Abstract
Rationale
Methamphetamine (mAMPH) administration in animals can lead to a variety of cognitive and behavioral deficits. We previously reported non-acute reversal learning impairments after a single-day administration of mAMPH, providing evidence of this drug’s selective effects on inhibitory control. Effortful decision-making (i.e., how much effort to invest in rewards) is an aspect of cognition that has not yet been explored after mAMPH.
Objectives
Given that frontostriatal circuitry mediating this type of choice is vulnerable to the effects of mAMPH, we tested the hypothesis that mAMPH may also affect decision-making involving effort, another form of cognitive flexibility.
Methods
We examined the non-acute effects of an experimenter-administered single day of mAMPH on effort discounting. In this task, rats previously treated with mAMPH or saline (SAL) could select a high reward at the cost of climbing over a tall barrier or a low reward with no barrier impeding its procurement.
Results
Following treatment, mAMPH rats were more work-averse than SAL rats. A control task showed there were no treatment group differences when the high and low rewards involved equal work: all rats chose the high reward preferentially. There were no significant treatment group differences in [125I]RTI-55 binding to dopamine and serotonin transporters (DAT, SERT) in any of the regions assayed; however, there were significant correlations of accumbens DAT and cingulate SERT with post-treatment performance.
Conclusions
These findings suggest that even modest dose mAMPH exposure has long-lasting effects on effortful decision-making and may do so through influences on forebrain monoaminergic systems.
Keywords: Cost, Reward processing, Decision-making, Cognitive flexibility, Dopamine transporter, Serotonin transporter
Introduction
Methamphetamine (mAMPH) is a widely abused and highly addictive psychostimulant drug that can result in behavioral and cognitive deficits. Cognitive deficits in human users range from learning, memory, and attentional problems to deficits in executive function, including inhibitory control and decision-making (Rogers et al. 1999; Ornstein et al. 2000; Simon et al. 2000; Salo et al. 2002; Kalechstein et al. 2003; Gonzalez et al. 2004). Animal models are useful in elucidating neural mechanisms and consequences of mAMPH use. Specifically, experimenter-administered regimens of mAMPH, consisting of “binge” doses; i.e., multiple doses over a single day that mimic human users’ binge style of consumption and, if administered at sufficiently high doses, result in brain monoamine-depleting effects similar to those observed in the human mAMPH-abusing population (Wilson et al. 1996; McCann et al. 1998; Sekine et al. 2001; Volkow et al. 2001; Kish et al. 2009).
The effects of binge mAMPH in animals include motor impairments (Walsh and Wagner 1992), motor learning impairments (Chapman et al. 2001; Daberkow et al. 2005), object and odor recognition memory impairments (Chapman et al. 2001; Bisagno et al. 2002; Schröder et al. 2003; Belcher et al. 2005; Daberkow et al. 2005; O’Dell et al. 2010), and cognitive flexibility impairments. The latter can be assessed by a variety of tasks such as spatial reversal (White et al. 2009), response reversal (Cheng et al. 2007), and discrimination reversal learning (Izquierdo et al. 2010), all of which are impaired after mAMPH administration. Binge mAMPH regimens can result in long-lasting depletions of dopamine (DA) and decreases of DA transporters (DAT) through striatal subregions (Eisch et al. 1992; Wagner et al. 1980; Ricaurte et al. 1982; Wagner and Walsh 1991; Fleckenstein et al. 1997; Volz et al. 2007), including the nucleus accumbens (NAc) core (Broening et al. 1997).
While DA and DAT changes are most pronounced after binge dose mAMPH administration, serotonin (5-HT) and 5-HT transporter (SERT) are typically affected as well (Hotchkiss and Gibb 1980). Significant decreases in SERT concentrations have been observed in human mAMPH users in orbitofrontal and occipital cortices (Kish et al. 2009) as well as after experimenter-administered mAMPH in rat perirhinal cortex (Belcher et al. 2005), hippocampus (Schröder et al. 2003), and striatum (Haughey et al. 2000). It remains unclear, however, which mAMPH-induced changes (DAergic or 5-HTergic) most correlate with cognitive inflexibility.
Some kinds of executive function such as working memory and attention are heavily attributed to DA modulation, while others such as response inhibition and impulse control are more closely linked to 5-HT function (Arnsten 1998; Robbins 2005; Brigman et al. 2010). One facet of cognition that has not yet been explored after mAMPH is effortful decision-making: i.e., reward choices made in the face of increasing effort (Walton et al. 2002). The primary circuitry involved in effortful choices includes the medial frontal cortex, the basolateral amygdala, and DA in the NAc (Cousins et al. 1996; Walton et al. 2002; Denk et al. 2005; Floresco and Ghods-Sharifi 2007; Hauber and Sommer 2009; Salamone et al. 2009). Because mAMPH is known to affect striatal DA and DAT, crucial moderators of forebrain circuitry subserving effortful decision-making, work aversion might be expected to occur in mAMPH-pretreated rats. This hypothesis is strengthened by reports that striatal DA depletions and administration of DA antagonists can impair performance on tasks that require cost–benefit assessment (Cousins et al. 1993; Salamone et al. 1994; Lindner et al. 1997). By contrast, there is evidence that 5-HT plays no role in effortful decision-making (Denk et al. 2005).
