Abstract
Bacterial urease activity in dental plaque and in saliva generates ammonia, which can increase the plaque pH and can protect acid-sensitive oral bacteria. Recent cross-sectional studies suggest that reduced ability to generate ammonia from urea in dental plaque can be an important caries risk factor. In spite of this proposed important clinical role, there is currently no information available regarding important clinical aspects of oral ureolysis in children.
OBJECTIVE
The objective of this study was to evaluate the distribution and pattern of urease activity in the dental plaque and in the saliva of children during a three-year period, and to examine the relationship of urease with some important caries risk factors.
METHODS
A longitudinal study was conducted with repeated measures over a three-year period on a panel of 80 children, ages three to six years at recruitment. The dynamics of change in urease activity were described and associated with clinical, biological, and behavioral caries risk factors.
RESULTS
Urease activity in plaque showed a trend to remain stable during the study period and was negatively associated with sugar consumption (P<0.05). Urease activity in unstimulated saliva increased with age, and it was positively associated with the levels of mutans streptococci in saliva and with the educational level of the parents (P<0.05).
CONCLUSIONS
The results of this study reveal interesting and complex interactions between oral urease activity and some important caries risk factors. Urease activity in saliva could be an indicator of mutans infection in children.
Keywords: urease, caries risk factors, children
INTRODUCTION
Ureases are large, multi-subunit enzymes, which catalyze the hydrolysis of urea into ammonia and carbonic acid. Ureases produced by bacteria in the gastrointestinal and the urinary tracts of humans, such as the Helicobacter pylori and the Ureaplasma urealyticum, respectively, are involved in the pathogenesis of gastritis, urolethiasis, urinary tract infections and other diseases (1, 2). In the oral cavity, there are several bacteria that produce urease, both in the dental plaque, and in the saliva (3). The best-characterized oral urease enzyme is the one produced by Streptococcus salivarius, which is currently regarded as the most important ureolytic species in saliva. Dental plaque has higher ureolytic activity than saliva, but the ureolytic bacteria and their relative contribution to this activity have not been definitively determined. Actinomyces naeslundii is considered as one of the most important ureolytic species in dental plaque, however, other species, which are currently unidentified, may contribute to the total urease activity expressed in dental biofilms (3–5).
Urea is secreted in the oral cavity through the salivary glands at concentrations similar to blood urea levels, about 3 to 10 mM in health individuals (6). The generation of ammonia from urea by the urease pathway can cause a significant increase in the pH of dental biofilms, which may help neutralize the acids generated from the glycolytic processing of dietary carbohydrates by dental plaque bacteria (7–10) and may also protect acid-sensitive bacteria from acid-induced damage (11, 12). For these reasons it has been hypothesized that urease activity in the dental plaque and in the saliva can prevent the development of dental caries (1,13). Indeed, recombinant Streptococcus mutans strains expressing the urease genes from S. salivarius were shown to be significantly less cariogenic in an animal caries model compared to the wild type, non-ureolytic strains (14).
A number of indirect clinical observations initially suggested that urease activity could play an important role in the development of dental caries. Patients with chronic renal failure, who have elevated urea concentrations in their blood and in their saliva, are relatively resistant to dental caries and have a more alkaline plaque both during fasting, and after sugar consumption, compared to healthy subjects (15). Three recent pilot cross-sectional showed a significant negative association between plaque urease activity in adults and children (16–18). No associations were observed between salivary urease activity and caries levels in these aforementioned cross-sectional studies.
Despite the increasing amount of clinical and laboratory data suggesting that urease activity may play an important role in pH homeostasis, in oral ecology and in caries resistance, there are fundamental questions regarding clinical aspects of oral ureolysis that have not been addressed. For example, it is not known what the normal levels of urease activity in the dental plaque and in the saliva of children are, how stable this activity is over time, and how it relates to other important caries risk factors. These questions need to be addressed to accurately determine the role of urease in caries development. To address these questions we conducted a longitudinal study with repeated measures over a three-year period on a panel of children ages three to six years at recruitment. The objective of this study was to evaluate the distribution and pattern of urease activity in the dental plaque and in the saliva of children over a three-year period and to examine its relationship with some important caries risk factors, including socio-demographic factors, sugar consumption, amount of plaque and mutans streptococci levels. This study also investigated the role of urease in caries development and in the oral ecology of the children. Due to the large amount of data obtained, these latter objectives will be presented in a separate manuscript.
MATERIALS AND METHODS
Study Design and Study Group
The study employed a prospective panel design with repeated measures every six months over a three-year period. The panel consisted of 80 healthy children, ages 3 to 6 years at recruitment. The rationale for selecting this particular age group was that the most drastic changes in the caries status of children are likely to occur around the time of the eruption of the first permanent molars (19). According to the design of this study, all the children in the study group went through that phase during the study period. The panel was balanced at baseline with respect to age, gender and caries experience (Table 1). This was achieved using a balanced-block recruitment strategy (20). The children were recruited from the dental clinics of the University of Puerto Rico School of Dental Medicine (UPR-SDM) and from the San Juan Metropolitan area using flyers and press-announcements in local newspapers. The children were not using orthodontic appliances and did not receive antibiotics for at least two months prior to each follow-up visit. In the event that a child took antibiotics during the two months prior to the scheduled follow-up visit, the visit was rescheduled to two months after the end of the antibiotic regimen. The study was approved by the Institutional Review Board (IRB) of the University of Puerto Rico Medical Sciences Campus (UPR-MSC). The children assented to participate in the study using an age-appropriate assent form. The parents or legal guardians of the children consented to the voluntary participation of the children by signing an informed consent approved by the IRB of the UPR-MSC. The children received the benefit of a free dental examination by a pediatric dentist every six months and referrals for treatment as needed.
