses within the AL sufficed to explain fast learning [25]. The model also revealed that olfactory coding equipped with fast learning is robust to noise in the input, as only responses to repeated, reliable stimuli become increasingly precise. This mechanism may also contribute to enduring increases in correlated spontaneous activity induced by odor presentations [16].
STDP and oscillatory synchronization
When examining responses elicited by the firing of KCs in their followers in the β-lobe of the MBs, Cassenaer and Laurent [26] found evidence for spike time-dependent plasticity (STDP), a process that can strengthen or weaken a synapse depending upon the precise timing of spikes in the pre- and post-synaptic neurons [27]. The connection between KCs and β-lobe neurons strengthened or weakened adaptively to maintain the precise timing of spikes in β-lobe neurons with respect to the phase of oscillations generated in the AL and transmitted throughout the olfactory system. Thus, STDP here maintains the fidelity of oscillatory synchronization of neurons as signals propagate through the brain.
3. Hours to days
Insects can learn to associate odors with food or other types of reward or punishment, and retain these memories for hours, days, or even years. In proboscis extension reflex (PER) conditioning, an insect (honeybee, moth, Drosophila) is trained to extend its proboscis, a drinking straw-like mouthpart, when it encounters an odor previously paired with a sucrose reward [28]. Morphological analyses, genetic manipulations [29**,30,31*], calcium imaging [32] and electrophysiological recordings [33] all support the idea that MBs are important for the formatting and perhaps storage of these associative memories. Furthermore, genetic analyses in Drosophila suggest that different subsets of neurons in the MBs, including the KCs, may participate in memory storage and retrieval at different times [29**,34,35]. Exactly how associations are stored and retrieved in the insect brain is an area of active inquiry.
Sparseness and memory
In sharp contrast to the voluble responses in populations of PNs, KCs – their followers – respond to odors with remarkable sparseness; recordings from locusts [24], moths [36], and flies [37] show only a small proportion of KCs responds to any given odor, and these responses consist of vanishingly few spikes (Figure 1b). This sparsening in KCs is caused by their high firing thresholds, feed-forward inhibition from GABAergic interneurons in the LH [24,38], and feedback inhibition from a unique, giant GABAergic neuron (M. Papadopoulou, G. Turner, and G. Laurent, 2009, Frontiers in Systems Neuroscience, conference abstract, 10.3389/conf.neuro.06.2009.03.106). Further, the strengths of synapses linking PNs to KCs may be adaptively regulated to function well across a broad range of conditions [39]. Sparse representations of sensory stimuli can be advantageous for storing memories, matching patterns, and forming new associations because they require relatively few sites to be modified, compared or associated, and representations are more distinct from one another [4].
Might STDP mediate odor learning? Ito et al. [36] recently ruled out STDP as the basis of odor associations in KCs. They used the PER paradigm in moths to show robust olfactory learning could occur even when reinforcement (sugar-water) was delivered several seconds after all odor-evoked spiking in KCs had ceased, a condition inconsistent with STDP’s timing requirements [27]. One open possibility is that the association of odor and reinforcement may occur downstream from KCs, possibly through STDP. Or, associations may be established in KCs but by a different mechanism.
Long term memories and the antennal lobe
Lengthy exposure to odors, even without conditioning, causes morphological and functional changes in the AL [40,41] and the MB [42]. Do associative memories also form within the AL? Anatomical and functional evidence suggest reward modulators including octopamine are released in the AL, implicating its role in associative learning [43,44,45]. Recording from AL neurons during conditioning is challenging because mouth part movements usually cause the brain to move as well [46,47]. Studies in which calcium fluctuations were imaged as measures of neural activity in glomeruli have produced conflicting results [48,49,50,51,52]. A recently introduced paradigm, sting extension reflex conditioning, avoids the potential movement confounds, but does not appear to induce modifications of glomerular activity [53].
4. Life-time memories
Odors experienced early in life can leave lasting impressions [54,55]. One example among insects is “imaginal conditioning” in Drosophila: a newly hatched imago exposed to an odor later shows altered preferences for that odor in adulthood, mediated by changes in ORNs [56]. To explore the effect of imaginal conditioning on odor coding, Iyengar et al. [57*] compared the responses of ORNs in odor-exposed and odor-deprived flies. Early exposure to an odor later increased the ORN’s sensitivity to that odor, and further, prior exposure to an odor-rich environment made temporal spiking patterns in ORNs more odor-specific [57*].