Although researchers have employed widely varying binge mAMPH doses, the possibility of extensive neurotoxicity and unwanted side effects with high mAMPH doses motivated us to examine effects of a moderate binge dose, which was expected to effect smaller changes in forebrain DA and 5-HT transmission. In the present study, rats were administered a binge dose of mAMPH, chosen for its selective action on a measure of cognitive flexibility in a recent study (Izquierdo et al. 2010), and subsequently assessed on effortful decision-making. This regimen was also used because it has modest long-term effects on DA transporter (DAT) density (Belcher et al. 2008; Izquierdo et al. 2010) and produces no noticeable motor deficits, even though it has marked acute physiological effects (as evidenced by hyperthermia). After behavioral testing was concluded, we quantified DAT in the striatum, the area most sensitive to the DA-depleting effects of mAMPH (Eisch et al. 1992; Cass 1997) and SERT in several areas of frontal cortex.
Materials and methods
Subjects
Subjects were 18 male Long Evans rats (Charles River Laboratories), individually housed, weighing between 230 and 290 g at the start of testing. Vivaria were maintained at a 12-h light/12-h dark cycle, with the temperature at 22°C. All behavioral testing took place 5–6 days per week between 0800 and 1600 h during the rats’ inactive period, consistent with previous and ongoing studies in our lab (Izquierdo et al. 2010). All procedures were accepted by the Institution for Animal Care and Use Committee at California State University, Los Angeles.
Behavioral testing apparatus
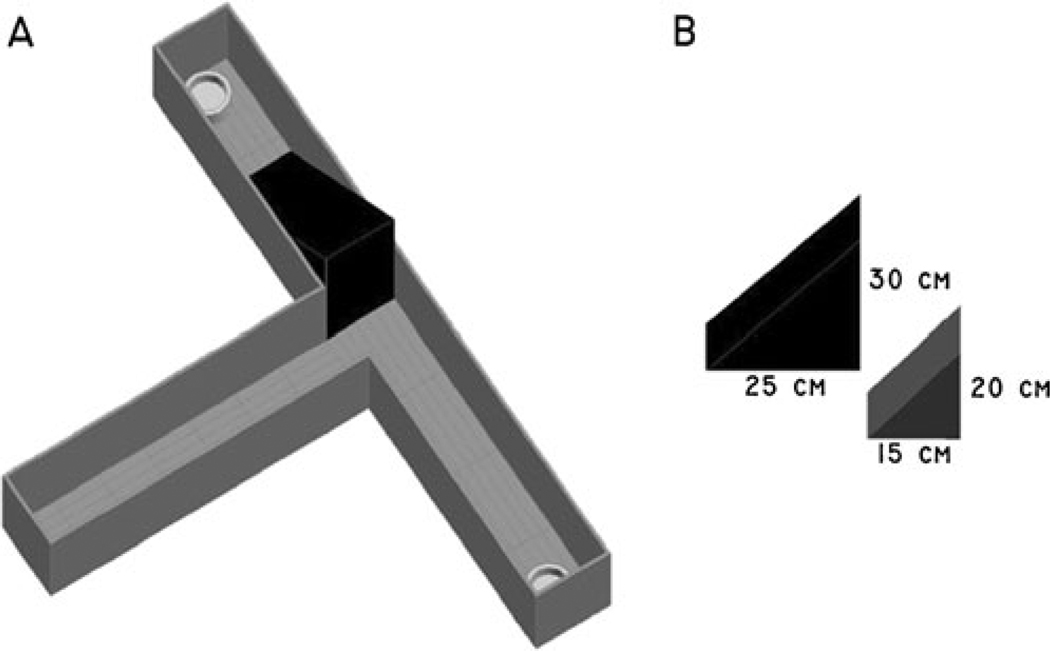
Our behavioral protocol was adapted from experimental procedures conducted by Walton et al. (2002). A commercially available t-maze (Stoelting Co., Wood Dale, Illinois) with one start arm and two goal arms was used (Fig. 1). Each goal arm measured 41.9 cm in length, 10.2 cm wide, with walls 20.3 cm high. The start arm measured 50.3 cm in length. Located at the end of each goal arm was a white ceramic bowl measuring 5.1 cm in diameter in which the food reward was placed. “Froot loops” (Kellogg NA Co., Battle Creek, MI) were given as food rewards during testing: a “high reward” (HR) consisted of four one half froot loops (i.e., two froot loops), while a “low reward” (LR) consisted of one one half froot loop. To increase effort for the HR, wooden triangular blocks of 15, 20, 25, and 30 cm heights were constructed in the lab and used to impede access to the food reward in the HR arm. Barrier heights were chosen from previous research conducted by Walton et al. (2002). The wooden triangular blocks (barriers) were placed in the t-maze such that the rat was required to climb straight up the side (90°) and down at an angle to the food reward located at the end of the goal arm. The angle of decline to the food reward averaged 44.5° across the barriers. Between trials, the rat was removed from the t-maze and placed in a 30 cm h × 25 cm diameter cylindrical glass holding tank.