Table 1.
Composition of the study group at the beginning and at the end of the study.
| Variable | Categories | At Baseline % (N=80) | After 36 Months % (N=52) |
|---|---|---|---|
| Gender | Males Females |
47.50 52.50 |
52.00 48.00 |
| Age at Recruitment | 3 years 4 years 5 years 6 years |
23.27 31.32 21.03 24.38 |
24.49 32.65 20.41 22.45 |
| Caries Status at Baseline | Low Caries (dmfs≤2) High Caries (dmfs>2) |
55.00 45.00 |
64.00 36.00 |
Sample size
The sample size for the study was calculated using data from a previous study in adults (17) for m (measurements per subject)=7 and ρ (intra-class correlation between two consecutive measurements)=0.60. Using these parameters it was calculated that a total of 40 subjects would be needed to complete the seven visits of the study for 90% power and α=0.05 (21). Expecting an annual attrition rate of 20%, it was estimated that 80 subjects should be recruited in order to achieve the desired sample size of 40 subjects at the end of the study period.
Clinical Procedures
All study visits took place during the morning hours. The children were asked to refrain from brushing their teeth the night before and in the morning before the study to allow for sufficient plaque to accumulate. In the first visit, a medical, dental, and social history was obtained by the parents using a self-administered questionnaire that was subsequently reviewed by the examiner. This questionnaire has been used in previous, closely related pilot studies (18). The purpose of this questionnaire was to determine eligibility and to establish the socio-demographic profile of the study group. In every visit, the parents also completed a 24-hour diet recall record, which the examiner later reviewed with the child and parent to confirm the consumption of sugar-containing foods. A sample of whole, unstimulated saliva (about 3 mls) was initially collected using a sputum trap attached to the dental suction (18). All saliva samples were collected between 8:00 and 10:00 AM. The teeth were briefly rinsed and dried with water-air spray. Supragingival plaque was then collected from all available smooth surfaces using a periodontal instrument and pooled in a pre-weighed micro-centrifuge tube. Plaque and saliva samples were kept on ice during collection. Following the collection of the plaque and saliva samples the teeth of the children were brushed with toothpaste and a dental exam was performed with the Fiber-Optic Trans-Illumination (FOTI) method (22). Caries lesions were scored using Ekstrand’s criteria as previously described (18) and the DMFS index was calculated to define overall caries status. “Decay” was defined at the level of dentin (d3), irrespective of the presence or absence of cavitation. All dental exams were performed by the same examiner, who was calibrated prior to the study by an expert reference examiner (inter-examiner kappa: 0.83).
Laboratory Procedures
Plaque and saliva samples were transferred to the adjacent laboratory of the Clinical Research Center of the UPR-MSC immediately after the study visit. 300 µl of saliva were used for enumeration of mutans streptococci (18). The remaining saliva was divided in 1 ml aliquots, snap-frozen in dry ice-ethanol bath and stored at −80°C. Plaque samples were centrifuged for 1 minute to pellet the plaque and weighed again on an analytical balance (Ohaus Corporation, Pine Brook, NJ, USA) to determine the amount of plaque collected. The pellet was resuspended in 300 µl of 10 mM sodium phosphate buffer, aliquoted, snap-frozen and stored at −80°C.
Study Variables
The main outcome variables in this study were urease activity in the dental plaque and urease activity in the saliva. Urease activity in the plaque and saliva samples was measured using a spectrophotometric method as previously described (16–18), and was expressed as µ moles urea hydrolyzed/min/mg protein.
The predictor variables included in the analysis were: age, gender, sugar consumption, amount of plaque, mutans streptococci in saliva, and fasting. Sugar consumption was measured with a method that is based on a 24-hour diet recall record, which was completed by the parent as described previously (“clinical procedures”). This method, originally described by Nizel and Pappas (23), has been utilized and described in previous closely related pilot studies by the same PI (18). The sugar score calculated with this method reflects the consistency of the sugary foods consumed (liquid, solid, slowly dissolving) and the frequency with which they consumed. The amount of plaque collected from each child was measured by weighing the plaque collection tubes before and after the sample collection and expressed as milligrams (wet plaque). Mutans streptococci in saliva were quantified by plating on selective media as previously described (18). Fasting was defined as follows; 0: child had nothing to eat or drink except water in the day of the study prior to the sample collection; 1: child had consumed non-sugary foods or beverages in the morning of the study visit prior to sample collection; 2: child had consumed sugar-containing foods or beverages prior to sample collection.
Analytical Procedures
Descriptive statistics, including means, medians, standard deviations and proportions were initially used to examine the distribution of urease activity levels at each time point. The non-parametric, Kruskal-Wallis and Mann-Whitney tests were used to compare the distribution of plaque and saliva urease activity in the different socio-economic groups. The Lowess curve was used to describe the trend of plaque and saliva urease activity levels by gender, age and initial caries status (24). A Generalized Linear Latent and Mixed Model (GLLAMM) (25) was used to describe the changes in the urease levels at the different time points in relation to the predictor variables. Interactions among the predictor variables were assessed using the Likelihood-Ratio test. The intraclass correlation coefficient (ρ) was used to assess the reproducibility of urease activity at different time points. Log-transformation and square-root transformation were performed for plaque and saliva urease, respectively, to assure the normality assumption in the model. Data were analyzed using the STATA version 10.0 software.