Conclusions
Responses of the insect nervous system to an odor stimulus depend not only on instantaneous input, but also on its history. These sensory memories, acting at multiple timescales - from milliseconds to life long - are fundamentally coupled to the dynamic computations at various stages of the olfactory circuit, including the perireceptor environment, receptors, AL, different parts of the MB, and beyond. As we begin to localize the traces of specific forms of memory [29**], it will be important to keep this connection between memory and computation in mind.
Highlights.
Temporal dynamics of a network provide a mechanism for encoding sensory memories.
Insect olfactory system exhibits memory at multiple timescales, from milliseconds to years.
Throughout the olfactory system different circuit elements exhibit different forms of memory.
Memory and computation are fundamentally intertwined, each shaping the other.
Acknowledgements
We thank Stopfer lab members for helpful discussions, and NICHD-NIH for intramural funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Buonomano DV, Maass W. State-dependent computations: spatiotemporal processing in cortical networks. Nature Reviews Neuroscience. 2009;10:113–125. doi: 10.1038/nrn2558. A review of recent brain models that argue for the role of transient dynamics of a network in maintaining the history of the stimulus.
- 2.Ganguli S, Huh D, Sompolinsky H. Memory traces in dynamical systems. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18970–18975. doi: 10.1073/pnas.0804451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jefferis GSXE, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nature Reviews Neuroscience. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- 5*.Raman B, Joseph J, Tang J, Stopfer M. Temporally diverse firing patterns in olfactory receptor neurons underlie spatiotemporal neural codes for odors. Journal of Neuroscience. 2010;30:1994–2006. doi: 10.1523/JNEUROSCI.5639-09.2010. This study shows the odor-encoding temporal firing patterns of PNs derive from the temporal spiking patterns of ORNs, and are elaborated upon by antennal lobe circuitry.
- 6**.Ito I, Bazhenov M, Ong RC-y, Raman B, Stopfer M. Frequency transitions in odor-evoked neural oscillations. Neuron. 2009;64:692–706. doi: 10.1016/j.neuron.2009.10.004. This study shows responses undergoing sensory adaptation are encoded differently than responses to stimuli of reduced intensity, and demonstrates profound effects of sensory adaptation on downstream olfactory coding.
- 7.Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. Journal of Neuroscience. 2009;29:8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 9.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nature Neuroscience. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- 11.Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. doi: 10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovich M, Huerta R, Laurent G. Transient dynamics for neural processing. Science. 2008;321:48–50. doi: 10.1126/science.1155564. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nature Neuroscience. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- 14.Mazor O, Laurent G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron. 2005;48:661–673. doi: 10.1016/j.neuron.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Geffen MN, Broome BM, Laurent G, Meister M. Neural encoding of rapidly fluctuating odors. Neuron. 2009;61:570–586. doi: 10.1016/j.neuron.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Galán RF, Weidert M, Menzel R, Herz AVM, Galizia CG. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Computation. 2006;18:10–25. doi: 10.1162/089976606774841558. [DOI] [PubMed] [Google Scholar]
- 17.Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nature Neuroscience. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- 19.Pelosi P. Perireceptor events in olfaction. Journal of Neurobiology. 1996;30:3–19. doi: 10.1002/(SICI)1097-4695(199605)30:1<3::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Kaissling KE, Zack Strausfeld C, Rumbo ER. Adaptation processes in insect olfactory receptors. Mechanisms and behavioral significance. Annals of the New York Academy of Sciences. 1987;510:104–112. doi: 10.1111/j.1749-6632.1987.tb43475.x. [DOI] [PubMed] [Google Scholar]
- 21.Störtkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. Journal of Neuroscience. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Larkin A, Karak S, Priya R, Das A, Ayyub C, Ito K, Rodrigues V, Ramaswami M. Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learning & Memory. 2010;17:645–653. doi: 10.1101/lm.1839010. A study of short-term olfactory habituation in Drosophila larvae, demonstrating that this memory cannot be explained by sensory adaptation, and possibly involves NMDAR-dependent synaptic changes in the antennal lobe.
- 23.Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 25.Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G. Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron. 2005;46:483–492. doi: 10.1016/j.neuron.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–713. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- 27.Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annual Review of Neuroscience. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 28.Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) Journal of Comparative Psychology. 1983;97:107–119. [PubMed] [Google Scholar]
- 29**.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. This review provides an overview of memory research in Drosophila, focusing on the localization of different types of memory traces using genetic approaches.
- 30.Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nature Reviews Neuroscience. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 31*.Hourcade B, Muenz TS, Sandoz J-C, Rössler W, Devaud J-M. Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? Journal of Neuroscience. 2010;30:6461–6465. doi: 10.1523/JNEUROSCI.0841-10.2010. This study found that the structural density of microglomeruli in the mushroom bodies increases upon formation of a long-term associative memory.