Fig. 1.
T-Maze apparatus. The effortful t-maze (a) was equipped with one start arm and two goal arms, each baited with either a high reward, HR (four one half froot loops) or a low reward, LR (one half froot loop). Wooden blocks (b) of varying heights 15, 20, 25, and 30 cm were used to increase the effort required to access the high reward arm
Behavioral testing and drug treatment
Handling and acclimation to food rewards
Each rat was handled for a minimum of 10 min once per day for 5 days prior to behavioral testing. During the last 2 days of handling, animals were transported for approximately 5 min to habituate them to the transport cart prior to the commencement of testing. Upon returning to the vivarium, they were fed ten froot loops in their homecage to accustom them to the food reward.
Food restriction
All rats were food-restricted to 85% of their free-feeding body weight to ensure motivation to work for food, while water was available ad libitum. Weights were monitored three times per week to ensure a healthy body weight. Following treatment with mAMPH, rats were given 3–4 days rest without behavioral testing.
Acclimation to the t-maze
A habituation and training protocol adapted from Walton et al. (2002) was used to habituate the rats to the t-maze and familiarize them with the froot loops. During the acclimation phase, each rat was individually placed into the t-maze and allowed to explore and eat froot loops freely for 10 min. Criterion for advancement to the next phase was consuming 15 one half froot loops within 10 min for two consecutive days.
Phase 1 discrimination training with free sampling
In this phase, one goal arm was baited with four one half froot loops (HR arm), and the other with one one half froot loop (LR arm). The rat was allowed to sample freely from both arms for five trials. Each trial lasted until the rat finished all the froot loops. Trials were separated by a 30-s intertrial interval (ITI) on day 1, and a 60-s ITI on day 2, during which time they were placed in an empty cylindrical glass holding tank. HR and LR arm designations were counter-balanced among rats (half the rats received HR in the left goal arm, the other half in the right) and remained constant for the duration of testing. This phase was administered for two consecutive days.
Phase 2 discrimination training with forced trials
For this phase, each rat was administered ten “forced” trials, in which either the HR or LR arm was blocked by a white cardboard insert, forcing the rat to one side or the other according to a Gellerman schedule. This phase marked the beginning of learning to visit only one arm as well as continuing to learn each arm’s associated reward values. Each trial was separated by a 30-s ITI, during which time rats were placed in an empty cylindrical glass holding tank. This phase was administered for two consecutive days.
Phase 3 discrimination training with free choice
Each rat was allowed to choose either the HR or LR arm and was removed from the t-maze upon eating the food reward from the chosen arm. Ten trials were administered per day, with a forced trial administered after trials 5 and 10, forcing the rat to the arm not chosen on the most recent previous trial to prevent side biases (i.e., if the HR was chosen on trial 5, the forced trial would be to the LR). A 30-s ITI was used between trials, during which time the t-maze was wiped clean with 70% ethanol solution to prevent the rat’s use of scent-guided choice. Criterion for the next phase was choosing the HR arm 90% or more for two consecutive days.
Training phase with barriers
During this phase, rats were required to climb successively larger wooden barriers (beginning at 15 cm) to achieve the HR. Each session consisted of ten free choice trials, with a forced trial after trials 5 and 10 to prevent side biases. Upon eating the food reward, the rat was placed in a holding tank for a 30-s ITI, during which the maze was wiped clean with 70% ethanol. This phase continued for 12 days of daily testing, with barrier heights increasing every third day from 15, 20, 25 to 30 cm, irrespective of the rat’s choice.
Drug treatment
Within 3 days following testing at the 30-cm barrier, rats were administered mAMPH or SAL. Drug treatment was similar to that described in Izquierdo et al. (2010). On the day of mAMPH injections rats were kept in large, clear Plexiglas boxes [40 cm (length) × 40 cm (width) × 38 cm (height)] containing bedding but with no lids. Ambient room temperature was kept warm at 24±3°C by overhead lights mounted on each box. Rats were given four injections of d-mAMPH (Sigma, St Louis, MO; 2 mg free base/kg, s.c.) or a physiological SAL solution (1 ml/kg, s.c.) at 2-h intervals on a single day (mAMPH n = 12, SAL n = 6). Animals’ body temperatures during the mAMPH binge regimen were monitored by rectal thermocouple probe 60 min after each injection.
Post-treatment testing
Following 3–4 days of no testing or food restriction, rats were placed back on food restriction and tested again on barriers of 15, 20, 25, and 30 cm, following a similar protocol used for the “Training phase with barriers” stage above.
Post-treatment control task
Following the last day of the post-treatment test phase with barriers (i.e., the next day), a control task in which work was equalized between the two arms was conducted. In this task, the barrier to the HR arm was removed, allowing free access to the HR (i.e., both the HR and the LR arms were accessible without barriers). Ten trials were recorded for each rat per session.