RESULTS
Description of the study panel and attrition during the study
The composition of the study panel with respect to age, gender and caries experience at the beginning and at the end of the study period is shown in Table 1. The attrition rate in the study was 13.8% after year 1, 14.7% after year 2 and 10.35% after year 3, much lower compared to the expected 20% annually. The final composition of the panel with respect to age and gender was similar to the baseline panel, indicating that there was no excessive loss of any particular age group or gender during the study period. There was somewhat higher attrition of children with high caries levels at baseline (dmfs>2, which was the median for the panel at baseline) compared to children with low caries levels at baseline. The proportion of children that was completely caries-free decreased from 44% at baseline to 38.5% at the end of the three-year study period.
The socio-economic profile of the study group, as self-reported by the parents/guardians at baseline is presented in Table 2. According to this Table, the study group included children from a wide-range of socio-economic backgrounds. No significant differences were observed in the distribution of plaque and saliva urease levels among the different socio-economic groups (Kruskal-Wallis P>0.05), with the exception of parental educational level. Children whose parents had college education had significantly lower urease levels in their saliva compared to the other groups (Kruskal-Wallis P=0.044, Mann-Whitney P=0.011). These children also had fewer caries lesions compared to the children whose parents did not have college education (Mann-Whitney P=0.025).
Table 2.
Socio-economic Profile of the Study Group
| Variable | Categories | % at Baseline (N=80) |
|---|---|---|
| Lives with | Both parents One parent Other |
65.82 32.91 1.27 |