- 32.Haehnel M, Menzel R. Sensory representation and learning-related plasticity in mushroom body extrinsic feedback neurons of the protocerebral tract. Frontiers in Systems Neuroscience. 2010;4:161. doi: 10.3389/fnsys.2010.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strube-Bloss MF, Nawrot MP, Menzel R. Mushroom body output neurons encode odor-reward associations. Journal of Neuroscience. 2011;31:3129–3140. doi: 10.1523/JNEUROSCI.2583-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Current Biology. 2009;19:1341–1350. doi: 10.1016/j.cub.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito I, Ong RC-Y, Raman B, Stopfer M. Sparse odor representation and olfactory learning. Nature Neuroscience. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. Journal of Neurophysiology. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 38.Assisi C, Stopfer M, Laurent G, Bazhenov M. Adaptive regulation of sparseness by feedforward inhibition. Nature Neuroscience. 2007;10:1176–1184. doi: 10.1038/nn1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finelli La, Haney S, Bazhenov M, Stopfer M, Sejnowski TJ. Synaptic learning rules and sparse coding in a model sensory system. PLoS Computational Biology. 2008;4:e1000062. doi: 10.1371/journal.pcbi.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devaud JM, Acebes A, Ferrús A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. Journal of Neuroscience. 2001;21:6274–6282. doi: 10.1523/JNEUROSCI.21-16-06274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Kremer MC, Christiansen F, Leiss F, Paehler M, Knapek S, Andlauer TFM, Förstner F, Kloppenburg P, Sigrist SJ, Tavosanis G. Structural Long-Term Changes at Mushroom Body Input Synapses. Current Biology. 2010:1938–1944. doi: 10.1016/j.cub.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 43.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 44.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learning & Memory. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 45.Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. Journal of Neuroscience. 2007;27:11132–11138. doi: 10.1523/JNEUROSCI.2712-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daly KC, Christensen Ta, Lei H, Smith BH, Hildebrand JG. Learning modulates the ensemble representations for odors in primary olfactory networks. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10476–10481. doi: 10.1073/pnas.0401902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denker M, Finke R, Schaupp F, Grun S, Menzel R. Neural correlates of odor learning in the honeybee antennal lobe. European Journal of Neuroscience. 2010;31:119–133. doi: 10.1111/j.1460-9568.2009.07046.x. [DOI] [PubMed] [Google Scholar]
- 48.Peele P, Ditzen M, Menzel R, Galizia CG. Appetitive odor learning does not change olfactory coding in a subpopulation of honeybee antennal lobe neurons. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2006;192:1083–1103. doi: 10.1007/s00359-006-0152-3. [DOI] [PubMed] [Google Scholar]
- 49.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 50.Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nature Neuroscience. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez PC, Locatelli FF, Person-Rennell N, Deleo G, Smith BH. Associative conditioning tunes transient dynamics of early olfactory processing. Journal of Neuroscience. 2009;29:10191–10202. doi: 10.1523/JNEUROSCI.1874-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hourcade B, Perisse E, Devaud J-M, Sandoz J-C. Long-term memory shapes the primary olfactory center of an insect brain. Learning & Memory. 2009;16:607–615. doi: 10.1101/lm.1445609. [DOI] [PubMed] [Google Scholar]
- 53.Roussel E, Sandoz J-C, Giurfa M. Searching for learning-dependent changes in the antennal lobe: simultaneous recording of neural activity and aversive olfactory learning in honeybees. Frontiers in Behavioral Neuroscience. 2010;4:1–12. doi: 10.3389/fnbeh.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arenas A, Giurfa M, Farina WM, Sandoz JC. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. European Journal of Neuroscience. 2009;30:1498–1508. doi: 10.1111/j.1460-9568.2009.06940.x. [DOI] [PubMed] [Google Scholar]
- 55.Blackiston DJ, Silva Casey E, Weiss MR. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PloS One. 2008;3:e1736. doi: 10.1371/journal.pone.0001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakraborty TS, Goswami SP, Siddiqi O. Sensory correlates of imaginal conditioning in Drosophila melanogaster. Journal of Neurogenetics. 2009;23:210–219. doi: 10.1080/01677060802491559. [DOI] [PubMed] [Google Scholar]
- 57*.Iyengar A, Chakraborty TS, Goswami SP, Wu C-F, Siddiqi O. Post-eclosion odor experience modifies olfactory receptor neuron coding in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9855–9860. doi: 10.1073/pnas.1003856107. This paper suggests that life-long olfactory memories in Drosophila, formed by early-life odor exposures, involve changes in olfactory coding in ORNs.