[125I]RTI-55 binding to DAT and SERT
Rats were euthanized an average of 4 weeks after mAMPH or SAL treatment, and an average of 2.5 weeks after barrier testing. Rats were given an overdose of sodium pentobarbitol (250 mg/kg, i.p.), decapitated, and their brains removed and frozen at −20°C by immersion in isopentane. Coronal sections (20 µm-thick) were cut on a cryostat at the level of the anterior striatum (AP coordinates +1.7 to +0.8 mm, according to Paxinos and Watson 2005), thaw-mounted on Vectabond-treated glass slides and stored at −20°C until used for autoradiography. Separate slides were used for determination of DAT and SERT binding. For determination of cortical SERT binding, warmed slides removed from the −20°C freezer were preincubated at room temperature (22°C) in a solution of assay buffer (10 mM NaPO4, 120 mM NaCl, 100 mM sucrose) for 5 min to remove endogenous ligands that could interfere with subsequent radioligand binding. After preincubation, the sections were incubated (also at room temperature, 22°C) in a solution of assay buffer containing 25 pM [125I]RTI-55 for 2 h. The sections were then rinsed twice for 2 min each at 4°C in assay buffer, then once for 10 s in 4°C distilled water. The rinsed slides were then rapidly dried under a stream of heated air. Determinations of striatal DAT were performed in much the same way as those for SERT with the exception that the preincubation and incubation media contained 100 nM fluoxetine to block [125I]RTI-55 binding to SERT (Boja et al. 1992). The dried slides and [14C]-containing autoradiographic standards were apposed to Hyperfilm MP (GE Healthcare) for 48 h before development.
Quantification of [125I]RTI-55 binding was done using an MCID image analyzer (InterFocus Imaging; Cambridge, England). Image densities were converted to [125I]RTI-55 binding levels using a calibration curve based on images of the standard slides packed with each film. Regional densities of RTI binding were obtained by outlining the desired structures on their respective [125I]RTI-55 images. Values obtained represented the average of measurements taken from both hemispheres in a total of four sections per animal. For DAT analysis, the images were first divided into caudate-putamen (CP) and nucleus accumbens septi (NAc) samples. The CP was then subdivided into four subregions consisting of: dorsomedial (dmCP), dorsolateral (dlCP), ventromedial (vmCP), and ventrolateral (vlCP) parts, which were separately quantified for [125I]RTI-55 binding. For SERTanalysis, samples encompassing all cortical layers were taken in cingulate, motor, somatosensory, and insular cortical.
Data analyses
Data were analyzed using StatView software. Statistical significance was noted when p values were equal to or less than 0.05. Barrier performance data (percentage of HR chosen) were analyzed using repeated measures ANOVA. Temperature, DAT, and SERT data were also analyzed using repeated measures ANOVA. Pearson product-moment correlation coefficients were generated for regional DAT and SERT depletion and post-treatment performance.
Results
Pre-treatment performance on barriers
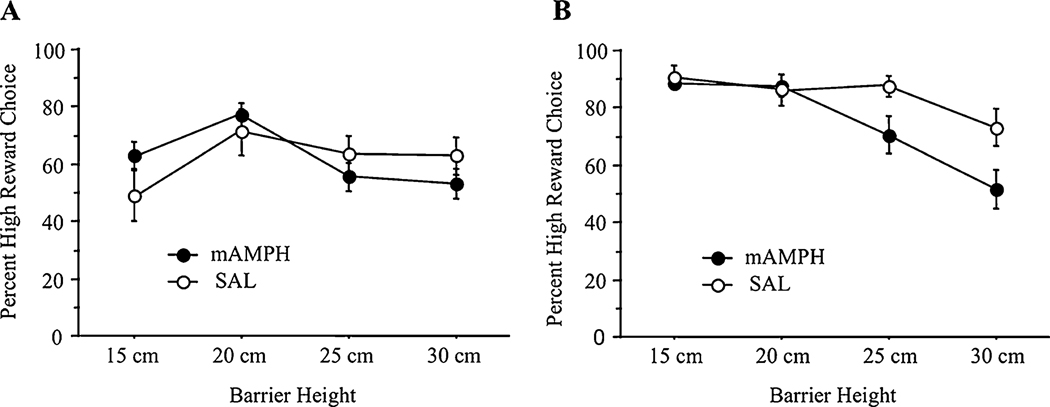
Effortful decision-making was measured as a percentage of HR choices for each barrier height (i.e., rats choosing the HR had higher scores, while work-averse rats had lower scores). A repeated measures analysis of variance (ANOVA) was conducted to test for differences in performance by treatment group (mAMPH vs. SAL) and barrier height (15, 20, 25, and 30 cm). A significant within-subject effect for barrier height was found (F11,165 = 3.15, p<0.01), though there was neither a significant main effect for treatment group nor an interaction of treatment group by barrier height. Therefore, there were no pretreatment differences in performance over barriers between prospective mAMPH and SAL rats.
Effect of mAMPH on effort: post-treatment performance on barriers
A repeated-measures ANOVA was conducted to analyze post-treatment performance differences by treatment group (mAMPH vs. SAL) and barrier height (15, 20, 25, and 30 cm). Though there was no significant main effect for treatment group, there was a significant main effect for barrier height (F11,99 = 3.89, p<0.01) and a significant interaction of treatment group by barrier height (F11,99 = 1.91, p = 0.05).