| Parent Education Level* | None Elementary School Intermediate School High School Professional School University |
2.53 0 1.27 34.18 6.33 55.70 |
| School | None Public Private |
16.46 55.70 27.85 |
| Medical Insurance | None Health Reform Private |
2.53 55.70 41.77 |
| Dental Insurance | None Health Reform Private |
5.06 54.43 40.51 |
Significant differences in saliva urease among these groups (Kruskal-Wallis: P=0.0441); significant differences in saliva urease among children who’s parents have University level education vs. the rest (Mann-Whitney: P=0.011)
Urease activity in dental plaque
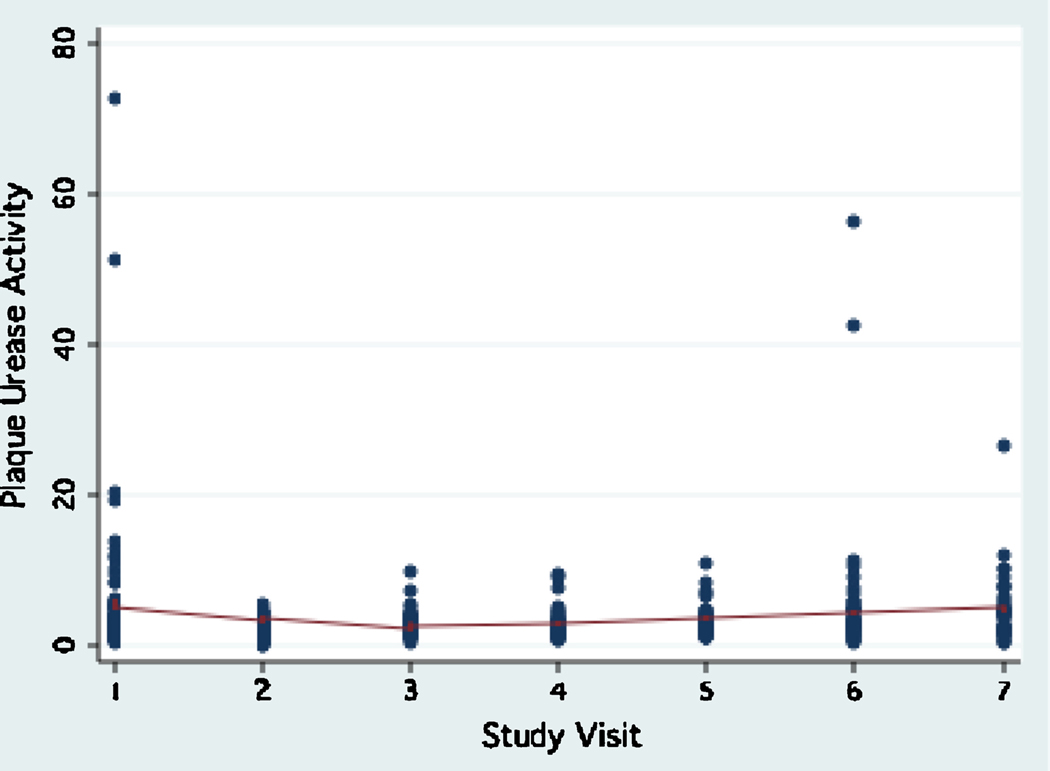
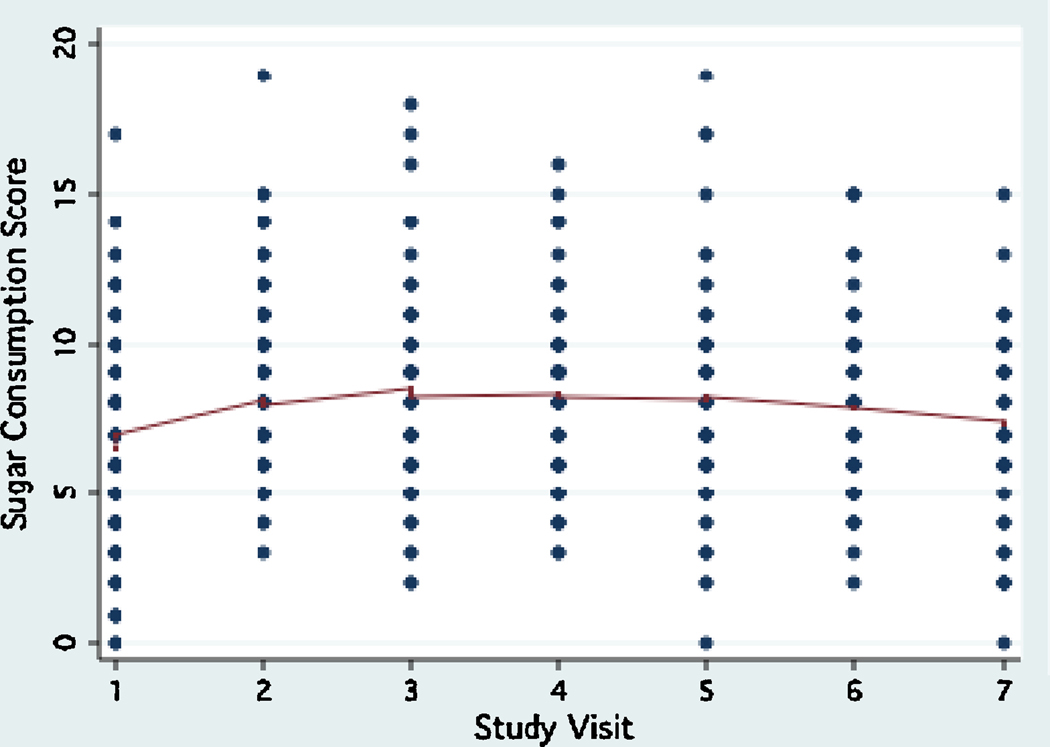
Urease activity in plaque ranged between 0 to 73.03 units, (interquartile range: 2.38, mean: 3.48±5.78, median: 2.33) during the study period. Plaque urease activity remained relatively stable during the study period, with a trend for a decrease between visit 2 and visit 4 (6 months to 18 months, Fig.1). During the same period, an opposite trend for sugar consumption to increase was observed (Fig. 2). No differences were observed in the trend of plaque urease activity by age group, gender, and baseline caries status (data not shown). The reproducibility of plaque urease activity in this study overall was poor (Intraclass correlation coefficient ρ : 0.07–0.08), but it was much better in fasting samples (Intraclass correlation coefficient ρ : 0.35).
Figure 1.
Trend of plaque urease activity during the three-year study period.
Figure 2.
Trend of sugar consumption during the three-year study period.
Plaque urease activity was negatively associated with sugar consumption (adjusted β : −0.03, 95% CI −0.048, −0.013, P=0.001) (Table 3). Age had a marginally significant positive association with plaque urease (0.05<P<0.1). The association of plaque urease with sugar consumption did not differ among the fasting samples vs. those collected after a sugary meal.