Bonferroni post-hoc comparisons revealed no significant mean differences at the 15-cm barrier height or 20-cm barrier height. However, post-hoc comparisons did reveal a significant difference at the 25 cm height (mAMPH = 70.40 vs. SAL = 87.50, p = 0.05) and at the 30-cm height (mAMPH = 51.60 vs. SAL = 73.13, p = 0.03), with mAMPH-treated rats showing more work aversion than SAL-treated rats (see Fig. 2).
Fig. 2.
Pre- and post-treatment performance over barriers. a Preference scores during pretreatment phase. Mean±SEM percent high reward (HR) choices by prospective treatment group as a function of increasing barrier heights (15, 20, 25, and 30 cm). There were no significant pre-existing group differences on effort (n = 18). b Preference scores during post-treatment phase. Mean±SEM percent high reward (HR) choices by treatment group as a function of increasing barrier heights (15, 20, 25, and 30 cm). Significant treatment group differences emerged only with increasing effort requirement (mAMPH n = 9, SAL n = 6)
Effect of mAMPH on discrimination of HR versus LR: post-treatment performance without barrier
A repeated-measures ANOVA was conducted to analyze group differences during a post-treatment-testing two-session control task in which no barrier impeded access to the HR. Under these conditions, both groups showed overwhelming preference for the HR arm (mAMPH = 97.1% vs. SAL = 97.5%). This ANOVA revealed no significant main effects for treatment group, nor a significant session by group interaction.
Effect of mAMPH on temperature: day-of-treatment temperatures
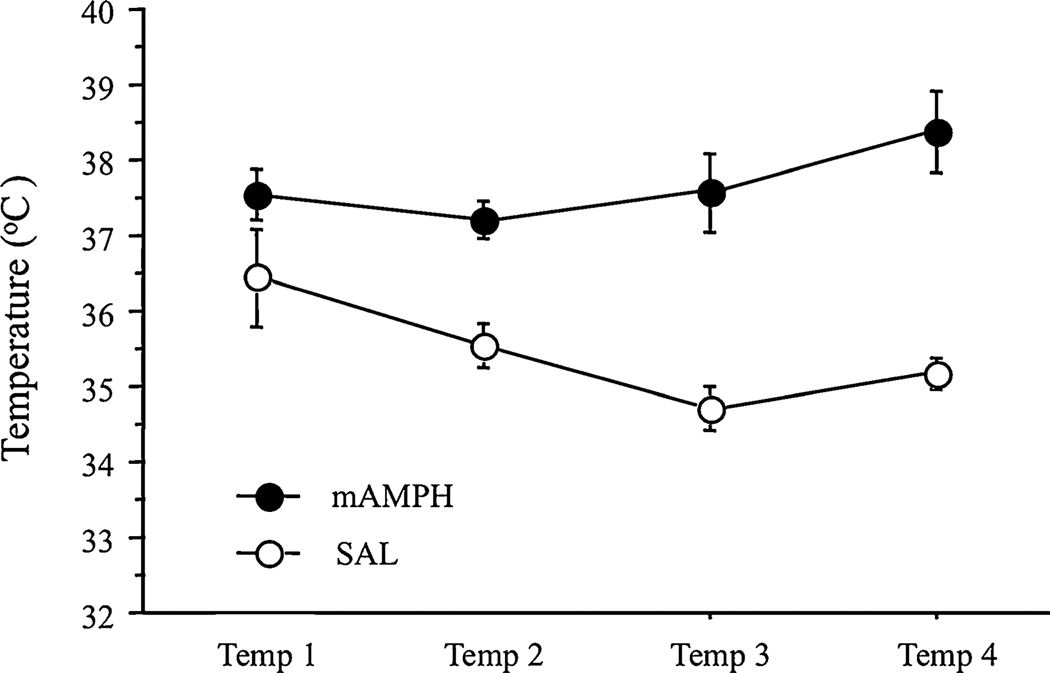
Body temperature elevation during the course of binge mAMPH treatment is commonly used to monitor the effectiveness of the treatment. Hyperthermia is one factor that can contribute to the neurotoxicity of mAMPH (Bowyer et al. 1994; Albers and Sonsalla 1995), which can influence later performance on tasks measuring cognitive function. For these reasons, temperature data were analyzed for group differences. Significant overall main effects for treatment group (F1,16 = 26.00, p<0.001) were found, with mAMPH-treated rats showing higher temperatures after each of the four injections when compared to SAL rats. There were no significant effects for either time of temperature reading or a treatment group by time interaction (see Fig. 3).
Fig. 3.
Body temperatures of rats during mAMPH treatment. Temperature readings taken after each of the four methamphetamine or saline injections (designated Temp 1 through Temp 4). Temperatures (°C) were recorded using a rectal thermocouple 1 h after injections, mAMPH n = 12, SAL n = 6
[125I]RTI-55 binding to DAT in the striatum, and post-treatment performance
Rats exposed to a binge mAMPH regimen (4 × 2 mg/kg) showed small, nonsignificant reductions in DAT binding in all striatal subregions. The NAc (−17%) and ventrolateral CP (−17%) regions were the most affected in the mAMPH-exposed group. An ANOVA was conducted to assess treatment group differences in striatal DAT levels. This analysis revealed no significant group differences in overall DAT binding in the NAc or caudate putamen (CP). However, DAT levels in the NAc were correlated with post-treatment performance over all barrier heights (i.e., all barrier data compacted into one variable) (r = 0.25, p < 0.002) and particularly with post-treatment performance over the 30 cm barrier height (r = 0.36, p<0.03). There were no significant correlations between post-treatment performance over barriers and DAT depletions in any of the CP subregions (Table 1).