Table 3.
Multivariate Linear Regression Models for Plaque and Saliva Urease
| Plaque Urease | Saliva Urease | |||
|---|---|---|---|---|
| Predictor | β (95% CI) | P-value | β (95% CI) | P-value |
| Age | 0.039 (−0.001, 0.079) | 0.058 | 0.074 (0.054, 0.093) | 0.000 |
| Gender (female vs. male) | −0.033 (−0.167, 0.102) | 0.635 | 0.053 (−0.03, 0.136) | 0.208 |
| Sugar score | −0.03 (−0.048, −0.013) | 0.001 | −0.0002 (−0.008, 0.007) | 0.955 |
| Plaque | −1.302 (−4.647, 2.042) | 0.445 | 1.177 (−0.182, 2.538) | 0.09 |
| Salivary mutans | −0.007 (−0.061, 0.047) | 0.804 | 0.03 (0.004, 0.055) | 0.022 |
| Non-sugary meal vs. fasting | 0.047 (−0.189, 0.282) | 0.699 | −0.037 (−0.133, 0.059) | 0.447 |
| Sugary-meal vs. fasting | −0.068 (−0.212, 0.077) | 0.359 | −0.083 (−0.143, −0.023) | 0.007 |
Urease activity in the saliva
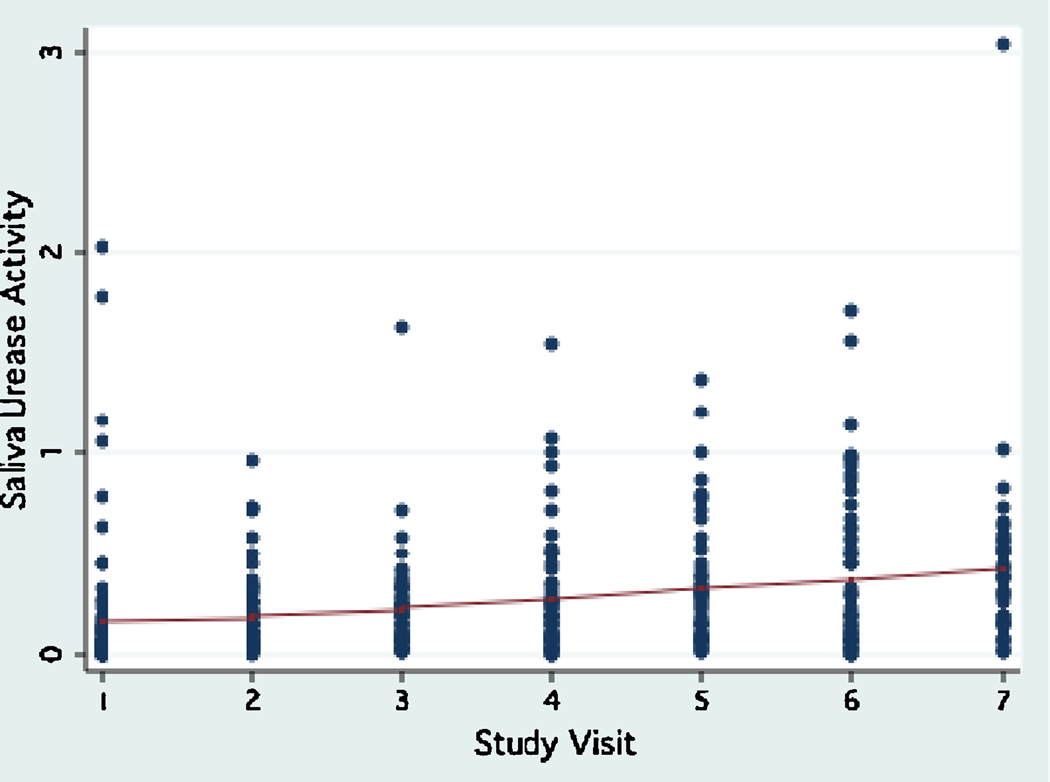
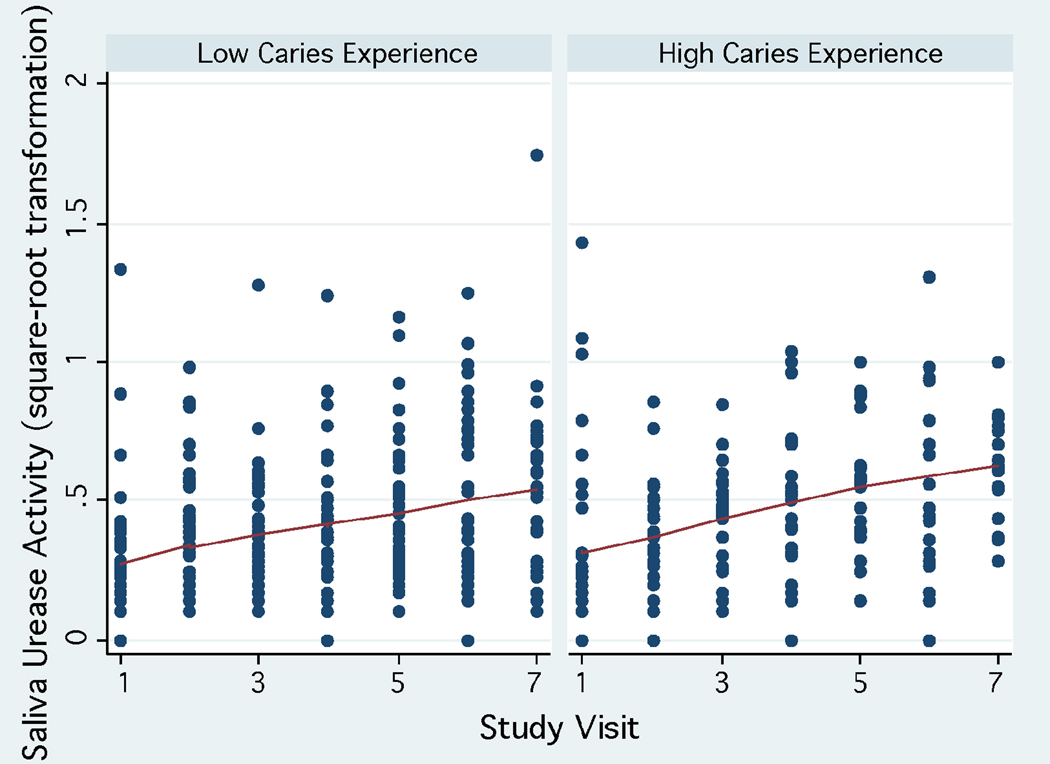
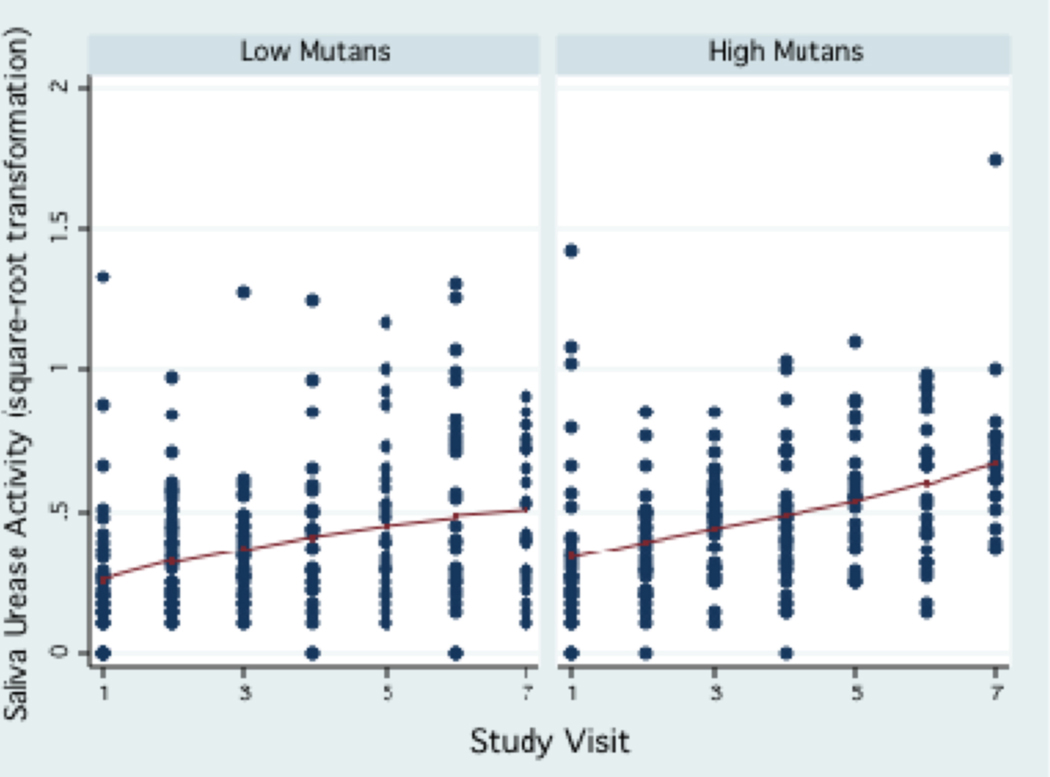
Urease activity in saliva was much lower compared to plaque urease, ranging between 0 to 3.03 units (interquartile range: 0.28, mean: 0.26±0.33, median: 0.15) during the study period. Urease activity levels in saliva showed a trend to increase over time (Fig. 3) in all age groups. A trend for higher saliva urease activity was observed in children in the high caries strata at baseline (dmfs>2, which was the median) (Fig. 4), in females, and in children with high levels of mutans streptococci in their saliva (≥104 CFU/ml, which was the median, Fig.5). The reproducibility of saliva urease activity was better than plaque urease (Intraclass correlation coefficient ρ : 0.33–0.34), and it was higher in the non-fasting samples than in the fasting samples. Urease activity in plaque was positively associated with urease activity in saliva (β : 0.34, 95% CI 0.14, 0.55, P=0.001).