Table 1.
Correlation matrix of striatal DAT and post-treatment barrier performance
| Post-Tx barriers | Post-Tx 25 cm barrier | Post-Tx 30 cm barrier | NAc DAT | CP Subregion DAT | ||||
|---|---|---|---|---|---|---|---|---|
| dmCP | dlCP | vmCP | vlCP | |||||
| Post-Tx barriers | 1.00 | 0.25*** | ns | ns | ns | ns | ||
| Post-Tx 25 cm barrier | 1.00 | 0.61*** | ns | ns | ns | ns | ns | |
| Post-Tx 25 cm barrier | 1.00 | 0.36* | ns | ns | ns | ns | ||
Scores are reported as r values showing levels of DAT binding in regions of the CP and NAc and their association with post-treatment performance over barriers
Post-Tx barriers post-treatment performance over all barrier heights, post-Tx 25 cm barrier post-treatment performance over the 25-cm barrier, post-Tx 30 cm barrier post-treatment performance over the 30-cm barrier, DAT dopamine transporter, CP caudate putamen, dmCP dorsomedial caudate putamen, dlCP dorsolateral caudate putamen, vmCP ventromedial caudate putamen, vlCP ventrolateral caudate putamen
p<0.05;
p<0.01;
p<0.001
[125I]RTI-55 binding to SERT in the frontal and parietal cortex, and post-treatment performance
MAMPH-exposed rats showed small, nonsignificant reductions in SERT binding in all frontal and parietal cortical areas sampled, ranging from −6% in cingulate cortex to −10% in insular cortex. An ANOVA was conducted to assess treatment group differences in frontoparietal cortical SERT levels. This analysis revealed no significant group differences in overall SERT depletions throughout the cingulate, motor, somatosensory, and insular cortices. However, significant correlations were found between post-treatment performance (i.e., all barrier heights compacted into one variable) and SERT in the cingulate cortex (r = −0.22, p<0.01), the somatosensory cortex (r = 0.32, p<0.0001), and the insular cortex (r = 0.32, p < 0.0001). Additionally, there were significant correlations between post-treatment performance over the 25-cm barrier and SERT in the somatosensory cortex (r = 0.47, p<0.01) and the insular cortex (r = 0.48, p<0.01), and post-treatment performance over the 30 cm barrier and SERT in the somatosensory cortex (r = 0.34, p<0.04) (see Table 2).
Table 2.
Correlation matrix of frontoparietal cortical SERT and post-treatment barrier performance
| Post-Tx barriers |
Post-Tx 25 cm barrier |
Post-Tx 30 cm barrier |
Brain region SERT | ||||
|---|---|---|---|---|---|---|---|
| Cingulate | Motor | Somato- sensory |
Insular | ||||
| Post-Tx barriers | 1.00 | −0.22** | ns | 0.32*** | 0.32*** | ||
| Post-Tx 25 cm barrier | 1.00 | 0.61*** | ns | ns | 0.47** | 0.48** | |
| Post-Tx 30 cm barrier | 1.00 | ns | ns | 0.34* | Ns | ||
Scores are reported as r values showing levels of SERT binding within regions of frontoparietal cortex and their association with post-treatment performance over barriers
Post-Tx barriers post-treatment performance over all barrier heights, post-Tx 25 cm barrier post-treatment performance over the 25-cm barrier, post-Tx 30 cm barrier post-treatment performance over the 30-cm barrier, SERT serotonin transporter
p<0.05,
p<0.01,
p<0.001
Discussion
Work-aversion after 4 × 2 mg/kg mAMPH treatment
In the present study, we observed an effect of a moderate binge dose of mAMPH on effortful decision-making; this extends the behavioral effects of this dose beyond impairments in reversal learning (Izquierdo et al. 2010). It is important to note that these effects on cognition are separate from the acute effects of the drug—i.e., the work-averse pattern of reward choices in the mAMPH-treated group occurred approximately 10–11 days post-treatment when rats encountered the 25- and 30-cm barrier heights. Rats pretreated with mAMPH increased their choices of the LR as barrier height increased. While there were no treatment group differences at lower barrier heights, significant work aversion at the two tallest barrier heights was observed. This work aversion pattern could not be explained by preexisting group differences. Additionally, motivation to consume the reward was unaffected by treatment; all rats readily ate food in their home cages and during testing, indicating appetite was unaffected.
Notably, pretreatment training over barriers resulted in lower frequencies of HR choices than what is seen post-mAMPH treatment. Presumably, this occurs as a result of a practice effect: scores hover around 50% HR during initial training over barriers (pre-mAMPH/SAL treatment) and rise after training on all barrier heights (i.e., after the 30-cm barrier). Upon resuming testing, post-mAMPH treatment, at the 15 cm height, rats appear to manage the obstacles with greater ease than during initial training. Only at the two highest barrier heights do we see mAMPH-treated rats display any aversion to the HR arm, which we interpret as work aversion, while the SAL rats display no such tendencies.