Figure 3.
Trend of saliva urease activity during the three-year study period.
Figure 4.
Trend of saliva urease activity during the three-year study period according to baseline caries status.
Figure 5.
Trend of saliva urease activity during the three-year study period by the median salivary mutans streptococci levels.
Saliva urease activity was positively associated with age (adjusted β : 0.074, 95% CI 0.054, 0.093, P<0.001) and salivary mutans levels (adjusted β : 0.03, 95% CI 0.004, 0.055, P=0.022) (Table 3). In the same model, saliva urease activity was significantly lower in samples collected after a sugary meal, compared to those collected under fasting conditions (adjusted β : −0.083, 95% CI: −0.143, −0.023, P=0.007). The association of saliva urease with age was similar in the fasting and non-fasting samples. However, fasting samples showed a strong positive association with the amount of plaque (adjusted β : 8.55, 95% CI: 1.827, 15.273, P=0.013), which was not observed in the non-fasting samples. The association of salivary urease with mutans levels was not significant in this stratified analysis (P>0.1).
DISCUSSION
A growing body of clinical and scientific evidence points to a potentially significant role of urease in caries development and in oral ecology. In order to determine more definitively this role, we need to first understand some important clinical aspects of oral urease activity. This longitudinal panel study gave us the opportunity to observe the dynamic changes in urease activity in the dental plaque and in the saliva of children over a three-year period, and to associate those changes with some biological, behavioral and socio-demographic caries risk factors.
The trend of plaque urease activity levels was relatively stable in the study panel, however urease activity in saliva showed a trend to increase with time. Part of this trend could be related to loss of urease activity during storage of the samples. The estimated maximum loss of urease activity in saliva samples was up to 60% over 12 months, and in plaque samples up to 25% in 6 months. Samples from the more recent time points were stored for shorter time compared to the samples from the earlier time points, therefore suffered less loss of activity. However, the regression analysis showed a significant positive association between age and urease levels in saliva even when the models were adjusted for length of storage. The mean salivary urease levels in this study were lower than the levels reported in older children 4 to 12 years (18), and in adults (16, 17). Although urease activity in these other studies was sometimes measured in plaque and saliva samples that had not been frozen, these observations together suggest that urease levels in saliva may increase with age. This increase could be associated with an increase in the numbers of ureolytic organisms in saliva, such as S. salivarius, as well as with a general increase in the complexity of the oral flora.
The most important factor affecting urease activity in dental plaque was sugar consumption, as demonstrated by the regression analysis and also by the opposite trends of urease levels and sugar scores during the study period. The negative association between plaque urease levels and sugar consumption observed in this study may reflect lower proportions of ureolytic bacteria in the plaque of subjects who consume high sucrose diets. It has been proposed that frequent sugar consumption can lead not only to an increase in the proportions of cariogenic bacteria, but also to a concomitant decrease in the proportions of beneficial alkali-producing species, including the ureolytic organisms which are usually less aciduric (1, 13, 12, 26). This hypothesis is supported by the data presented here, and it is currently being more thoroughly evaluated using molecular techniques.
Urease levels in saliva were positively associated with the salivary levels of mutans streptococci, a somewhat unexpected observation based on what was discussed previously. A possible explanation for this finding is that the urease of S. salivarius, which is the most significant ureolytic organism in saliva, has been shown to be repressed in neutral pH, and becomes de-repressed in acidic environments (27–29). It is possible that saliva from subjects with high levels of mutans streptococci may have a more acidic pH than the saliva from subjects with low mutans levels, resulting in stronger induction of the urease genes. A negative correlation was indeed observed between mutans streptococci levels in saliva and salivary pH in the non-fasting samples in this study (Spearman ρ=−0.106, P=0.034); however, this hypothesis needs to be further investigated. The positive relationship between saliva urease and salivary mutans levels could have important clinical applications. Simple, chair-side tests for measuring urease activity in saliva can be easily developed and they could be used as an indicator of salivary mutans infection and possibly caries risk. The advantage of such biochemical tests is that they can give much faster results compared to the currently available microbiological chair-side tests for S. mutans.
Salivary urease levels were significantly lower in children who had eaten sugar-containing foods prior to sample collection, compared to children who had not eaten anything since the night before. This observation is likely linked to the previous observation of a positive association between saliva urease activity and salivary mutans levels, because children who had eaten had significantly lower levels of mutans streptococci in their saliva compared to those who were fasting (Kruskal-Wallis P=0.0046). The increased numbers of mutans streptococci in the un-stimulated saliva of fasting children may be related to reduced salivary flow due to lack of stimulation from eating and reduced clearance from the mouth overnight. This could also explain the significant association between saliva urease and plaque levels that was observed in the fasting samples. It could also explain why the association between saliva urease and mutans levels was no longer significant when the analysis was stratified by fasting.
Urease measurements in plaque and in saliva had overall low reproducibility in this study. This low reproducibility can be attributed to the multiple factors that were shown to have a significant association with urease activity in this clinical study, as discussed previously. In vitro studies have also shown that urease enzymes, including those from oral bacteria, are rarely constitutively expressed. In most cases the expression of urease enzymes in bacteria is highly regulated in response to environmental factors such as substrate availability, nitrogen and carbohydrate availability and pH (2, 27–30). In the case of plaque urease, the low reproducibility could also be attributed to the fact that the samples were pooled from multiple teeth and tooth sites. These observations suggest that in order to improve the reproducibility of urease measurements in future studies it will be necessary to control for multiple factors and to use a more site-specific method for plaque collection.
In conclusion, the results of this study reveal some important clinical and epidemiological aspects of oral ureolysis in children and demonstrate interesting and complex interactions between oral urease activity and some important caries risk factors. Of particular clinical interest is the unexpected positive association between urease activity and mutans streptococci in saliva, which suggests that saliva urease could be used as an indicator of mutans infection in children. We are currently finalizing the analysis of the caries data and the microbiological data from this longitudinal study, which will clarify the role of urease in caries development and in the oral ecology of children.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the staff of the Clinical Research Center at the University of Puerto Rico Medical Sciences Campus and the UPR students Omar Chacon, Joel Caraballo, Joel Diaz, Guillermo Ramirez, Valerie Garcia, Monica Rodriguez and Aixa Morales for their help in this study. The study described was partially supported by a Career Development Award, K23 DE015285 from the National Institute for Dental and Craniofacial Research, by RCMI grant #G12 RR 03051, and by an RCMI Clinical Research Infrastructure Initiative (RCRII) Award 1P20 RR 11126, from the National Center for Research Resources (NCRR). This publication was also possible by Grant Number 1U54RR026139-01A1 from NCRR, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
REFERENCES
- 1.Burne RA, Chen YY. Bacterial ureases in infectious diseases. Microbes Infect. 2000 Apr;2(5):533–542. doi: 10.1016/s1286-4579(00)00312-9. Review. PubMed PMID: 10865198. [DOI] [PubMed] [Google Scholar]
- 2.Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. Review. PubMed PMID: 2651866; PubMed Central PMCID: PMC372718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salako NO, Kleinberg I. Incidence of selected ureolytic bacteria in human dental plaque from sites with differing salivary access. Arch Oral Biol. 1989;34(10):787–791. doi: 10.1016/0003-9969(89)90029-0. PubMed PMID: 2610614. [DOI] [PubMed] [Google Scholar]
- 4.Sissons CH, Anderson SA, Wong L, Coleman MJ, White DC. Microbiota of plaque microcosm biofilms: effect of three times daily sucrose pulses in different simulated oral environments. Caries Res. 2007;41(5):413–422. doi: 10.1159/000104801. PubMed PMID: 17713343. [DOI] [PubMed] [Google Scholar]
- 5.Sissons CH, Hancock EM, Perinpanayagam HE, Cutress TW. The bacteria responsible for ureolysis in artificial dental plaque. Arch Oral Biol. 1988;33(10):727–733. doi: 10.1016/0003-9969(88)90006-4. PubMed PMID: 3075450. [DOI] [PubMed] [Google Scholar]
- 6.Kopstein J, Wrong OM. The origin and fate of salivary urea and ammonia in man. Clin Sci Mol Med. 1977 Jan;52(1):9–17. doi: 10.1042/cs0520009. PubMed PMID: 23916. [DOI] [PubMed] [Google Scholar]
- 7.Dibdin GH, Dawes C. A mathematical model of the influence of salivary urea on the pH of fasted dental plaque and on the changes occurring during a cariogenic challenge. Caries Res. 1998;32(1):70–74. doi: 10.1159/000016432. PubMed PMID: 9438574. [DOI] [PubMed] [Google Scholar]
- 8.Imfeld T, Birkhed D, Lingström P. Effect of urea in sugar-free chewing gums on pH recovery in human dental plaque evaluated with three different methods. Caries Res. 1995;29(3):172–180. doi: 10.1159/000262065. PubMed PMID: 7621491. [DOI] [PubMed] [Google Scholar]