After the testing phase, in which mAMPH-treated rats displayed work aversion at the 25- and 30-cm barrier heights, rats were tested on a “control task,” where work was equalized for the two options by removing the barrier, allowing free access to both the HR and LR arms. All rats chose the HR arm 90–100% of the time, and there were no treatment group differences. This suggests that the performance difference between SAL and mAMPH-treated rats was due to an aversion to climbing the barrier, and not other factors, such as an inability to discriminate between the HR and LR. Given equal work, mAMPH- and SAL-treated rats performed comparably. Long-lasting motor impairments are unlikely following this modest dose. Wallace et al. (1999) employed a binge regimen fivefold higher than the present dose regimen (four injections of 10 mg/kg every 2 h) and found only a slight reduction of spontaneous locomotor activity 1 week after treatment even though the DA content of the CP and NAc were reduced by 56% and 30%, respectively.
Our findings indicate that pretreatment with mAMPH has the potential to alter choice behavior away from actions or rewards that require more work, even when the reward magnitude associated with the more effortful response outweighs the alternative option. These results suggest that mAMPH-treated animals are able to perform simple, familiar tasks normally, with the emergence of deficits only in goal-directed, effortful behaviors at higher response demands. These findings are in accord with the impairments observed by Izquierdo et al. (2010) where retention of a familiar visual discrimination problem was unaffected after mAMPH and an impairment observed only when the task became more difficult (i.e., reversal of reward contingencies). Future studies should investigate if increasing reward magnitude or enhancing reward palatability affects mAMPH-pretreated animals’ ability to engage in effortful behavior.
DA Changes and Work Aversion after 4 × 2 mg/kg mAMPH Treatment
Animal studies have clearly established that binge administration of moderate-to-high doses of mAMPH decreases striatal DA content, DAT protein, and radioligand binding, as well as other markers of dopaminergic terminal integrity (e.g., tyrosine hydroxylase protein—for review, see Volz et al. 2007; Yamamoto et al. 2010; Cadet and Krasnova 2009). The dose regimen employed here (4 × 2 mg/kg) was below the range (3 or 4 × 4–10 mg/kg) typically used to achieve dopaminergic neurotoxicity. Although small DAT reductions were observed in NAc and all CP subregions, these were not statistically significant.
Though our present design precludes establishing a causal relationship, we were able to observe a significant positive correlation of NAc DAT with post-treatment barrier performance, while finding no such correlation with performance in any other striatal subregion. The possibility that mAMPH’s effects on effortful responding involve changes in striatal dopamine is supported by studies using either dopamine receptor ligand or striatal neurotoxic lesion techniques. Specifically, one recent study showed significant LR, low-effort preference in rats treated with a DA antagonist while rats treated with a DA agonist showed a HR, high-effort preference (Bardgett et al. 2009). Additionally, DA levels in the NAc appear to be a mediating factor in effortful choice behavior: rats with 6-hydroxydopamineinduced DA depletions in this region show impairments on tasks with high fixed ratio response requirements, with no significant impairments seen at low fixed ratio requirements (Aberman and Salamone 1999; Correa et al. 2002). Here, though we cannot conclude that the changes in behavior occurred as a result of DAT changes in the NAc, there does appear to be a mild correlation between these two variables.
Behavioral changes may occur even without accompanying monoaminergic neurotoxicity and have been observed using different treatment regimens and testing other realms of cognitive function. For example, a 3-week sensitizing regimen of mAMPH (3 mg/kg, every other day) produced impairments in object recognition memory 1 week after the final mAMPH dose without producing any loss of CP or NAc DAT or cortical or hippocampal SERT (Belcher et al. 2006). Additionally, a loss of object recognition memory is seen in rats that undergo extended (6 h/day for 14 days) mAMPH self-administration and are tested for memory 1 week after the last mAMPH injection (Rogers et al. 2008), a mAMPH exposure regimen reported not to produce a loss of dopamine or serotonin in CP, NAc, or prefrontal cortex (Schwendt et al. 2009). In aggregate, these findings as well as those of Izquierdo et al. (2010) suggest that mAMPH-induced impairments in memory and executive functions may occur under conditions of minimal or no damage to dopamine terminals. The animal model employing binge administration of lower mAMPH doses may be relevant to understanding the cognitive impairments that can occur even before human abusers commence long-term, higher-dose use of this psychostimulant drug.
Temporal Discounting, Impulsivity, and SERT
Future studies of performance measures in mAMPH-pretreated rats would benefit by incorporating a measure of temporal (or delay) discounting. Although the time spent climbing over the barrier is minimal, an argument could be made that the aversion to the HR choice seen by the mAMPH rats was due to the additional time required to climb the barrier, rather than the additional effort. If so, then this task could be interpreted as having measured delay discounting and impulsivity, not effortful behavior. Impulsivity and increased sensitivity to delayed reinforcement has been well documented in rats lesioned in such areas as the NAc (Cardinal et al. 2001) and orbitofrontal cortex (Mobini et al. 2002), both of which can be affected by mAMPH. Consequently, although the likelihood of this task measuring delay discounting and not effort is minimal, it cannot be ruled out. Additionally, higher levels of temporal discounting and impulsivity are seen in individuals with addictions to a variety of substances, including opioids (Madden et al 1997; Kirby et al 1999), cocaine (Coffey et al 2003), and alcohol (Richards et al 1999; Petry 2001). It is likely, then, that delay discounting and impulsivity play a role in addiction, helping to maintain drug-using behaviors instead of behavioral states that have the effect of supporting reward deferral and long-term rewards like sobriety (as reviewed in Jentsch and Taylor 1999). It is, of course, possible that both an aversion to work and delay play independent roles as factors in mAMPH-treated rats’ choice behavior.
The degree to which frontal cortex can modulate quick, low-effort responses is greatly related to impulsivity. A single-day of binge mAMPH has been reported to result in cortical changes, affecting metabolism and gene expression in several cortical areas (Pontieri et al 1990; Belcher et al 2009). Such functional 5-HT changes can occur secondarily to changes in DA transmission (Marshall et al. 2007; Belcher et al. 2009) and might predispose the organism to work and/or delay aversion. Serotonin in the frontal cortex is also altered after mAMPH (Hotchkiss and Gibb 1980). Though we did not observe significant treatment group differences in SERT in the frontal cortex, we did observe significant correlations of widespread frontocortical SERT levels with post-treatment barrier performance. The most interesting association was negative: in cingulate cortex. A previous report established no 5-HT involvement in effortful decision-making (Denk et al. 2005), yet there is a large body of data showing that 5-HT is involved in impulsive choice (Robbins 2005) and delay discounting (Denk et al. 2005).
Conclusions
Ongoing experiments are aimed at comparing the effects of repeated and binge exposure to mAMPH on decision-making and determining if any impairments are associated with a differential pattern of DA and 5-HT changes in the brain. A thorough account of the effects of these dosing regimens would capture profiles of use (both binge and escalating daily use) seen in the human mAMPH user and are complementary to self-administration models in studying neurobiological mechanism and consequences of psychostimulant addiction.
Acknowledgements
This work was supported by 1SC2MH087974 (Izquierdo) and 1RO1 DA012204 (Marshall). We thank the CSULA Animal Care staff and Dr. Andrew Holmes for comments on an earlier version of the paper.
Footnotes
Disclosure/conflicts of interest There is nothing to disclose, nor are there any conflicts of interest.
Contributor Information
Alisa R. Kosheleff, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA
Millie Grimes, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA.
Steve J. O’Dell, Department of Neurobiology and Behavior, University of California, Irvine, CA 92617, USA
John F. Marshall, Department of Neurobiology and Behavior, University of California, Irvine, CA 92617, USA
Alicia Izquierdo, Email: aizquie@calstatela.edu, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Green L. Dopamine modulates effort-based decision-making in rats. Behav Neurosci. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. Long-term changes in dopamine-stimulated gene expression after single-day methamphetamine exposure. Synapse. 2009;63:403–412. doi: 10.1002/syn.20617. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. High-affinity binding of [125]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12:27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Cheng R-K, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007;1186:255–266. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behavioural Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- Cousins M, Atherton A, Turner L, Salamone J. Nucleus accumbens dopamine depletions after relative response allocation in a t-maze cost/benefit task. Behavioural Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MFS, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O’Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision-making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19:2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR. The effects of methamphetamine on serotonin transporter activity. J Neurochem. 2000;75:1608–1617. doi: 10.1046/j.1471-4159.2000.0751608.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wichman DJ, Sherwin A, Tong J. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Francis JM, Blaney TJ, Salamone JD, Emerich DF. Rats with partial striatal dopamine depletions exhibit robust and long-lasting behavioral deficits in a simple fixed-ratio bar-pressing task. Behavioural Brain Res. 1997;86:25–40. doi: 10.1016/s0166-4328(96)02240-1. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102:61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11c]win-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behavioural Brain Res. 2010;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. United States: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Crane AM, Seiden LS, Kleven MS, Porrino LJ. Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology. 1990;102:175–182. doi: 10.1007/BF02245919. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, De Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JFW, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;15:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:1–12. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possinc K, Leamon M, Gibsond DR, Galloway GP, Flynnd NM, Henikf A, Pfefferbaum A, Sullivang EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Schröder N, O’Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See R, Pacchioni A, Mcginty J, Kalivas P. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102 Suppl:44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Walsh SL. Evaluation of the effects of inhibition of monoamine oxidase and senescence on methamphetamine-induced neuronal damage. Int J Dev Neurosci. 1991;9:171–174. doi: 10.1016/0736-5748(91)90008-a. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MFS. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, Minamoto T, Odell JR, Mayhorn J, White W. Brief exposure to methamphetamine (meth) and phencyclidine (pcp) during late development leads to long-term learning deficits in rats. Brain Res. 2009;1266:72–86. doi: 10.1016/j.brainres.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinksy KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N YAcad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]