- 9.Kleinberg I. Effect of urea concentration on human plaque pH levels in situ. Arch Oral Biol. 1967 Dec;12(12):1475–1484. doi: 10.1016/0003-9969(67)90183-5. PubMed PMID: 5237332. [DOI] [PubMed] [Google Scholar]
- 10.Sissons CH, Cutress TW. In-vitro urea-dependent pH-changes by human salivary bacteria and dispersed, artificial-mouth, bacterial plaques. Arch Oral Biol. 1987;32(3):181–189. doi: 10.1016/0003-9969(87)90132-4. PubMed PMID: 3478020. [DOI] [PubMed] [Google Scholar]
- 11.Morou-Bermudez E, Burne RA. Genetic and physiologic characterization of urease of Actinomyces naeslundii. Infect Immun. 1999 Feb;67(2):504–512. doi: 10.1128/iai.67.2.504-512.1999. PubMed PMID: 9916052; PubMed Central PMCID: PMC96348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu M, Browngardt CM, Chen YY, Burne RA. Role of urease enzymes in stability of a 10-species oral biofilm consortium cultivated in a constant-depth film fermenter. Infect Immun. 2003 Dec;71(12):7188–7192. doi: 10.1128/IAI.71.12.7188-7192.2003. PubMed PMID: 14638814; PubMed Central PMCID: PMC308945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000 Dec 1;193(1):1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. Review. PubMed PMID: 11094270. [DOI] [PubMed] [Google Scholar]
- 14.Clancy KA, Pearson S, Bowen WH, Burne RA. Characterization of recombinant, ureolytic Streptococcus mutans demonstrates an inverse relationship between dental plaque ureolytic capacity and cariogenicity. Infect Immun. 2000 May;68(5):2621–2629. doi: 10.1128/iai.68.5.2621-2629.2000. PubMed PMID: 10768953; PubMed Central PMCID: PMC97468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson S, Woodhead J, Crall J. Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res. 1985 Aug;19(8):796–799. doi: 10.1203/00006450-198508000-00003. PubMed PMID: 3898000. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009 Apr;24(2):89–95. doi: 10.1111/j.1399-302X.2008.00477.x. Erratum in: Oral Microbiol Immunol. 2009 Jun;24(3):264. PubMed PMID: 19239634; PubMed Central PMCID: PMC2742966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu M, Morou-Bermudez E, Suárez-Pérez E, Rivera-Miranda C, Browngardt CM, Chen YY, Magnusson I, Burne RA. The relationship between dental caries status and dental plaque urease activity. Oral Microbiol Immunol. 2007 Feb;22(1):61–66. doi: 10.1111/j.1399-302X.2007.00325.x. PubMed PMID: 17241172. [DOI] [PubMed] [Google Scholar]
- 18.Toro E, Nascimento MM, Suarez-Perez E, Burne RA, Elias-Boneta A, Morou-Bermudez E. The effect of sucrose on plaque and saliva urease levels in vivo. Arch Oral Biol. 2010 Mar;55(3):249–254. doi: 10.1016/j.archoralbio.2009.12.007. Epub 2010 Jan 21. PubMed PMID: 20096398; PubMed Central PMCID: PMC2853032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopycka-Kedzierawski DT, Billings RJ. A longitudinal study of caries onset in initially caries-free children and baseline salivary mutans streptococci levels: a Kaplan-Meier survival analysis. Community Dent Oral Epidemiol. 2004 Jun;32(3):201–209. doi: 10.1111/j.1600-0528.2004.00153.x. PubMed PMID: 15151690. [DOI] [PubMed] [Google Scholar]
- 20.Beltrán-Aguilar ED, Estupiñán-Day S, Báez R. Analysis of prevalence and trends of dental caries in the Americas between the 1970s and 1990s. Int Dent J. 1999 Dec;49(6):322–329. doi: 10.1111/j.1875-595x.1999.tb00532.x. PubMed PMID: 10907429. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Liang KY. Sample size calculations for studies with correlated observations. Biometrics. 1997 Sep;53(3):937–947. PubMed PMID: 9290224. [PubMed] [Google Scholar]
- 22.Pine CM. Fiber-optic trans-illumination (FOTI) in caries diagnosis. In: Stookley GK, editor. Early detection of dental caries: Proceedings of the 1st annual Indiana Conference; Indianapolis: Indiana University School of Dentistry; 1996. pp. 51–65. [Google Scholar]
- 23.Nizel AE, Papas AS. Nutrition in Clinical Dentistry. 3rd edition. W.B. Saunders Company; pp. 277–308. [Google Scholar]
- 24.Hastie T, Tibshirani . Generalized Additive Models. USA: Chapman & Hall/CRC; 1990. Ed. [DOI] [PubMed] [Google Scholar]
- 25.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling using Stata. Second Edition. College Station, TX: Stata Press; 2008. [Google Scholar]
- 26.Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med. 2002;13(12):108–125. doi: 10.1177/154411130201300202. Review. PubMed PMID: 12097354. [DOI] [PubMed] [Google Scholar]
- 27.Chen YY, Burne RA. Analysis of Streptococcus salivarius urease expression using continuous chemostat culture. FEMS Microbiol Lett. 1996 Jan 15;135(2–3):223–229. doi: 10.1111/j.1574-6968.1996.tb07993.x. PubMed PMID: 8595861. [DOI] [PubMed] [Google Scholar]
- 28.Li YH, Chen YY, Burne RA. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ Microbiol. 2000 Apr;2(2):169–177. doi: 10.1046/j.1462-2920.2000.00088.x. PubMed PMID: 11220303. [DOI] [PubMed] [Google Scholar]
- 29.Sissons CH, Perinpanayagam HE, Hancock EM, Cutress TW. pH regulation of urease levels in Streptococcus salivarius. J Dent Res. 1990 May;69(5):1131–1137. doi: 10.1177/00220345900690050301. PubMed PMID: 2110582. [DOI] [PubMed] [Google Scholar]
- 30.Morou-Bermudez E, Burne RA. Analysis of urease expression in Actinomyces naeslundii WVU45. Infect Immun. 2000 Dec;68(12):6670–6676. doi: 10.1128/iai.68.12.6670-6676.2000. PubMed PMID: 11083780; PubMed Central PMCID: PMC97765. [DOI] [PMC free article] [PubMed] [Google Scholar]